Abstract
Background: Rift Valley fever (RVF) outbreaks have been associated with periods of widespread and above-normal rainfall over several months. Knowledge on the environmental factors influencing disease transmission dynamics has provided the basis for developing models to predict RVF outbreaks in Africa. From 2008 to 2011, South Africa experienced the worst wave of RVF outbreaks in almost 40 years. We investigated rainfall-associated environmental factors in southern Africa preceding these outbreaks.
Methods: RVF epizootic records obtained from the World Animal Health Information Database (WAHID), documenting livestock species affected, location, and time, were analyzed. Environmental variables including rainfall and satellite-derived normalized difference vegetation index (NDVI) data were collected and assessed in outbreak regions to understand the underlying drivers of the outbreaks.
Results: The predominant domestic vertebrate species affected in 2008 and 2009 were cattle, when outbreaks were concentrated in the eastern provinces of South Africa. In 2010 and 2011, outbreaks occurred in the interior and southern provinces affecting over 16,000 sheep. The highest number of cases occurred between January and April but epidemics occurred in different regions every year, moving from the northeast of South Africa toward the southwest with each progressing year. The outbreaks showed a pattern of increased rainfall preceding epizootics ranging from 9 to 152 days; however, NDVI and rainfall were less correlated with the start of the outbreaks than has been observed in eastern Africa.
Conclusions: Analyses of the multiyear RVF outbreaks of 2008 to 2011 in South Africa indicated that rainfall, NDVI, and other environmental and geographical factors, such as land use, drainage, and topography, play a role in disease emergence. Current and future investigations into these factors will be able to contribute to improving spatial accuracy of models to map risk areas, allowing adequate time for preparation and prevention before an outbreak occurs.
Key Words: : Rift Valley fever, Southern Africa, Environmental factors, Geographic factors, Normalized difference vegetation index data
Introduction
Rift Valley fever (RVF) was first identified in 1931 in Kenya's Great Rift Valley following a large epizootic among sheep (Daubney 1931). Since that time, outbreaks have been reported across Africa and the Arabian Peninsula (Davies 1975, Swanepoel 1976, Meegan and Bailey 1989, Davies et al. 1992, Arthur et al. 1993, Madani et al. 2003, Mohamed et al. 2010, Shieh et al. 2010, Hassan et al. 2011). This disease was first recognized in South Africa in 1951, an epidemic that resulted in 500,000 abortions and 100,000 deaths among sheep (Gear et al. 1951, Woods et al. 2002). Since then, it has continued to occur every 3–15 years, with the last severe outbreak before 2008 occurring in 1970 (Pienaar and Thompson 2013). The most recent multiyear epizootic starting in 2008 and ending in 2011 resulted in approximately 303 human cases, including 26 deaths and over 19,000 cases in livestock (National Health Laboratory Systems 2011).
RVF infects a variety of wild and domestic ruminants, but livestock, specifically sheep, cattle, and goats, are the most impacted (Meegan and Bailey 1989). In sheep, abortion rates can reach 100%, with young animals and foreign breeds being most at risk for severe disease and death. In adult animals, mortality ranges from 5% to 60%. Several livestock vaccines exist; however, they all have limitations and are costly, preventing widespread use (Dungu et al. 2013).
RVF impacts a community in multiple ways. First, outbreaks are associated with huge economic loss. This is due to a combination of livestock deaths and strict trade bans imposed and enforced by the World Organization for Animal Health (OIE) preventing animal export during outbreaks (Little 2009, Rich and Wanyoike 2010). In addition to this, humans can become infected, usually resulting in mild flu-like symptoms with a 1–5% risk of developing severe complications or death (Bird et al. 2009). Finally, outbreaks commonly are accompanied with flooding, which is associated with infected vector emergence (Linthicum et al. 1999). These communities often are vulnerable, with low resilience to disease and disaster, making it imperative for public health officials to recognize and prepare for these outbreaks (Dar et al. 2013).
Current prediction models exist to create RVF risk maps based on several satellite-derived measurements including sea surface temperatures, outgoing longwave radiation, normalized difference vegetation index (NDVI), and rainfall (Anyamba et al. 2009, 2010). Excess rainfall results in flooding and hatch of dormant Aedes virus-infected mosquito eggs in dambo habitats that then infect livestock and humans (Linthicum et al. 1984).
NDVI data have been proven to be a good surrogate for rainfall along with soil moisture, soil type, and energy availability in the tropical semiarid regions of Sub-Saharan Africa (Nicholson et al. 1990, Tucker and Nicholson 1999). Thus, these data are used as an indicator of breeding and upsurge patterns of some insect pests and vectors of disease, including locusts and mosquitoes (Hielkema et al. 1986, Linthicum et al. 1990). In this study, we sought to apply these parameters to southern Africa's varied subtropical climate, ranging from dry desert-like conditions in the west (NDVI values of 0.1–0.2 and annual rainfall between 200 and 300 mm) to wetter and highly vegetative regions in the east (NDVI values of 0.6 and annual rainfall as high as 600–800 mm) to observe correlations with the RVF outbreaks of 2008–2011.
