Abstract
Introduction
We previously reported the prognostic significance of the lung adenocarcinoma immune microenvironment. In this study, we preformed comprehensive analysis of immune markers and their associations with prognosis in patients with lung squamous cell carcinoma.
Methods
We reviewed surgically resected, solitary lung squamous cell carcinoma patients (n = 485; 1999 to 2009) that were randomly split into a training cohort (n = 331) and validation cohort (n = 154). We constructed tissue microarrays and performed immunostaining for CD3, CD45RO, CD8, CD4, FoxP3, CD20, CD68, CXCL12, CXCR4, CCR7, IL-7R, and IL-12Rβ2. Overall survival (OS) was analyzed using the log-rank test and the Cox proportional hazards model.
Results
Analysis of single immune cell infiltration revealed that high tumor-infiltrating CD10+ neutrophils were associated with worse prognoses in the training cohort (P = 0.021). Analysis of biologically relevant immune cell combinations identified that patients with high CD10+ neutrophil and low CD20+ lymphocyte had a significantly worse OS (5-year OS, 42%) than those with other combinations of CD10 and CD20 (5-year OS, 62%; P < 0.001); this was confirmed in the validation cohort (P = 0.032). For the multivariate analysis, high CD10/low CD20 immune cell infiltration was an independent predictor of OS in both the training cohort (HR = 1.61, P = 0.006) and validation cohort (HR = 1.75; P = 0.043).
Conclusions
High CD10+/low CD20+ immune cell infiltration ratio is a significant prognostic factor of lung squamous cell carcinoma. Immunomodulatory therapy of tumor-specific neutrophil and B lymphocyte responses may have applicability in the treatment of lung squamous cell carcinoma.
Keywords: tumor infiltrating lymphocytes, immune response, non-small cell lung cancer, survival
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality worldwide for men and the second leading cause for women.1, 2 Two major histologic types of lung cancer are squamous cell carcinoma and adenocarcinoma. While squamous cell carcinoma currently comprises a higher proportion of diagnosed lung cancers in males,3 the rate of squamous cell carcinoma has been increasing among females and declining among males in North America.4
To date, tumor-node-metastasis (TNM) stage is the most reliable prognostic predictor and dictates therapeutic decisions for solid malignancies, including non-small cell lung cancer (NSCLC).5 Prognostic value of tumor-infiltrating immune cells has been proven in solid malignancies, which is influenced by type, density, and location of immune cells.6–12 Tumor-infiltrating immune cells have also been identified as a prognostic factor in NSCLC, but these reports included heterogeneous cohorts in tumor histologic types.13–18
Recently, using a homogeneous large cohort of stage I lung adenocarcinoma patients, we have identified high tumor-infiltrating FoxP3/CD3 lymphocytes ratio in tumor-related stroma, overexpression of interleukin-7 receptor (IL-7R), and loss of IL-12Rβ2 expression in tumor cells as independent prognostic factors of stage I lung adenocarcinoma.19 However, prognostic utility of immune markers, such as tumor-infiltrating immune cell and cytokine (receptors) expression remains unknown in lung squamous cell carcinoma. In this study, we investigated whether immune markers, including type and density of tumor-infiltrating immune cells and tumoral cytokine (receptor) expression, correlated with clinical outcomes in a uniform cohort of patients with resected lung squamous cell carcinoma. Additionally, we also evaluated peripheral blood immune markers and inflammation scores on H&E slides.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK). We reviewed patients with therapy-naïve, solitary lung squamous cell carcinoma who had undergone surgical resection at MSK between 1999 and 2009; 485 of those patients had tumor slides that were available for histologic evaluation. Clinical data were collected from the prospectively maintained Thoracic Surgery Service Lung Carcinoma Database, and disease stage was assigned based on the 7th edition of the American Joint Committee on Cancer TNM Staging Manual.20
Preoperative peripheral blood cell count test results—which included total white blood cell, neutrophil, and lymphocyte counts—were available for 466 patients. The neutrophil to lymphocyte ratio (NLR) was defined as absolute neutrophil count divided by absolute lymphocyte count, as previously reported.21–29
Histologic evaluation
All available hematoxylin and eosin (H&E) stained slides were reviewed by two pathologists (K.K. and W.D.T.), who were blinded to patient clinical outcomes, using an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan) with a standard 22-mm diameter eyepiece.
With regard to histologic inflammation scores based on H&E-stained slides,30 acute inflammation (neutrophil infiltration), chronic inflammation (lymphocyte and plasma cell infiltration), and lymph follicle (any lymphoid nodule) count were tabulated for each case. Percentage of acute inflammation area was recorded using 3 high-power fields (HPF) at ×400 magnification of the region with the most severe inflammatory reaction; this was done after all tumor slides were scanned using intermediate-power fields at ×100 magnification. Percentage of chronic inflammation area and lymph follicle count were recorded using 3 low-power fields at ×40 magnification of the region with the most severe inflammatory reaction, after reviewing all tumor slides at scanning magnification.
Additionally, pleural invasion, which was classified as absent (PL0) or present (PL1, PL2 and PL3),20 and lymphovascular invasion were investigated.
