Abstract
Gram-negative resistance has reached a crucial point, with emergence of pathogens resistant to most or all available antibiotics. Ceftazidime-avibactam is a newly approved agent combining ceftazidime and a novel β-lactamase inhibitor with activity against multidrug-resistant gram-negative bacteria. Avibactam has increased potency and expanded spectrum of inhibition of class A and C β-lactamases relative to available β-lactamase inhibitors, including extended-spectrum β-lactamase, AmpC, and Klebsiella pneumoniae carbapenemase (KPC) enzymes. Avibactam expands ceftazidime's spectrum of activity to include many ceftazidime- and carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa. Early clinical data indicate that ceftazidime-avibactam is effective and well tolerated in patients with complicated urinary tract infections (cUTI) and complicated intraabdominal infections (cIAI). In a phase II trial of patients with cUTI, ceftazidime-avibactam produced similar rates of clinical and microbiologic success compared with imipenem-cilastatin (70.5% and 71.4% microbiologic success rates, respectively). Likewise, patients receiving ceftazidime-avibactam plus metronidazole in a phase II study of patients with cIAI had similar response rates to those receiving meropenem (91.2% and 93.4% clinical success rates, respectively). Based on available in vitro, in vivo, and phase II trial data, as well as preliminary phase III trial results in ceftazidime-resistant, gram-negative cUTI and cIAI, ceftazidime-avibactam received United States Food and Drug Administration approval for treatment of cUTI, including pyelonephritis, and cIAI, in combination with metronidazole, in adult patients with limited or no alternative treatment options. The approved dosage, ceftazidime 2 g–avibactam 0.5 g administered as a 2-hour infusion every 8 hours, was selected based on pharmacodynamic analysis and available clinical data. This dosage is under further investigation in patients with cUTI, cIAI, and nosocomial or ventilator-associated pneumonia. The current body of evidence suggests that ceftazidime-avibactam is a promising addition to our therapeutic armamentarium with potential to answer an urgent unmet medical need. Further data in highly resistant gram-negative infections, particularly those caused by KPC-producing Enterobacteriaceae, are needed. As it is introduced into clinical use, careful stewardship and rational use are essential to preserve ceftazidime-avibactam's potential utility.
Keywords: β-lactamase, gram-negative, resistance, carbapenemase, ceftazidime-avibactam, Enterobacteriaceae, Klebsiella, Escherichia, Pseudomonas, ESBL, KPC
Since penicillin was introduced into clinical use in the early 1940s, a biological chess match has ensued between medical science and microbes. Nearly 80 years later, antibiotic resistance has reached a critical point, leaving some to speculate that we are nearing the “postantibiotic era.” Gram-negative resistance is at the forefront of the discussion, with the Centers for Disease Control and Prevention's list of top antibiotic resistance threats dominated by gram-negative organisms.1 This includes the major gram-negative nosocomial pathogens such as multidrug-resistant (MDR) Acinetobacter species and Pseudomonas aeruginosa, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and carbapenem-resistant Enterobacteriaceae. Among the numerous resistance mechanisms these organisms often possess, their proclivity to produce various β-lactamase enzymes confers much of their acquired resistance to our cornerstone antibiotics—the β-lactams. Enzymes such as the Ambler class C cephalosporinases (e.g., AmpC, CMY-2) and ESBLs (CTX-M-15, TEM-3, SHV-2) confer resistance to a majority of the available β-lactams. More concerning is the emergence of carbapenemase (Klebsiella pneumoniae carbapenemase [KPC]-2, OXA-48, VIM, IMP, New Delhi metallo–β-lactamase-1 [NDM-1])-producing organisms, with the potential for resistance to all β-lactams. When coupled with additional resistance mechanisms, it is not surprising to see pandrug-resistant organisms begin to emerge.2
Escalating the apprehension surrounding these essentially untreatable infections is the dwindling antibiotic pipeline. In response to a 2009 report demonstrating a dearth of novel antibiotics in later stage clinical development, the Infectious Diseases Society of America launched the multi-organizational “10 × '20” initiative aimed to develop 10 new antibiotics by 2020 and lay the foundation of a continued and sustainable pipeline of new antimicrobials.3 Seven new antibiotics have been approved by the United States Food and Drug Administration (FDA) since the program's inception, marking tremendous progress in contemporary antibiotic development. However, until recently, none of these novel agents had appreciable activity against MDR gram-negative organisms.
In an attempt to answer this unmet medical need, drug developers have turned to advancement of a proven strategy to combat β-lactamase–mediated resistance: the β-lactamase inhibitors. By combining novel high-potency, expanded-spectrum β-lactamase inhibitors with existing β-lactam antibiotics, in vitro susceptibility to a variety of resistant gram-negative pathogens, including many carbapenemase producers, can be achieved.4 Ceftazidime-avibactam (Avycaz; Actavis plc, Dublin, Ireland) is one such combination product recently granted expedited FDA approval for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, and complicated intraabdominal infections (cIAI), in combination with metronidazole, in adult patients with limited or no alternative treatment options. Through the addition of avibactam (formerly NXL 104, AVE1330A), the spectrum of activity of ceftazidime is greatly expanded to include highly resistant gram-negative pathogens including many AmpC-, ESBL-, and KPC carbapenemase–producing strains.5,6 Given its potential to answer this unmet medical need, it was granted approval based on experimental model and phase II clinical data as a Qualified Infectious Disease Product under the Generating Antibiotics Incentives Now Act. This review will focus on the available published data regarding ceftazidime-avibactam, with emphasis on spectrum of activity, as well as in vitro, in vivo animal model, and clinical data.
Chemical Structures of Ceftazidime and Avibactam
Ceftazidime
As seen with many third-generation cephalosporins, ceftazidime possesses an R1 side chain with a 2-aminothiazole group (Figure 1). This not only increases binding affinity for penicillin-binding protein (PBP)-3 among a multitude of gram-negative organisms but also makes ceftazidime a poor substrate for some β-lactamases such as early (non–extended-spectrum) TEM, OXA, and SHV variants.7 Unlike other agents from this generation, however, ceftazidime has an α-carbon dimethylacetic acid rather than the more common methoxyamino group. This substitution is responsible for the significantly enhanced potency against Pseudomonas species, modest potency gains against some Enterobacteriaceae, and reduced potency against gram-positive organisms.8 The inclusion of a propylcarboxy side chain group also increases affinity for pseudomonal PBP while also reducing the induction of some class A and class C β-lactamases (relative to other second- and third-generation cephalosporins). Ceftazidime, however, is generally unstable in the presence of Ambler class C β-lactamases, ESBLs, and carbapenemases (Figure 2).
Figure 1.
Chemical structure of ceftazidime. PBP = penicillin-binding protein.
Figure 2.

β-Lactamase-catalyzed hydrolysis of ceftazidime.
Avibactam
Unlike other currently approved β-lactamase inhibitors, avibactam is a non–β-lactam β-lactamase inhibitor. Rather than a β-lactam core, as seen with derivatives of the naturally occurring clavulanic acid or synthetic penicillanic acid sulfones such as tazobactam, avibactam was designed around a diaza-bicyclo octane structure (Figure 3).9 This bridged bicyclic core has been meticulously designed to maintain structural similarities with β-lactams while also incorporating a more rigid transition state scaffold and additional sites for hydrogen bonding with β-lactamase active site residues. Unlike some other investigational non–β-lactam inhibitors, this is achieved without dramatically increasing the molecular weight or structure size.9,10
Figure 3.
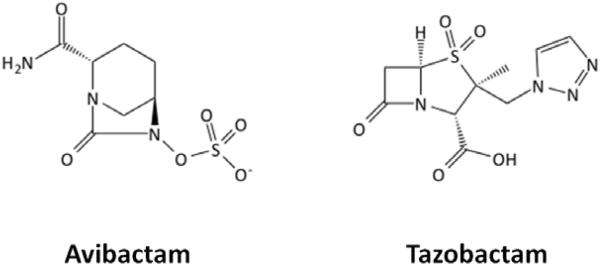
Chemical structures of avibactam and tazobactam.
Mechanism of Action
Ceftazidime
β-Lactams, including ceftazidime, exert a primarily bactericidal effect through binding of PBP and the inhibition of cell wall synthesis. The bacterial cell wall is composed of peptidoglycan, which consists of a web of alternating polysaccharides, N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG), with pentapeptide chains cross-linking NAM residues of neighboring chains. PBPs constitute a group of enzymes, likely originating from serine proteases, responsible for catalyzing the transpeptidation reactions that link peptidoglycan chains. Inhibition of this cross-linking by β-lactam antibiotics greatly compromises the structural integrity of the bacterial cell wall and leads to aberrant cellular morphology and in sufficient concentrations, cell lysis, and death.11
Avibactam
The previously available β-lactam–based β-lactamase inhibitors exert their activity by binding to the active site of β-lactamases. Through either transient competitive occupancy of the active site (acyl-enzyme intermediate preceding hydrolysis) or formation of irreversible inactivation products (suicide inhibition), the relative quantity of enzyme available for hydrolysis of antimicrobial β-lactams is decreased.8 Whether the acyl-enzyme intermediate proceeds with hydrolysis, restoring the active enzyme, or irreversible inhibition occurs, is highly dependent on the specific enzyme and inhibitor involved.12 β-Lactam–based inhibitors, particularly clavulanic acid and tazobactam, have been found to potently inhibit most Ambler class A β-lactamases (excluding carbapenemases such as KPC-2), but not those from Ambler classes B, C, or D.12
The mechanism of β-lactamase inhibition used by avibactam is similar to that of previous β-lactamase inhibitors. Through covalent binding of the β-lactamase active site hydroxyl group, avibactam diminishes the availability of active enzyme for hydrolysis and thus decreases inactivation of the β-lactam antibiotic. In contrast to the acyl-enzyme intermediate formed with β-lactam based inhibitors, avibactam forms a carbamate linkage when transitioning to the enzyme intermediate upon opening of the diaza-bicyclo octane ring structure.10 High-resolution x-ray crystallography of avibactam complexed with Ambler class A and C β-lactamases has revealed this complex to be more rigidly positioned within the active site. Inflexibility along the carbamate bridge, the presence of multiple hydrogen bonds with enzyme residues, and displacement of the water molecule required for hydrolysis allows for this intermediate structure to persist without degradation.10 Although it has been shown that 50% of TEM-1 initially inhibited by clavulanic acid is restored to its active form within 7 minutes, deacylation of 50% of the avibactam-TEM-1 complex took 7 days in the same assay (Figure 4).13 In addition to a lack of hydrolysis, avibactam has been shown recyclyze to its original active form upon the slow release of β-lactamase enzymes.14 In addition to potent inhibitory activity against Ambler class A enzymes inhibited by clavulanic acid and tazobactam, avibactam also possesses expanded inhibitory activity against class A carbapenemases (KPC), class C, and some class D β-lactamases.13,15
Figure 4.