Materials and Methods
Livestock data
RVF animal case data were obtained from the OIE's World Animal Health Information Database (WAHID) by searching monthly reports from mid-2007 through 2013 (WAHID 2008, 2009a, 2009b, 2010a, 2010b, 2010c, 2011a, 2011b, 2012). Each report is location and species specific. Data from outbreaks in South Africa, Namibia, Botswana, and Swaziland were included. Epidemic years were synchronized to the seasonal rainfall cycle preceding RVF outbreaks so that 2008 contained all outbreaks between October, 2007, and September, 2008; 2009 contained all outbreaks between October, 2008 and September, 2009, and so on. Animals were tested and cases were identified using methods described by the OIE Manual (World Organization for Animal Health 2011).
Statistics, epidemiological curves, and mapping
Average case fatality rate and risk were calculated by year. Case fatality was defined as the number of deaths divided by number of cases for each species-specific report, and risk was defined as the number of cases divided by the total number of susceptible animals for each report. Average risk is the average percent of each species infected at each location. Incidence rates were calculated by province using animal density data from the Global Livestock Production and Health Atlas (GLIPHA) (Food and Agriculture Organization of the United Nations 2013).
Epidemic curves were created by summing the total number of cases by week for each epidemic year beginning with week 40, correlating to October 1st, and ending with week 39, correlating to the last week of September the following year. All data management, summary statistics, and epidemic curves were done using Stata 12.0 (StataCorp 2011). Epidemic curves were created and defined using methodology described by the Centers for Disease Control and Prevention (2014).
Case locations were mapped using ArcGIS 10.0 (Environmental Systems Research Institute 2011). Data were imported, then transformed using the given latitude and longitude coordinates and projected to a geographic coordinate system using the World Geodetic System (WGS) 1984 datum. Base maps were provided by the Environmental Systems Research Institute (Esri) for the case location map and the Database of Global Administrative Boundaries (GADM) via DIVA-GIS–provided administrative boundaries for the risk maps.
Rainfall and NDVI data
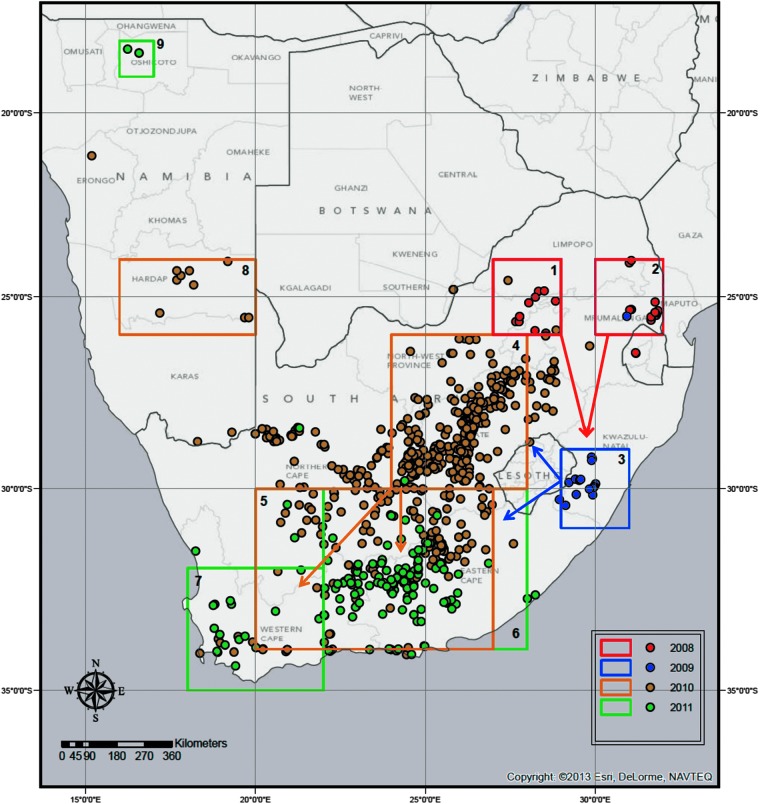
Rainfall and NDVI data were extracted and analyzed for nine predefined regions, (Fig. 1), corresponding to areas with a high density of RVF cases. Rainfall data were obtained from the National Oceanic and Atmospheric Administration's (NOAA's) Climate Prediction Center (CPC) Africa Rainfall Climatology (ARC) archives. These data are derived from geostationary infrared (IR) measurements centered over Africa created by the European Organization for the Exploitation of Meteorological Satellites (EUMETSAT) combined with quality controlled Global Telecommunication System (GTS) 24-h rainfall gauge observations across Africa (Novella and Thiaw 2013). Daily and long-term mean, minimum, and maximum rainfall and anomaly values were extracted for each region from 2007 to 2011.
FIG. 1.
Rift Valley fever (RVF) case locations for 2008–2011 epidemic years and outlined regions with high density of cases used for the rainfall and normalized difference vegetation index (NDVI) analysis.
NDVI data was derived from measurements made by the National Aeronautic and Space Administration's (NASA's) Earth Observing System Moderate Resolution Imaging Spectroradiometer (MODIS) instrument aboard the Terra (EOS AM-1) spacecraft (Townshend and Justice 2002). For this study, we used MOD13C2 NDVI data aggregated from 16 day 250 m MODIS NDVI to global monthly 0.05 degree (5600 m) NDVI data (US Geological Survey, no date). Monthly and long-term mean, minimum, and maximum NDVI and anomaly values were extracted for each region from 2007 to 2011.
Cumulative daily rainfall and monthly NDVI means were calculated by summing up all values between October 1st and each succeeding time point for the epidemic year corresponding with each region. All analyses and graphs were computed and plotted using Stata 12.0 (StataCorp 2011).