Tissue microarray
In our recent publications on lung adenocarcinoma, both tumoral and stromal cores were used for tissue microarrays.19 However, on review of H&E stained slides, we evaluated only tumoral cores because the tumoral component was intermingled with the stromal component at a greater level in squamous cell carcinoma than in adenocarcinoma; also, it was difficult to construct tissue microarrays composed of purely tumoral or stromal cores.
Formalin-fixed, paraffin-embedded tumor specimens (n = 451) were used for tissue microarray construction. We marked 3 representative tumor areas with the most severe inflammatory reaction on H&E stained slides and, using an automated tissue arrayer ATA-27 (Beecher Instruments, Sun Prairie, WI, USA), we arrayed cylindrical 0.6 mm tissue cores from corresponding paraffin blocks into recipient blocks; this resulted in 5 tissue microarray blocks. In total, there were 447 available cases with adequate cores for immunohistochemical analysis.
Immunohistochemistry and scoring of immune markers
Briefly, 4-μm sections from the tissue microarray blocks were deparaffinized in xylene and dehydrated in graded alcohols. Standard avidin-biotin-peroxidase complex technique was used to immunohistochemically stain the immune markers (CD3, CD45RO, CD8, CD4, FoxP3, CD20, CD68, CXCL12, CXCR4, CCR7, IL-7R, and IL-12Rβ2), as we previously reported.19 In addition, the anti-CD10 antibody (56C6, Vector Laboratories; diluted at 1:50) was used as a neutrophil granulocyte marker.31, 32 Sections were stained using a Ventana Discovery XT Automated Immunohistochemical Stainer (Ventana Medical Systems, Tucson, AZ, USA), according to the manufacturer's guidelines. Diaminobenzidine was used as the chromogen and hematoxylin was used as the nuclear counterstain. Positive control tissues were stained in parallel with the study cases.
Under HPF at ×200 magnification, the amount of tumor-infiltrating immune cells that were positive for each marker was counted in each core. The score for each patient was determined by averaging the number of positive cells for all cores that were available for analysis.19 Additionally, the ratio of T lymphocyte subtypes (CD45RO, CD8, CD4, and FoxP3) to pan T cells (CD3) was calculated.
Scoring of cytokines and cytokine receptors was based on distribution and intensity of staining.19, 33 Distribution was scored as 0 (0%), 1 (1–50%), and 2 (>50%) to indicate percentage of positive cells in each core. Intensity of the staining was scored as 0 (no expression), 1 (mild expression), 2 (intermediate expression), and 3 (strong expression). Distribution and intensity scores were summed into a total score (0–5), and the average score of all available cores was considered the score for each patient.
Statistical analysis
The entire cohort was randomly split into a training cohort (n = 331) and validation cohort (n = 154) at a 2:1 ratio, and they were stratified by temporal intervals of surgery and pathologic stage. Associations between variables were analyzed using Fisher's exact test (for categorical variables) and the Wilcoxon test (for continuous variables).
We investigated two endpoints—overall survival (OS) and cumulative incidence of recurrence (CIR). OS was estimated using the Kaplan-Meier method and associations between factors and OS were analyzed using the log-rank test and stratified by pathologic stage. Using the cutoffs from our previous studies, immune cells that were positive for each marker were dichotomized into low and high groups.19, 32 Peripheral blood cell count, histologic inflammation score, ratio of T lymphocyte subtypes (CD45RO, CD8, CD4, and FoxP3) to pan T cells (CD3), and tumoral cytokine (receptor) expression were dichotomized using the optimal cut-point method, and P-values were adjusted for optimal search.34 OS was defined as time from surgery to death or last follow-up. A multivariate analysis was performed using the Cox proportional hazards regression model. Multivariate models were built to include all significant factors on univariate analyses. Any associations between pathologic factors were checked and, if any strong associations were discovered, only one factor at any given time was included in the model. Associations between factors and risk of recurrence were evaluated using competing risks methods.35, 36 CIR was estimated using a cumulative incidence function that accounted for death without recurrence as a competing event. Patients were censored if they were alive without a documented recurrence at most recent follow-up. Differences in CIR between groups were assessed using the Gray method (univariate nonparametric analysis).37
All statistical tests were 2-sided, and 5% was set as the level of significance. Statistical analyses were performed using R (version 3.0.1; R Development Core Team, Vienna, Austria) with the “maxstat,” “survival,” and “cmprsk” packages.
RESULTS
Patient clinicopathologic characteristics and their associations with OS
Clinicopathologic characteristics of patient in the training cohort are summarized in Table 1. Median age of patients was 72 years (range, 39 to 88 years) and most had pathological stage I disease (57%), followed by stage II disease (28%), and stage III disease (15%). With regard to surgical procedure, 84% had undergone lobectomy and 16% had undergone limited resection. Eighteen percent of patients received adjuvant therapy. There were no significant differences between patient clinicopathologic characteristics identified between the training and validation cohorts.
Table 1.