Acetylation of β-lactamases by avibactam.
Microbiologic Spectrum of Activity
Enterobacteriaceae
The addition of avibactam to ceftazidime expands the gram-negative spectrum of activity to include many Enterobacteriaceae resistant to ceftazidime alone. Data regarding the in vitro susceptibility of various aerobic gram-negative bacteria to ceftazidime-avibactam and select comparator agents are summarized in Table 1.4,15–23 Extrapolating the Clinical and Laboratory Standards Institute (CLSI) breakpoint for ceftazidime and Enterobacteriaceae (≤ 4 mg/L),23 nearly all tested E. coli and Klebsiella species isolates were susceptible. This included resistant phenotypes, notably meropenem-nonsusceptible (NS) K. pneumoniae. It should be noted that this is a conservative measure of ceftazidime-avibactam susceptibility for Enterobacteriaceae, as a susceptibility breakpoint of 8 mg/L was set following FDA review.24 Excluding cohorts of isolates already highly susceptible to ceftazidime (with a minimum inhibitory concentration for 90% of tested isolates [MIC90] ≤ 2 mg/L), the addition of avibactam resulted in 16–1024-fold reductions in the MIC90 for E. coli and Klebsiella species. The majority of other tested Enterobacteriaceae were susceptible to ceftazidime-avibactam, with > 95% of tested isolates susceptible, and addition of avibactam to ceftazidime resulted in large reductions in the MIC90.
Table 1.
In Vitro Susceptibility of Various Aerobic Gram-Negative Bacteria to Ceftazidime-Avibactam and Comparator Agents
| Piperacillin-Tazobactam | Cefepime | Meropenem | Ceftazidime | Ceftazidime-Avibactam | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism (no. of isolates) | MIC50 (mg/ L) |
MIC90 (mg/ L) |
MIC Range (mg/L) |
% Susc epti ble |
MIC50 (mg/ L) |
MIC90 (mg/ L) |
MIC Range (mg/L) |
% Susc epti ble |
MIC50 (mg/ L) |
MIC90 (mg/ L) |
MIC Range (mg/L) |
% Susc epti ble |
MIC50 (mg/ L) |
MIC90 (mg/ L) |
MIC Range (mg/L) |
% Susc epti ble |
MIC50 (mg/L) |
MIC90 (mg/L) |
MIG Range (mg/L) |
% Susc epti blea |
Fold Reduction in MIC90 |
| Escherichia coli | |||||||||||||||||||||
| E. coli (n=2,767) 16 | 2 | 8 | 95.2 | ≤0.06 | ≤0.06 | 99.9 | 0.12 | 2 | 91.8 | 0.06 | 0.12 | 100 | 16 | ||||||||
| ESBL phenotypeb (n=328) 16 | 8 | >64 | 76.8 | ≤0.06 | ≤0.06 | 98.8 | 16 | >32 | 30.8 | 0.12 | 0.25 | 100 | >128 | ||||||||
| E. coli (n=375)17 | 0.06 | 0.12 | ≤0.06–4 | 100 | |||||||||||||||||
| ESBL phenotypeb (n=328) 17 | 0.12 | 0.25 | ≤0.06–4 | 100 | |||||||||||||||||
| E. coli (n=25)18 | 1 | 64 | 0.064–>128 | NR | 0.125 | 0.5 | 0.064–0.5 | 128 | |||||||||||||
| CAZ-NS and/or CTX-NS (n=18)18 | 2 | 64 | 0.5–>128 | NR | 0.25 | 0.5 | 0.064–0.5 | 128 | |||||||||||||
| ESBL (n=161)19 | 8 | 64 | 0.06–>64 | 67.7 | 0.03 | 0.06 | 0.015–0.12 | 100 | 16 | 64 | 0.5–>64 | 65 | 0.12 | 0.25 | ≤0.004–2 | 100 | 256 | ||||
| AmpC (94)19 | 0.25 | 0.5 | 0.03–4 | 100 | 0.03 | 0.06 | 0.015–0.25 | 100 | 16 | 64 | 1–>64 | 35.1 | 0.12 | 0.5 | 0.004–2 | 100 | 128 | ||||
| ESBL and AmpC (n=8)19 | 16 | 32 | 05–32 | 37.5 | 0.03 | 0.06 | 0.015–0.06 | 100 | 32 | >64 | 2–>64 | 25 | 0.12 | 0.12 | 0.015–0.06 | 100 | >256 | ||||
| CTX-M-15 (n=20)15 | 16 | 32 | 4–32 | 20 | 32 | 32 | 2–64 | 25 | <0.008 | <0.008 | <0.008 | 100 | >256 | ||||||||
| Klebsiella spp. | |||||||||||||||||||||
| K. pneumoniae (n=1,847)16 | 4 | >64 | 86.6 | ≤0.06 | ≤0.06 | 93.8 | 0.12 | 32 | 85.4 | 0.12 | 0.5 | 99.9 | 64 | ||||||||
| ESBL phenotypb (n=296) 16 | >64 | >64 | 24.4 | ≤0.06 | >8 | 61.1 | >32 | >32 | 8.8 | 0.5 | 1 | 99.3 | >32 | ||||||||
| MEM-NS (n=115)16 | >64 | >64 | 0 | >8 | >8 | 0 | >32 | >32 | 0 | 0.5 | 2 | 98.3 | >16 | ||||||||
| K. pneumoniae (n=254)17 | 0.12 | 0.5 | ≤0.06–4 | 100 | |||||||||||||||||
| ESBL phenotypeb (n=84)17 | 0.25 | 1 | ≤0.06–4 | 100 | |||||||||||||||||
| MEM-NS (n=12)17 | 0.5 | 2 | ≤0.06–4 | 100 | |||||||||||||||||
| KPC (n=42)4 | ≥512 | ≥512 | 32–≥512 | 0 | 0.25 | 1 | ≤0.06–1 | 100 | ≥512 | ||||||||||||
| K. pneumoniae (n=25)18 | 1 | 64 | 0.125–>128 | 0.125 | 1 | 0.064–2 | 64 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=14)18 | 4 | 64 | 0.5–>128 | 0.125 | 1 | 0.064–2 | 64 | ||||||||||||||
| ESBL (n=29)19 | 8 | 16 | 0.12–64 | 51.2 | 0.06 | 0.06 | 0.03–0.12 | 100 | 64 | >64 | 0.12–>64 | 31 | 0.5 | 1 | 0.06–2 | 100 | >64 | ||||
| OXA-48 (n=25)15 | 32 | 512 | 0.5–512 | 8 | 256 | 512 | 0.5–512 | 8 | 0.25 | 0.5 | <0.008–1 | 100 | 1024 | ||||||||
| CTX-M-15 (n=12)15 | 16 | 64 | 2–256 | 8 | 8 | 64 | 2–256 | 25 | 0.06 | 0.25 | <0.008–0.25 | 100 | 256 | ||||||||
| K. oxytoca (n=442) 16 | 2 | 8 | 92.5 | ≤0.06 | ≤0.06 | 99.3 | 0.12 | 0.5 | 96.7 | 0.06 | 0.25 | 100 | 2 | ||||||||
| ESBL phenotypeb (n=44) 16 | >64 | >64 | 25 | ≤0.06 | ≤0.06 | 93.2 | 1 | >32 | 68.2 | 0.25 | 1 | 100 | >32 | ||||||||
| K. oxytoca (n=42)17 | 0.06 | 0.5 | ≤0.06–0.5 | 100 | |||||||||||||||||
| K. oxytoca (n=25)18 | 0.25 | 2 | 0.064–4 | NR | 0.25 | 05 | 0.064–0.5 | 4 | |||||||||||||
| Proteus spp. | |||||||||||||||||||||
| P. mirabilis (n=683) 16 | ≤0.5 | 1 | 99.7 | ≤0.06 | 0.12 | 100 | 0.06 | 0.12 | 99.1 | 0.03 | 0.06 | 100 | 2 | ||||||||
| ESBL phenotypeb (n=33) 16 | 1 | 4 | 100 | ≤0.06 | 0.12 | 100 | 2 | 8 | 81.8 | 0.06 | 0.12 | 100 | 64 | ||||||||