Results
There were 759 reports of RVF in southern Africa between January, 2008, and December, 2011. A total of 716 reports were from sheep, goats, or cattle, accounting for 96.8% of cases (18,767 cases out of 19,390 total cases) reported from 2008 to 2011. A total of 608 cases occurred in buffalo, camels, mixed livestock populations, or other wild ruminants. Reports of abortions and sudden or acute deaths in young lambs were also recorded.
The 2008 and 2009 outbreaks were small in magnitude compared to 2010 and 2011. In 2008, cattle accounted for 81% of cases out of 496 total livestock cases. The average number of animals infected at each location was highest during 2008, about 17%, but case fatality was lower than the following years, only about 60% versus 75–80%, and incidence rates were lower during these years ranging from 2.33 to 23.89/100,000 animals in cattle to 1.98 to 91.97/100,000 in goats. In 2009, 201 out of 210 cases occurred in cattle, and the risk of infection was only 2% (Tables 1 and 2).
Table 1.
Rift Valley Fever Outbreak Statistics by Year of Occurrence and Species
| Cattle | Goats | Sheep | Other | Total | |
|---|---|---|---|---|---|
| 2008 | |||||
| Outbreak reports | 23 | 2 | 1 | 3 | 29 |
| Cases | 403 | 67 | 26 | 13 | 509 |
| Average riska,b | 17.2% | 29.3% | 8.1% | 17.4% | |
| Average case fatalityc | 61.1% | 53.2% | 61.5% | 60.5% | |
| 2009 | |||||
| Outbreak reports | 17 | 0 | 1 | 0 | 18 |
| Cases | 201 | 0 | 9 | 0 | 210 |
| Average riska,b | 2.0% | 9.4% | 2.5% | ||
| Average case fatalityc | 60.3% | 0.0% | 57.0% | ||
| 2010 | |||||
| Outbreak reports | 153 | 21 | 371 | 27 | 572 |
| Cases | 782 | 324 | 13,016 | 337 | 14,459 |
| Average riska,b | 9.0% | 10.7% | 10.0% | 9.8% | |
| Average case fatalityc | 72.4% | 61.9% | 74.0% | 73.1% | |
| 2011 | |||||
| Outbreak reports | 23 | 12 | 92 | 13 | 140 |
| Cases | 63 | 400 | 3,491 | 258 | 4,212 |
| Average riska,b | 11.0% | 10.8% | 11.2% | 11.1% | |
| Average case fatalityc | 94.7% | 77.2% | 85.9% | 86.6% | |
| Total outbreak reports | 216 | 35 | 465 | 43 | 759 |
| Total sum of cases | 1449 | 791 | 16,542 | 608 | 19,390 |
| Average risk | 9.2% | 11.3% | 10.2% | 10.0% | |
| Average case fatality | 72.6% | 66.6% | 76.2% | 74.6% | |
A total of 663 livestock reports containing total susceptible species counts available for risk analysis.
Average risk=mean (cases/susceptibles) from reports.
Average case fatality=mean (deaths/cases) from reports.
Table 2.
Rift Valley Fever Incidence by Province (per 100,000 Animals)
| 2008 | 2009 | 2010 | 2011 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Province | Cattle | Goats | Sheep | Cattle | Goats | Sheep | Cattle | Goats | Sheep | Cattle | Goats | Sheep |
| Limpopo | 2.33 | 0.02 | ||||||||||
| Gauteng | 12.60 | 91.97 | 0.38 | 8.56 | ||||||||
| Mpumalanga | 23.89 | 1.98 | 3.38 | 11.77 | ||||||||
| Kwazulu-Natal | 7.22 | |||||||||||
| Northwest | 4.54 | 1.45 | 0.68 | 24.11 | ||||||||
| Free State | 17.22 | 29.38 | 187.97 | |||||||||
| Eastern Cape | 1.16 | 0.99 | 6.50 | 1.58 | 3.81 | 26.67 | ||||||
| Northern Cape | 5.07 | 8.22 | 344.18 | 0.06 | 120.99 | |||||||
| Western Cape | 38.69 | 15.49 | 12.54 | 2.16 | 108.18 | 25.45 | ||||||
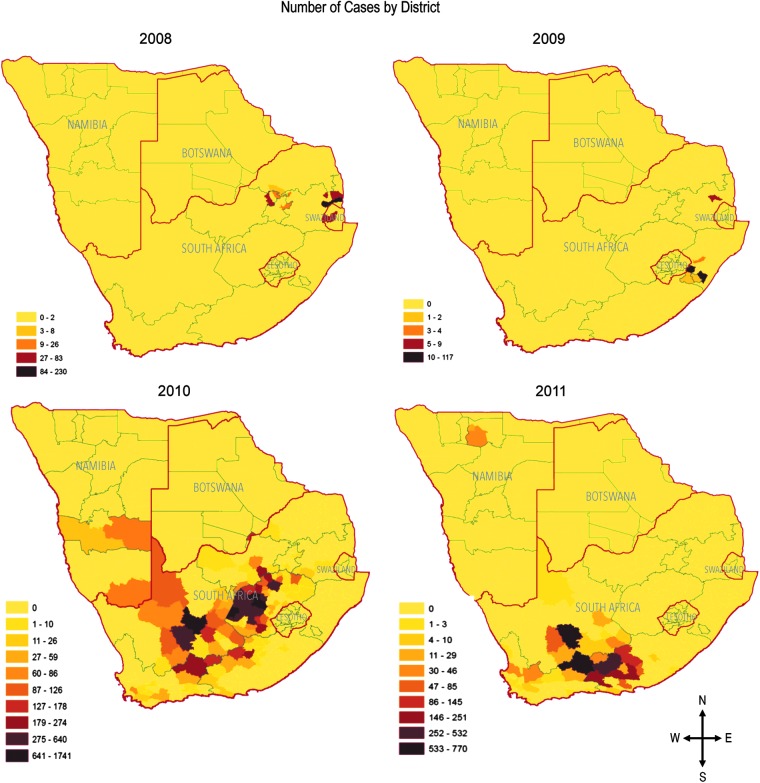
In 2010 there were 14,122 cases reported in livestock; 92% occurred in sheep. The average risk of infection was 10%. In the 2011 epizootic year, there were 3954 cases in livestock, 88% in sheep. The highest incidence of infection occurred during these years in the Northern Cape province in sheep, reaching 344 cases/100,000 and 121 cases/100,000 animals in 2010 and 2011, respectively (Table 2). The average risk of infection was the same among all species and similar to 2010; however, case fatality was highest during this year for all species, reaching 95% and 86% in cattle and sheep, respectively (Table 1). Camels, buffalo, waterbuck, and a variety of other wild species were infected in addition to livestock. Overall, more of these wild species were identified during 2010 and 2011 when the outbreaks were larger in magnitude.
