Abstract
Background: The use of chronic opioid therapy (COT) has risen dramatically in recent years, especially among women. However, little is known about factors influencing overall pain and function (global pain status) among COT users. Characterizing the typical experiences of COT patients by age–sex group could help clinicians and patients better weigh the risks and benefits of COT. Thus, we sought to characterize global pain status among COT users in community practice by age and sex.
Methods: Telephone survey of 2,163 health plan members aged 21–80 years using COT. We assessed average/usual pain (0–10 scale); pain-related interference (0–10); activity limitation days, last 3 months; and pain impact, last 2 weeks (0–11). Status on each indicator was classified as low (better pain/function), moderate, or high (worse pain/function). Global pain status was categorized as favorable if 2–4 indicators were low and 0–1 was high and unfavorable if 2–4 indicators were high and 0–1 was low.
Results: Among female COT patients, 15% (vs. 26% of males) had favorable global pain status and 59% (vs. 42% of males) had unfavorable status. Under age 65 years, women fared more poorly than men on every indicator. Among 65- to 80-year-olds, women and men had similar global pain status.
Conclusions: Although pain and function among COT users vary considerably, only one in five reported low pain levels and high levels of function. Young and middle-aged women seem to be at particularly high risk for unfavorable global pain status. More research is needed about how to best manage pain in this group.
Introduction
Over the past two decades, use of opioids for chronic noncancer pain has increased substantially.1,2 Approximately 4%–5% of Americans are now using opioids long term over months or years on a daily or intermittent basis.3 Although increases in chronic opioid therapy (COT) have occurred in all adult age groups and in both sexes, rates of COT use rise with age and are higher in women than in men in all age groups.4 It is estimated that 5%–6% of women aged 45–64 years and 8%–9% of women over age 65 without cancer are using COT.4 Nevertheless, little is known about overall levels of pain and social role function/disability (i.e., global pain status) among COT users and whether global pain status varies across age–sex groups.
Research on sex differences in responses to prescription opioids has focused on the short-term effects of a range of opioids for acute pain. A recent meta-analysis examining studies of patient-controlled analgesia using mu opioids for postoperative pain found a significantly greater opioid effect in women than in men.5 A separate meta-analysis in the same article found mixed action mu/kappa opioids to also be significantly more effective in women than in men for post-operative pain. However, to our knowledge, there are no studies examining sex differences in the effectiveness of long-term opioid use, an important question given the higher rates of COT use among women.
Elderly women are the age group most likely to be prescribed opioids long term, but a 2009 American Geriatrics Society guideline panel6 stated that, “evidence of long-term effectiveness [of opioids] for persistent noncancer pain conditions in all age groups is lacking” (p. 1338). Although opioid use among COT patients is often sustained for years, Furlan et al.7 reported that 74% of randomized clinical trials (RCTs) of opioid therapy had durations of less than 6 weeks, 24% lasted 11–16 weeks, and only one lasted longer (24 weeks). RCTs evaluating COT include only 1,500 person-years of observation,7 compared to 1,800,000 person-years for trials on antihypertensive agents, 753,000 for statins, and 117,000 for nonsteroidal inflammatory drugs.8,9 Thus, these trials have been too brief and their sample sizes too small to adequately evaluate effects of COT on global pain status or on safety outcomes.10 Although 63% of the participants in clinical trials have been women,7 the small sample sizes in these studies have also precluded subgroup analysis by sex.
Furthermore, few observational studies have evaluated pain and functional status among those using opioids in the community.11–13 Two studies11,12 found that those using opioids for chronic pain had poorer pain and disability status than those with chronic pain not using opioids, although it is not possible to determine causality for these findings (e.g., whether patients with higher pain and disability to begin with were more likely to be given opioids, and/or if opioids caused poorer outcomes). A third study focused on quality of life in daily opioid users13 and found that those on moderate opioid doses (20–40 mg daily morphine equivalent dose [MED]) had significantly better quality of life than those on higher doses and those with chronic pain not on opioids. However, none of these studies examined the association of COT patient sex or age with patient report of pain and function.
Thus, the aims of the current study were to: (1) characterize global pain status, including pain intensity and functional status, among COT users in community practice; and (2) describe the variation in global pain status across age–sex groups. In the absence of data from large, long-term randomized clinical trials in community practice settings, characterizing the pain and functional status of typical COT patients of a given age and sex may help patients, providers, and policy makers weigh the likely outcomes experienced by COT patients, the majority of whom are female, against the potential risks.