| P. mirabilis (n=24)18 | 0.064 | 64 | 0.064–>128 | 0.064 | 0.25 | 0.064–1 | 256 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=11)18 | 0.25 | >128 | 0.064–>128 | 0.064 | 0.25 | 0.064–1 | >512 | ||||||||||||||
| P. vulgaris (n=153) 16 | ≤0.5 | 1 | 99.3 | ≤0.06 | 0.12 | 100 | 0.06 | 0.12 | 100 | 0.06 | 0.06 | 100 | 2 | ||||||||
| P. vulgaris (n=27)18 | 0.064 | 0.5 | 0.064–1 | 0.064 | 0.125 | 0.064–0.125 | 4 | ||||||||||||||
| Enterobacter spp. | |||||||||||||||||||||
| Enterobacter spp. (n=159)17 | 0.12 | 1 | ≤0.06–≥32 | 98.1 | |||||||||||||||||
| E. cloacae (n=951) 16 | 2 | 64 | 85 | ≤0.06 | ≤0.06 | 99.5 | 0.25 | >32 | 79 | 0.12 | 05 | 100 | >64 | ||||||||
| CAZ-NS (n=200) 16 | 64 | >64 | 29.1 | ≤0.06 | 0.25 | 97.5 | >32 | >32 | 0 | 0.5 | 1 | 100 | >32 | ||||||||
| E. cloacae (n=26)18 | 0.5 | 64 | 0.125–128 | 0.25 | 0.5 | 0.125–1 | 128 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=10)18 | 16 | 64 | 0.5–128 | 0.5 | 1 | 0.125–1 | 64 | ||||||||||||||
| E. aerogenes (n=357) 16 | 4 | 64 | 80.6 | ≤0.06 | ≤0.06 | 99.4 | 0.25 | 32 | 77 | 0.12 | 0.25 | 99.7 | 128 | ||||||||
| CAZ-NS (n=82) 16 | 64 | >64 | 22 | ≤0.06 | 0.12 | 97.6 | 32 | >32 | 0 | 0.25 | 0.5 | 98.8 | >64 | ||||||||
| E. aerogenes (n=26) 18 | 0.5 | 64 | 0.064–128 | 0.25 | 0.5 | 0.064–0.5 | 128 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=13)18 | 16 | 64 | 0.5–128 | 0.5 | 1 | 0.125–0.5 | 64 | ||||||||||||||
| Citrobacter spp. | |||||||||||||||||||||
| Citrobacter spp. (n=176)17 | 0.12 | 0.5 | ≤0.06–≥232 | 98.3 | |||||||||||||||||
| C. koseri (n=186) 16 | 2 | 8 | 98.4 | ≤0.06 | ≤0.06 | 100 | 0.12 | 0.5 | 98.4 | 0.06 | 0.12 | 100 | 4 | ||||||||
| C. freundii (n=185) 16 | 4 | 64 | 82.1 | ≤0.06 | ≤0.06 | 97.8 | 0.5 | >32 | 76.8 | 0.12 | 0.5 | 99.5 | >64 | ||||||||
| C. freundii (n=24)18 | 8 | 128 | 0.064–>128 | 0.25 | 1 | 0.064–2 | 128 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=13)18 | 32 | >128 | 1–256 | 0.5 | 1 | 0.125–2 | >128 | ||||||||||||||
| C. diversus (n=27)18 | 0.125 | 0.5 | 0.064–64 | NR | 0.125 | 0.25 | 0.064–0.5 | 2 | |||||||||||||
| Serratia marcescens | |||||||||||||||||||||
| S. marcescens (n=506) 16 | 2 | 4 | 96.6 | ≤0.06 | ≤0.06 | 99.2 | 0.25 | 0.5 | 97.4 | 0.12 | 0.5 | 99.6 | 0 | ||||||||
| S. marcescens (n=67)17 | 0.25 | 1 | ≤0.06–≥32 | 95.5 | |||||||||||||||||
| S. marcescens (n=25)18 | 0.25 | 8 | 0.125–8 | 0.125 | 1 | 0.125 | 1 | 8 | |||||||||||||
| Morganella morganii | |||||||||||||||||||||
| M. morganii (n=295) 16 | ≤0.5 | 2 | 96.6 | ≤0.06 | 0.12 | 100 | 0.12 | 16 | 85.8 | 0.06 | 0.12 | 99.7 | 128 | ||||||||
| M. morganii (n=127)17 | 0.06 | 0.12 | ≤0.06–0.5 | 100 | |||||||||||||||||
| M. morganii (n=25)18 | 1 | 64 | 0.64–128 | 0.125 | 1 | 0.064–1 | 64 | ||||||||||||||
| CAZ-NS and/or CTX-NS (n=11)18 | 8 | 128 | 0.5–128 | 0.125 | 1 | 0.064–1 | 128 | ||||||||||||||
| Providencia spp. | |||||||||||||||||||||
| Providencia spp. (n=268) 16 | 1 | 8 | 94.4 | ≤0.06 | 0.12 | 99.2 | 0.12 | 4 | 90.7 | 0.12 | 0.5 | 95.9 | 8 | ||||||||
| Providencia spp. (n=12)18 | 0.5 | >128 | 0.064–>128 | 0.5 | 1 | 0.064–>128 | >128 | ||||||||||||||
| Pseudomonas aeruginosa | |||||||||||||||||||||
| P. aeruginosa (n=1,967) 16 | 8 | >64 | 78.3 | 0.5 | 8 | 82 | 2 | 32 | 83.2 | 2 | 4 | 96.9 | 8 | ||||||||
| MEM-NS (n=115)16 | 64 | >64 | 36.4 | 8 | >8 | 0 | 16 | >32 | 46.6 | 4 | 16 | 87.3 | >2 | ||||||||
| CAZ-NS (n=330)16 | >64 | >64 | 4.5 | 4 | >8 | 45.3 | 32 | >32 | 0 | 4 | 16 | 82.1 | >2 | ||||||||
| P. aeruginosa (n=80)17 | 2 | 32 | 0.25–≥32 | 83.8 | |||||||||||||||||
| CAZ-NS (n=26)17 | 8 | >32 | 1–≥32 | 53.8 | |||||||||||||||||
| MEM-NS (n=26)17 | 8 | >32 | 1–≥32 | 53.8 | |||||||||||||||||
| P. aeruginosa (n=470)20 | 4 | 64 | ≤1–>512 | 90.9 | 4 | 16 | ≤1–>64 | 81.3 | 0.5 | 8 | ≤0.03–>32 | 88.1 | 4 | 32 | ≤0.25–>32 | 82.1 | 2 | 8 | ≤0.25–>16 | NR | 4 |
| P. aeruginosa (n=126)21 | 16 | >128 | 1–>128 | 74.6 | 8 | 64 | 0.5–>128 | 65.1 | 4 | 8 | 0.5–64 | 93.7 | 8 | ||||||||
| PER-1 (n=14)15 | 32 | 32 | 8–32 | 29 | 128 | 128 | 4–256 | 14 | 4 | 16 | 0.25–16 | 86 | 8 | ||||||||
| P. aeruginosa (n=25)18 | 4 | 16 | 0.5–64 | 4 | 8 | 1–16 | 2 | ||||||||||||||
| CAZ and/or ATM-NS (n=18)18 | 8 | 16 | 2–64 | 4 | 8 | 2–16 | 2 | ||||||||||||||
| MEM-NS (n=11)18 | 8 | 8 | 2–16 | 4 | 8 | 2–8 | 0 | ||||||||||||||
| Cysticfibrosis isolates (n=334)22 | 32 | 512 | 36 | 4 | 64 | 76 | 8 | ||||||||||||||
| Acinetobacter spp. | |||||||||||||||||||||
| Acinetobacter spp. (n=321) 16 | >64 | >64 | 41.3 | 8 | >8 | 47 | 32 | >32 | 41.7 | 16 | >32 | 31.2 | 0 | ||||||||
| A. baumannii (n=12)18 | 8 | >128 | 2–>128 | 8 | 32 | 1–>128 | >4 | ||||||||||||||
| CAZ and/or ATM-NS (n=13)18 | 128 | >128 | 32–>128 | 16 | 32 | 4–128 | >4 | ||||||||||||||
| MEM-NS (n=11)18 | 128 | >128 | 32–>128 | 16 | 32 | 16–128 | >4 | ||||||||||||||
| PER-1, OXA-51, OXA-58 (n=20)15 | 64 | 128 | 16–128 | 0 | 32 | 256 | 4–256 | 0 | 32 | 256 | 8–256 | 20 | 0 | ||||||||
MIC50 and MIC90 = minimum inhibitory concentration for 50% and 90% of tested isolates, respectively; CAZ = ceftazidime; CTX = cefotaxime; MEM = meropenem; NS = nonsusceptible.
Percent susceptible based on Clinical and Laboratory Standards Institute breakpoint for ceftazidime alone: ≤ 4 mg/L for Enterobacteriaceae, ≤ 8 mg/L for Pseudomonas aeruginosa23.??
Minimum inhibitory concentration > 1 mg/L for ceftazidime, ceftriaxone, or aztreonam.