Associations between clinicopathologic characteristics and overall survival in the training cohort
| Variables | n | % | 5-year OS | P | HR | (95% CI) |
|---|---|---|---|---|---|---|
| Age, years | 0.050 | |||||
| ≤65 | 85 | 26 | 63% | ref. | ||
| >65 | 246 | 74 | 56% | 1.44 | (1.00 – 2.07) | |
| Sex | 0.066 | |||||
| Female | 134 | 40 | 65% | ref. | ||
| Male | 197 | 60 | 53% | 1.32 | (0.98 – 1.78) | |
| Smoking pack-year | 0.004 | |||||
| ≤90 | 268 | 81 | 62% | ref. | ||
| >90 | 63 | 19 | 41% | 1.64 | (1.17 – 2.31) | |
| Surgery | 0.15 | |||||
| Lobectomy | 278 | 84 | 59% | ref. | ||
| Limited resection | 53 | 16 | 52% | 1.32 | (0.91 – 1.93) | |
| Adjuvant therapy | 0.90 | |||||
| No | 273 | 82 | 58% | ref. | ||
| Yes | 58 | 18 | 58% | 1.03 | (0.69 – 1.54) | |
| T classification | < 0.001 | |||||
| T1 | 146 | 44 | 67% | ref. | ||
| T2 | 142 | 43 | 54% | 1.26 | (0.92 – 1.72) | |
| T3+4 | 43 | 13 | 39% | 2.23 | (1.45 – 3.43) | |
| N classification | 0.020 | |||||
| N0 | 239 | 72 | 61% | ref. | ||
| N1 | 58 | 18 | 51% | 1.38 | (0.95 – 2.00) | |
| N2 | 34 | 10 | 45% | 1.76 | (1.12 – 2.74) | |
| Pathologic stage | < 0.001 | |||||
| Stage I | 190 | 57 | 65% | ref. | ||
| Stage II | 93 | 28 | 52% | 1.43 | (1.03 – 1.98) | |
| Stage III | 48 | 15 | 39% | 2.30 | (1.55 – 3.43) | |
| Pleural invasion | 0.010 | |||||
| Absent (PLO) | 280 | 85 | 60% | ref. | ||
| Present (PL123) | 51 | 15 | 45% | 1.49 | (1.09 – 2.05) | |
| Lymphovascular invasion | 0.013 | |||||
| Absent | 104 | 31 | 69% | ref. | ||
| Present | 227 | 69 | 52% | 1.62 | (1.12 – 2.33) |
Significant P-values are shown in bold.
OS, overall survival; HR, hazard ratio; CI, confidence interval
In the training cohort, 30% of patients (n = 99) experienced a recurrence and 57% (n = 188) died from any cause during the follow-up period (median, 49 months; range, 0.1 to 156). In the validation cohort, 26% of patients (n = 40) experienced a recurrence and 60% (n = 93) died from any cause during the follow-up period (median, 54 months; range, 0.1 to 152).
The associations between patient clinicopathologic characteristics and OS in the training cohort are summarized in Table 1. With regard to pathologic stage, 5-year OS was the worst for patients with stage III disease, followed by patients with stage II disease, and stage I disease (5-year OS, 39%, 52%, and 65%, respectively; P < 0.001). On univariate OS analysis, history of heavier smoking (>90 smoking pack-year; P = 0.004), pleural invasion (P = 0.010), and lymphovascular invasion (P = 0.013) were associated with worse OS.
Associations between white blood cell counts in peripheral blood and OS
In the training cohort, low percentage of lymphocyte (P = 0.005) and high NLR (P = 0.001) were associated with worse OS (Table 2). However, these findings were not reproduced in the validation cohort.
Table 2.
Associations between peripheral blood cell count test or histologic inflammation scores and overall survival in the training cohort
| Variables | n | % | 5-year OS | P * | HR | 95% CI |
|---|---|---|---|---|---|---|
| Complete blood count | ||||||
| White blood cell, No. | 0.32 | |||||
| Low (≤7) | 125 | 39 | 62% | ref. | ||
| High (>7) | 194 | 61 | 55% | 1.17 | (0.86 – 1.59) | |
| Neutrophil, % | 0.26 | |||||
| Low (≤77) | 264 | 83 | 60% | ref. | ||
| High (>77) | 55 | 17 | 45% | 1.24 | (0.85 – 1.79) | |
| Neutrophil, No. | 0.92 | |||||
| Low (≤4) | 84 | 26 | 57% | ref. | ||
| High (>4) | 235 | 74 | 58% | 1.02 | (0.72 – 1.43) | |
| Lymphocyte, % | 0.005 | |||||
| Low (≤15) | 71 | 22 | 38% | ref. | ||
| High (>15) | 248 | 78 | 63% | 0.62 | (0.45 – 0.87) | |
| Lymphocyte, No. | 0.26 | |||||
| Low (≤2) | 257 | 81 | 55% | ref. | ||
| High (>2) | 62 | 19 | 69% | 0.80 | (0.54 – 1.18) | |
| Neutrophil/lymphocyte ratio | 0.001 | |||||
| Low (≤5.5) | 266 | 83 | 62% | ref. | ||
| High (>5.5) | 53 | 17 | 34% | 1.82 | (1.26 – 2.62) | |
| Histologic inflammation scores | ||||||
| Acute inflammation, % | 0.013 | |||||
| Low (<5%) | 212 | 64 | 60% | ref. | ||
| High (≥5%) | 119 | 36 | 53% | 1.46 | (1.08 – 1.97) | |
| Chronic inflammation, % | 0.71 | |||||
| Low (<10%) | 196 | 59 | 59% | ref. | ||
| High (≥10%) | 135 | 41 | 56% | 1.06 | (0.79 – 1.42) | |
| Lymph follicles, No. | 0.041 | |||||
| Low (<5) | 188 | 57 | 62% | ref. | ||
| High (≥5) | 143 | 43 | 52% | 1.35 | (1.01 – 1.81) |
P-values adjusting for pathologic stage. Significant P-values are shown in bold.