Timing and location of outbreaks
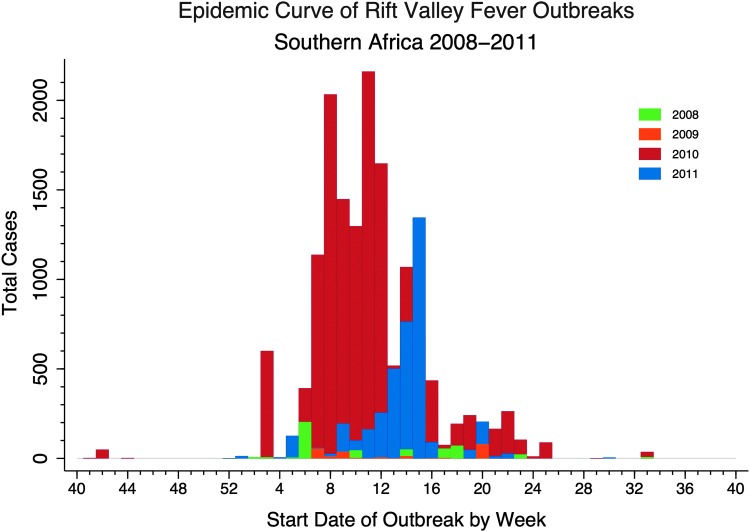
RVF cases were detected by the end of January, except in 2009, and the bulk of the cases each year occurred within a 20-week timeframe between February and June (Fig. 2). Unlike most RVF epidemics that begin and end within 1 year, this series of outbreaks continued over 4 years defined by the rainy season. In 2008 and 2009, cases occurred more sporadically and the circulating RVF virus was lineage C, but in 2010 and 2011 the outbreaks were RVF lineage H and a defined epidemic wave was evident (Grobbelaar et al. 2011).
FIG. 2.
Overlapping epidemic curves for each year Rift Valley fever (RVF) occurred in southern Africa including all species. Week 40 corresponds with October 1, 2007, 2008, 2009, and 2010.
In 2008, outbreaks began in mid-January and continued through August, affecting the northeastern regions of South Africa and Swaziland. The 2009 outbreak lasted from February to May and was mostly concentrated in a small area in the south of Kwazulu-Natal Province. In 2010, the outbreak began in October, 2009, and continued through September, 2010, spanning most of South Africa, but was particularly concentrated in the interior regions, with some cases reported further west in Namibia and further north in Botswana. The 2011 epidemic began in December, 2010 and lasted through August, 2011; however, cases peaked in late April as compared to late February and mid-March as they had during the previous years. Most cases occurred in eastern and western Cape Provinces in the southwest of South Africa, but there was one outbreak reported in northern Namibia (Figs. 1 and 3).
FIG. 3.
Number of Rift Valley fever (RVF) cases aggregated by subprovince for each epidemic year.
Environmental factors
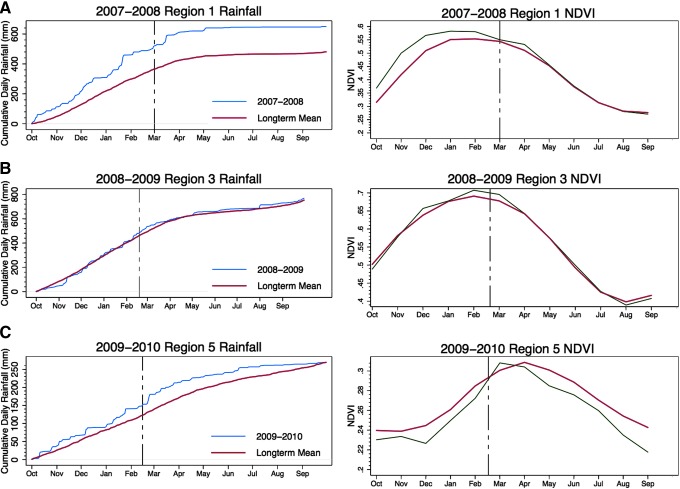
RVF followed periods of extended anomalously high rainfall (>60 days) in seven out of the nine predefined regions (Table 3). The average time between rainfall accumulation above average and the first case report was 115 days, ranging from 85 to 152 days, validating the findings from Kenya (Linthicum et al. 1985). However, in regions 3 and 6, rainfall was above average only for a short period of time before the first case appeared (Fig. 4). The amount of accumulated rainfall also varied widely between regions (Table 3).