Pseudomonas aeruginosa and Acinetobacter Species
In contrast to the effect of avibactam on the susceptibility of Enterobacteriaceae, the observed MIC90 reductions for P. aeruginosa were more modest, ranging from 2–8-fold (Table 1). Susceptibility of P. aeruginosa to ceftazidime-avibactam was variable and depended on the cohort of organisms and resistance phenotype subgroup examined. Overall susceptibility of P. aeruginosa to ceftazidime-avibactam ranged from 80–90% and was improved relative to ceftazidime alone in most studies. However, susceptibility among ceftazidime-NS and meropenem-NS P. aeruginosa urinary isolates from a global surveillance program was only slightly above 50%.17 Despite reductions in MIC90 in some studies, Acinetobacter species are largely resistant to ceftazidime-avibactam.15,16,18 This likely represents the variable activity of avibactam against the Ambler class D OXA β-lactamases and the multiple alternate resistance mechanisms commonly employed by Acinetobacter species.6,15
Specific β-Lactamase–Producing Organisms
In vitro susceptibility data examining the activity of ceftazidime-avibactam against organisms producing characterized β-lactamases demonstrate reliable activity against Ambler class A and C β-lactamases. Castanheira and colleagues tested 701 ESBL phenotype isolates collected from 72 United States hospitals in 2012 for various β-lactamase genes.25 Susceptibility results from this study demonstrated a ceftazidime-avibactam MIC90 of 2 mg/L among 118 KPC-producing organisms and 0.25–0.5 mg/L for various ESBL (CTX-M-14–like producers, CTX-M-15–like producers, SHV-ESBLs) and Ambler class C (CMY-2–like producers) organisms. Endimiani and colleagues also showed potent in vitro activity of ceftazidime-avibactam against 42 KPC-producing e, with an MIC90 of 1 mg/L.4 The activity of ceftazidime and ceftazidime-avibactam against individual well-characterized strains producing specific β-lactamases are shown in Table 2.5,6,15,18,26–29 The addition of avibactam resulted in vast reductions in the MIC (4–1204-fold) for both Enterobacteriaceae and P. aeruginosa strains producing Ambler class A and C β-lactamases, including AmpC, EBSL, and KPC enzymes.5,6,18,26–29 In contrast, the addition of avibactam to ceftazidime had very little, if any, impact on the MIC for various Ambler class B and D enzyme-producing strains.6,15,18,27,28 One notable exception is Ambler class D OXA-48 carbapenemase. Aktas and colleagues demonstrated activity of ceftazidime-avibactam against a single strain of OXA-48–producing E. coli (MIC <0.008 mg/L) and 25 strains of K. pneumoniae (MIC range <0.008–1 mg/L).15
Table 2.
In Vitro Susceptibility of Select Gram-Negative Bacteria Strains Producing Various β-Lactamases to Ceftazidime and Ceftazidime-Avibactam
| Organism and Enzymes | Ceftazidime MIC (mg/L) | Ceftazidime-Avibactam MIC (mg/L) | Fold Reduction in MIC |
|---|---|---|---|
| Ambler class C | |||
| Escherichia coli | |||
| AmpC overproducera6 | 128 | 1 | 128 |
| CMY-246 | 64 | 0.5 | 128 |
| ACT-15 | 16 | 0.12 | 128 |
| Klebsiella pneumoniae | |||
| CMY-86 | 64 | 2 | 32 |
| MOX-16 | 64 | 1 | 64 |
| Enterobacter cloacae | |||
| High-level AmpCb 26 | 256 | 1 | 256 |
| AmpC mutant26 | >512 | 64 | >8 |
| Citrobacter freundii | |||
| AmpC overproducera6 | 64 | <0.12 | >512 |
| Pseudomonas aeruginosa | |||
| AmpC derepressed27 | 64 | 4 | 16 |
| Ambler class A | |||
| Escherichia coli | |||
| CTX-M-276 | 8 | 0.25 | 32 |
| CTX-M-556 | 128 | 0.5 | 256 |
| CTX-M-656 | 128 | 2 | 64 |
| SHV-25 | >128 | 1 | >128 |
| SHV-75 | >128 | 0.12 | >1024 |
| SHV-526 | 128 | 2 | 64 |
| TEM-96 | >128 | 0.5 | >256 |
| TEM-285 | 128 | 0.25 | 512 |
| TEM-715 | 32 | ≤0.06 | 512 |
| KPC-328 | 64 | 0.25 | 256 |
| KPC-2, TEM-129 | 64 | 0.25 | 256 |
| Klebsiella pneumoniae | |||
| SHV-35 | >128 | 2 | >64 |
| SHV-45 | >128 | 1 | >128 |
| SHV-185 | >128 | 0.5 | >256 |
| TEM-36 | 128 | 1 | 128 |
| TEM-10, TEM-125 | >128 | 1 | >128 |
| TEM-26, SHV-15 | 128 | 1 | 128 |
| KPC-229 | 1024 | 1 | 1024 |
| KPC-329 | 512 | 0.5 | 1024 |
| KPC-2, CMY-2, SHV-11, CTX-M-1418 | >128 | 1 | >128 |
| KPC-2, SHV-12, TEM-type5 | >128 | 1 | >128 |
| KPC-3, SHV-11, TEM-15 | >128 | 1 | >128 |
| Klebsiella oxytoca | |||
| KPC-2, TEM-type5 | >128 | 1 | >128 |
| Enterobacter cloacae | |||
| KPC-2, TEM-1, KLUC-229 | 512 | 8 | 64 |
| Citrobacter freundii | |||
| KPC-2, TEM-118 | 64 | 1 | 64 |
| Pseudomonas aeruginosa | |||
| TEM-118 | 8 | 2 | 4 |
| VEB27 | >128 | 64 | >2 |
| PER-127 | >128 | 8 | >16 |
| Ambler class B | |||
| Escherichia coli | |||
| IMP-115 | 256 | 64 | 4 |
| IMP-418 | >128 | >128 | 0 |
| NDM28 | >256 | >256 | 0 |
| Citrobacter freundii | |||
| IMP-4, TEM-118 | >128 | >128 | 0 |
| Ambler class D | |||
| Escherichia coli | |||
| OXA-26 | 0.25 | 0.25 | 0 |
| OXA-36 | 0.5 | 0.12 | 4 |
| OXA-4815 | 4 | <0.008 | >512 |
| Pseudomonas aeruginosa | |||
| OXA-1127 | >128 | >128 | 0 |
| OXA-1427 | >128 | >128 | 0 |
| OXA-1527 | >128 | 64 | >2 |
| Acinetobacter baumannii | |||
| OXA-406 | >128 | >128 | 0 |
| OXA-696 | >128 | >128 | 0 |
| OXA-956 | 128 | 64 | 2 |
| Multiple Ambler classes | |||
| Escherichia coli | |||
| CTX-M-15, OXA-15 | 16 | ≤0.06 | 256 |
| KPC-3, VIM-1, TEM-15 | >128 | 4 | >32 |
| Klebsiella pneumoniae | |||
| IMP-4, TEM-118 | 64 | 1 | 64 |
| IMP-4, DHA-118 | >128 | >128 | 1 |
| Enterobacter cloacae | |||
| AmpC, NDM-1, OXA-1, OXA-9, CTX-M-15, LAP-2 26 | >512 | >256 | 2 |
| KPC-3, TEM-1, OXA-929 | 1024 | 8 | 128 |
| VIM-1, TEM-15 | >128 | >128 | 1 |
| Citrobacter freundii | |||
| KPC-2, TEM-1, CMY-218 | 128 | 0.5 | 256 |
| Pseudomonas aeruginosa | |||
| PER-1, OXA-1627 | >128 | 32 | >4 |
MIC = minimum inhibitory concentration.
AmpC overproduction confirmed by DNA sequencing for presence of mutations in promoter region and boronic acid inhibition test.
AmpC expression levels confirmed by mRNA expression levels.
Anaerobic and Gram-Positive Bacteria
Ceftazidime-avibactam's activity against anaerobic bacteria is limited. Available data suggest that the addition of avibactam to ceftazidime results in a small 2–4-fold reductions in the MIC90 for many gram-negative and gram-positive anaerobic organisms.30,31 However, the MIC90 remained above the CLSI susceptibility breakpoint of 16 mg/L set for other cephalosporins in a majority of cases23, thus, it does not appear that ceftazidime-avibactam possesses clinically relevant anaerobic coverage by itself. When metronidazole was combined with ceftazidime-avibactam, further reduction of the MIC90 was noted for many species.30,31 This suggests that combination regimens of ceftazidime-avibactam and metronidazole would be effective for the treatment of polymicrobial infections involving anaerobic organisms. Ceftazidime-avibactam also possesses a limited spectrum of activity against gram-positive pathogens, similar to ceftazidime. Data indicate poor activity against Staphylococcus aureus (MIC90 >32 mg/L) but consistent activity against β-hemolytic streptococci (MIC90 0.5 mg/L).17
Resistance to Ceftazidime-Avibactam
In addition to limited activity against strains producing Ambler class B and D β-lactamases and Acinetobacter species, resistance to ceftazidime-avibactam among otherwise susceptible bacteria exists. Winkler and colleagues examined 10 archived clinical P. aeruginosa isolates resistant to ceftazidime-avibactam to elucidate potential mechanisms of resistance.32 Through a series of analyses, decreased inhibition of Pseudomonas-derived cephalosporinases and AmpC variants by avibactam and varying AmpC expression levels were ruled out as causes of the observed resistance. The presence of alternative β-lactamases and changes in PBP function or expression were also excluded. Whole genome sequencing demonstrated that the OprD porin was absent or nonfunctional in a majority of the isolates, potentially decreasing cell permeability. However, susceptibility of other OprD mutants to avibactam suggested that this was not the primary mechanism of resistance. Up-regulation of tripartite resistance-nodulation-division efflux pumps was also explored but could not be conclusively demonstrated. Through the use of efflux pump inhibitors and examination of susceptibility patterns to other classes of antibiotics, the authors concluded that the observed resistance was likely due to diminished outer membrane permeability and/or overexpressed efflux pumps.
