Abstract
Postnatal pancreas is a potential source for progenitor cells to generate endocrine β-cells for treating type 1 diabetes. However, it remains unclear whether young (1-week-old) pancreas harbors multipotent progenitors capable of differentiating into duct, acinar, and endocrine cells. Laminin is an extracellular matrix (ECM) protein important for β-cells' survival and function. We established an artificial extracellular matrix (aECM) protein that contains the functional IKVAV (Ile-Lys-Val-Ala-Val) sequence derived from laminin (designated aECM-lam). Whether IKVAV is necessary for endocrine differentiation in vitro is unknown. To answer these questions, we cultured single cells from 1-week-old pancreas in semi-solid media supplemented with aECM-lam, aECM-scr (which contains a scrambled sequence instead of IKVAV), or Matrigel. We found that colonies were generated in all materials. Individual colonies were examined by microfluidic reverse transcription-polymerase chain reaction, immunostaining, and electron microscopy analyses. The majority of the colonies expressed markers for endocrine, acinar, and ductal lineages, demonstrating tri-lineage potential of individual colony-forming progenitors. Colonies grown in aECM-lam expressed higher levels of endocrine markers Insulin1, Insulin2, and Glucagon compared with those grown in aECM-scr and Matrigel, indicating that the IKVAV sequence enhances endocrine differentiation. In contrast, Matrigel was inhibitory for endocrine gene expression. Colonies grown in aECM-lam displayed the hallmarks of functional β-cells: mature insulin granules and glucose-stimulated insulin secretion. Colony-forming progenitors were enriched in the CD133high fraction and among 230 micro-manipulated single CD133high cells, four gave rise to colonies that expressed tri-lineage markers. We conclude that young postnatal pancreas contains multipotent progenitor cells and that aECM-lam promotes differentiation of β-like cells in vitro.
Introduction
Type 1 diabetes (T1D) is a chronic disease caused by autoimmune destruction of insulin-secreting β-cells. β-cells and other endocrine cells, such as the glucagon-secreting α-cells, are located in the pancreas in discrete clusters, termed islets of Langerhans, with diameters of 116±80 μm [1]. β-cells function by sensing elevated glucose concentrations in the blood, such as after meals, and in response secrete appropriate amount of insulin. The absence of β-cells causes hyperglycemia, which in turn leads to long-term complications in T1D patients. End-stage T1D patients can be effectively managed by allogeneic islet cell transplantation [2]; however, the lack of cadaveric organs limits the number of patients who may benefit from this promising treatment. Therefore, there is a critical need to generate therapeutic β-like cells from alternative sources such as stem or progenitor cells.
Pancreas is composed of endocrine, acinar, and duct cell lineages that differentiate from progenitor cells in the developing embryo [3]. Early progenitor cells that arise around embryonic day (E) 8.5 in the foregut region are committed to a pancreas fate by upregulation of the transcription factor pancreatic and duodenal homeobox 1 (Pdx1) [4,5]. Before E12.5, pancreatic progenitor cells are located in the ductal epithelium and are multipotent [6]. As the differentiation program continues, progenitor cells become restricted in lineage potential and committed to endocrine lineage by upregulating the transcription factor neurogenin 3 (Ngn3) [4,7,8]. From E13.5 onward Ngn3+ endocrine progenitors delaminate from the ducts and migrate to form endocrine cells [9,10]. By late gestation (around E18.5), the endocrine cells are loosely arranged as small clusters; at this stage β-cells cannot sense glucose and secrete insulin [11,12].
Immediately after birth, β-cells undergo extensive proliferation and functional maturation [13,14]. Progenitor cells may linger in the postnatal pancreas, as suggested by lineage-tracing experiments that showed that a portion of duct cells labeled with sex-determining region box 9 (Sox9) [15] or carbonic anhydrase II could contribute to new endocrine cells [16]. However, whether dedicated progenitor cells exist in the pancreas after birth remains controversial. In vivo lineage-tracing studies using ductal markers Sox9, pancreas-specific transcription factor 1a (Ptf1a), or hepatocyte nuclear factor 1 β (Hnf1β) showed that tripotent progenitors lose their tri-lineage differentiation capacities before or soon after birth [15,17,18].
On the other hand, tri-lineage potential was demonstrated for adult centroacinar cells (enriched by high aldehyde dehydrogenase 1 enzymatic activity) [19] and adult ductal cells (enriched by CD133 and Sox9 co-expression) [20]. These cells can be isolated, expanded, and differentiated in vitro into all three pancreatic lineages, which include glucose-responsive β-like cells [19,20]. The results from these studies and others rationalized the use of in vitro assays not only for the generation of insulin-producing cells for cell replacement therapy, but as a means to identify and characterize pancreatic progenitors particularly from the understudied adult and postnatal stage.
Earlier, we and others have devised 3D colony assays (also known as organoid culture) to study differentiation of progenitor-like cells from pancreas of adult (2–4 months old) mice [20,21] and humans [22], and those from murine fetal pancreas [23]. We have designated a progenitor cell that is capable of forming a colony in vitro a “pancreatic colony-forming unit (PCFU)”. Our colony assays provide quantitative means to characterize self-renewal and differentiation of PCFUs [20]. In a recent publication, we demonstrated that postnatal (1-week-old) liver and pancreas contained CFU-Dark, a class of rare progenitors that give rise to morphologically distinct insulin-expressing colonies [24]. In addition to Dark colonies, other types of postnatal colonies with cystic or compact structures from pancreas were observed but had not been characterized [24]. In this study, we sought to determine the lineage potentials of these young postnatal progenitors.
Extracellular matrix (ECM) proteins are adhesive proteins that provide sites of cell attachment and signaling cues that promote survival and function of various cells, including β-cells [25,26]. A variety of ECM proteins are present in the pancreas such as laminins, collagen type IV, fibronectin, heparin sulfate proteoglycans, and nidogen/entactin [27]. Among these, laminins were found to induce Insulin gene expression and increase β-cell proliferation in islets from VEGF-A tissue-specific knockout mice that do not have normal amounts of laminin surrounding the β-cells [26]. Laminins also enhance survival and function (glucose-stimulated insulin secretion) of isolated islets [28–30]. Laminins are complex proteins composed of three subunits (α, β and γ) with sizes ranging from 400 to 800 kDa (depending on subunit composition) [31,32].
IKVAV (Ile-Lys-Val-Ala-Val) is a peptide sequence derived from the laminin α1 subunit that mediates cell-matrix binding [33]. Encapsulation of MIN6 β-cells in a polyethylene glycol hydrogel containing the IKVAV sequence was found to enhance viability and insulin secretion [34]. We previously incorporated the IKVAV sequence into an elastin-based artificial extracellular matrix (aECM) protein [35–37]. Elastin was used for several practical reasons: (1) as an ECM protein, it mimics the rigidity of soft tissue, (2) physical properties of the hybrid aECM protein such as solubility and pH responsiveness can be controlled by varying the elastin sequence [38,39] without perturbation of the IKVAV sequence, and (3) because elastins are thermally responsive, the hybrid aECM protein can be purified by repeated thermal cycling. This method, called “inverse temperature cycling,” is a low cost, technically simple approach for purifying large quantities of recombinant proteins made in bacterial expression systems [38]. We have previously demonstrated positive effects of an aECM protein containing the IKVAV sequence on the differentiation of adult murine PCFUs into β-like cells in culture [20]. However, whether the IKVAV sequence in our aECM is required for β-cell differentiation from PCFUs has not been determined.
In this report, we tested whether postnatal (1-week-old) pancreas contains PCFUs with tri-lineage differentiation capacity in vitro, and whether the IKVAV sequence is necessary for the differentiation of postnatal PCFUs into β-like cells. We also compared the effects of aECM to Matrigel, a commercial source of a mixture of ECM proteins derived from a murine sarcoma cell line.
We found that (1) young postnatal murine pancreas contains PCFUs that give rise to colonies containing acinar, duct, and endocrine-like cells, (2) aECM containing the IKVAV sequence enhances differentiation of postnatal PCFUs into functional β-like cells and, surprisingly, (3) Matrigel is inhibitory to insulin expression in colonies generated in the presence of aECM containing the IKVAV sequence in vitro. Our results demonstrate that postnatal pancreas contains multipotent progenitor-like cells, and these progenitor-like cells may be useful for future cell replacement therapy of T1D.
