Abstract
Background
Trichinella spiralis infection induces protective immunity against re-infection in animal models. Identification of the antigens eliciting acquired immunity during infection is important for vaccine development against Trichinella infection and immunodiagnosis.
Methods and Findings
The T. spiralis adult cDNA library was immunoscreened with sera from pigs experimentally infected with 20,000 infective T. spiralis larvae. Total 43 positive clones encoding for 28 proteins were identified; one of the immunodominant proteins was 20 kDa Ts-ES-1 secreted by Trichinella stichocytes and existing in the excretory/secretory (ES) products of T. spiralis adult and muscle larval worms. Ts-ES-1 contains 172 amino acids with a typical signal peptide in the first 20 amino acids. The expression of Ts-ES-1 was detected in both the adult and muscle larval stages at the mRNA and protein expression levels. Mice immunized with recombinant Ts-ES-1 (rTs-ES-1) formulated with ISA50v2 adjuvant exhibited a significant worm reduction in both the adult worm (27%) and muscle larvae burden (42.1%) after a challenge with T. spiralis compared to the adjuvant control group (p<0.01). The rTs-ES-1-induced protection was associated with a high level of specific anti-Ts-ES-1 IgG antibodies and a Th1/Th2 mixed immune response.
Conclusion
The newly identified rTs-ES-1 is an immunodominant protein secreted by Trichinella stichocytes during natural infection and enables to the induction of partial protective immunity in vaccinated mice against Trichinella infection. Therefore, rTs-ES-1 is a potential candidate for vaccine development against trichinellosis.
Introduction
Trichinella spiralis is a tissue-dwelling nematode that infects a wide variety of vertebrate hosts including humans, in most areas of the world [1,2]. Human infection occurs by eating raw or undercooked meat containing infective Trichinella spiralis larvae [3]. Because of changes in diet and cooking practices and an increase in the consumption of meat, trichinellosis caused by T. spiralis infection is regarded as an emerging or re-emerging infectious disease [4,5]. It has been estimated that more than 11 million people could be infected in the world [6]. Outbreaks of trichinellosis in humans have been regularly reported in different areas of the world [6,7]. This zoonosis is both a public health challenge and an economic issue in porcine animal production and food safety [8,9]. Therefore, the development of vaccines against Trichinella infection in livestock and humans is needed as an effective approach to control this disease.
All of the developmental stages in the life-cycle of T. spiralis occur in the same host, including the adult worm in the small intestine and the larval stage that develops in the muscle to form cysts [10]. Protective immunity induced by primary T. spiralis infection has been observed in different infected animals [11–13]. Infection-induced resistance to secondary infection is related to a potent Th2 response and high antibody titer [11,12]. However, the complete mechanism of protective immunity and which antigens induce protective immunity in the host remain unknown. Therefore, identification of the antigens produced by T. spiralis that elicit host protective immunity is critical for understanding the protective mechanism and targeting these antigens for vaccine or drug development for the control of trichinellosis.
To identify the protective antigens during infection, the adult cDNA library of T. spiralis was immunoscreened with T. spiralis-infected swine sera. More than forty positive clones were recognized by the T. spiralis-infected sera, and one 20-kDa protein secreted by T. spiralis muscle larvae and adult worms was cloned and characterized. Significant protection was induced in immunized mice against T. spiralis infection. Here, we describe the screening, molecular characterization and evaluation of the protective efficacy against Trichinella infection induced by this antigen in a murine model.
Materials and Methods
Parasites and antigen preparation
T. spiralis (ISS533) was maintained in female ICR mice. Muscle larvae (ML) were recovered from infected mice using a modified pepsin-hydrochloric acid digestion method as previously described [14]. Adult worms were collected from the intestines of infected mice four days following larval challenge. Mice were euthanized prior to these procedures for collection of parasite. Crude somatic extracts of ML and adult worms were prepared with conventional homogenizing methods [11]. The excretory-secretory products of ML (MES) were prepared and collected as previously described [15]. Briefly, freshly collected T. spiralis ML were washed three times with phosphate-buffered saline (PBS) and then incubated in RPMI-1640 medium supplemented with 100 U/ml penicillin, 100 U/ml streptomycin and 0.1% bile bovine (Sigma,USA) at 37°C and 5% CO2 for 48 hours. The culture supernatant was collected by centrifugation and was filtered through a 0.45-micron syringe filter and buffer exchanged into PBS. The excretory-secretory products of adult worms (AES) were obtained with the same method as MES except for absence of bile bovine stimulation [16]. The protein concentrations of the prepared worm antigens were determined using a BCA assay (Pierce, USA).