Spontaneous resistance to ceftazidime-avibactam has only been demonstrated in vitro and has yet to manifest during animal experiments or clinical trials.33 During in vitro hollow-fiber experiments simulating human exposures of ceftazidime 2 g-avibactam 0.5 g as a 2-hour infusion every 8 hours by Crandon and colleagues, two of seven P. aeruginosa strains exhibited significant regrowth at 24 hours accompanied by a 32-fold increase in ceftazidime-avibactam MIC.34 Both of these strains had a ceftazidime-avibactam MIC at the susceptibility breakpoint MIC of 8 mg/L at baseline.24 In contrast, all four of the strains with a baseline MIC of 4 mg/L exhibited a maximum bactericidal response, and no resistant populations arose when the strains were studied with ceftazidime-avibactam in neutropenic mouse thigh experiments. When interpreting these experiments, it is important to note that the in vitro hollow-fiber system represents a rigorous test of pharmacodynamics given its complete lack of host immunity, even relative to an in vivo model of neutropenia.
To further investigate the mechanisms of ceftazidime-avibactam resistance in P. aeruginosa, Lahiri and colleagues conducted a series of spontaneous resistance frequency experiments in three strains with varying levels of AmpC expression.35 The observed resistance frequency was low, between 1.2 × 10−9 and 2.5 × 10−8 at 4-fold the agar dilution MIC, and substantially less than that of imipenem and meropenem in similar experiments.36 The resistant variants exhibited 8–32-fold increases in the ceftazidime-avibactam MIC. Whole genome sequencing characterized the nature of the mutations of the resultant variants, along with the mutant strains from the hollow-fiber experiments by Crandon and colleagues.34 A majority of the isolates possessed one of two deletions in the AmpC coding region resulting in changes to the Ω-loop of the enzyme. Interestingly, many of the mutant strains produced had reduced carbapenem MICs with some becoming susceptible to imipenem and/or doripenem.
Resistance to ceftazidime-avibactam has also been demonstrated in vitro in Enterobacteriaceae.33 Similar to the findings of Lahiri and colleagues, spontaneous resistance frequencies in single-passage experiments were low, ranging from 2.2 × 10−9 to 2.2 × 10−8 in strains of K. pneumoniae, E. coli Citrobacter freundii, and Enterobacter cloacae producing various Ambler class A β-lactamases. This included two KPC-producing strains of K. pnuemoniae and one KPC-producing strain of E. cloacea with alterations in the blaKPC gene occurring in or near the Ω-loop region. Spontaneous resistance in Enterobacteriaceae has also been demonstrated in avibactam–β-lactam combinations other than ceftazidime.37 Of interest, one analysis by Papp-Wallace and colleagues demonstrated emergence of resistant mutants with ampicillin-avibactam but not ceftazidime-avibactam. This suggests that the spontaneous resistance potential of avibactam is dependent on the β-lactam partner.
Pharmacokinetics
The pharmacokinetics of ceftazidime-avibactam have been examined in phase I and II studies. No significant pharmacokinetic interaction between ceftazidime and avibactam has been observed. At steady state, ceftazidime 2 g–avibactam 0.5 g given as a 2-hour prolonged infusion every 8 hours achieves a peak serum concentration (Cmax) of 90 and 15 mg/L for ceftazidime and avibactam, respectively.33 The half-life of both agents is approximately 2.7 hours following repeated administration. Ceftazidime is predominantly (83%) renally cleared. Avibactam also undergoes extensive renal clearance, with > 97% of a 500-mg dose excreted unchanged in the urine within 12 hours of administration.38 Protein binding for each agent is low, 21% and 8% for ceftazidime and avibactam, respectively.39 Additional pharmacokinetic parameters are shown in Table 3.33,40
Table 3.
Pharmacokinetic Parameters of Ceftazidime and Avibactam at Steady State
| Parameter | Ceftazidime 2 g Every 8 Hours33,40,a | Avibactam 500 mg Every 8 Hours33,a |
|---|---|---|
| Vd (L) | 17.0 | 22.2 |
| Cmax (mg/L) | 90.4 | 14.6 |
| t1/2 (hours) | 2.7 | 2.7 |
| AUC0–tau (mg•hr/L) | 291 | 38.2 |
| fAUC0–tau (mg•hr/L) | 230 | 35.1 |
| Protein binding | 21% | 8% |
| Elimination | 83% urine | >97% urine |
| ELF penetration | 21% | 25–35% |
Vd: volume of distribution; Cmax: peak serum concentration; t½: half- life; AUC0-tau: area under the curve over the dosing interval;fAUC0-tau: free area under the curve over the dosing interval; ELF: epithelial lining fluid
Administered as a 2-hour infusion.
The pharmacokinetics of each of these agents has also been examined in patients with varying degrees of renal dysfunction. Ceftazidime clearance has been shown to strongly correlate with creatinine clearance (CrCl), and dose adjustment for patients with a creatinine clearance less than 50 mL/minute is recommended to attain comparable drug exposure.41 Avibactam displays similarly reduced clearance among patients with renal insufficiency. Patients with mild (CrCl 50– 79mL/min), moderate (CrCl 30– 49mL/min) and severe (CrCl 15– 29mL/min) renal dysfunction displayed 40%, 26%, and 15% of the total avibactam clearance of patients with normal renal function (14.6 L/hr).42
The current FDA-approved dosage, ceftazidime 2 g–avibactam 0.5 g as a 2-hour prolonged infusion every 8 hours, is higher than the dosages used in phase II studies.24 This dosage was selected after considerable evaluation of the pharmacodynamics data concerning the combination and is under further phase III trial investigation. No dosage adjustments are required for patients with any degree of hepatic impairment; however, dosage adjustments are recommended for patients with renal impairment and are listed in Table 4.24 Currently, there are no explicit labeled recommendations for dosing in intermittent hemodialysis, although both ceftazidime and avibactam are dialyzable and should be given after dialysis on dialysis days (55% removed during a 4-hr hemodialysis session for each agent).24 To our knowledge, there are no published data for the pharmacokinetics of ceftazidime-avibactam in patients with other renal replacement therapy modalities such as peritoneal dialysis, continuous renal replacement therapy, or sustained low-efficiency dialysis.
Table 4.
Ceftazidime-Avibactam Dosage Recommendations for Patients with Varying Degrees of Renal Function
| Creatinine Clearance (mL/min) | Dosage Recommendation |
|---|---|
| > 50 | Ceftazidime 2 g–avibactam 0.5 g every 8 hours |
| 31–50 | Ceftazidime 1 g–avibactam 0.25 g every 8 hours |
| 16–30 | Ceftazidime 0.75 g–avibactam 0.19 g every 12 hours |
| 6–15 | Ceftazidime 0.75 g–avibactam 0.19 g very 24 hours |
| ≤ 5 | Ceftazidime 0.75 g–avibactam 0.19 g every 48 hours |
Pharmacodynamics
The pharmacodynamics of ceftazidime have been well described. Similar to other cephalosporins, the percentage of time of the dosing interval that free drug concentration is in excess of the MIC (%fT>MIC) is the best predictor of antimicrobial activity. For ceftazidime, a %fT>MIC of approximately 50 has been shown to both predict bactericidal activity in vitro and correlate with successful treatment of gram-negative nosocomial pneumonia.43
Numerous pharmacodynamic targets have been proposed for β-lactamase inhibitors.44,45 The currently prevailing target for avibactam is the percentage of time that free inhibitor concentration is above the threshold concentration (%fT>CT).46 The CT is the concentration of inhibitor that must be maintained, concomitantly with a β-lactam, to inhibit bacterial growth. Thus, inhibitor concentrations ≥ CT sufficiently inactivate β-lactamases as they are produced and allow the β-lactam antibiotic to act on PBP targets in the absence of functional β-lactamases. Once inhibitor concentrations drop below CT, newly produced β-lactamases begin to overcome the available inhibitor. A period of continued bacterial growth inhibition by the β-lactam antibiotic is observed after inhibitor concentrations fall below CT, referred to as the postinhibitor effect. The duration of this effect appears variable and is dependent on the concentration of β-lactam available and the isolate being tested. With concentrations of inhibitor ≥ CT, the antimicrobial activity of a β-lactam and β-lactamase inhibitor combination is thought to be dictated by the pharmacodynamic target of the β-lactam.47 It is important to note that it is thought that CT is affected by several factors, including the β-lactam agent the inhibitor is paired with, the dose of β-lactam used, and the bacterial inoculum present.