Materials and Methods
Mice
Postnatal (P) 7- or 8-day-old C57BL/6J inbred (The Jackson Laboratory) or CD-1 outbred mice (Charles River Laboratory) were used in this study. All mice were maintained under specific pathogen-free conditions, and animal experiments were conducted according to the Institutional Animal Care and Use Committee at the City of Hope.
Preparation of single cell suspensions
Murine pancreata were dissected, cleared of fat tissue under a dissecting microscope, and rinsed thrice in cold DPBS containing 0.1% bovine serum albumin, penicillin, and streptomycin (PBS/BSA). Whole pancreata, in a dry Petri dish placed on ice, were minced using spring scissors for ∼2 min or until finely minced. The triturated tissue was transferred to a 15 mL conical tube, washed once, resuspended in PBS/BSA containing collagenase B (2–4 mg/mL) (Roche) and DNase I (2,000 U/mL) (Calbiochem), and incubated at 37°C for 20 min to yield a mostly single cell suspension. To hasten the digestion, tissue was gently disrupted every 5–10 min using a 16½ G syringe needle. Cells were then washed twice in cold PBS/BSA supplemented with 2,000 U/mL DNase I, which was used to prevent reaggregation of dissociated cells, and filtered through a 60 μm nylon mesh (BD Biosciences).
Expression and purification of aECM protein
DNA fragments encoding an elastin-laminin hybrid sequence (aECM-lam) or elastin-scrambled sequence (aECM-scr) were cloned into a pET28a plasmid. To express the proteins, chemically competent E. coli strain BL21 DE3 (Qiagen, Valencia, CA) was transformed with the required recombinant pET28a plasmid (pET28a/aECM-lam or pET28a/aECM-scr) and pLysS (to prevent leaky expression) and grown in 10 L of TB (terrific broth) medium in a 10-L Bioflo 3000 reactor (New Brunswick Scientific) in the presence of 50 μg/L kanamycin and 35 μg/L chloramphenicol. The culture was grown to an OD600 of 3 before induction with 2.5 mM isopropyl β-D-1-thiogalactopyranoside, then grown to an OD of 10, with oxygen and pH control as previously described [40,41]. Bacterial cells were pelleted by centrifugation (10,000 g, 15 min, 4°C) and lysed in TEN buffer (10 mM Tris-HCl, 1 mM EDTA, and 0.1 M NaCl, pH 8) supplemented with 50 μg/mL phenylmethylsulfonyl fluoride (Sigma Aldrich) and 10 μg/mL each of ribonuclease A and deoxyribonuclease 1 (Sigma Aldrich). Cells were lysed by a freeze-thaw cycle followed by sonication at 4°C. Lysates were centrifuged (35,000 g, 2 h, 4°C), and the soluble fraction was adjusted to pH 9 in 1 M NaCl. Each thermal cycle consisted of a temperature shift to 37°C, followed by centrifugation (35,000 g, 2 h), resolubilization of the pellet in pH 9 water at 4°C, and centrifugation (35,000 g, 2 h). After three thermal cycles, aECM proteins were dialyzed in water at 4°C and the product lyophilized. A solution of 0.25 mg/mL of protein was subjected to electrophoresis on a Novex 12% Bis-Tris polyacrylamide gel, labeled with colloidal blue (both Invitrogen), and imaged on a Typhoon 9400 molecular imager (GE Healthcare).
MALDI-TOF mass spectrometry
Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) was performed on a Voyager DE-Pro MALDI TOF-MS (Applied Biosystems). Protein solutions at 30 mg/mL were added to matrix solution consisting of 10 mg/mL sinapinic acid in 0.07% trifluoroacetic acid and 30% acetonitrile. A matrix to protein ratio of 20:1 was used.
Lower critical solution temperature measurements
Lower critical solution temperature (LCST) measurements were performed at a protein concentration of 10 mg/mL in PBS (pH 7.4). Absorbance at 300 nm was measured on a DU7400 diode array UV-visible spectrophotomer (Beckman Coulter).
Colony assays
Dissociated cells were plated in 24-well ultra-low protein-binding plates (Corning Incorporated Life Sciences) at a density of 2.5×103 cells/well/0.5 mL for Matrigel-supplemented cultures or 10×103 cells/well/0.5 mL for aECM-supplemented cultures. For in vitro glucose challenge assays, 25×103 cells/well were plated. Growth factor reduced Matrigel (Corning) was used. Culture media contained DMEM/F12, 1% methylcellulose (Sinetsu Chemical), 35% conditioned media from mouse embryonic stem cell-derived pancreatic-like cells, 5% fetal calf serum (FCS), 10 mM nicotinamide (Sigma), 10 ng/mL human recombinant activin B, and 0.1 nM exendin-4 (Sigma). When indicated, either 5% (vol/vol) Matrigel or 100 μg/mL of aECM-lam or aECM-scr was added to medium. Cells were incubated in a humidified 5% CO2 atmosphere and the resulting colonies were analyzed 7–8 days post-plating.
Cell sorting and single cell manipulation
Cell suspension from dissociated postnatal pancreas was first incubated with anti-mouse CD16/32 (10 μg/mL; BioLegend) for 5 min on ice to diminish nonspecific binding. Biotin-conjugated anti-mouse CD133 (clone 13A4, 1 μg/mL; eBioscience) or the control biotin-conjugated rat immunoglobin (Ig)G1 isotype (1 μg/mL; eBioscience) antibodies were added, and the cells incubated on ice for 20 min. After washing twice, cells were treated with streptavidin-labeled allophycocyanin (2 μg/mL; BioLegend) on ice for 15 min. Cells were washed twice, resuspended in PBS/BSA/DNase I containing DAPI (0.2 μg/mL), and filtered through 40 μm mesh before sorting. Cell sorting was performed on an Aria-special order research product (Becton Dickinson). All analyses included an initial gating of forward and side scatters to exclude cell debris. Sorting further excluded doublets by gating on forward scatter width and side scatter width, and live cells were selected by DAPI negative staining (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). For single cell manipulation, freshly sorted cells were mixed in 1% methylcellulose and 15% FCS at a density of 3,000 cells/mL and placed in a 35-mm Petri dish. Individual cells were visualized under a microscope and lifted one by one using a fine Pasteur pipet with a diameter of ∼30 μm at the opening. Each cell was plated into one well in a low-binding 96-well plate containing 100 μL of semi-solid media supplemented with 5% Matrigel. Presence of a single cell per well was confirmed by visualization under a microscope. For conventional colony assays, sorted cells were plated at a density of 10×103 cells/well/0.5mL of Matrigel-supplemented media.
Conventional or microfluidic quantitative reverse transcription–polymerase chain reaction
Total RNA extraction, reverse transcription and conventional quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analyses were performed as previously described [20]. Duplicate samples were used in all analyses. Microfluidic qRT-PCR was performed using the BioMark™ Dynamic Array real-time PCR system (Fluidigm). Single colonies were lifted one by one from the methylcellulose-containing medium under direct microscopic visualization by using a 10 μL Eppendorf pipette, collected in 10 μL reaction buffer, and subjected to a preamplification step (14 cycles) according to the manufacturer's instructions (Fluidigm). Amplified cDNA was loaded onto a 48×48 microwell plate using the NanoFlex integrated fluidic circuit controller (Fluidigm). Threshold cycle (Ct), defined as the cycle where the fluorescence intensity is above the common threshold, was determined by the BioMark PCR analysis software (Fluidigm) and expressed as a heat map or delta Ct compared to β-actin. All experiments were performed with negative (water) and positive controls (P7 or P8 pancreatic cells from C57BL/6J mice). TaqMan probes (Life Technologies) and their catalog numbers are listed in Supplementary Table S1.
Electron microscopy
Single colonies were collected, pooled, and fixed in 2% glutaraldehyde in 0.1 M Cacodylate buffer [Na(CH3)2AsO2•3H2O; pH 7.2] at 4°C overnight. Colonies were washed thrice with 0.1 M Cacodylate buffer (pH 7.2), post-fixed with 1% OsO4 in 0.1 M Cacodylate buffer for 30 min, and washed thrice with 0.1 M Cacodylate buffer. The samples were then dehydrated, embedded in EMbed 812 (Electron Microscopy Sciences), and polymerized at 64°C for 48 h. Ultrathin sections (70-nm thickness) were cut using a Leica ultramicrotome with a diamond knife, transferred to 200-mesh EM grids, and stained with 2% uranyl acetate in 70% ethanol for 1 min followed by Reynold's lead citrate for 1 min. Electron microscopy was carried out on an FEI Tecnai 12 transmission electron microscope equipped with a Gatan Ultrascan 2K CCD camera operated at 120 keV.