OS, overall survival; HR, hazard ratio; CI, confidence interval
Associations between histologic inflammation scores and OS
In the training cohort, high degree of acute inflammation (P = 0.013) and high lymph follicle count (P = 0.041) in tumors were associated with worse OS (Table 2). However, these findings were not reproduced in the validation cohort.
Associations between immunohistochemical immune markers and OS or CIR
In the training cohort, OS of patients with high tumor-infiltrating CD10+ neutrophils was significantly worse (n = 111; 5-year OS, 52%) than those with low tumor-infiltrating CD10+ neutrophils (n = 191; 5-year OS, 60%; P = 0.021) (Table 3); however, this finding was not confirmed in the validation cohort (5-year OS, 55% [high CD10] vs. 64% [low CD10]; P = 0.37). The other single immune markers were not associated with OS.
Table 3.
Associations between tumor-infiltrating immune cell subtypes and overall survival in the training cohort
| Variables | n | % | 5-year OS | P * | HR | 95% CI |
|---|---|---|---|---|---|---|
| No. of positive cell count | ||||||
| CD3, No. | 0.38 | |||||
| Low (<50) | 91 | 30 | 61% | ref. | ||
| High (≥50) | 212 | 70 | 55% | 1.16 | (0.83 – 1.61) | |
| CD45RO, No. | 0.44 | |||||
| Low (<50) | 160 | 52 | 58% | ref. | ||
| High (≥50) | 146 | 48 | 56% | 1.13 | (0.84 – 1.51) | |
| CD8, No. | 0.52 | |||||
| Low (<50) | 165 | 54 | 59% | ref. | ||
| High (≥50) | 141 | 46 | 55% | 1.10 | (0.82 – 1.48) | |
| CD4, No. | 0.42 | |||||
| Low (<20) | 146 | 48 | 58% | ref. | ||
| High (≥20) | 160 | 52 | 57% | 1.13 | (0.84 – 1.53) | |
| FoxP3, No. | 0.29 | |||||
| Low (<20) | 135 | 44 | 59% | ref. | ||
| High (≥20) | 171 | 56 | 55% | 1.18 | (0.87 – 1.60) | |
| CD20, No. | 0.65 | |||||
| Low (<20) | 216 | 71 | 56% | ref. | ||
| High (≥20) | 87 | 29 | 62% | 0.92 | (0.66 – 1.29) | |
| CD68, No. | 0.23 | |||||
| Low (<50) | 73 | 24 | 53% | ref. | ||
| High (≥50) | 230 | 76 | 59% | 0.81 | (0.58 – 1.14) | |
| CD10, No. | 0.021 | |||||
| Low (<10) | 191 | 63 | 60% | ref. | ||
| High (≥10) | 111 | 37 | 52% | 1.43 | (1.06 – 1.94) | |
| Ratio to CD3 positive cells | ||||||
| CD45RO, % | 0.079 | |||||
| Low (≤19) | 31 | 10 | 78% | ref. | ||
| High (>19) | 272 | 90 | 55% | 1.72 | (0.93 – 3.17) | |
| CD8, % | 0.067 | |||||
| Low (≤31) | 62 | 20 | 65% | ref. | ||
| High (>31) | 241 | 80 | 55% | 1.42 | (0.97 – 2.07) | |
| CD4, % | 0.15 | |||||
| Low (≤4) | 48 | 16 | 69% | ref. | ||
| High (>4) | 255 | 84 | 55% | 1.39 | (0.88 – 2.20) | |
| FoxP3, % | 0.32 | |||||
| Low (0) | 247 | 82 | 55% | ref. | ||
| High (>0) | 56 | 18 | 66% | 0.82 | (0.55 – 1.21) | |
| Tumoral cytokine (receptor) | ||||||
| CXCL12 | 0.22 | |||||
| Low (≤4) | 180 | 59 | 59% | ref. | ||
| High (>4) | 123 | 41 | 54% | 1.21 | (0.89 – 1.63) | |
| CXCR4 | 0.52 | |||||
| Low (≤4) | 231 | 76 | 56% | ref. | ||
| High (>4) | 72 | 24 | 60% | 0.88 | (0.61 – 1.29) | |
| CCR7 | 0.21 | |||||
| Low (≤4) | 114 | 38 | 51% | ref. | ||
| High (>4) | 190 | 63 | 61% | 0.82 | (0.61 – 1.12) | |
| IL-7R | 0.34 | |||||
| Low (≤4) | 143 | 47 | 58% | ref. | ||
| High (>4) | 163 | 53 | 56% | 0.86 | (0.64 – 1.16) | |
| IL-12Rβ2 | 0.26 | |||||
| Low (≤2) | 49 | 16 | 62% | ref. | ||
| High (>2) | 255 | 84 | 56% | 1.27 | (0.84 – 1.94) |
P-values adjusting for pathologic stage. Significant P-values are shown in bold.