A series of in vitro experiments performed by Colemann and colleagues using hollow-fiber pharmacokinetic-pharmacodynamic models have examined the activity of ceftazidime in combination with avibactam against Enterobacteriaceae with characterized β-lactamase production.46 All organisms tested possessed ceftazidime MICs ≥ 64mg/L but had ceftazidime-avibactam MICs ≤ 4mg/L when tested with avibactam 4 mg/L. When using ceftazidime continuously infused to produce a concentration of either 8 or 16 mg/L to remain above the ceftazidime-avibactam MIC but below that of ceftazidime alone, and continuous avibactam concentrations of 1, 2, or 4 mg/L, bactericidal killing was maintained for the entire 24-hour study period in all but one experiment (where regrowth occurred at 22 hours). Notably, the regrowth occurred in the experiment where the avibactam concentration was fixed at 4 mg/L, which was at, rather than in excess of, the E. cloacae ceftazidime-avibactam MIC of 4 mg/L. Experiments using ceftazidime concentrations simulating 1- or 2-g doses infused over 30 minutes every 8 hours in a patient with normal renal function and various avibactam regimens against a KPC-3–producing K. pneumoniae isolate and an E. cloacae isolate exhibiting stably derepressed AmpC were also conducted. Against the E. cloacae isolate, the ceftazidime 2-g regimen with avibactam 0.25 mg/L being continuously maintained over 24 hours only inhibited growth for 8 hours. In contrast, avibactam 0.5 mg/L continuously maintained with the ceftazidime 1-g regimen provided bactericidal activity for the entire 24 hour period. When avibactam 0.5 mg/L was maintained for 4.5 hours in combination with human simulated ceftazidime concentrations, approximately 9 and 12 hours of bactericidal growth reductions of E. cloacae and K. pneumoniae, respectively, were observed. These data led the investigators to conclude that for Enterobacteriaceae treated with ceftazidime 2 g every 8 hours, the CT for avibactam appears to be ≤ 0.5 mg/L, and that avibactam concentrations of 0.25–0.5 mg/L should be targeted for at least 50% of the 8-hour dosing interval for maintained suppression of bacterial regrowth.
Berkhout and colleagues conducted similar ceftazidime-avibactam pharmacodynamic studies in murine models of P. aeruginosa infection.48,49 In a murine neutropenic thigh infection model, animals were inoculated with 106 colony-forming units (CFU) of P. aeruginosa possessing ceftazidime MICs ranging from 32–128mg/L. Ceftazidime was administered every 2 hours at the maximum dose allowing 1–2 log10 CFU/thigh of bacterial regrowth over the 24-hour study period when given as monotherapy. Avibactam, in fractionated doses, was then administered in combination with this dose of ceftazidime, and %fT>CT targets were calculated using various CT thresholds. In this model, it was determined that the %fT>CT using a CT of 1 mg/L was the strongest predictor of reduced bacterial burden. The average %fT>CT required for inhibition of P. aeruginosa growth at 24 hours was around 40% when using a CT of 1 mg/L, but it varied by isolate tested(15– 70%). It was also shown that increasing doses of ceftazidime lowered the %fT>CT required for avibactam-associated growth suppression. Experiments using neutropenic murine models of P. aeruginosa pneumonia were also conducted. Using a very similar experimental design, the %fT>CT using a CT of 1 mg/L was the strongest predictor of avibactam associated suppression of bacterial growth. However, the %fT>CT required for bacterial stasis was found to be lower, at approximately 20– 25%.
Using this pharmacodynamic profile for avibactam when used in combination with ceftazidime, Monte Carlo simulations were conducted to inform pharmacodynamic target selection using the proposed dosing regimen. When applying conservative pharmacodynamic targets for ceftazidime (50%fT>MIC) and avibactam (50%fT>CT, CT = 1 mg/L), it was determined that the proposed dose of ceftazidime 2 g-avibactam 0.5 g infused over 2 hours every 8 hours would achieve >98% probability of target attainment (PTA) for ceftazidime-avibactam MICs as high as 8 mg/L. The PTA drops to 51% and 1% for MICs of 16 and 32mg/L, respectively.33
Another important pharmacodynamic consideration regarding ceftazidime-avibactam is the potential for drug-drug interactions. In particular, the potential for microbiologic antagonism with other antimicrobials with which it may be used in combination clinically. This was investigated by Dallow and colleagues, who found no microbiologic interaction between ceftazidime-avibactam and a variety of antibiotic agents, including colistin, tobramycin, tigecycline, levofloxacin, vancomycin, and linezolid in checkerboard experiments.50
Early Clinical Efficacy Data
Published data regarding the efficacy of ceftazidime-avibactam are currently limited to phase II clinical trials. The first study was a prospective, multicenter, randomized, double-blind, comparative study of ceftazidime 500 mg–avibactam 125 mg given as a 30-minute intravenous infusion every 8 hours versus imipenem 500 mg–cilastatin 500 mg given as a 30-minute intravenous infusion every 6 hours for the treatment of cUTI, including pyelonephritis.51 Patients were to receive a minimum of 4 days of intravenous study therapy before switching to oral therapy, with a minimum and maximum of 7 and 14 total days of antibiotics, respectively, based on investigator discretion. Patients were excluded if the cUTI was caused by a pathogen know to be resistant to either study drug, effectively excluding infections caused by carbapenemase-producing organisms. The primary endpoint was microbiologic response at a test-of-cure (TOC) visit 5–9 days following the last dose of study medication in the microbiologically evaluable (ME) population. Secondary endpoints included microbiologic response at the end of intravenous therapy and at late follow-up (LFU), 4–6 weeks post-therapy in the ME population, as well as clinical response at TOC and LFU within the clinically evaluable (CE) population.
One hundred thirty-seven patients were randomized; the average age was approximately 47 years, and the population was predominantly (~75%) female. Nearly two thirds of patients in both arms carried a diagnosis of pyelonephritis, and the most common pathogen was E. coli (92.6% in the ceftazidime-avibactam arm; 94.3% in the imipenem-cilastatin arm). Three patients (6.5%) in the ceftazidime-avibactam group had a positive blood culture at baseline, all of which were E. coli. No isolates were resistant to imipenem-cilastatin, whereas 20 were nonsusceptible (intermediate or resistant according to CLSI standards) to ceftazidime, with 8 of these occurring in the ceftazidime-avibactam group. Approximately 30% of patients in both treatment arms had a baseline pathogen possessing the blaCTX-M-15 gene and thus the ability to produce ESBLs.33 In the ceftazidime-avibactam arm, 27 and 46 patients were included ME and CE populations, respectively. Microbiologic and clinical response rates for primary and secondary outcomes were similar between both treatment groups. Favorable microbiological response at TOC, the primary outcome, was observed in 19 (70.4%) of the 27 patients in the ceftazidime-avibactam arm and 25 (71.4%) of 35 patients in the imipenem-cilastatin arm. The TOC microbiologic response rates were also similar when the cohort was stratified by diagnosis and those infected with E. coli (76% ceftazidime-avibactam; 69.7% imipenem-cilastatin) and ceftazidime-NS organisms (85.7% ceftazidime-avibactam; 81.8% imipenem-cilastatin). Microbiologic response rates were 57.7% (15/26 patients) and 60% (18/30 patients) at LFU in the ceftazidime-avibactam and imipenem-cilastatin arms respectively.
The second published phase II study was a prospective, multicenter, randomized, double-blind, controlled trial of ceftazidime 2 g–avibactam 0.5 g given as a 30-minute intravenous infusion every 8 hours plus metronidazole 500 mg given as a 1-hour intravenous infusion every 8 hours versus meropenem 1 g given as a 30-minute intravenous infusion every 8 hours for patients with cIAI.52 Patients were to receive between 5 and 14 days of study therapy, and were allowed concomitant vancomycin, linezolid, or daptomycin for suspected or confirmed gram-positive co-infection. Subjects with evidence of cIAI requiring surgical intervention and antibiotic therapy were eligible for inclusion. Notable exclusions included those with an Acute Physiological Assessment and Chronic Health Evaluation (APACHE) II score > 25, significantly elevated liver enzyme levels or chronic liver disease, creatinine clearance < 50 mL/min, and various immune compromised populations (acquired immunodeficiency syndrome, metastatic or hematologic malignancy requiring chemotherapy, neutropenia [absolute neutrophil count < 1500 cells/mm3], or chronic corticosteroid use [> 50 mg of prednisone equivalents/day]). Like the cUTI trial, patients with an infection suspected or confirmed to be caused by a pathogen resistant to either study drug at baseline were excluded. The primary endpoint was clinical response at a TOC visit in the ME population. Secondary outcomes included clinical response at the end of intravenous therapy, TOC, and LFU visit in the CE population.
Two hundred four patients were randomized, with the cohort predominantly male and a majority having an APACHE II score ≤ 10. The most common sites of origin of infection were the appendix and stomach or duodenum, and the predominant diagnosis was peritonitis (>80%). More than one third of patients had a polymicrobial infection, with E. coli the most common organism isolated from both the cIAI site and bloodstream. In the ceftazidime-avibactam arm, 7 (8.2%) of 85 modified microbiologic intent-to-treat (mMITT) patients had bacteremia at baseline. Overall, there were 6 organisms (3 K. pneumoniae, 2 P. aeruginosa, 1 A. baumannii) with a ceftazidime-avibactam MIC > 8 mg/L, and two meropenem-resistant organisms (1 P. aeruginosa, 1 A. baumannii). An ESBL gene was exhibited in 44 (25.3%) of 174 patients in the mMITT population, with CTX-M-15 most common.33 Although two meropenem-resistant pathogens were isolated, neither was reported to produce a carbapenemase enzyme. The primary outcome of favorable clinical response in the ME population at TOC was met in 62 (91.2%) of 68 patients in the ceftazidime-avibactam plus metronidazole arm and 71 (93.4%) of 76 patients in the meropenem arm. Similar clinical response rates were also seen between treatment groups at the end of intravenous therapy and at LFU visit in the ME, CE, and mMITT populations as well as all in the subgroup analyses based on site of infection and severity of illness. Additionally, all patients with bacteremia at baseline were deemed clinical cures at TOC. Among the 43 patients infected with a ceftazidime-NS pathogen alone at baseline, the response rates were 96.2% (25/26 patients) and 94.1% (16/17 patients) in the ceftazidime-avibactam plus metronidazole and meropenem arms, respectively.