Materials and Methods
We conducted a cross-sectional survey of patients taking opioids long-term for pain in two integrated health care delivery systems. Data on pain intensity and functional status as well as a composite index of global pain status derived from these measures are presented by age-sex group.
Settings and opioid use data
The data presented here were collected by telephone survey as part of the CONSORT (Consortium to Study Opioid Risks and Trends) study, which investigated long-term use of opioids for noncancer pain among adults enrolled in Group Health (GH) in Washington State, and in Kaiser Permanente of Northern California (KPNC).14 These two health plans serve over 1% of the United States population (approximately 4 million people). All CONSORT procedures were approved by the institutional review boards of both organizations.
Both GH and KPNC maintain automated encounter data for all covered pharmacy and medical services. Over 90% of enrollees at both health plans obtain nearly all their prescription medications through their health plan pharmacies.15,16 Pharmacy files include generic drug name, dose, quantity, date, and days' supply dispensed, as well as other information. Descriptors of opioid use were estimated from these data using methods described in detail elsewhere.14 To estimate average daily dose, the total morphine equivalent dose for the 90 days prior to each participant's interview date was divided by 90.
Sample
Potential participants were identified from automated health care data and considered eligible if they: (1) were 21–80 years old; (2) had been continuously enrolled in the health plan for at least 1 year prior to the sample selection date; (3) had filled an opioid prescription in the 30 days prior to the selection date; and (4) had filled at least 10 prescriptions for opioids and/or received at least 120 days' supply of an opioid in the year before the sample selection date, with at least 90 days between the first and last opioid dispensing in that year. Minors were excluded to avoid asking them about illegal alcohol use. Persons over age 80 years were excluded to ensure sufficient sample size at younger ages to study risk factors for prescription opioid abuse (which is more common among younger people), as this was a primary aim of this National Institute on Drug Abuse-funded CONSORT study. Enrollees with a cancer diagnosis other than nonmelanoma skin cancer, as determined from local cancer registries, or who had two or more cancer diagnoses in automated visit records for the year prior to sample selection were excluded.
Within the pool of potentially eligible patients in each health plan, stratified sampling was used to select an equal number of patients in each of three opioid dose strata: 1–49 mg, 50–99 mg, and 100 or more mg MED per day. Because most COT patients receive relatively low doses, this approach oversamples patients in the high dose groups. To obtain estimates representative of the populations from which the sample was drawn, observations were weighted in data analyses by the inverse of the probability of selection.
Survey protocol
Potential participants were mailed a letter explaining the study, along with a modest ($2–$5 value) pre-incentive. Interviewers then called potential participants, asking them to participate in the telephone interview and to allow the study team to access their electronic health care data. Consenting participants completing the interview received $20 cash (GH) or a $50 gift card (KPNC). The different payment levels were based on prior experience in each survey center concerning the incentives needed to achieve an acceptable response rate.
Measures
Demographic and health variables
Data on age and sex were gathered from the electronic records and confirmed by the interviewer. Level of education, smoking status, height, and weight (for calculation of body mass index) and current employment status were collected by interview. We categorized participants into two employment groups: (1) employed full time, in school or vocational training, or homemaker; and (2) unemployed, laid off, or looking for work, permanently disabled or unable to work for health reasons, or temporarily unable to work for health reasons. Retired individuals were not considered in calculating employment status frequencies.
Depression was assessed using the eight-item version of the Patient Health Questionnaire (PHQ-8), a validated, widely used measure of depressive symptoms.17,18 A score of 10 or greater on the PHQ-8 is considered indicative of likely major depression.
Finally, participants were asked, “Over the past month, how helpful have you found opiate pain medicines in relieving your pain?” Response categories were as follows: not at all helpful, a little helpful, moderately helpful, very helpful, and extremely helpful.
Pain and functional status
Participants were asked about the presence of pain in the past 3 months at each of 11 specific body sites, pain at any other site, and widespread pain. Detailed questions were asked about whichever pain problem the participant considered most bothersome. Four measures of pain and functional status were assessed and a composite index of global pain status was derived from these measures, as described below.