Table 3.
Environmental Factors and Case Numbers by Region
| Year | Region | Datea | RVFb | Daysc | Rainfall amountd | NDVI above average | Outbreak locations | Cases |
|---|---|---|---|---|---|---|---|---|
| 2008 | 1 | 1-Oct-08 | 1-Mar-08 | 152 | 158.00 | Yes | 12 | 135 |
| 2 | 1-Oct-08 | 14-Jan-08 | 105 | 82.43 | Yes | 15 | 328 | |
| 2009 | 3 | 9-Feb-09 | 18-Feb-09 | 9 | 20.52 | Yes | 17 | 200 |
| 2010 | 4 | 1-Oct-09 | 19-Jan-10 | 110 | 57.77 | Yes | 265 | 9824 |
| 5 | 11-Oct-09 | 15-Feb-10 | 127 | 27.60 | No | 137 | 2812 | |
| 8 | 2-Jan-10 | 10-May-10 | 128 | 12.52 | No | 9 | 37 | |
| 2011 | 6 | 15-Dec-10 | 19-Jan-11 | 35 | 32.58 | Yes | 105 | 3888 |
| 7 | 15-Dec-10 | 10-Mar-11 | 85 | 24.21 | Yes | 15 | 128 | |
| 9 | 28-Dec-10 | 4-Apr-11 | 97 | 317.32 | Yes | 2 | 73 |
Date daily cumulative mean rainfall exceeded average cumulative mean rainfall.
Date of first RVF case.
Days since daily cumulative mean rainfall exceeded average cumulative mean rainfall.
Cumulative rainfall amount above average since October 1st.
RVF, Rift Valley fever; NDVI, normalized difference vegetation index.
FIG. 4.
Long-term mean and daily cumulative rainfall (blue lines) and long-term mean and monthly cumulative normalized difference vegetation index (NDVI) (green lines) compared to first Rift Valley fever (RVF) case date (vertical black dotted line) for regions 1 (A), 3 (B), and 5 (C).
NDVI analyses revealed a pattern further from what was expected. Although seven of nine regions showed NDVI values above average prior to the first RVF case, they were not the same regions with above-average rainfall. Both regions 3 and 6, which had few days of above-average rainfall before the first RVF case was identified, had above-average NDVI at this time. Conversely, regions 5 and 8, which had anomalous rainfall at the time of the first RVF case, had below-average NDVI at that time (Fig. 4). This adds strength to the argument that other elements such as soil, topography, or manmade structures played a role in the 2008–2011 outbreaks. It also may suggest that there is a different NDVI threshold associated with an outbreak in southern Africa than eastern Africa.
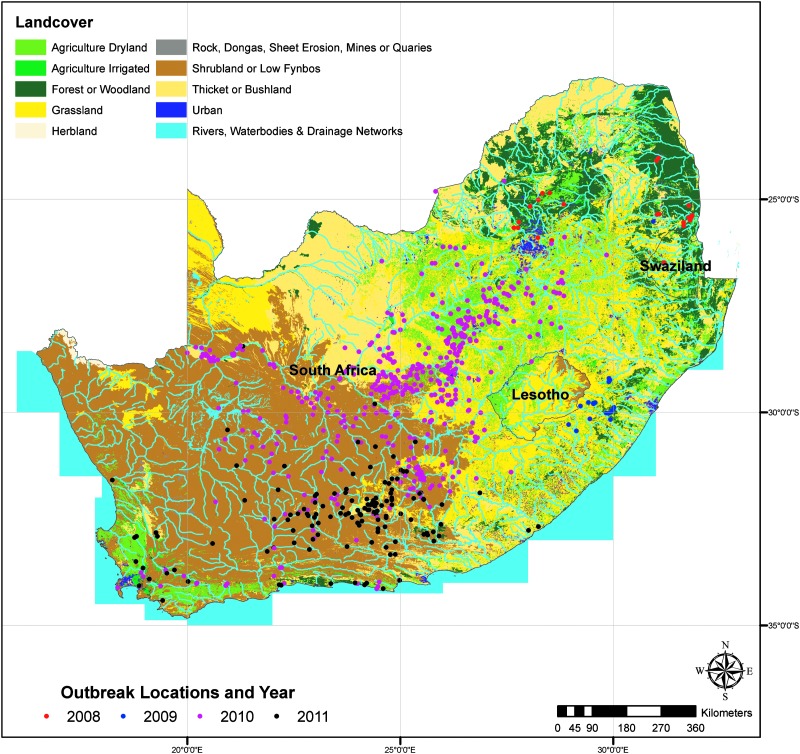
Subsequent land use analyses revealed some differences between years; however, the majority of outbreaks occurred in shrubland, low fynbos, grassland, or agricultural areas. Even in regions where rainfall and NDVI were inconsistent, land use at case locations was similar to other regions. Overall, 37% of locations were areas of shrubland, low fynboes, or herbland—all from 2010 and 2011. Grassland regions were the next highest represented area, accounting for 20.6% of locations, followed by agriculture dryland (16.2%) and agriculture irrigated land (10.8%). The land use at outbreak locations in 2008 was notably different from the following years; more than half (55.2%) of the locations were categorized as forest, woodland, and forest plantation. This is compared to 2010 and 2011, where case locations were predominantly shrubland and grassland, accounting for 58.2% and 67.9% of locations, respectively (Table 4). Another feature that is evident is the proximity to water bodies, including rivers and drainage areas (Fig. 5). One example is the clustering of cases around the Orange River in 2010.