In addition to the published phase II studies, a number of phase III trials are currently underway or awaiting publication of results. These include additional cIAI (NCT01499290, NCT01500239, NCT01726023) and cUTI (NCT01595438, NCT01599806) trials, as well as nosocomial pneumonia and ventilator-associated pneumonia (NCT01808092). Although the results of these trials have not been published to date, preliminary results of a phase III cIAI trial led to a warning of decrease clinical response in patients with a baseline creatinine clearance of 30 –50 mL/min in the ceftazidime-avibactam FDA label.24 Decreased clinical cure was noted in this subgroup of patients in both the ceftazidime-avibactam plus metronidazole and meropenem arms. However, the observation was more pronounced in the ceftazidime-avibactam plus metronidazole arm, with a clinical cure rate of 45% (14/31 patients) versus 74% (26/35 patients) in the meropenem arm.24 It is noted in the package insert that patients treated with ceftazidime-avibactam at this strata of creatinine clearance were receiving a 33% lower daily dose than the currently recommended dose for such populations.24 Although this finding should be interpreted with caution given the limited patient sample, final results of this trial and others are needed to inform the use of ceftazidime-avibactam in patients with renal impairment.
The most anticipated study is a phase III trial of ceftazidime-avibactam for treatment of cUTI or cIAI infections due to ceftazidime-resistant gram-negative organisms (NCT01644643). This trial compares ceftazidime 2 g–avibactam 0.5 g given as a prolonged 2-hour infusion every 8 hours with “best available therapy,” which was at investigator discretion based on local standard of care and susceptibility patterns. Preliminary data presented to the FDA in December 2014 in support of the new drug application included 4 mMITT patients with cIAI and 44 mMITT patients with cUTI. A numerically higher response rate in favor of ceftazidime-avibactam (71.4% vs 47.8%) was observed.33 Notably, this trial includes the first infection due to a confirmed carbapenemase producer, a KPC-producing strain of K. pneumoniae, in a patient treated with ceftazidime-avibactam who had a favorable outcome at TOC and LFU.
Safety and Tolerability
The available body of evidence suggests that ceftazidime-avibactam is well tolerated and has a safety profile similar to that of ceftazidime alone. This is supported by the lack of a pharmacokinetic interaction observed between ceftazidime and avibactam, as well as the published phase II clinical data.51,52 In these trials, the occurrence of treatment-emergent adverse events and serious adverse events was similar among ceftazidime-avibactam and comparator patients. Common treatment-emergent adverse events observed in at least 5% of ceftazidime-avibactam–treated patients along with their occurrence in the comparator arms are listed in Table 5.33,51,52 Serious adverse events attributed to ceftazidime-avibactam included diarrhea, acute renal failure, elevated liver enzyme levels, and accidental overdose occurring in a single patient each. Although none of these patients were found to have Clostridium difficile–associated diarrhea (CDAD),33 it is an important consideration and labeled warning for ceftazidime-avibactam, as with all systemic antibacterial drugs.24 Further comparative clinical study and investigation of ceftazidime-avibactam's impact on bowel flora are needed to further describe the associated risk of CDAD. Along with the phase II data, a phase I safety analysis designed to evaluate the potential impact of ceftazidime-avibactam on QTc interval in healthy subjects indicates that ceftazidime-avibactam has little potential to cause electrocardiogram abnormalities.51–53 It should be noted that no dose-related adverse effects have been demonstrated.33 Although it has not been observed with ceftazidime-avibactam to date, 30 years of clinical experience with ceftazidime suggest that neurologic toxicity should also be a consideration, especially in patients with reduced renal function.54
Table 5.
Treatment-Emergent Adverse Events Occurring in at Least 5% of Patients Treated with Ceftazidime-Avibactam in Phase II Study Safety Populations33,51,52
| Complicated Intraabdominal Infection | Complicated Urinary Tract Infection | |||
|---|---|---|---|---|
| Adverse Event | Ceftazidime-avibactam + metronidazole (n=101) | Meropenem (n=102) | Ceftazidime-avibactam (n=68) | Imipenem-cilastatin (n=67) |
| Nausea | 10 (9.9) | 6 (5.9) | 0 | 2 (3.0) |
| Vomiting | 14 (13.9) | 5 (4.9) | 0 | 0 |
| Constipation | 4 (4.0) | 1 (1.0) | 7 (10.3) | 2 (3.0) |
| Diarrhea | 5 (5.0) | 4 (4.9) | 6 (8.8) | 7 (10.4) |
| Abdominal pain | 8 (7.9) | 3 (2.9) | 10 (14.7) | 4 (6) |
| Headache | 3 (3.0) | 3 (2.9) | 13 (19.1) | 21 (31.3) |
| Dizziness | 0 | 2 (2.0) | 4 (5.9) | 0 |
| Insomnia | 0 | 2 (2.0) | 4 (5.9) | 4 (6.0) |
| Hypertension | 2 (2.0) | 3 (2.9) | 4 (5.9) | 2 (3.0) |
| Pyrexia | 9 (8.9) | 11 (10.8) | 0 | 1 (1.5) |
| Chest pain | 1 (1.0) | 1 (1.0) | 4 (5.9) | 3 (4.5) |
| Infusion-site reaction | NR | NR | 5 (7.4) | 16 (23.9) |
| Hypertension | 2 (2.0) | 3 (2.9) | 4 (5.9) | 2 (3) |
| Cough | 6 (5.9) | 4 (3.9) | 1 (1.5) | 1 (1.5) |
| Pyruria | 5 (5.0) | 5 (4.9) | 0 | 0 |
| Increased ALT level | 8 (7.9) | 13 (12.7) | 2 (2.9) | 4 (6) |
| Increased AST level | 9 (8.9) | 15 (14.7) | 2 (2.9) | 3 (3.4) |
| Increased ALP level | 9 (8.9) | 7 (6.9) | 2 (2.9) | 1 (1.5) |
| Increased WBC count | 5 (5) | 6 (5.9) | 0 | 0 |
Data are no. (%) of patients.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; WBC: white blood cell.
Conclusion
Through the addition of avibactam, ceftazidime's activity is expanded to many ceftazidime-resistant and carbapenem-resistant Enterobacteriaceae and P. aeruginosa. This includes isolates producing a variety of Ambler class A and C β-lactamases, including AmpC, ESBLs, and KPC, as well as select class D OXA enzymes. In contrast, ceftazidime-avibactam does not possess any appreciable activity against the Ambler class B metallo–β-lactamases. Positive experimental model and clinical data resulted in priority FDA approval of ceftazidime-avibactam for a limited use indication of cUTI, including pyelonephritis, and cIAI, in combination with metronidazole, in patients with limited or no alternative treatment options. Based on extensive pharmacodynamic analysis and early clinical data, a dosage of ceftazidime 2 g–avibactam 0.5 g given as a 2-hour infusion every 8 hours was selected. This dosage is under further study in patients with cUTI, cIAI, and nosocomial pneumonia, including ventilator-associated pneumonia. These data in patients with serious infections outside of cUTI and cIAI, including patients with gram-negative bacteremia, will further delineate the role of ceftazidime-avibactam. Additional data in patients with highly resistant gram-negative infections, such as KPC-producing Enterobacteriaceae, are also needed to definitively demonstrate ceftazidime-avibactam's efficacy where it is needed most. According to the Centers for Disease Control and Prevention, carbapenem-resistant Enterobacteriaceae is the most urgent unmet medical need that can potentially be addressed by ceftazidime-avibactam.1 Through potential improvements in clinical efficacy and spared use of more toxic treatment alternatives, such as polymyxin-containing regimens, ceftazidime-avibactam may have a profound impact on the outcomes of patients with carbapenem-resistant Enterobacteriaceae infections. The antimicrobial stewardship implications associated with ceftazidime-avibactam's introduction into clinical use are also of crucial consideration. Careful judgment should be exercised in making the decision to deploy ceftazidime-avibactam. Although spontaneous resistance to ceftazidime-avibactam has yet to emerge clinically or in vivo, efforts to preserve its susceptibility profile will be essential to its continued utility.
ACKNOWLEDGMENT
The authors acknowledge the contribution of Deven M. Millay for her diligent work in the preliminary literature search for this manuscript.
Disclosures: Michael Rybak has received grant support, or has consulted or participated on speaking bureaus for Cempra, Cubist, Actavis and The Medicines Company. In addition, he is supported in part by NIH R21 AI109266-01.