Whole-mount immunostaining
Colonies were manually picked, pooled, fixed in 4% paraformaldehyde at 4°C overnight, and washed with PBS before immunostaining. Colonies were first incubated in blocking buffer (PBS supplemented with 5% donkey, 5% goat serum, and 0.1% Triton X-100), then stained with primary antibodies diluted in blocking buffer. Both incubations were done overnight, at 4°C, with gentle shaking. Primary antibodies were detected with goat- or donkey-raised secondary antibodies conjugated with DyLight488, DyLight649, or Cy3 (Jackson Immunoresearch). Images were captured on a Zeiss LSM510 Meta 2-photon Inverted Microscope. Figures were prepared with LSM Image Browser and Axiovision LE softwares (Carl Zeiss MicroImaging GmbH). Antibodies used are listed in Supplementary Table S2.
In vitro glucose challenge assay
Colonies were handpicked (n=50 from Matrigel, n=150 from aECM-scr or aECM-lam), pooled, and incubated overnight with Krebs-Ringer Buffer Solution (KRBS; 129 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5 mM NaHCO3, 10 mM HEPES, and 0.1% BSA) containing 10% FCS and 2.5 mM d-glucose. The next day, colonies were washed thrice with KRBS containing 2% FCS and 2.5 mM d-glucose, and then sequentially incubated with 2.5 mM and 16.7 mM d-glucose (37°C, 0.1 mL/well, 2 h). Concentrations of C-peptide were measured using the murine C-peptide ELISA kit, U-Type (Shibayaji Co.), which has a detection limit of 30 pg/mL. The extent of stimulation was expressed as the fold-change of C-peptide concentrations in the high glucose buffer compared to the low glucose buffer of the same well.
Statistical analysis
All values are shown as mean±standard deviation (SD). Significance was determined by Student's two-tailed t-test. P<0.05 was considered significant.
Results
Design and characterization of aECM proteins
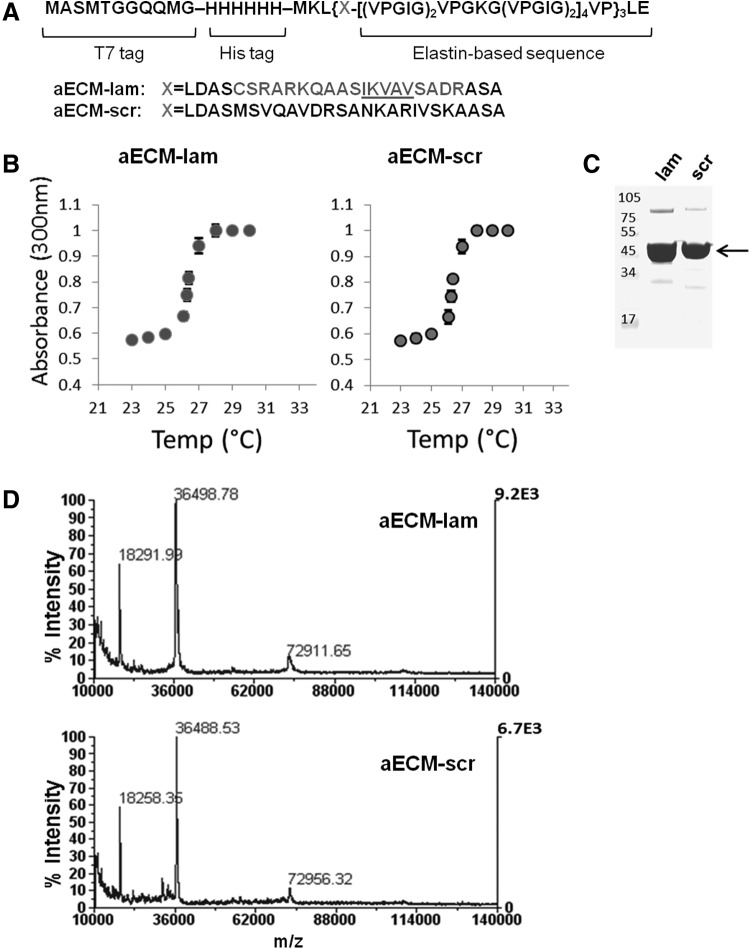
aECM-lam is prepared by bacterial expression of an artificial gene that encodes an elastin-laminin hybrid sequence (Fig. 1A). Previous work demonstrated that viability and function of β-cells in vitro were improved when cells were encapsulated in a hydrogel bearing the IKVAV sequence derived from the α1 chain of laminin [34]. However, whether the IKVAV sequence is necessary for endocrine differentiation and/or survival of PCFUs in our colony assay is unknown. To test this, we generated a second artificial gene, which encodes a scrambled IKVAV sequence within the same elastin backbone; the resulting protein was designated aECM-scr (Fig. 1A). Solutions of elastin-like proteins exhibit characteristic LCST, above which phase separation occurs [38,42]. Consistent with this behavior, both aECM proteins formed coacervate phases at temperatures above 27°C, as indicated by abrupt increases in solution turbidity (Fig. 1B). Cycling through the LCST provided both aECM proteins in good purity as shown by Colloidal blue staining of SDS-PAGE gels (Fig. 1C, arrow). MALDI-TOF analysis confirmed the molar mass of the aECM proteins to be 36.5 g/mol, as expected (Fig. 1D).
FIG. 1.
Purification and characterization of aECM proteins. (A) aECM proteins were cloned into pET28a and expressed under the control of a T7 promoter in BL21 (DE3) E. coli. The laminin-elastin and scrambled-elastin hybrid sequences are shown. (B) Turbidity of aECM solutions as a function of temperature was measured at 300 nm to determine the LCST of aECM-lam and aECM-scr. (C) Purified aECM proteins were separated by SDS-PAGE and stained by Colloidal blue. Arrow indicates that the major bands migrated with the expected mobility of the aECM proteins. Note also the presence of dimer at around 72 kDa. (D) Both samples were analyzed with MALDI-TOF, showing major peaks at ∼36.5 kDa, 18.3 kDa, and 73 kDa. The peak at 18.2 kDa is likely due to double-charging of the protein; that at 72.9 kDa is due to dimerization. aECM, artificial extracellular matrix; LCST, lower critical solution temperature.
Dissociated single cells from postnatal pancreas form colonies in vitro
Colony assays developed in our laboratory and others have been employed as an ex vivo tool to analyze progenitor cell activity. Cells are grown in a three-dimensional (3D) culture with factors that support progenitor survival and differentiation. Inclusion of ECM proteins such as Matrigel allows the colonies or organoids to form complex structures [43], which is not possible in a two-dimensional (2D) culture system. Our colony assay has another advantage in that it interrogates the capacity of a single cell to form a colony and differentiate into multiple lineages. This is achieved by incorporating methylcellulose, a biologically inert material that increases viscosity, thereby immobilizing dissociated cells and preventing aggregation. A single cell capable of giving rise to a colony is termed a PCFU.