Pain intensity
Participants rated the intensity of their usual pain (for the most bothersome pain) on a scale from 0 to 10, where 0 is “no pain” and 10 is “pain as bad as could be.” Using previously validated cut points,19 we categorized responses to this question as low/mild (pain rated 0–3), intermediate/moderate (4–6), or high/severe pain (7–10).
Functioning
Pain-related activity interference was calculated as the mean of three items assessing how much, in the past 3 months, pain has interfered with (a) daily activities; (b) ability to take part in recreational, social, and family activities; and (c) ability to work, including housework, rated on a 0–10 scale where 0 is “no interference” and 10 is “unable to carry out any activities.”20 This measure has good internal consistency, test–retest reliability and validity.21 Because there is little research on categorizing this measure, we used the same cut offs as for pain intensity [i.e., low (scores of 0–3), moderate (>3 but <7), and high pain-related activity interference (7–10)].
Pain impact on daily activities was captured using 11 yes–no items adapted from the Sickness Impact Profile.22 These items (e.g., “In the past two weeks because of pain have you stayed in bed more?”) are associated with the highest levels of disability experienced by persons with pain in the general population.21 The number of “yes” responses was summed, resulting in a score of 0–11, which was categorized as low (scores of 0–3), moderate (4–6), or high (7–11).
As an additional measure of function, participants reported the number of days in the past 3 months they had been kept from their usual activities because of pain. Persons with 5 or fewer activity limitation days (i.e., one work week or less) were categorized as having a low number, those with 6–29 days as having a moderate number and those with 30–90 days as having a high number of disability days.
Global pain status
Finally, we derived a composite measure of global pain status for each participant based on the individual indicators of pain and disability—pain intensity, pain-related interference, pain impact, and activity limitation days. Persons with 2–4 individual indicators classified as low and no more than one indicator classified as high were assigned an overall status of “favorable.” Persons with 2–4 of the individual indicators classified as high and no more than one indicator classified as low were assigned an overall status of “unfavorable.” All individuals not meeting criteria for favorable or unfavorable status were classified as having intermediate status.
Statistical analysis
Descriptive data were compiled for six age–sex groups (i.e., separately for men and women in each of three age groups: 21–44 years old, 45–64 years old, and 65–80 years old). Previous research has not identified specific age thresholds related to opioid outcomes, but it has indicated some differences in use at advanced ages.23,24 We selected age groups in order to account for general sociocultural life changes, involving the transition from early to middle adulthood at about age 45 years, and the transition to retirement at about age 65. These cutoffs have been used in other research on opioid prescription trends.25
Analyses used SAS (version 9.2) PROC SURVEYMEANS, PROC SURVEYFREQ, and PROC SURVEYREG commands, which account for the stratified random sampling design when computing variance estimates. All analyses were weighted to adjust for oversampling of patients in the high-dose groups and for differential response based on dose stratum (see results). Thus, the analyses produce weighted estimates representative of the population sampled. For demographic and opioid use characteristics, within-sex comparisons across age groups were assessed by one-way analysis of variance for continuous variables and chi-square tests for categorical variables. Sex differences within age groups were assessed by t-tests for continuous variables and by chi-square for categorical variables.
Results
Of the 3,605 eligible patients approached, 2,163 were interviewed (60% response rate). Response rates increased with average daily opioid dose at KPNC (58% for <50 mg MED; 66% for 50 to <100 mg MED; 71% for 100+ mg MED), but not at GH. As described in the statistical analysis section, differential response rates by dose were adjusted for in the weighted analyses.
In general, participants from the two study sites were similar demographically (percent female: GH, 62.5%; KPNC, 62.6%; mean age: GH, 55.8 years; KPNC, 54.8 years; percent completing some college: GH, 69.2%; KPNC, 65.7%). However, the KPNC sample had a higher percentage of minorities (GH, 89.5% non-Hispanic white; KPNC, 76.4% non-Hispanic white; chi square p<0.0001).
Table 1 shows the distribution of COT patient characteristics and opioid use for each age–sex group. A larger proportion in the oldest group reported that they were unemployed, laid off, or not working for health reasons, compared with younger persons of the same sex. The percentage of persons on an opioid dose of 120 mg or more MED also differed by age for both sexes, with higher rates of high-dose usage in younger men and younger and middle-aged women. Accounting for sampling and nonresponse rates by dose, there were no statistically significant sex differences in patient characteristics or opioid use variables for the 65- to 80-year-olds.