Table 4.
Land Use at Unique Case Locations by Year for Outbreaks In South Africaa
| 2008 | 2009 | 2010 | 2011 | Total | |
|---|---|---|---|---|---|
| Shruband, low fynbos, herbland | 171 (34.1%) | 82 (61.2%) | 253 (37.0%) | ||
| Grassland | 2 (6.9%) | 9 (47.4%) | 121 (24.1%) | 9 (6.7%) | 141 (20.6%) |
| Agriculture dryland | 5 (17.2%) | 2 (10.5%) | 98 (19.5%) | 6 (4.5%) | 111 (16.2%) |
| Agriculture irrigated | 5 (17.2%) | 7 (36.8%) | 44 (8.8%) | 18 (13.4%) | 74 (10.8%) |
| Thicket and bushland | 1 (3.4%) | 1 (5.3%) | 41 (8.2%) | 14 (10.4%) | 57 (8.3%) |
| Other | 27 (5.4%) | 5 (3.7%) | 32 (4.7%) | ||
| Forest, woodland, forest plantation | 16 (55.2%) | 16 (2.3%) | |||
| Total | 29 | 19 | 502 | 134 | 684 |
Given as number of unique case locations n(%).
FIG. 5.
Reported Rift Valley fever (RVF) case locations in relation to Land Use/Land Cover.
Discussion
From 2008 to 2011, South Africa experienced the worst wave of RVF outbreaks in over 30 years. Throughout this 4-year period, every province in the country reported disease in livestock. In 2008 and 2009, the epidemics were smaller, associated with RVF lineage C, and the main species affected was cattle in the eastern exterior provinces, where there is a higher cattle density. The 2010 and 2011 epidemics were larger, associated with lineage H, and mainly affected sheep in the interior and southern provinces of South Africa (Grobbelaar et al. 2011). The case fatality rate was consistently higher among sheep every year with the exception of 2011.
The year 2011 marked the first time a large outbreak of RVF was detected and recorded in the Western Cape regions; disease had only occurred here once before during a small outbreak in 1983–1984 (Pienaar and Thompson 2013). Although 2011 was not as large as the outbreak in 2010, the disease did seem to be more severe, indicated by a high case fatality rate for all species. It is suspected that because it was the first appearance of disease in this region, immunity was lower due to a combination of first exposure, lack of vaccination, and more naïve and vulnerable breeds present in this region.
Although the potential for outbreaks existed, current methods using rainfall, NDVI, sea surface temperatures, and outgoing longwave radiation were unable to predict precisely areas that would be affected, especially in 2010 and 2011, indicating differences in environmental and RVF dynamics not only between eastern and southern Africa but also within southern Africa itself. We found that above-average rainfall for a period of 85–152 days preceded RVF outbreaks in seven out of nine regions over the 4 years. However, in two regions, RVF occurred less than 40 days after rainfall exceeded the long-term average. This is not indicative of RVF risk as previously seen in East Africa, where outbreaks have tended to occur after 50–60 days of anomalous rainfall (Anyamba et al. 2012). In addition to this, NDVI in regions 5 and 8 was below average, and there was some incongruence between NDVI and rainfall within the regions. This observation was unexpected considering the strong association between rainfall and NDVI in other parts of Africa and findings on RVF in East Africa (Linthicum et al. 1990, Nicholson et al. 1990, Tucker and Nicholson 1999, Anyamba et al. 2009).
One explanation for this is the large aggregated areas that were used to make rainfall and NDVI comparisons. Each of the nine regions was analyzed as if there was constant rainfall and NDVI. Although analyses comparing NDVI and rainfall at individual locations to the aggregated regions revealed similar trends, it has been reported that NDVI and rainfall correlations are highest at small scales because NDVI is also dependent on other factors, such as topography and soil type, that vary within our regions (Nicholson et al. 1990). It is plausible that water in shallow areas had accumulated, but was undetectable using large regions. Small geographic differences, such as being located at the bottom of an incline where water accumulates or near a water basin, could also increase the chance of mosquito breeding and RVF transmission. One place this is seen is along waterways in the Northern Cape province (Fig. 5). Future research aimed at characterizing these other factors, such as soil type, proximity to water, and topography, is needed.
Two other limitations were potentially missed data and no control group for further analyses. It is likely that some cases were missed, including asymptomatic infections. These animals would greatly add to the knowledge of risk factors but would not be captured. There also is a possibility of underreporting, especially in remote areas of Namibia and Botswana, where surveillance is weak. Two important factors not included were age of the animals that died and the abortion rate among herds of different species. Abortion and death in young animals are the most common outcomes of RVF. Adult fatality may be less correlated with RVF and its predictors than animal abortions would be and may be more indicative of breed or some other variable. If feasible, it would be helpful to collect more detailed information, including age of the animals, breed, and overall abortion rates. If we had his information on cases and controls, analyses could be conducted to identify risk factors for infection.