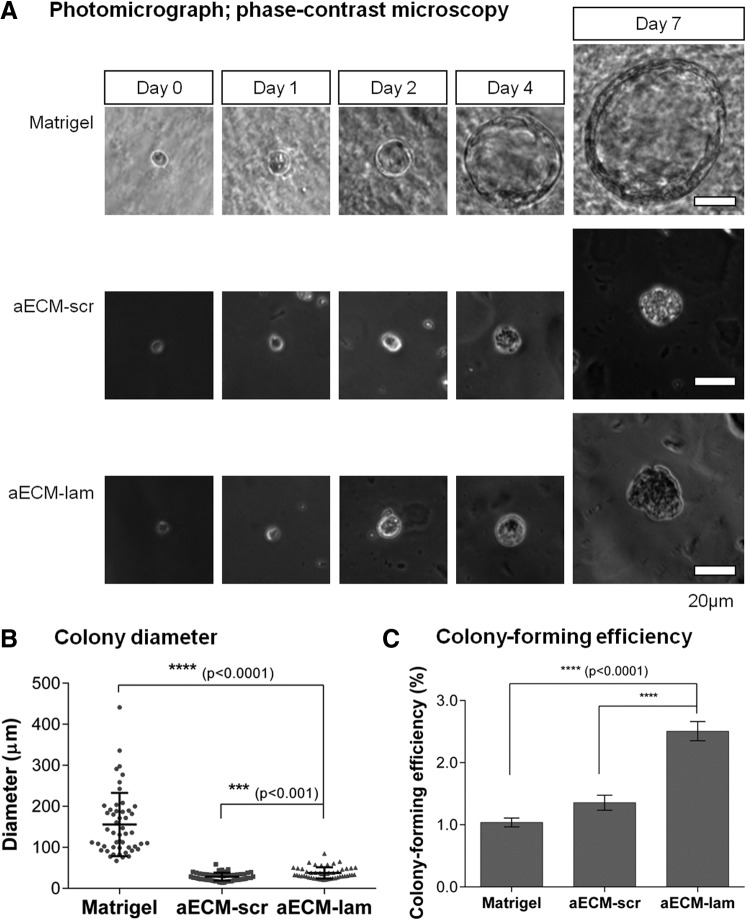
Pancreata from 7-day-old mice were dissociated into single cell suspension by treating with collagenase and plated into our 3D colony assays containing Matrigel, aECM-scr, or aECM-lam. Single cells did not reaggregate and remained solitary 1 h post-plating in all three conditions (Fig. 2A; day 0). In the culture containing Matrigel, small cysts with a central lumen were detected by day 1, and these structures continued to grow in size over time. In contrast, in the presence of aECM-lam or aECM-scr individual cells remained single by day 1, and only small cell clusters were observed at day 7 post-plating (Fig. 2A). Analysis of colony size, as indicated by the diameter of the colonies, confirmed that colonies grown in Matrigel were larger than those grown in aECM-lam or aECM-scr at day 7 (Fig. 2B). Thus, Matrigel and aECM induced the formation of colonies with different morphologies as shown by phase-contrast microscopy.
FIG. 2.
Murine postnatal pancreas contains colony-forming progenitor cells. Pancreata (n=2 or 3) procured from 7- or 8-day-old mice were dissociated into single cell suspensions and plated into semisolid media containing either Matrigel (5%), aECM-scr (100 μg/mL), or aECM-lam (100 μg/mL). (A) Photomicrographs of representative colonies over the course of 7 days after plating. (B) Diameters of 7-day-old colonies (n=50). Individual point represents diameter of each colony. Horizontal lines represent mean±SD. (C) Colonies were counted 7 days post-plating. Colony-forming efficiency was determined by dividing the number of resulting colonies by the number of cells plated. Data are expressed as mean±SD from triplicate wells. SD, standard deviation.
Next we examined colony-forming efficiency, defined as the number of colonies divided by the number of plated cells. Approximately 1% of total postnatal cells were PCFUs when grown in the presence of Matrigel, a frequency that is similar to that observed in the adult pancreas [20]. aECM-lam supported the formation of a higher number of colonies from postnatal cells, compared with Matrigel and aECM-scr (Fig. 2C). These results suggest that aECM-lam supports the survival of postnatal PCFUs better than Matrigel or aECM-scr. Alternatively, aECM-lam, aECM-scr, and Matrigel may each support a different population of PCFUs from postnatal pancreas, with that supported by aECM-lam being the largest.
aECMs preferentially support the formation of endocrine and acinar lineages while Matrigel supports ductal cells
To determine what lineage cells were developed in culture, 7-day-old colonies were pooled and examined by qRT-PCR analysis (Fig. 3). Colonies supported by aECM-scr or aECM-lam expressed β- (Insulin1 and Insulin2), α- (Glucagon) and acinar (Amylase2A) cell markers, whereas expression of these endocrine and acinar genes is lower in colonies grown in Matrigel. In contrast, the ductal marker Cytokeratin7 was expressed more in colonies grown in Matrigel compared with aECM-scr or aECM-lam. Taken together, these results demonstrate that both aECM proteins preferentially support the formation of endocrine and acinar lineage cells, while Matrigel enhances ductal lineage development in vitro.
FIG. 3.
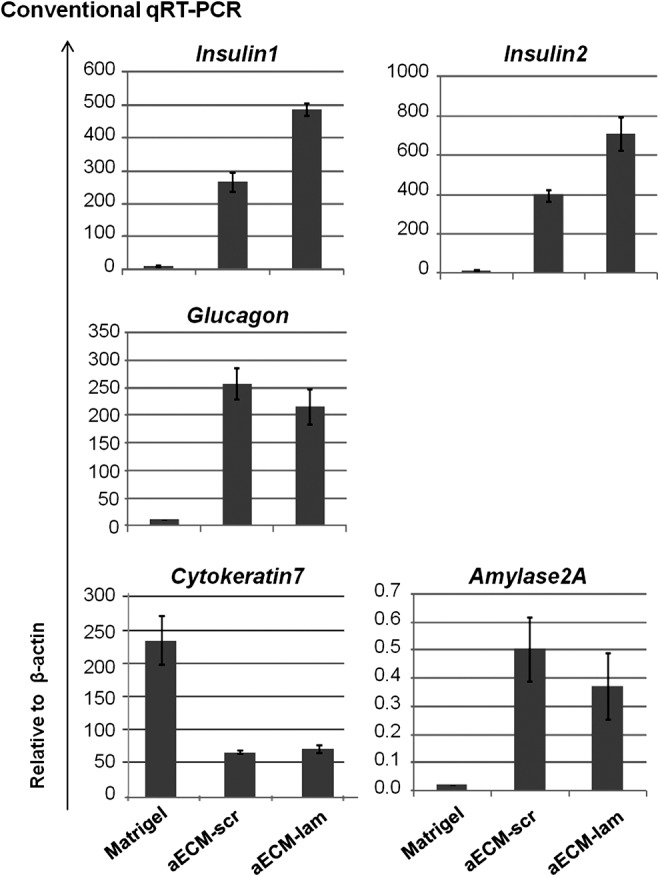
Postnatal cells grown in aECM-lam and aECM-scr express higher levels of endocrine and acinar markers, whereas those in Matrigel express higher ductal markers. Dissociated 7-day-old pancreatic cells were plated. Total cells, including colonies, from cultures containing designated materials were collected on day-7 post-plating. Gene expression was analyzed by conventional qRT-PCR. qRT-PCR, quantitative reverse transcription–polymerase chain reaction.