Table 1.
Participant Characteristics by Age and Sex
| Age 21–44 years | Age 45–64 years | Age 65–80 years | ||||
|---|---|---|---|---|---|---|
| Participant characteristic | Men (n=126) | Women (n=289) | Men (n=522) | Women (n=799) | Men (n=158) | Women (n=269) |
| Education: partial college or more (%) | 62.3% | 72.3% | 65.6% | 70.4% | 68.5% | 61.1% |
| Employment (%)a,b | ||||||
| Working/student/homemaker | 75.0% | 72.4% | 65.8% | 56.6% | 38.2% | 52.2% |
| Unemployed/laid off/not working for health reasons | 25.0% | 27.6% | 34.2%c | 43.4%c | 61.8% | 47.8% |
| [% “missing” employment status (retired)] | [1.0%] | [2.2%] | [8.0%] | [10.5%] | [65.7%] | [50.8%] |
| Mean BMI | 29.4 | 29.9 | 30.3c | 31.9c | 29.8 | 30.2 |
| Current smoker (%)a,b | 32.8% | 27.5% | 25.5% | 27.2% | 13.1% | 14.7% |
| Depressed (PHQ-8 score ≥10) (%)a | 35.1%c | 52.2%c | 33.9%c | 48.8%c | 26.9% | 32.1% |
| Using opioids for >1 pain condition (%) | 61.8% | 67.4% | 60.3% | 65.0% | 54.0% | 63.5% |
| Daily opioid dose ≥120 mg MED (%)a,b | 17.2% | 14.9% | 15.3% | 13.2% | 7.3% | 7.7% |
n's are unweighted; percentages and means are weighted for stratified sampling and differential response rate by dose.
Significant (p<0.05) age difference within men.
Significant (p<0.05) age difference within women.
Significant (p<0.05) sex difference within age group.
BMI, body mas index; MED, morphine equivalent dose; PHQ-8, eight-question Patient Health Questionnaire.
As shown in Table 2, only 11.4% of COT patients (9.5% of women and 14.6% of men) reported average pain in the mild range (0–3 out of 10). In every age group, women were more likely than men to report high pain intensity. Among women aged 65 years or older, high pain intensity (7–10) was the norm.
Table 2.
Usual Pain Intensity, Last Three Months
| Age 21–44 years | Age 45–64 years | Age 65–80 years | All Ages | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual pain (0–10 scale) | Men | Women | Men | Women | Men | Women | Men | Women | Total |
| Low (0–3) | 8.0% | 7.0% | 17.1% | 9.6% | 11.3% | 11.5% | 14.6% | 9.5% | 11.4% |
| Moderate (4–6) | 72.8% | 51.3% | 56.0% | 56.7% | 50.0% | 42.2% | 57.1% | 52.4% | 54.2% |
| High (7–10) | 19.2% | 41.8% | 27.0% | 33.7% | 38.7% | 46.3% | 28.3% | 38.1% | 34.4% |
| Mean | 5.2 | 5.9 | 5.4 | 5.9 | 5.8 | 6.2 | 5.4 | 5.9 | 5.8 |
Percentages and means are weighted for stratified sampling and differential response rate by dose.
Table 3 shows the distributions of the measures of pain-related function. Overall, 47% of COT patients rated interference with usual activities to be moderate and 37% reported high interference. About 60% of patients overall, including more than half the patients in each age–sex group, endorsed 7–11 of the 11 Sickness Impact Profile items. In every age group, women were more likely to report high pain impact than their male counterparts were. Almost half of patients (49%) reported experiencing 30–90 pain-related activity limitation days in the past 3 months. About one-quarter of both women and men reported that pain limited their activity every day.
Table 3.