OS, overall survival; HR, hazard ratio; CI, confidence interval
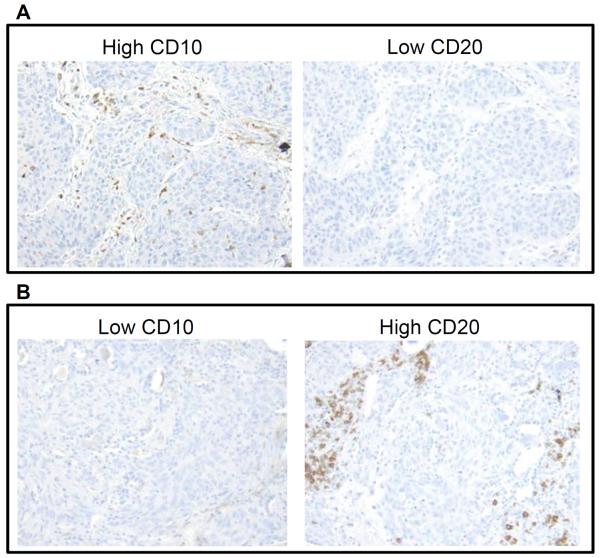
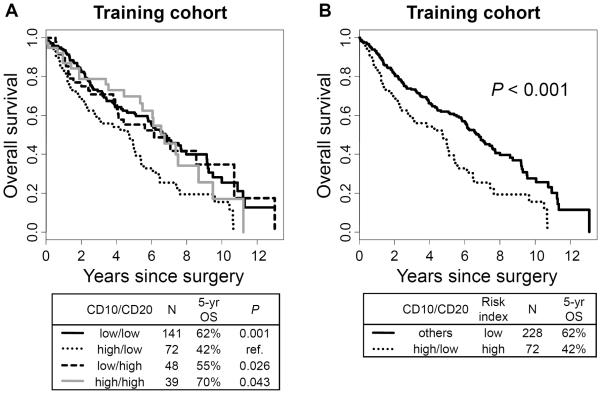
We then analyzed prognostic value by the biologically relevant combinations of two types of tumor-infiltrating immune cells and identified the combination of tumor-infiltrating CD10+ neutrophils and CD20+ lymphocytes as a powerful prognostic factor. Figure 1 represents CD10+ neutrophil and CD20+ lymphocytes infiltrating tumors. The OS of patients with high CD10+ neutrophil and low CD20+ lymphocyte infiltration (5-year OS, 42%) was significantly worse compared with those with low CD10/low CD20 (5-year OS, 62%; P = 0.001; hazard ratio [HR] = 0.55; 95% confidence interval [CI] = 0.38 – 0.79), low CD10/high CD20 (5-year OS, 55%; P = 0.026; HR = 0.58; 95% CI = 0.36 – 0.94), and high CD10/high CD20 (5-year OS, 70%; P = 0.043; HR = 0.60; 95% CI = 0.36 – 0.98) (Fig. 2A). On the basis of this observation, CD10/CD20 risk index was established as a strong predictor of OS—“high-risk” for high CD10/low CD20 and “low risk” for low CD10/low CD20, low CD10/high CD20 and high CD10/high CD20. Using this risk index in the training cohort, OS of patients with high CD10/CD20 risk index was significantly worse (n = 72; 5-year OS, 42%) than those with low risk index (n = 228; 5-year OS, 62%; P < 0.001; HR = 0.56; 95% CI = 0.4–0.78) (Fig. 2B). This finding was confirmed in the validation cohort. The OS of patients with high CD10+ neutrophil and low CD20+ lymphocyte infiltration (5-year OS, 46%) was relatively worse compared with those with low CD10/low CD20 (5-year OS, 58%; P = 0.069; HR = 0.61; 95% CI = 0.36–1.04), low CD10/high CD20 (5-year OS, 84%; P = 0.10; HR = 0.55; 95% CI = 0.27–1.13), and high CD10/high CD20 (5-year OS, 71%; P = 0.092; HR = 0.52; 95% CI = 0.24–1.11) (Fig. 3A). OS of patients with high CD10/CD20 risk index was significantly worse (n = 30; 5-year OS, 46%) than those with low risk index (n = 112; 5-year OS, 66%; P = 0.032; HR = 0.58; 95% CI = 0.35–0.96) (Fig. 3B).
Figure 1.
CD10+ neutrophil and CD20+ lymphocyte infiltration in tumors
(A) Tumor with high CD10+ neutrophil infiltration and low CD20+ lymphocyte infiltration. (B) Tumor with low CD10+ neutrophil infiltration and high CD20+ lymphocyte infiltration.
Figure 2.