REFERENCES
- 1.(CDC) CfDCaP [Accessed Feb 13, 2015];Antibiotic Resistance Threats in the United States, 2013. 2014 http://www.cdc.gov/drugresistance/threat-report-2013/
- 2.Pontikis K, Karaiskos I, Bastani S, et al. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. International journal of antimicrobial agents. 2014 Jan;43(1):52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Benjamin DK, Jr., et al. 10 × '20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013 Jun;56(12):1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endimiani A, Choudhary Y, Bonomo RA. In vitro activity of NXL104 in combination with beta-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother. 2009 Aug;53(8):3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. In vitro Susceptibility of Characterized beta-Lactamase-Producing Strains Tested with Avibactam Combinations. Antimicrob Agents Chemother. 2014 Dec 22; doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizumi A, Ishii Y, Aoki K, Testa R, Nichols WW, Tateda K. In vitro susceptibility of characterized beta-lactamase-producing Gram-negative bacteria isolated in Japan to ceftazidime-, ceftaroline-, and aztreonam-avibactam combinations. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2015 Feb;21(2):148–151. doi: 10.1016/j.jiac.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Neu HC, Labthavikul P. Antibacterial activity and beta-lactamase stability of ceftazidime, an aminothiazolyl cephalosporin potentially active against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Jan;21(1):11–18. doi: 10.1128/aac.21.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garzone P, Lyon J, Yu VL. Third-generation and investigational cephalosporins: I. Structure-activity relationships and pharmacokinetic review. Drug intelligence & clinical pharmacy. 1983 Jul-Aug;17(7–8):507–515. doi: 10.1177/106002808301700703. [DOI] [PubMed] [Google Scholar]
- 9.Leonard DA, Bonomo RA, Powers RA. Class D beta-lactamases: a reappraisal after five decades. Accounts of chemical research. 2013 Nov 19;46(11):2407–2415. doi: 10.1021/ar300327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahiri SD, Mangani S, Durand-Reville T, et al. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC beta-lactamases. Antimicrob Agents Chemother. 2013 Jun;57(6):2496–2505. doi: 10.1128/AAC.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutmann L, Vincent S, Billot-Klein D, Acar JF, Mrena E, Williamson R. Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis of Escherichia coli by some beta-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam) Antimicrob Agents Chemother. 1986 Dec;30(6):906–912. doi: 10.1128/aac.30.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clinical microbiology reviews. 2010 Jan;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnefoy A, Dupuis-Hamelin C, Steier V, et al. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor. The Journal of antimicrobial chemotherapy. 2004 Aug;54(2):410–417. doi: 10.1093/jac/dkh358. [DOI] [PubMed] [Google Scholar]
- 14.Ehmann DE, Jahic H, Ross PL, et al. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jul 17;109(29):11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktas Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. International journal of antimicrobial agents. 2012 Jan;39(1):86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014;58(3):1684–1692. doi: 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flamm RK, Sader HS, Farrell DJ, Jones RN. Ceftazidime-avibactam and comparator agents tested against urinary tract isolates from a global surveillance program (2011) Diagnostic microbiology and infectious disease. 2014 Nov;80(3):233–238. doi: 10.1016/j.diagmicrobio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhang F, Zhao C, et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother. 2014;58(3):1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagace-Wiens PR, Tailor F, Simner P, et al. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother. 2011 May;55(5):2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walkty A, DeCorby M, Lagace-Wiens PR, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study) Antimicrob Agents Chemother. 2011 Jun;55(6):2992–2994. doi: 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levasseur P, Girard AM, Claudon M, et al. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2012 Mar;56(3):1606–1608. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalhoub H, Tunney M, Elborn JS, et al. Avibactam confers susceptibility to a large proportion of ceftazidime-resistant Pseudomonas aeruginosa isolates recovered from cystic fibrosis patients. The Journal of antimicrobial chemotherapy. 2015 Jan 14; doi: 10.1093/jac/dku551. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Sixteenth Informational Supplement. CLSI; Wayne, PA: 2014. M100-S24: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI Document M100-S20 ed. [Google Scholar]
- 24.Avycaz (Ceftazidime-Avibactam) Package Insert. Forest Pharmaceuticals; Cincinnati, OH: 2015. [Google Scholar]
- 25.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother. 2014;58(2):833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahiri SD, Giacobbe RA, Johnstone MR, Alm RA. Activity of avibactam against Enterobacter cloacae producing an extended-spectrum class C beta-lactamase enzyme. The Journal of antimicrobial chemotherapy. 2014 Nov;69(11):2942–2946. doi: 10.1093/jac/dku237. [DOI] [PubMed] [Google Scholar]
- 27.Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. The Journal of antimicrobial chemotherapy. 2010 Nov;65(11):2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 28.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011 Jan;55(1):390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stachyra T, Levasseur P, Pechereau MC, et al. In vitro activity of the {beta}-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. The Journal of antimicrobial chemotherapy. 2009 Aug;64(2):326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citron DM, Tyrrell KL, Merriam V, Goldstein EJ. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother. 2011 Jul;55(7):3616–3620. doi: 10.1128/AAC.01682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubreuil LJ, Mahieux S, Neut C, Miossec C, Pace J. Anti-anaerobic activity of a new beta-lactamase inhibitor NXL104 in combination with beta-lactams and metronidazole. International journal of antimicrobial agents. 2012 Jun;39(6):500–504. doi: 10.1016/j.ijantimicag.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Winkler ML, Papp-Wallace KM, Hujer AM, et al. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015 Feb;59(2):1020–1029. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerexa I. a subsidiary of Actavis plc. Ceftazidime-Avibactam for Injection Anti-Infective Drugs Advisory Committee. Administration USFaD, ed. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425459.pdf2014.
- 34.Crandon JL, Schuck VJ, Banevicius MA, et al. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012 Dec;56(12):6137–6146. doi: 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahiri SD, Walkup GK, Whiteaker JD, et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. The Journal of antimicrobial chemotherapy. 2015 Feb 1; doi: 10.1093/jac/dkv004. [DOI] [PubMed] [Google Scholar]
- 36.Tanimoto K, Tomita H, Fujimoto S, Okuzumi K, Ike Y. Fluoroquinolone enhances the mutation frequency for meropenem-selected carbapenem resistance in Pseudomonas aeruginosa, but use of the high-potency drug doripenem inhibits mutant formation. Antimicrob Agents Chemother. 2008 Oct;52(10):3795–3800. doi: 10.1128/AAC.00464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papp-Wallace KM, Winkler ML, Taracila MA, Bonomo RA. Variants of the KPC-2 beta-lactamase which are resistant to inhibition by avibactam. Antimicrob Agents Chemother. 2015 Feb 9; doi: 10.1128/AAC.04406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vishwanathan K, Mair S, Gupta A, et al. Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug metabolism and disposition: the biological fate of chemicals. 2014 May;42(5):932–942. doi: 10.1124/dmd.113.055335. [DOI] [PubMed] [Google Scholar]
- 39.Lam YW, Duroux MH, Gambertoglio JG, Barriere SL, Guglielmo BJ. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob Agents Chemother. 1988 Mar;32(3):298–302. doi: 10.1128/aac.32.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boselli E, Breilh D, Rimmele T, et al. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive care medicine. 2004 May;30(5):989–991. doi: 10.1007/s00134-004-2171-2. [DOI] [PubMed] [Google Scholar]
- 41.Welage LS, Schultz RW, Schentag JJ. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob Agents Chemother. 1984 Feb;25(2):201–204. doi: 10.1128/aac.25.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merdjan H, Tarral A, Haazen W, Evene E, Robertson M, C S. Pharmacokinetics and tolerability of NXL104 in normal subjects and patients with varying degrees of renal insufficiency. Paper presented at: 20th European Congress of Clinical Microbiology and Infectious Diseases2010; Vienna, Austria. [Google Scholar]
- 43.MacVane SH, Kuti JL, Nicolau DP. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob Agents Chemother. 2014;58(3):1359–1364. doi: 10.1128/AAC.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhagunde P, Chang KT, Hirsch EB, Ledesma KR, Nikolaou M, Tam VH. Novel modeling framework to guide design of optimal dosing strategies for beta-lactamase inhibitors. Antimicrob Agents Chemother. 2012 May;56(5):2237–2240. doi: 10.1128/AAC.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strayer AH, Gilbert DH, Pivarnik P, Medeiros AA, Zinner SH, Dudley MN. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob Agents Chemother. 1994 Oct;38(10):2351–2356. doi: 10.1128/aac.38.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman K, Levasseur P, Girard AM, et al. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother. 2014 Jun;58(6):3366–3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley MN. Combination beta-lactam and beta-lactamase-inhibitor therapy: pharmacokinetic and pharmacodynamic considerations. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 1995 Mar 15;52(6 Suppl 2):S23–28. doi: 10.1093/ajhp/52.6_Suppl_2.S23. [DOI] [PubMed] [Google Scholar]
- 48.Berkhout J, Melchers MJ, Van Mill CH, et al. Exposure response relationships of Ceftazidime and Avibactam in a Neutropenic Thigh Model. Paper presented at: 53rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy2013; Denver, CO. [Google Scholar]
- 49.Berkhout J, Melchers MJ, Van Mill CH, et al. Pharmacodynamics of Ceftazidime and Avibactam in a Neutropenic Mouse Lung Model. Paper presented at: 53rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy2013; Denver, CO. [Google Scholar]
- 50.Dallow J, Otterson LG, Huband MD, Krause KM, Nichols WW. Microbiological interaction studies between ceftazidime-avibactam and pulmonary surfactant and between ceftazidime-avibactam and antibacterial agents of other classes. International journal of antimicrobial agents. 2014 Dec;44(6):552–556. doi: 10.1016/j.ijantimicag.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez JA, Gonzalez Patzan LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Current medical research and opinion. 2012 Dec;28(12):1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 52.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trial. The Journal of antimicrobial chemotherapy. 2013 May;68(5):1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 53.Das S, Armstrong J, Mathews D, Li J, Edeki T. Randomized, placebo-controlled study to assess the impact on QT/QTc interval of supratherapeutic doses of ceftazidime-avibactam or ceftaroline fosamil-avibactam. Journal of clinical pharmacology. 2014 Mar;54(3):331–340. doi: 10.1002/jcph.199. [DOI] [PubMed] [Google Scholar]
- 54.Chow KM, Szeto CC, Hui AC, Wong TY, Li PK. Retrospective review of neurotoxicity induced by cefepime and ceftazidime. Pharmacotherapy. 2003 Mar;23(3):369–373. doi: 10.1592/phco.23.3.369.32100. [DOI] [PubMed] [Google Scholar]