Measures of Pain-Related Function: Pain-Related Interference, Impact on Daily Activities, and Pain-Related Disability Days
| Age 21–44 years | Age 45–64 years | Age 65–80 years | All Ages | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | Total | |
| Pain-related interferencea | |||||||||
| Low (0–3) | 25.6% | 8.3% | 19.7% | 12.0% | 21.5% | 20.6% | 20.9% | 13.1% | 16.0% |
| Moderate (>3–<7) | 47.5% | 46.7% | 54.1% | 46.4% | 35.2% | 43.0% | 49.1% | 45.7% | 47.0% |
| High (7–10) | 26.9% | 45.0% | 26.2% | 41.6% | 43.3% | 36.5% | 30.0% | 41.1% | 37.0% |
| Mean | 5.0 | 6.3 | 5.1 | 5.9 | 5.4 | 5.7 | 5.2 | 5.9 | 5.6 |
| Pain impact on daily activitiesb | |||||||||
| Low (0–3) | 18.7% | 17.2% | 24.6% | 13.6% | 33.9% | 24.0% | 25.8% | 16.6% | 20.0% |
| Moderate (4–6) | 27.1% | 17.4% | 21.9% | 18.4% | 15.6% | 21.4% | 21.3% | 18.9% | 19.8% |
| High (7–11) | 54.2% | 65.4% | 53.4% | 68.0% | 50.5% | 54.6% | 52.9% | 64.5% | 60.2% |
| Mean | 6.5 | 7.4 | 6.2 | 7.3 | 5.4 | 6.4 | 6.1 | 7.1 | 6.7 |
| Pain-related disability daysc | |||||||||
| Low (0–5) | 37.1% | 23.1% | 45.2% | 23.8% | 34.2% | 32.2% | 41.7% | 25.5% | 31.6% |
| Moderate (5–29) | 19.0% | 24.6% | 17.9% | 21.4% | 9.3% | 17.8% | 16.3% | 21.3% | 19.4% |
| High (30–90) | 43.9% | 52.3% | 36.8% | 54.8% | 56.5% | 50.0% | 42.0% | 53.2% | 49.0% |
| Mean | 30.1 | 35.2 | 30.5 | 41.3 | 45.2 | 38.5 | 33.5 | 39.4 | 37.3 |
Percentages and means are weighted for stratified sampling and differential response rates by dose.
Pain-related interference in the last 3 months, rated on a 0–10 scale.
Impact on daily activities in the last 2 weeks, rated on a 0–11 scale.
Pain-related disability days in the last 3 months (0–90 days).
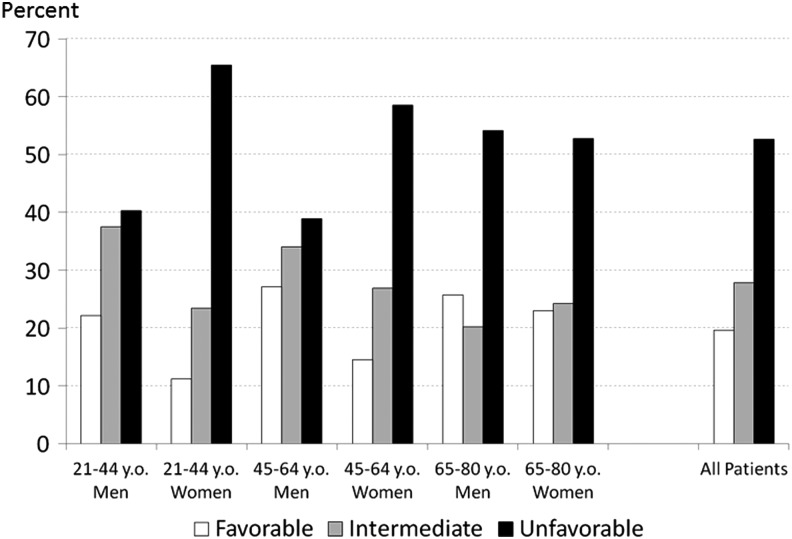
Only 19.6% of COT patients were classified as having a favorable global pain status, compared with 27.8% with intermediate status and 52.6% with unfavorable status (Fig. 1). In all age-sex groups, the most common overall status was “unfavorable.” Within the two younger age groups, women were substantially more likely than men to have unfavorable status (66% of women vs. 40% of men among 21- to 44-year-olds; 59% of women vs. 39% of men in those 45–64 years). Among the oldest group, global pain status in men and women was similar, with slightly more than half of both men and women experiencing unfavorable status. For women, the percent with an unfavorable overall pain status decreased across the age groups, whereas for men, the probability of an unfavorable global pain status was highest in the oldest group.
FIG. 1.
Percentage of patients with favorable, intermediate, and unfavorable global pain status, by age and sex. y.o., years old.