Overall survival (OS) of combination of CD10+ neutrophil and CD20+ lymphocyte infiltration in the training cohort
(A) OS of patients with high CD10+ neutrophil and low CD20+ lymphocyte infiltration (5-year OS, 42%) was significantly worse compared with those with low CD10/low CD20 (5-year OS, 62%; P = 0.001; hazard ratio [HR] = 0.55; 95% confidence interval [CI] = 0.38–0.79), low CD10/high CD20 (5-year OS, 55%; P = 0.026; HR = 0.58; 95% CI = 0.36–0.94), and high CD10/high CD20 (5-year OS, 70%; P = 0.043; HR = 0.60; 95% CI = 0.36–0.98). (B) OS of patients with high CD10/CD20 risk index was significantly worse (n = 72; 5-year OS, 42%) than those with low risk index (n = 228; 5-year OS, 62%; P < 0.001; HR = 0.56; 95% CI = 0.4–0.78).
Figure 3.
Overall survival (OS) of combination of CD10+ neutrophil and CD20+ lymphocyte infiltration in the validation cohort
(A) OS of patients with high CD10+ neutrophil and low CD20+ lymphocyte infiltration (5-year OS, 46%) was relatively worse compared with those with low CD10/low CD20 (5-year OS, 58%; P = 0.069; HR = 0.61; 95% CI = 0.36–1.04), low CD10/high CD20 (5-year OS, 84%; P = 0.10; HR = 0.55; 95% CI = 0.27–1.13), and high CD10/high CD20 (5-year OS, 71%; P = 0.092; HR = 0.52; 95% CI = 0.24–1.11). (B) OS of patients with high CD10/CD20 risk index was significantly worse (n = 30; 5-year OS, 46%) than those with low risk index (n = 112; 5-year OS, 66%; P = 0.032; HR = 0.58; 95% CI = 0.35–0.96).
For the multivariate analysis of OS, adjusting for patient age, sex, smoking status (pack-year), surgical procedure, pathologic stage, and lymphovascular invasion in the training cohort (Table 4), CD10/CD20 risk index was an independent predictor of OS (high-risk vs. low-risk; HR = 1.61, P = 0.006). This finding was reproduced in the validation cohort (HR = 1.75; P = 0.043) (Table 4). Among the other variables, only pathologic stage (stage III vs. stage I) was confirmed as an independent prognostic factor in both the training (HR = 2.15; P = 0.001) and validation cohorts (HR = 2.20; P = 0.040).
Table 4.
Multivariate analysis of overall survival
| Variables | Training cohort |
Validation cohort |
|||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | ||
| Age | >65 vs. ≤65 | 1.29 | (0.87 – 1.91) | 0.20 | 2.51 | (1.36 – 4.64) | 0.003 |
| Sex | males vs. females | 1.24 | (0.89 – 1.71) | 0.20 | 1.43 | (0.88 – 2.31) | 0.15 |
| Smoking pack-year | >90 vs. ≤90 | 1.46 | (1.00 – 2.14) | 0.048 | 0.84 | (0.45 – 1.55) | 0.57 |
| Surgery | limited resection vs. lobectomy | 1.42 | (0.94 – 2.15) | 0.096 | 1.15 | (0.58 – 2.26) | 0.69 |
| Pathological stage | Stage II vs. Stage I | 1.43 | (0.98 – 2.10) | 0.064 | 1.19 | (0.67 – 2.10) | 0.56 |
| Stage III vs. Stage I | 2.15 | (1.35 – 3.43) | 0.001 | 2.20 | (1.04 – 4.66) | 0.040 | |
| Lymphovascular invasion | present vs. absent | 1.06 | (0.75 – 1.50) | 0.73 | 2.14 | (1.21 – 3.77) | 0.009 |
| CD10/CD20 risk index | high-risk vs. low-risk | 1.61 | (1.15 – 2.27) | 0.006 | 1.75 | (1.02 – 3.01) | 0.043 |
Significant P-values are shown in bold.
HR, hazard ratio; CI, confidence interval
Next, we analyzed the correlation between CD10/CD20 risk index and risk of recurrence in stage I patients. We determined that CD10/CD20 risk index was not associated with risk of recurrence (5-year CIR, 23% for high risk index vs. 22% for low risk index; P = 0.72).
DISCUSSION
In this study, using two independent cohorts (training and validation) of patients with resected lung squamous cell carcinoma, we performed comprehensive analyses of immune factors such as, NLR (based on preoperative peripheral blood cell count), histologic immune scores (based on H&E stained slides), and immunohistochemical immune markers (based on the tissue microarray method) that included tumor-infiltrating immune cells and tumoral cytokine (receptor) expressions. We identified the tumor-infiltrating CD10/CD20 immune cell risk index (coincidence of high CD10+ neutrophil infiltration and low CD20+ B lymphocyte infiltration in tumors) as a significant prognostic factor for OS that is independent of pathologic TNM stage.
With regard to type of tumor-infiltrating lymphocyte evaluated by immunohistochemistry, we have recently reported that high FoxP3 to CD3+ tumor-infiltrating lymphocyte ratio in tumor-related stroma was an independent prognostic factor for patients with stage I lung adenocarcinoma.19 Additionally, previous studies by others have demonstrated that CD4+ or CD8+ tumor-infiltrating lymphocytes, as well as coincidence of CD4+ and CD8+ lymphocytes, were favorable prognostic factors for NSCLC.13, 15, 16 However, in our current study of lung squamous cell carcinoma no single type of tumor-infiltrating lymphocytes nor any combination of tumor-infiltrating lymphocytes (including combinations of FoxP3/CD3 or CD4/CD8) were significantly associated with patient clinical outcomes.