Conclusion
In conclusion, above-normal rainfall and NDVI are still indicative of possible RVF disease activity; however, other ecological factors probably play a significant role in South Africa in relation to outbreak potential, such as geographic location relative to water bodies and surrounding land use. It will be important to identify these because vaccination is not recommended after the onset of an outbreak and is too expensive for many countries to maintain. An early warning system is currently the best method to prepare and take preventive action. If a warning is issued, countries have the power to prioritize and prepare, enabling proactive prevention like vaccination versus a reaction to fight an epidemic. As climate continues to change and shift toward more extremes, it is likely that vector populations will expand to new geographic locations, bringing with them many diseases that put human health and economic well-being at risk (Martin et al. 2008). Therefore, this work could have future applications, especially for diseases for which there is no effective vaccine.
Acknowledgments
Margaret Glancey was funded by Universities Space Research Association's (USRA) Goddard Earth Sciences Technology and Research (GESTAR) 2013 Summer Internship Fellowship at NASA Goddard Space Flight Center. The Rift Valley fever Monitoring and Risk Mapping project is funded by the Department of Defense–Armed Forces Health Surveillance Center, Division of Global Emerging Infections Surveillance and Response System (GEIS) Operations. We wish to acknowledge Jennifer Small for extracting the NDVI and Rainfall data used in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- Anyamba A, Chretien J-P, Small J, Tucker CJ, et al. . Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA 2009; 106:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyamba A, Linthicum KJ, Small J, Britch SC, et al. . Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. Am J Trop Med Hyg 2010; 83(2 Suppl):43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyamba A, Linthicum KJ, Small JL, Collins KM, et al. . Climate teleconnections and recent patterns of human and animal disease outbreaks. PLoS Negl Trop Dis 2012; 6:e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur R, Cope S, Botros B, Hibbs R. Recurrence of Rift Valley fever in Egypt. Lancet 1993; 342:1149–1150 [DOI] [PubMed] [Google Scholar]
- Bird BH, Ksiazek TG, Nichol ST, MacLachlan J. Rift Valley Fever Virus. Vet Med Today Zoonosis Update 2009; 234:883–893 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Quick Learn: Create an Epi Curve. 2014. Available at www.cdc.gov/training/QuickLearns/createepi/index.html
- Dar O, Mcintyre S, Hogarth S, Heymann D. Rift Valley fever and a new paradigm of research and development for zoonotic disease control. Emerg Infect Dis 2013; 19:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubney R. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol 1931; 34:545–579 [Google Scholar]
- Davies F. Observations on the epidemiology of Rift Valley fever in Kenya. J Hyg (Lond). 1975; 75:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F, Kilelu E, Linthicum J, Pegram RG. Patterns of Rift Valley fever activity in Zambia. Epidemiol Infect 1992; 108:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungu B, Donadeu M, Bouloy M. Vaccination for the control of Rift Valley Fever in enzootic and epizootic situations. Dev Biol (Basel) 2013; 135:61–72 [DOI] [PubMed] [Google Scholar]
- Environmental Systems Research Institute (Esri). ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute, 2011 [Google Scholar]
- Food and Agriculture Organization of the United Nations. GLIPHA. Livest. Prod. Rome, Italy: FAO, 2013 [Google Scholar]
- Gear J, De Meillon B, Measroch V, Harwin R, et al. . Rift Valley Fever in South Africa 2. The Occurrence of human cases in the Orange Free State, The North-Western Cape Province, The Western and Southern Transvaal. S Afr Med J 1951; 25:908–912 [PubMed] [Google Scholar]
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, et al. . Molecular epidemiology of Rift Valley fever virus. Emerg Infect Dis 2011; 17:2270–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan O, Ahlm C, Sang R, Evander M. The 2007 rift valley fever outbreak in Sudan. PLoS Negl Trop Dis 2011; 5:e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hielkema J, Roffey J, Tucker C. Assessment of ecological conditions associated with the 1980/81 desert locust plague upsurge in West Africa using environmental satellite data. Int J Remote Sens 1986; 7:1609–1622 [Google Scholar]
- Linthicum K, Davies F, Bailey C, Kairo A. Mosquito species encountered in a flooded grassland dambo in Kenya. Mosq News, 1984. Available at http://cat.inist.fr/?aModele=afficheN&cpsidt=8913484
- Linthicum K, Davies F, Kairo A, Bailey C. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J Hyg (Lond) 1985; 95:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum K, Bailey C, Tucker C, Mitchell K, et al. . Application of polar-orbiting, meteorological satellite data to detect flooding of Rift Valley Fever virus vector mosquito habitats in Kenya. Med Vet Entomol 1990; 4:433–438 [DOI] [PubMed] [Google Scholar]
- Linthicum K, Anyamba A, Tucker C. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science (80-) 1999. Available at www.sciencemag.org/content/285/5426/397.short [DOI] [PubMed]