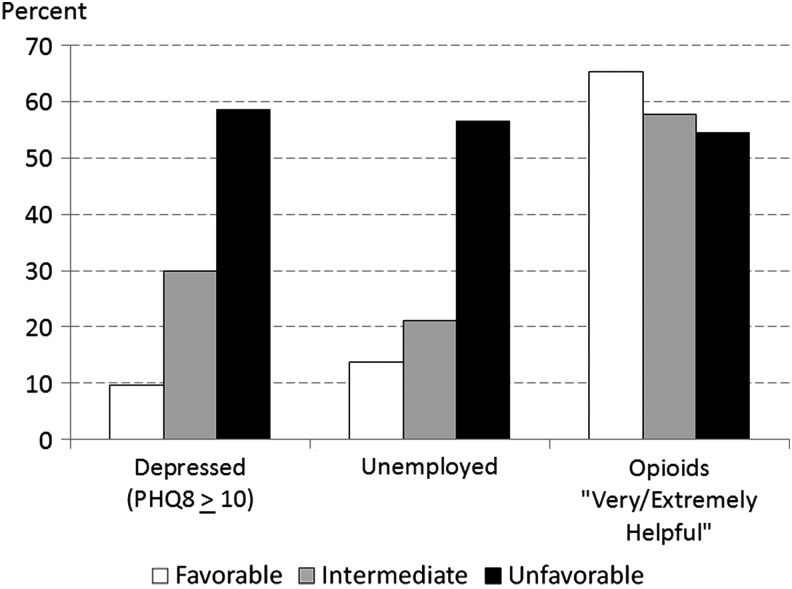
We assessed differences in other health and function indicators for patients categorized as having favorable, intermediate or unfavorable global pain status (Fig. 2). In the sample as a whole, COT patients with unfavorable global pain status were more likely to be doing poorly on other indicators of health and function. Over 58% of patients with unfavorable global pain status met PHQ criteria for major depression, and 56.6% were unemployed, laid off, or not working for health reasons. Among those with favorable global pain status, rates of depression (9.6%) and unemployment (13.7%) were substantially lower. The associations of depression and unemployment with unfavorable global pain status held across all age–sex groups. However, regardless of global pain status, in every age–sex group, the majority of patients rated opioids as very or extremely helpful in relieving pain.
FIG. 2.
Association of depression, unemployment and self-rated opioid effectiveness with global pain status. “Unemployed” includes persons who were laid off or not working for health reasons. Ratings of opioid helpfulness were in response to the question, “Over the past month, how helpful have you found opiate pain medicines in relieving your pain?” PHQ-8, eight-item Patient Health Questionnaire.
Discussion
We found substantial variability in global pain status among persons using opioids long-term for chronic noncancer pain. About one in five COT patients had favorable global pain status, slightly more than a quarter had intermediate status and over half had an unfavorable status. There were notable differences by sex and age. Women under 65 years of age had worse pain and functional status than men of comparable age, with approximately 6 in 10 having unfavorable status. Among patients aged 65–80 years, pain and functional status indicators among men and women were generally similar, with slightly over half of both men and women categorized as having unfavorable global pain status. Persons with unfavorable global pain status had high rates of depression and unemployment. Although patients with poor global pain status were less likely to rate opioids as helpful, the majority in every age–sex group still reported that they found opioids very or extremely helpful for relieving pain.
To our knowledge, this is the first study to report global pain status or to examine variability by sex among adult COT patients in community practice. A limitation of the CONSORT study, from which these data come, is that it did not include the entire age spectrum (minors and COT patients over 80 years of age were excluded.) In addition, this study is limited by its cross-sectional design and by the lack of a comparison group of patients with pain not using COT. Given cross-sectional data, we could not assess whether pain and function had improved or deteriorated from the time these patients began using opioids. We also do not know what the status of these individuals would have been had they not been using COT. By the patients' own report, opioids were usually perceived to be helpful in relieving pain. However, average pain levels were moderate to severe for the large majority of patients. A recent study26 found that pain intensity levels of 4 or greater on a 0–10 scale were considered unacceptable by patients with a range of pain conditions. By this patient-centered criterion, only 9.5% of women (and 14.6% of men) in this study had an acceptable pain outcome.