Neutrophil count in pretreatment peripheral blood cell count has been identified as an independent prognostic factor using large cohorts of patients with advanced NSCLC.38, 39 Moreover, recent studies have reported increased NLR in preoperative or pretreatment peripheral blood cell count as a poor prognostic factor in various solid cancers,21–26 including NSCLC.27–29 Our findings, which are based on a large cohort of patients with resected lung squamous cell carcinoma, were compatible with the previous studies. OS of patients with high NLR was much lower than OS of those with low NLR (5-year OS, 34% vs. 62%) and OS of patients with low lymphocyte percentage was much lower than those with high lymphocyte percentage (5-year OS, 38% vs. 63%) in the training cohort. Since peripheral blood testing is inexpensive, reproducible, preoperatively measurable, and widely available in clinical practice at different institutions, evaluation of NLR may have potentially valuable implications as a clinical biomarker—with regard to preoperative (e.g., surgical procedures and neoadjuvant therapy) and postoperative decision-making (e.g., adjuvant therapy)—for improvement of patient clinical outcomes. This warrants further investigation based on large external cohorts of patients with lung squamous cell carcinoma.
The tumor immune microenvironment can be directly evaluated more effectively using H&E stained slides from resected tumors. In a previous study, an increased level of tumor-infiltrating lymphocytes correlated with improved recurrence-free survival in patients with early stage NSCLC.40 In our study using H&E stained slides of resected lung squamous cell carcinomas, chronic inflammation (tumor-infiltrating lymphocytes and plasma cells) in tumors did not correlate with prognosis, whereas acute inflammation (total tumor-infiltrating neutrophil) was associated with poor prognoses in the training cohort. This suggests that tumoral acute inflammation may play a more important role than chronic inflammation in tumor cell progression in lung squamous cell carcinoma.
Tumor-associated neutrophils (TAN) play an important role in tumor biology. Neutrophils can promote tumor progression by activating angiogenesis41, 42 and tumor metastasis by facilitating tumor cell transendothelial migration.43 On the other hand, neutrophils can also suppress tumor progression via direct tumor cytotoxicity.44 However, prognostic utility of TANs remains unknown in lung cancers. We identified that tumor-related neutrophils have unfavorable prognostic value in lung squamous cell carcinoma on the basis of 3 different aspects: (1) NLR measured by peripheral blood cell count; (2) tumoral acute inflammation (total neutrophil infiltration) evaluated by H&E stained slides; and (3) tumor-infiltrating neutrophil (especially neutrophil/lymphocyte ratio) analyzed by immunohistochemistry. Recently, TANs have been classified into two different phenotypes—antitumorigenic (N1), induced by interferon β, and protumorigenic type (N2), driven by transforming growth factor (TGF) β.45, 46 Interestingly, Eruslanov et al demonstrated that in early-stage lung cancer, TANs were not immunosuppressive, but rather stimulate T cell responses.47 Mishalian et al reported that TANs had more cytotoxic effects on tumor cells in early-stage tumor development while they acquired a more protumorigenic phenotype at later stages, thereby suggesting that TANs display different functions in different tumor microenvironments.48 There is no widely accepted immunohistochemical marker for detecting TAN phenotypes (N1 or N2). As a possible marker for N2 phenotype TAN, tumor-infiltrating CD66b+ neutrophil, when referring to a specific activated subtype, was described as a poor prognostic immune factor of NSCLC, renal cell carcinoma, melanoma, and head and neck squamous cell carcinoma.49–52 Additionally, tumor-infiltrating CD10+ (cell surface-zinc-dependent metalloprotease) neutrophil was reported to be a poor prognostic factor in patients with colorectal cancer and was associated with tumoral TGF-β expression. This suggests that CD10+ neutrophils may represent the N2 TAN phenotype that is activated by tumor cell-producing TGF-β.31 Similarly, in our study OS of patients with high CD10+ tumor-infiltrating neutrophils was lower than those with low CD10+ neutrophils in both independent cohorts (5-year OS, 52% vs. 60% for training cohort; 55% vs. 64% for validation cohort).
The interaction between neutrophils and lymphocytes plays an important role in the tumor immune microenvironment and non-tumoral inflammatory process. Antitumorigenic TANs (N1) may increase activation of CD8+ cytotoxic T cells, while protumorigenic type TANs (N2) may inhibit cytotoxic response of CD8+ T cells; this allows tumor cells to escape immune surveillance.45 Moreover, the tumor-infiltrating neutrophil to lymphocyte (CD8+ T-cell) ratio has been recently reported as an independent poor prognostic factor in patients with NSCLC.49 In our study, we have demonstrated that the tumor-infiltrating neutrophil (CD10+ immune cells) to lymphocyte ratio (CD20+ B cell) is an independent prognostic factor in patients with lung squamous cell carcinoma. The biological interaction of TANs with CD20+ B cells in the tumor immune microenvironment is unclear; however, it was demonstrated that B cells inhibit neutrophil migration and, in contrast, B cell deficiency leads to increased infiltration of neutrophil in the non-tumoral inflammatory process.53, 54 This suggests that B cells—especially regulatory B cells—have a suppressive effect on neutrophil inflammatory response. Therefore, we speculate that increased infiltration of B cells may suppress TANs, while decreased infiltration of B cells may upregulate TANs (possible N2 phonotype neutrophils) in the tumor immune microenvironment. This will, in turn, lead to tumor progression and poor clinical outcomes in lung squamous cell carcinoma.