- Little P. Hidden value on the hoof: Cross-Border Livestock Trade in Eastern Africa. Common Mark East South Africa Compr African Agric Dev Program. Policy Brief 2, 2009 [Google Scholar]
- Madani T, Al-Mazrou Y, Al-Jeffri M, Mishkhas A, et al. . Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin Infect Dis 2003; 37:1084–1092 [DOI] [PubMed] [Google Scholar]
- Martin V, Chevalier V, Ceccato P, Anyamba A, et al. . The impact of climate change on the epidemiology and control of Rift Valley fever. Rev Sci Tech 2008; 27:1–14 [PubMed] [Google Scholar]
- Meegan J, Bailey C. Rift Valley fever. In Arboviruses: Epidemiology and Ecology, vol. IV Monath TP, ed. Boca Raton, FL: CRC Press Inc., 1989:51–76 [Google Scholar]
- Mohamed M, Mosha F, Mghamba J, Zaki S, et al. . Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg 2010; 83(Suppl 2):22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Laboratory Systems. National Institute for Communicable Diseases | Rift Valley Fever Outbreak; 2011. Available at www.nicd.ac.za/?page=rift_valley_fever_outbreak&id=94 [Google Scholar]
- Nicholson S, Davenport M, Malo A. A comparison of the vegetation response to rainfall in the Sahel and East Africa, using normalized difference vegetation index from NOAA AVHRR. Clim Change 1990; 17:209–241 [Google Scholar]
- Novella NS, Thiaw WM. African rainfall climatology version 2 for famine early warning systems. J Appl Meteorol Climatol 2013; 52:588–606 [Google Scholar]
- Pienaar NJ, Thompson PN. Temporal and spatial history of Rift Valley fever in South Africa: 1950 to 2011. Onderstepoort J Vet Res 2013; 80:1–13 [DOI] [PubMed] [Google Scholar]
- Rich K, Wanyoike F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am J Trop Med Hyg 2010; 83(Suppl 2):52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W, Paddock C, Lederman E, Rao C, et al. . Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am J Trop Med Hyg 2010; 83(Suppl 2):38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011 [Google Scholar]
- Swanepoel R. Studies on the epidemiology of Rift Valley Fever. J S Afr Vet Assoc 1976; 47:93–94 [PubMed] [Google Scholar]
- Townshend J, Justice C. Towards operational monitoring of terrestrial systems by moderate-resolution remote sensing. Remote Sens Environ 2002; 83:351–359 [Google Scholar]
- Tucker C, Nicholson S. Variations in the size of the Sahara Desert from 1980 to 1997. Ambio 1999; 28:587–591 [Google Scholar]
- US Geological Survey. Vegetation Indices Monthly L3 Global 0.05Deg CMG MOD13C2. Available at https://lpdaac.usgs.gov/products/modis_products_table/mod13c2
- Woods C, Karpati A, Grein T, McCarthy N, et al. . An outbreak of Rift Valley fever in northeastern Kenya, 1997–98. Emerg Infect Dis. 2002; 8:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Animal Health Information Database (WAHID). Interface (2008) Rift Valley Fever [Internet]. Follow-up report No. 5. Report reference, OIE Ref: 7370, Report Date: 23/09/2008, Country: Swaziland. 2008. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000007370_20080923_171407.pdf
- World Animal Health Information Database (WAHID). Interface (2009) Rift Valley Fever [Internet]. Follow-up report No. 7. Report reference, OIE Ref: 7891, Report Date: 16/03/2009, Country: South Africa. 2009a. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000007891_20090317_124907.pdf
- World Animal Health Information Database (WAHID). Interface (2009) Rift Valley Fever [Internet]. Follow-up report No. 5. Report reference: RVF KZN 2009, OIE Ref: 8312, Report Date: 23/07/2009, Country: South Africa. 2009b. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000008312_20090723_175454.pdf
- World Animal Health Information Database (WAHID). Interface (2010) Rift Valley Fever [Internet]. Follow-up report No. 17. Report reference: Free State Bultfontein, OIE Ref: 9982, Report Date: 29/11/2010, Country: South Africa. 2010a. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000009982_20101129_173322.pdf
- World Animal Health Information Database (WAHID). Interface (2010) Rift Valley Fever [Internet]. Follow-up report No. 3. Report reference, OIE Ref: 9983, Report Date: 29/11/2010, Country: Botswana. 2010b. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000009983_20101129_172841.pdf
- World Animal Health Information Database (WAHID). Interface (2010) Rift Valley Fever [Internet]. Follow-up report No. 3. Report reference: Zwaardraai, OIE Ref: 8937, Report Date: 08/02/2010, Country: South Africa. 2010c. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000008937_20100208_164327.pdf
- World Animal Health Information Database (WAHID). Interface (2011) Rift Valley Fever [Internet]. Follow-up report No. 3. Report reference:, OIE Ref: 10294, Report Date: 25/02/2011, Country: Namibia. 2011a. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000010294_20110225_165922.pdf
- World Animal Health Information Database (WAHID). Interface (2011) Rift Valley Fever [Internet]. Follow-up report No. 14. Report reference: RVF2011, OIE Ref: 11073, Report Date: 28/09/2011, Country: South Africa. 2011b. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000011073_20110929_143957.pdf
- World Animal Health Information Database (WAHID). Interface (2012) Rift Valley Fever [Internet]. Follow-up report No. 2. Report reference: RVF 1, OIE Ref: 11783, Report Date: 20/03/2012, Country: Namibia. 2012. Available at www.oie.int/wahis_2/temp/reports/en_fup_0000011783_20120320_180935.pdf
- World Organization for Animal Health. Terrestrial Animal Health Code General provisions. 2011