Young and middle-aged women and older adults of both sexes were the groups most likely to report high pain intensity and poor function when using opioids long term. The reasons for these results are not clear. It is possible that there are age and/or sex effects on opioid pharmacokinetics (PK) or pharmacodynamics (PD). The literature on age and sex differences in PK and PD for opioids used clinically is sparse.27 One high-quality PKPD analysis28 of morphine analgesia found similar morphine pharmacokinetics and similar metabolism of the drug in young men and women. However, morphine potency was 80%–100% greater in women, and time course (over 7 hours) was different with both slower analgesia onset and slower analgesia offset in women. If these findings generalized to other opioids, one would expect that women would have a more favorable response to opioids than men do, as seems to be the case in the short term.5 However, the PKPD differences do not explain our findings with COT patients, which indicate a less favorable picture for women. In terms of age, a recent systematic review27 found eight studies with data on opioid effect in postmenopausal women versus similarly aged men. Two of the studies reported the loss of sex differences after age 60 years, whereas six studies found that sex differences persisted. Clearly, more research is needed in this area.
Another possible reason for our observed findings is hormonal. The endogenous opioid systems of reproductive-aged men and women differ,29 and estrogen has been found to interact with the mu opioid system in women.30 Thus, it is reasonable to expect that responses to exogenous opioids may differ as well. In addition, it may be that psychosocial role norms allow women to express more pain and disability than men. However, opioids appear to be more effective in women than in men in the short term,5 which would suggest that gender-based response tendencies cannot entirely account for the differences observed in our study. We speculate that opioid medications may become less effective in reproductive-aged women when used long term, possibly because of their interaction with endogenous opioid systems. It is also possible that opioids are equally effective or ineffective for both sexes, but that women have higher levels of pain prior to starting opioid use. This would certainly be plausible, given that women, on average, report higher levels of pain than men in response to most laboratory pain stimuli.31 Women might also be at greater risk than men for experiencing opioid-induced hyperalgesia,32 although there is little research on this topic and the relevance of opioid-induced hyperalgesia to clinical pain remains controversial.33 Finally, the higher rates of depression in women may predispose them to a more unfavorable global pain status. Unfortunately, our cross-sectional study design does not permit analyses assessing whether specific factors (e.g., depression) mediate the observed relationships between age–sex group and global pain status. This topic should be examined in future studies.
In summary, this study of global pain status among community-practice COT patients suggests that, although pain and function were highly variable across patients, the global pain status of the majority of patients was unfavorable. Sex and age differences in pain and function were also apparent. Younger women and both men and women over age 65 receiving COT experience substantial pain and disability, but even among younger men, moderate-to-severe pain is typical and high levels of disability are not uncommon.
Conclusions
Clinicians and patients with chronic pain are faced with deciding among treatment options, including long-term use of opioids, with inadequate evidence from randomized clinical trials regarding long-term safety and effectiveness. Our observational data indicate that for typical COT patients in community practice the probability of experiencing good pain control and favorable levels of functioning is relatively low. Young and middle-aged women seem to be at particularly high risk for unfavorable global pain status. Given the unique risks that opioid use poses to women in this age group (e.g., reduced fertility and potential effects on the neonate associated with maternal use during pregnancy),34 these unfavorable pain and disability ratings in the presence of patient report of opioid helpfulness make decisions about opioid use in women of reproductive age particularly problematic. In all age groups, the most likely outcomes experienced by patients using opioids long term need to be balanced against known risks of opioid use in both sexes, including opioid misuse, addiction and overdose,35 depression, endocrinopathy, and chronic constipation.36
Acknowledgments
This study was supported by National Institutes of Health Grants R01 DA022557 (Drs. Campbell, Merrill, Von Korff and Ms. Saunders), R01 AG034181 (Drs. LeResche, Shortreed, Thielke, Von Korff and Ms. Saunders), and K23 AG028954 and R03 AG042930 (Dr. Dublin).
Author Disclosure Statement
Dr. Von Korff and Ms. Saunders have been supported on grants to Group Health Research Institute (GHRI) from Pfizer Inc. Dr. Shortreed has received salary support through grants funded by Bristol Meyers Squibb and Pfizer to GHRI. Dr. Campbell has been supported as an Investigator on a Division of Research subcontract portion of a grant to the Center for Health Research (Kaiser-Permanente Northwest) funded by Purdue Pharmaceuticals. Ms. Saunders owns stock in Merck. Dr. Dublin received a Merck/American Geriatrics Society New Investigator Award. Drs. LeResche, Thielke and Merrill have no potential conflicts of interest.