In conclusion, in this first large-scale study of the tumor immune microenvironment of patients with resected lung squamous cell carcinoma, we have identified prognostic immune markers. CD10/CD20 risk index (CD10+ neutrophil infiltration to CD20+ B lymphocytes ratio) was an independent prognostic factor using two independent cohorts. More importantly, this finding may provide a crucial foundation for future investigations into immunomodulatory therapies for lung squamous cell carcinoma.
ACKNOWLEDGMENTS
We thank Joe Dycoco of the MSK Thoracic Surgery Service for assisting with the Thoracic Service of the Department of Surgery's Lung Cancer Database; Irina Linkov of the MSK Pathology Core Facility for technical assistance with immunohistochemistry; and Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
FUNDING SUPPORT This work is supported by grants from the National Institutes of Health (R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P30 CA008748, P50 CA086438-13, and P30 CA008748), and the U.S. Department of Defense (LC110202).
Footnotes
CONFLICT OF INTEREST All authors affirm no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. Journal of thoracic oncology. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. Journal of thoracic oncology. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 6.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 11.Ujiie H, Kadota K, Nitadori J, et al. Tumoral and Stromal Immune Microenvironment in Epithelioid Malignant Pleural Mesothelioma: Tumoral IL-7 Receptor, Tumor-Infiltrating CD20+ Lymphocytes and Their Ratio to Tumor-Associated Macrophages are Independent Predictors of Survival. Oncoimmunology. 2015 doi: 10.1080/2162402X.2015.1009285. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5247–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer science. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trojan A, Urosevic M, Dummer R, et al. Immune activation status of CD8+ T cells infiltrating non-small cell lung cancer. Lung Cancer. 2004;44:143–147. doi: 10.1016/j.lungcan.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. British journal of cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 18.Lee M-C, Buitragoa DH, Kadota K, et al. The Tumor Immune Microenvironment in Octogenarians with Stage I Non-Small Cell Lung Cancer. Oncoimmunology. 2015;3:e967142. doi: 10.4161/21624011.2014.967142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: Tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th ed Springer; New York, NY: 2009. pp. 253–270. [Google Scholar]
- 21.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Annals of surgical oncology. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 22.Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 23.Jankova L, Dent OF, Chan C, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC cancer. 2013;13:442. doi: 10.1186/1471-2407-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Annals of surgery. 2013;258:301–305. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 25.Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. British journal of cancer. 2013;108:901–907. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szkandera J, Stotz M, Eisner F, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PloS one. 2013;8:e78225. doi: 10.1371/journal.pone.0078225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Tomita M, Shimizu T, Ayabe T, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer research. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 29.Yao Y, Yuan D, Liu H, et al. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer immunology, immunotherapy : CII. 2011;60:1721–1728. doi: 10.1007/s00262-011-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanh do T, Mekata E, Mukaisho K, et al. Prognostic role of CD10(+) myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer science. 2011;102:1724–1733. doi: 10.1111/j.1349-7006.2011.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadota K, Villena-Vargas J, Nitadori J, et al. Tumoral CD10 Expression Correlates with Aggressive Histology and Prognosis in Patients with Malignant Pleural Mesothelioma. Ann Surg Oncol. Jan 22;2015 doi: 10.1245/s10434-015-4374-x. 2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshizawa A, Fukuoka J, Shimizu S, et al. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:240–248. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Statistics in medicine. 2000;19:113–132. doi: 10.1002/(sici)1097-0258(20000115)19:1<113::aid-sim245>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Chappell R. Competing Risk Analyses: How Are They Different and Why Should You Care? Clin Cancer Res. 2012;18:2127–2129. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 36.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 38.Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13:1221–1230. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 39.Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. European journal of cancer (Oxford, England : 1990) 2009;45:1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. The Journal of surgical research. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 41.Shojaei F, Singh M, Thompson JD, et al. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu QD, Wang JH, Condron C, et al. Human neutrophils facilitate tumor cell transendothelial migration. American journal of physiology Cell physiology. 2001;280:C814–822. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 44.Hicks AM, Riedlinger G, Willingham MC, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of clinical investigation. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishalian I, Bayuh R, Levy L, et al. Tumor-associated neutrophils (TAN) develop protumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 50.Jensen TO, Schmidt H, Moller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476–2485. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 51.Jensen HK, Donskov F, Marcussen N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 52.Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 53.Kondratieva TK, Rubakova EI, Linge IA, et al. B Cells Delay Neutrophil Migration toward the Site of Stimulus: Tardiness Critical for Effective Bacillus Calmette-Guérin Vaccination against Tuberculosis Infection in Mice. J Immunol. 2010;184:1227–1234. doi: 10.4049/jimmunol.0902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smelt SC, Cotterell SE, Engwerda CR, et al. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]