Tri-lineage potential of postnatal PCFUs
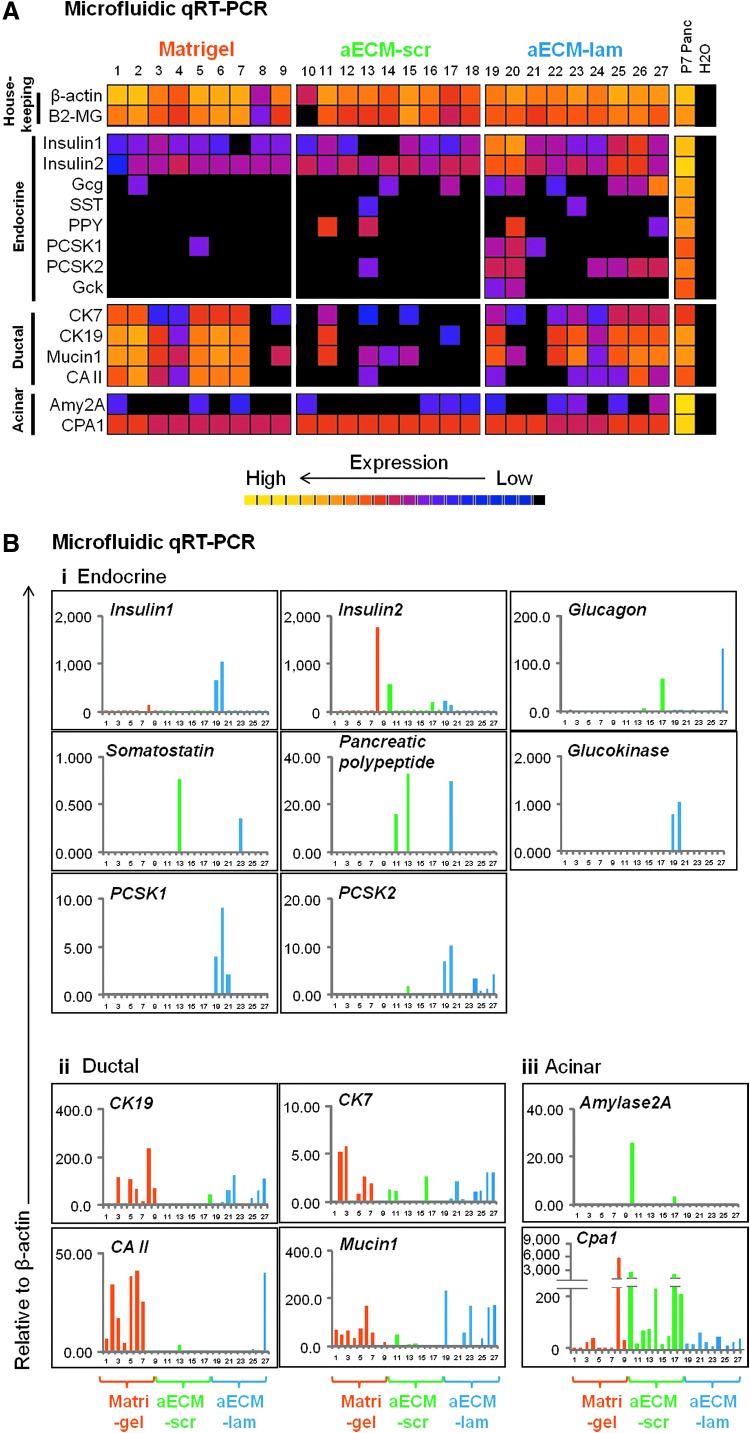
To further discern the lineage composition of individual colonies and thereby determine the lineage potential of the originating PCFUs, we performed single colony microfluidic RT-PCR analysis. Microfluidic qRT-PCR uses an automated system to combine samples and reagents at nano-liter volumes, thus allowing the examination of gene expression at the single colony level. A total of nine colonies from each culture condition were individually handpicked under direct microscopic visualization and examined for expression of a panel of housekeeping, endocrine, ductal, and acinar marker genes. Consistent with the observation that Matrigel-supported colonies were larger (Fig. 2A, B), the levels of housekeeping genes were higher (Fig. 4A; warmer color) in Matrigel-grown colonies than those grown in either aECM-scr or aECM-lam. Of the 27 colonies analyzed, 21 expressed tri-lineage markers while the remaining 6 (colonies 8, 10, 12, 16, 18, and 21) expressed endocrine and acinar, but not ductal markers (Fig. 4A). Taken together, these results suggest that the majority of postnatal PCFUs supported by Matrigel, aECM-scr, or aECM-lam were tripotent. The tri-lineage potential of individual postnatal PCFUs was confirmed by single-cell micromanipulation, as described in a later section.
FIG. 4.
Individual postnatal colonies express multiple lineage markers by microfluidic RT-PCR analyses. Dissociated 7-day-old pancreatic cells were plated. Seven-day-old colonies grown in the presence of designated materials were individually handpicked (n=9 per group) and gene expression analyzed by microfluidic qRT-PCR. Results are expressed as a heatmap (A) or expression relative to the housekeeping gene β-actin (B). Each column or bar indicates an individual colony.
To compare the levels of expression of endocrine, acinar, and ductal genes in individual colonies, we analyzed the data presented in Fig. 4A relative to expression of β-actin (Fig. 4B). Many individual colonies grown in aECM-scr or aECM-lam expressed higher levels of endocrine markers (Insulin, Glucagon, Somatostatin, and Pancreatic polypeptide) than those supported by Matrigel. Insulin2 expression was surprisingly higher in some of the colonies grown in aECM-scr and Matrigel. However, only colonies from aECM-lam expressed Insulin genes in coordination with genes that are essential for β-cell function such as prohormone convertase 1 and 2 (Pcsk1 and Pcsk2), and glucokinase (Gck) (Fig. 4A). In contrast, only one colony grown in Matrigel and aECM-scr expressed Pcsk1 or Pcsk2 (colonies 5 and 13, respectively), and none expressed Gck (Fig. 4A). This suggests that colonies in aECM-lam contain insulin-expressing cells that may be capable of responding to glucose stimuli. In a later section, we will demonstrate that the aECM-lam supported colonies are functional in glucose-stimulated insulin secretion in vitro. Furthermore, consistent with the results of the conventional qRT-PCR analysis, the majority of colonies in Matrigel expressed ductal genes at higher levels (Fig. 4B). Acinar markers Amylase2A (Amy2A) and Carboxypeptidase A1 (Cpa1) were expressed at low levels in all colonies, with the highest expression found in colonies grown in aECM-scr (Fig. 4B). Taken together, these results demonstrate that Matrigel promoted ductal marker expression in postnatal colonies, while aECM-lam enhanced differentiation of PCFUs toward a mature β-cell phenotype.
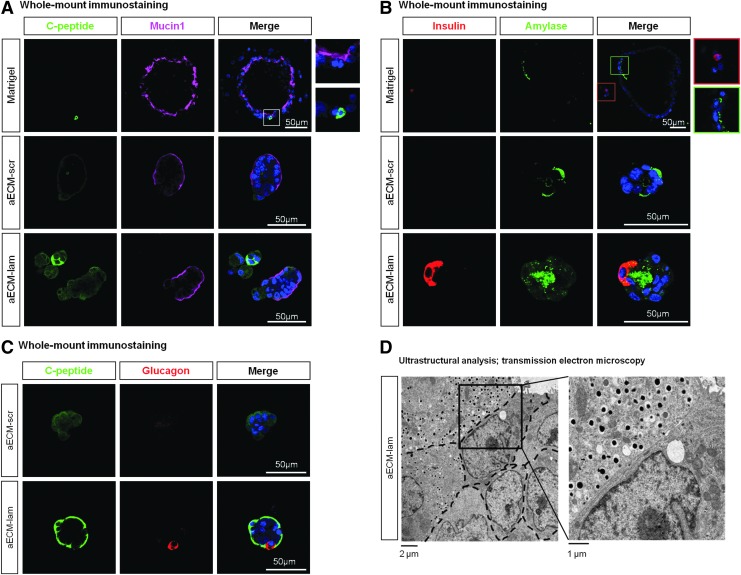
Whole-mount immunostaining confirmed protein expression of ductal (Mucin1), endocrine (C-peptide, Insulin, and Glucagon), and acinar (Amylase) markers in colonies grown in all materials (Fig. 5A–C). Notably, double immunostaining of C-peptide and Glucagon demonstrated that these markers were not co-expressed in the same cells of the colonies grown in aECM-lam and aECM-scr (Fig. 5C and Supplementary Video S1A, B). Transmission electron microscopy further demonstrated the presence of insulin granules in a colony grown in aECM-lam (Fig. 5D). Taken together, these results demonstrate that aECM-lam is able to induce differentiation of insulin-expressing cells from postnatal PCFUs in vitro.
FIG. 5.
Whole-mount immunostaining and electron microscopy analyses of individual colonies. Dissociated 7-day-old pancreatic cells were plated in the presence of Matrigel, aECM-scr, or aECM-lam. After 7 days, colonies were collected, fixed, and co-stained with antibodies against markers for (A) ductal (Mucin1) and β-cells (C-peptide), (B) acinar (Amylase) and β-cells (Insulin), and (C) α- (Glucagon) and β-cells (C-peptide). Nuclei were stained with DAPI (blue). The white box in (A) was enlarged to show a C-peptide+Mucin1− cell and several C-peptide−Mucin1+ cells within a cystic colony supported by Matrigel. The green and red boxes in (B) were enlarged to show Insulin−Amylase+ cells and an Insulin+Amylase− cell in the cystic colonies supported by Matrigel. (C) C-peptide+Glucagon− and C-peptide−Glucagon+ cells were detected in colonies supported by aECM-scr or aECM-lam. (D) Ultrastructural analysis by transmission electronmicroscopy of a colony supported by aECM-lam. Dashed lines represent cell membranes. Highlighted area is enlarged on the right, showing dense core, insulin-like granules in one cell and multilobed nucleus in a neighboring cell, indicating a ductal phenotype.