References
- 1.Kenan K, Mack K, Paulozzi L. Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med 2012;6:e41–e47 [PMC free article] [PubMed] [Google Scholar]
- 2.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician 2010;13:401–435 [PubMed] [Google Scholar]
- 3.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell CI, Weisner C, LeResche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health 2010;100:2541–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, Sarton E. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 2010;151:61–68 [DOI] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331–1346 [DOI] [PubMed] [Google Scholar]
- 7.Furlan A, Chaparro LE, Irvin E, Mailis-Gagnon A. A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag 2011;16:337–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: A review of the evidence. Clin J Pain 2008;24:469–478 [DOI] [PubMed] [Google Scholar]
- 11.Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain 2006;125:172–179 [DOI] [PubMed] [Google Scholar]
- 12.Fredheim OM, Mahic M, Skurtveit S, Dale O, Romundstad P, Borchgrevink PC. Chronic pain and use of opioids: A population-based pharmacoepidemiological study from the Norwegian Prescription Database and the Nord-Trøndelag Health Study. Pain 2014;155:1213–1321 [DOI] [PubMed] [Google Scholar]
- 13.Dillie KS, Fleming MF, Mundt MP, French MT. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. Am Board Fam Med. 2008;21:108–117 [DOI] [PubMed] [Google Scholar]
- 14.Von Korff M, Saunders K, Ray GT, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom B, ed. Pharmacoepidemiology, 4th ed. West Susgender, England: John Wiley and Sons, 2005:223–239 [Google Scholar]
- 16.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom B, ed. Pharmacoepidemiology, 4th ed. West Susgender, England: John Wiley and Sons, 2005:241–259 [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A systematic review. Gen Hosp Psychiatry 2010;32:345–359 [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173 [DOI] [PubMed] [Google Scholar]
- 19.Jensen MP, Smith DG, Ehde DM, Robinsin LR. Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and severe pain. Pain 2001;91:317–322 [DOI] [PubMed] [Google Scholar]
- 20.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133–149 [DOI] [PubMed] [Google Scholar]
- 21.Von Korff M. Epidemiological and survey methods: Assessment of chronic pain. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. New York: The Guilford Press, 2001:603–618 [Google Scholar]
- 22.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: Development and final revision of a health status measure. Med Care 1981;19:787–805 [DOI] [PubMed] [Google Scholar]
- 23.Auret K, Schug SA. Underutilisation of opioids in elderly patients with chronic pain: Approaches to correcting the problem. Drugs Aging 2005;22:641–654 [DOI] [PubMed] [Google Scholar]
- 24.Sale JE, Thielke S, Topolovec-Vranic J. Who is addicted to, and dying from, prescription opioids? J Am Geriatr Soc 2010;58:1401–1402 [DOI] [PubMed] [Google Scholar]
- 25.Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med 2010;11:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tubach F, Ravaud P, Martin-Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: Results from a prospective multinational study. Arthritis Care Res 2012;64:1699–1707 [DOI] [PubMed] [Google Scholar]
- 27.Niesters M, Dahan A, Aarts L, Sarton E. Sex differences in analgesic response. In: Chin ML, Fillingim RB, Ness TJ, eds. Pain in women. New York: Oxford University Press, 2013:54–66 [Google Scholar]
- 28.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: An experimental study in healthy volunteers. Anesthesiology 2000;93:1245–1254 [DOI] [PubMed] [Google Scholar]
- 29.Zubieta JK, Smith YR, Bueller JA, et al. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci 2002;22:5100–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 2006;26:5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain 2009;10:447–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479–496 [DOI] [PubMed] [Google Scholar]
- 33.Tompkins DA, Campbell CM. Opioid-induced hyperalgesia: Clinically relevant or extraneous research phenomenon. Curr Pain Headache Rep 2011;15:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med 2012;131:1181–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC). Vital signs: Overdoses of prescription opioid pain relievers and other drugs among women—United States, 1999–2010. MMWR Morb Mortal Wkly Rep 2013;62:537–542 [PMC free article] [PubMed] [Google Scholar]
- 36.Baldini A, Von Korff M, Lin EHB. A review of potential adverse effects of long-term opioid therapy: A practitioner's guide. Prim Care Companion CNS Disord 2012;14:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]