Matrigel enhances differentiation of ductal lineage at the expense of endocrine cells
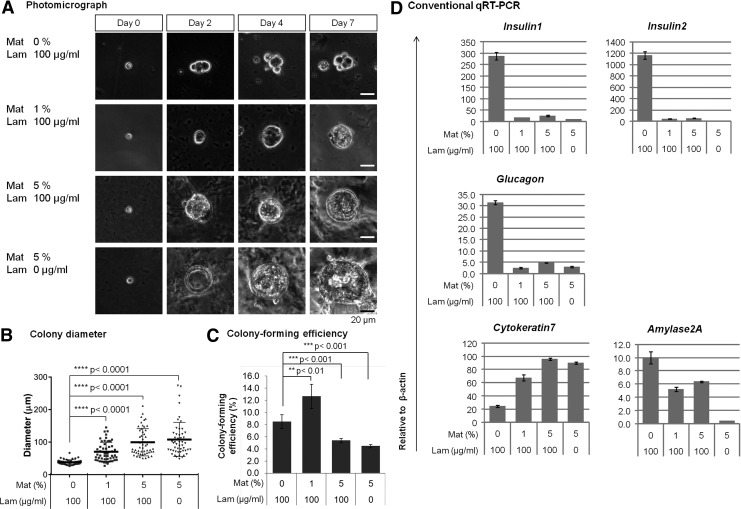
The opposing effects of Matrigel versus aECM-lam in supporting ductal versus endocrine lineage cell differentiation from PCFUs (Figs. 3 and 4B) prompted us to examine whether Matrigel is inhibitory to β-cell differentiation. To test this, postnatal cells were cultured in the presence of aECM-lam (100 μg/mL) with graded doses (0%, 1%, and 5% vol/vol) of Matrigel added to the cultures. A control culture received only 5% Matrigel, which was the dose used in the previous experiments. We found that Matrigel caused a dose-dependent increase of the size of colonies supported by aECM-lam (Fig. 6A, B). Colony numbers were significantly reduced in cultures with higher (5%) Matrigel concentration, but low Matrigel concentration (1%) promoted colony formation (Fig. 6C). Importantly, qRT-PCR analysis of the pooled colonies showed that as little as 1% Matrigel significantly decreased the levels expression of Insulin1, Insulin2, Glucagon, and Amylase2A in cultures that contained aECM-lam (Fig. 6D). In contrast, Matrigel increased ductal marker expression in a dose-dependent manner (Fig. 6D). Taken together, these results show that Matrigel enhances differentiation of ductal lineage at the expense of endocrine cells.
FIG. 6.
Matrigel inhibits formation of endocrine and acinar cells in colonies supported by aECM-lam. Dissociated 7-day-old pancreatic cells were plated in the presence of designated doses of Matrigel and aECM-lam. (A) Photomicrographs of representative colonies over the course of 7 days. (B) Diameters of 7-day-old colonies (n=50) were increased by addition of Matrigel into aECM-lam containing culture. Individual points represent the diameter of each colony. Horizontal lines represent mean±SD. (C) Colony-forming efficiency in each condition was determined by dividing the number of colonies by the number of cells plated. Data represent the mean percentage±SD from triplicate wells. Addition of Matrigel at 1% enhanced, and at 5% inhibited, colony formation of postnatal progenitor cells when cultured in the presence of aECM-lam. (D) Conventional qRT-PCR analysis on total cells collected from 7-day-old cultures. Addition of Matrigel decreased expression of markers for endocrine and acinar cells, but increased ductal marker expression in colonies supported by aECM-lam. Data represent expression relative to β-actin. Mat, Matrigel; Lam, aECM-lam.
Colonies supported by aECM-lam secrete insulin in response to glucose stimulation in vitro
To determine whether the insulin-expressing cells grown in the presence of aECM-lam were functional, an in vitro glucose challenge assay was performed. Pooled colonies grown from aECM-lam were treated with a low concentration (2.5 mM) and then a high concentration (16.7 mM) of d-glucose, and the amount of C-peptide released at the end of each incubation was measured by ELISA. Colonies grown from aECM-lam, but not those grown from Matrigel or aECM-scr, secreted higher amounts of C-peptide in response to 16.7 mM d-glucose (2.01-fold from aECM-lam vs. 0.42-fold and 0.25-fold from Matrigel and aECM-scr, respectively) (Fig. 7). These results suggest that the insulin-expressing colonies supported by aECM-lam, but not Matrigel or aECM-scr were functional in glucose-stimulated insulin-secretion in vitro.
FIG. 7.
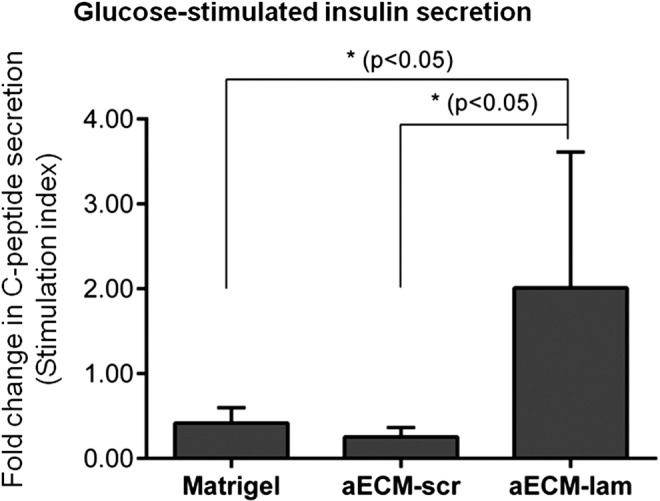
Colonies grown in aECM-lam, but not Matrigel or aECM-scr, are glucose-responsive in vitro. Eight-day-old colonies supported by either Matrigel, aECM-scr, or aECM-lam were handpicked, pooled, washed, and sequentially treated with 2.5 mM glucose followed by 16.7 mM glucose. Supernatant was collected from each well (n=6) after treatments. C-peptide concentration was determined by ELISA. Data are presented as stimulation index, which is the fold-change in C-peptide concentration in supernatant from high glucose treatment relative to low glucose for each well.
Tripotent postnatal PCFUs can be enriched by CD133high selection
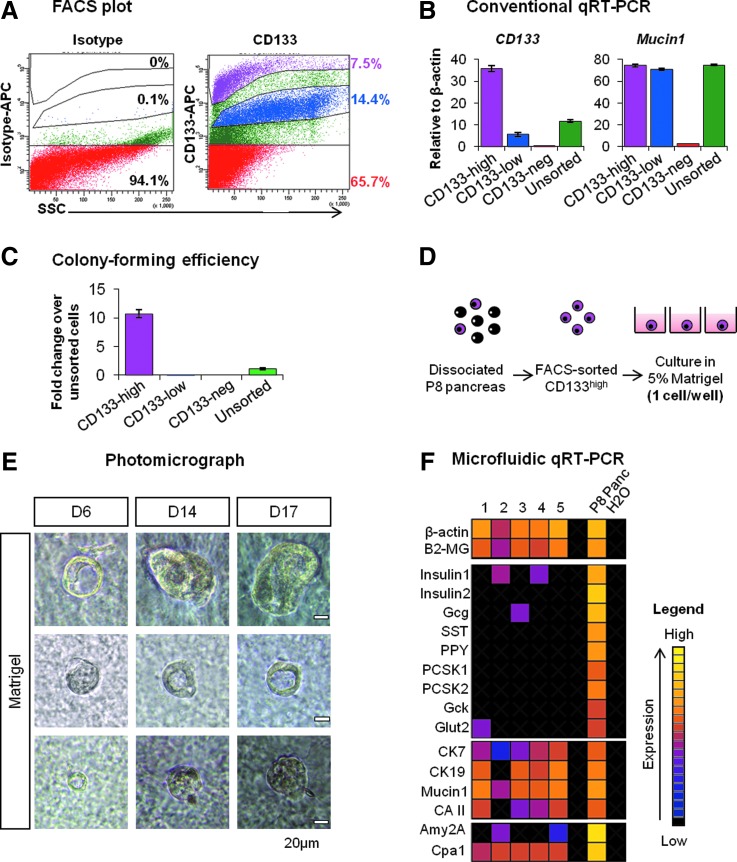
To characterize the colony-forming progenitors, postnatal pancreatic cells were analyzed according to cell surface expression of CD133, a ductal cell marker [20,44]. We have previously shown that single CD133+ cells isolated from adult (2–4 months old) pancreas can efficiently form colonies in the Matrigel-supplemented culture [45]. In the young postnatal pancreas, we further separated the CD133+ population into the CD133high and CD133low fractions, accounting for 8.1%±1.4% and 14.2%±0.8% (average±SD from seven independent experiments) of total live cells, respectively (Fig. 8A). Gene expression analysis demonstrated that both CD133high and CD133low, but not CD133negative fractions expressed ductal marker Mucin1, therefore confirming that CD133+ selection enriches for the ductal lineage (Fig. 8B). However, when CD133high, CD133low, and CD133negative cells were plated into Matrigel-containing media, only CD133high formed large cystic colonies that were previously observed with the unsorted population (Fig. 2). Colony-forming efficiency was enhanced by 11-fold in CD133high compared with unsorted cells (11.1% vs. 1%; Fig. 8C). Taken together, these results demonstrate that CD133high cells in the postnatal pancreas are enriched for colony-forming progenitors.
FIG. 8.
Postnatal PCFUs with tri-lineage potential can be enriched by CD133high expression. (A) Representative flow cytometry analysis of CD133 expression on postnatal pancreatic cells. Three distinct populations were observed: CD133high, CD133low, and CD133negative. (B) qRT-PCR analysis of CD133 and Mucin1 genes in freshly sorted CD133high, CD133low, CD133negative cells, and unsorted pancreatic cells from postnatal mice. (C) Cyst colony-forming efficiency in Matrigel-supplemented culture from CD133high, CD133low, and CD133negative cells, expressed as fold change over unsorted cells. (D) Schematic of single cell micromanipulation experiment. (E) Representative images of colonies derived from a single CD133high cell, grown over 17 days in cultures containing 5% Matrigel. (F) Microfluidic qRT-PCR analysis of five individual colonies formed from single CD133high cells, collected after 17 days in culture.
To determine whether CD133high progenitors are tripotent, we performed single cell manipulation of freshly sorted CD133high cells and plated one cell per well in Matrigel-supplemented semi-solid medium (Fig. 8D). Absence of contaminating cells in each well was verified by direct visualization under a microscope. After 6 days, colonies with cystic morphology with large central lumens were observed in five wells out of a total of 230 micromanipulated single cells (Fig. 8E; three representative colonies are shown). The colonies were monitored for an additional 11 days (for a total of 17 days in culture) before further analysis. Microfluidic qRT-PCR results demonstrated that four out five colonies (colonies 1–4) expressed endocrine, ductal, and acinar markers (Fig. 8F), confirming that a majority of the CD133high cells are tripotent.
To determine whether ECM proteins modulate the differentiation of CD133high cells, we plated them in aECM-lam alone or in the presence of increasing doses of Matrigel (Supplementary Fig. S2). Consistent with the finding shown in Fig. 6, colonies derived from CD133high cells cultured in the presence of aECM-lam alone expressed Insulin1, Insulin2, and Glucagon (Supplementary Fig. S2). Addition of Matrigel into the aECM-lam-containing culture diminished the expression of these endocrine genes (Supplementary Fig. S2). On the other hand, Cytokeratin7 was induced by Matrigel in a dose-dependent manner (Supplementary Fig. S2). Taken together, these data provide strong evidence that tripotent PCFUs in postnatal mice can be prospectively isolated using CD133high expression. Furthermore, differentiation of postnatal PCFUs toward the endocrine lineage is enhanced by aECM-lam but inhibited by Matrigel.
Discussion
We utilized methylcellulose-based colony assays to investigate colony formation and differentiation capacities of single pancreatic cells from young (1-week-old) postnatal mice. Single cells formed cystic colonies in Matrigel and small clusters in the aECM-based cultures (Fig. 1A). Our cystic colonies were morphologically similar to organoids derived from embryonic or adult pancreata, without having to use high concentrations of Matrigel (>33% vol/vol) as was used by others [21–23]. In our assay, the highest Matrigel concentration used in the semi-solid media was 5%; we have found this to be sufficient for postnatal (current study) and adult (2–4 months old) cystic colony formation [20].
The use of methylcellulose-based semi-solid media has several advantages: (1) colonies are evenly distributed and “embedded” throughout the well, facilitating qualitative and quantitative assessments of morphology, colony number, and colony size; (2) colonies can be individually handpicked for further analysis; (3) up to 2.5×104 cells can be plated in 500 μL media (per well of a 24-well plate) thereby allowing analysis of potentially rare progenitor populations; (4) media change is not necessary and cells can survive for up to 3 weeks [20]. Our colony assay thus provides a convenient and effective platform for ex vivo analysis of pancreatic progenitors at the postnatal stage, which offers insight on the transitional stage between embryos and adults.
The existence of multipotent progenitors in the young and adult pancreas is still debated. Much of our knowledge on progenitors stems from in vivo lineage tracing experiments. In these studies, cells expressing a known progenitor marker, such as Sox9, are irreversibly labeled with a reporter and examined at various developmental stages. Labeling of Sox9+ cells beginning at postnatal day 5 (P5) revealed that a small number of endocrine cells were generated from the Sox9+ compartment, but no acinar or β-cell neogenesis was observed [15]. However, by following the fates of carbonic anhydrase II+ (CAII) cells, Inada et al. showed that duct, acinar, and endocrine islets (including β-cells) were generated from the CAII+ ductal compartment between P0 and P28 [16]. Using a 2D culture assay, Oshima et al. showed that the CD133+ c-Met+ ductal cell population from neonatal pancreas (P0 or P1) has the capacity to differentiate into endocrine, acinar, and duct cells [44]. Using a 3D colony assay, we now show many individual colonies are composed of tri-lineage cells, indicating that many PCFUs from 1-week-old mice are tripotent. By micromanipulation of individual CD133high cells, we further demonstrate that a single cell is sufficient to form a cystic colony with tri-lineage expression (Fig. 8D–F), providing compelling evidence that these progenitor cells are multipotent. Taken together, these studies support the notion that the postnatal pancreas contains tripotent progenitor cells. From our results, it is likely that postnatal progenitors may originate from the ductal compartment, but additional studies using ductal lineage tracers (eg, Sox9) will be necessary to address this hypothesis.
In our prior studies we fractionated adult (2–4 months old) pancreatic cells based on CD133+ and CD133− expression, and we found that among CD133+ cells ∼5% were cystic colony-forming progenitor cells [45]. In this study, we subdivided the postnatal CD133+ population into CD133high and CD133low fractions, and we were able to enhance the colony-forming efficiency of CD133high cells to ∼10% (Fig. 8C). It remains to be determined whether colony-forming efficiency can be further improved by combining CD133high selection with other CD markers in the young postnatal pancreas. In addition, a direct comparison of the CD133high cells between adult and 1-week-old pancreas will be needed to further elucidate the role of ageing in the maintenance of pancreatic progenitor pools.
Previously, we demonstrated that postnatal pancreas and liver contain CFU-Dark. The frequency of CFU-Dark is 0.03% of the total postnatal pancreatic cells and 0.1% of liver cells [24]. In comparison, the PCFUs described in this study comprised at least 1.0% of pancreatic cells (Figs. 2C and 6C). Whether one type of colony may give rise to the other, or if they develop independently is unknown and requires further investigation.
ECM proteins play instructive and supportive roles in progenitor survival and differentiation [46]. Cell–matrix interactions can be recapitulated in vitro either by culturing on ECM-coated plates, or by encapsulation of cells in hydrogels functionalized with ECM-derived peptides such as Arg-Gly-Asp (RGD) and IKVAV. For example, RGD-functionalized hydrogels improve survival of human mesenchymal stem cells [47], while laminin-5 induced differentiation of these cells toward the osteogenic lineage [48]. In this report, we showed that IKVAV, a peptide sequence derived from the laminin α1 chain, promotes the differentiation of postnatal PCFUs into β-like cells that not only express insulin, but also contain dense core granules and secrete C-peptide in response to high glucose. Laminin is the most abundant (60%) ECM protein in Matrigel [49]. However, the colonies or organoids grown in Matrigel expressed β-cell markers at low or undetectable levels and were not responsive to glucose (current study) [21]. This suggests that there may be other factors in Matrigel that inhibit endocrine cell formation. Our results (Fig. 6 and Supplementary Fig. S2) were consistent with this idea, and showed that Matrigel inhibited endocrine gene expression dose dependently. Collagen is the second most abundant ECM protein in Matrigel (20%) [49]. The ratio of laminin to collagen has been shown to influence differentiation of neuronal subtypes and cardiac valve interstitial cells [50,51]. Varying the ratio of laminin to collagen also modulates β-cell survival and function in vitro, suggesting that β-cells respond to changes in ECM composition [52]. Whether collagen is the negative regulator responsible for the effect of Matrigel in our colony assay requires further investigation.
In conclusion, we extend previous findings and demonstrate that murine postnatal young pancreas harbors tripotent colony-forming progenitors. Individual colonies expressed markers of acinar, ductal, and endocrine lineages. Furthermore, an aECM protein containing the IKVAV sequence of laminin promotes differentiation toward functional β-like cells. Our results underscore the therapeutic potential of postnatal progenitor cells as a source of transplantable cells for T1D.
Supplementary Material
Acknowledgments
This work is supported in part by National Institutes of Health (NIH) grants R01DK081587 and R01DK099734 to H.T.K.; U01DK089533 to A.D.R; and California Institute for Regenerative Medicine (CIRM) grant RB5-07398 to D.A.T. N.G. is supported by a CIRM predoctoral fellowship as part of an institutional grant to City of Hope. We thank the Light Microscopy Core, Electron Microscopy Core, and the Analytical Cytometry Core at City of Hope for providing technical assistance.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P. and Hara M. (2009). Islet architecture: a comparative study. Islets 1:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM. and Rajotte RV. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 3.Gu G, Brown JR. and Melton DA. (2003). Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 120:35–43 [DOI] [PubMed] [Google Scholar]
- 4.Gu G, Dubauskaite J. and Melton DA. (2002). Direct evidence for the pancreatic lineage: NGN3+cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 5.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV. and Teitelman G. (1995). Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development 121:11–18 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA. and Melton DA. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13:103–114 [DOI] [PubMed] [Google Scholar]
- 7.Gradwohl G, Dierich A, LeMeur M. and Guillemot F. (2000). neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G. and Grapin-Botton A. (2007). Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 12:457–465 [DOI] [PubMed] [Google Scholar]
- 9.Gouzi M, Kim YH, Katsumoto K, Johansson K. and Grapin-Botton A. (2011). Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn 240:589–604 [DOI] [PubMed] [Google Scholar]
- 10.Miyatsuka T, Kosaka Y, Kim H. and German MS. (2011). Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci U S A 108:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan FC. and Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 240:530–565 [DOI] [PubMed] [Google Scholar]
- 12.Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang SB. and Rupnik M. (2009). Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann N Y Acad Sci 1152:53–62 [DOI] [PubMed] [Google Scholar]
- 13.Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A. and Melton DA. (2012). Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 30:261–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller K, Kim A, Kilimnik G, Jo J, Moka U, Periwal V. and Hara M. (2009). Islet formation during the neonatal development in mice. PLoS One 4:e7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J. and Sander M. (2011). Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138:653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A. and Bonner-Weir S. (2008). Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ. and Wright CV. (2002). The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32:128–134 [DOI] [PubMed] [Google Scholar]
- 18.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L. and Ferrer J. (2009). Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 17:849–860 [DOI] [PubMed] [Google Scholar]
- 19.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP. and Leach SD. (2010). Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A 107:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin L, Feng T, Shih HP, Zerda R, Luo A, Hsu J, Mahdavi A, Sander M, Tirrell DA, Riggs AD. and Ku HT. (2013). Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A 110:3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, et al. , (2013). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32:2708–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, et al. , (2013). Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M. and Grapin-Botton A. (2013). Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140:4452–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Feng T, Chai J, Ghazalli N, Gao D, Zerda R, Li Z, Hsu J, Mahdavi A, et al. , (2014). Colony-forming progenitor cells in the postnatal mouse liver and pancreas give rise to morphologically distinct insulin-expressing colonies in 3D cultures. Rev Diabet Stud 11:35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger B. and Yamada KM. (2011). Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, et al. , (2006). The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 10:397–405 [DOI] [PubMed] [Google Scholar]
- 27.Kragl M. and Lammert E. (2010). Basement membrane in pancreatic islet function. Adv Exp Med Biol 654:217–234 [DOI] [PubMed] [Google Scholar]
- 28.Pinkse GG, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA. and de Heer E. (2006). Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes 55:312–317 [DOI] [PubMed] [Google Scholar]
- 29.Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA. and Bosco D. (2006). Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes 55:1413–1420 [DOI] [PubMed] [Google Scholar]
- 30.Weber LM. and Anseth KS. (2008). Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol 27:667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domogatskaya A, Rodin S. and Tryggvason K. (2012). Functional diversity of laminins. Annu Rev Cell Dev Biol 28:523–553 [DOI] [PubMed] [Google Scholar]
- 32.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, et al. , (2005). A simplified laminin nomenclature. Matrix Biol 24:326–332 [DOI] [PubMed] [Google Scholar]
- 33.Yamada KM. (1991). Adhesive recognition sequences. J Biol Chem 266:12809–12812 [PubMed] [Google Scholar]
- 34.Weber LM, Hayda KN, Haskins K. and Anseth KS. (2007). The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28:3004–3011 [DOI] [PubMed] [Google Scholar]
- 35.Fong E. and Tirrell DA. (2010). Collective cell migration on artificial extracellular matrix proteins containing full-length fibronectin domains. Adv Mater 22:5271–5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrico IS, Maskarinec SA, Heilshorn SC, Mock ML, Liu JC, Nowatzki PJ, Franck C, Ravichandran G. and Tirrell DA. (2007). Lithographic patterning of photoreactive cell-adhesive proteins. J Am Chem Soc 129:4874–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong E, Tzlil S. and Tirrell DA. (2010). Boundary crossing in epithelial wound healing. Proc Natl Acad Sci U S A 107:19302–19307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer DE. and Chilkoti A. (1999). Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol 17:1112–1115 [DOI] [PubMed] [Google Scholar]
- 39.Mackay JA, Callahan DJ, Fitzgerald KN. and Chilkoti A. (2010). Quantitative model of the phase behavior of recombinant pH-responsive elastin-like polypeptides. Biomacromolecules 11:2873–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilshorn SC, DiZio KA, Welsh ER. and Tirrell DA. (2003). Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials 24:4245–4252 [DOI] [PubMed] [Google Scholar]
- 41.Nowatzki PJ. and Tirrell DA. (2004). Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials 25:1261–1267 [DOI] [PubMed] [Google Scholar]
- 42.Christensen T, Trabbic-Carlson K, Liu W. and Chilkoti A. (2007). Purification of recombinant proteins from Escherichia coli at low expression levels by inverse transition cycling. Anal Biochem 360:166–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ. and Clevers H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265 [DOI] [PubMed] [Google Scholar]
- 44.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N. and Taniguchi H. (2007). Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 132:720–732 [DOI] [PubMed] [Google Scholar]
- 45.Jin L, Feng T, Zerda R, Chen CC, Riggs AD. and Ku HT. (2014). In vitro multilineage differentiation and self-renewal of single pancreatic colony-forming cells from adult C57BL/6 mice. Stem Cells Dev 23:899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keung AJ, Kumar S. and Schaffer DV. (2010). Presentation counts: microenvironmental regulation of stem cells by biophysical and material cues. Annu Rev Cell Dev Biol 26:533–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benoit DS, Tripodi MC, Blanchette JO, Langer SJ, Leinwand LA. and Anseth KS. (2007). Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J Biomed Mater Res A 81:259–268 [DOI] [PubMed] [Google Scholar]
- 48.Klees RF, Salasznyk RM, Kingsley K, Williams WA, Boskey A. and Plopper GE. (2005). Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol Biol Cell 16:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corning Incorporated. (2013). Corning® Matrigel® Matrix: Frequently Asked Questions. Corning Incorporated, Corning, NY [Google Scholar]
- 50.Raghavan S. and Bitar KN. (2014). The influence of extracellular matrix composition on the differentiation of neuronal subtypes in tissue engineered innervated intestinal smooth muscle sheets. Biomaterials 35:7429–7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gould ST, Darling NJ. and Anseth KS. (2012). Small peptide functionalized thiol-ene hydrogels as culture substrates for understanding valvular interstitial cell activation and de novo tissue deposition. Acta Biomater 8:3201–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber LM, Hayda KN. and Anseth KS. (2008). Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A 14:1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.