Animals
Female BALB/c mice aged 6–8 weeks and free of specific pathogens were obtained from the Laboratory Animal Services Center of Capital Medical University (Beijing, China). The mice were maintained under specific pathogen-free condition with suitable humidity and temperature.
All experimental procedures were approved by the Capital Medical University Animal Care and Use Committee and comply with the NIH Guidelines for the Care and Use of Laboratory Animals.
Sera preparation
T. spiralis-infected rabbit sera were obtained from two New Zealand white rabbits orally infected with 4,000 T. spiralis muscle larvae and euthanized with exsanguination after being anaesthetized with 25 mg/kg of Ketamine. Infected swine sera were obtained from four Wuzhishan pigs each orally infected with 20,000 T. spiralis ML and then euthanized with exsanguination after being anaesthetized with 25 mg/kg of Ketamine. Infected mice sera were obtained from BALB/c mice orally infected with 500 T. spiralis ML and euthanized with CO2 inhalation using methods described [17]. Some of mice showed some weight loss and rough hair coat, but all tolerated for the challenge. Animals were monitored by research personnel every day for general appearance, hunched posture, rough haircoat, labored breathing, lethargy, lameness, ataxia, diarrhea, abnormal vocalization and abnormal discharge from the eyes or nose. If any animals have bleeding diarrhea, labored breathing, severe leg injuries or have become moribund they will be euthanized immediately by CO2 inhalation. All infected sera were collected 45 days post infection (dpi) and pooled. All human sera were collected from patients with agreement to donate sera for diagnostic and research purpose. All procedures were approved by the Capital Medical University Animal Care and Use Committee (approval number: 2012-X-108). Infected human sera were collected from T. spiralis-infected patients living in the endemic area of Yunnan Province of China during an outbreak and were confirmed by positive serological examination and typical clinical symptoms after being excluded from other parasite infections through fecal or blood examination. All human blood samples were collected for routine care and epidemiological investigation and not for the purposes of this specific study, without revealing any identity of patients, according to the protocol approved by the Institutional Review Board (IRB) of Capital Medical University.
Immunoscreening the adult cDNA library of T. spiralis
The T. spiralis adult (5-day old) λZAPⅡcDNA expression library was immunoscreened with T. spiralis-infected swine sera according to conventional methods. Briefly, 5x104 recombinant plaques on each petridish were incubated at 37°C with IPTG-soaked nitrocellulose membrane (NC) discs (Amersham Biosciences, UK) overnight. Each membrane disc was then blocked with 5% dry milk-PBST (PBS+0.05% Tween 20) overnight at 4°C and subsequently probed with pooled sera from pigs infected with T. spiralis (1:10,000) for 1 h at room temperature. After washing, the membranes were incubated for 1 h with horseradish peroxidase (HRP)-conjugated anti-swine IgG antibody. Immunoreactions were revealed using ECL (Amersham, USA). Positive plaques were rescreened twice until single positive clones were obtained.
DNA sequencing
The positive clones were excised into phagemids according to the manufacturer’s instructions (Stratagene, USA). The phagemid DNAs were extracted and sequenced using vector primers (T7, T3 promoter). The sequences of positive clones were compared with existing sequences in GenBank by BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Identification of the full length cDNA sequence of Ts-ES-1 by 5’-RACE
The full-length Ts-ES-1 cDNA was obtained by 5’-RACE PCR from adult T. spiralis cDNA using a 5’-Full RACE kit (Takara, Japan), according to the manufacturer's instructions using Ts-ES-1 gene-specific primer GSP1: 5’-CCATTCAATTTTGCGTCACA-3’ and GSP2: 5’-CTTGCACAGCAACGTTGCAT-3’.
Expression and purification of recombinant Ts-ES-1 protein (rTs-ES-1)
The DNA encoding the full-length Ts-ES-1 without the signal peptide (22–172 amino acids) was amplified from T. spiralis adult total cDNA by PCR with the forward primer (5’-CGGGATCCgcgaaatcactggatgccgt-3’) and the reverse primer (5’-cgGAATTCctgtaatccattcaattttg-3’), then subcloned into the pET-28b (+) expression vector (Novagen) using the BamHI and EcoRI sites. After being transformed into Escherichia coli BL21 (DE3) cells, the expression of rTs-ES-1 with a His-tag expressed at the N- and C-termini was induced with IPTG at a final concentration of 1 mM at 37°C for 4 h. After ultrasonic decomposition, the fractions of the induced cells were collected and analyzed by SDS-PAGE. The rTs-ES-1 was expressed as an insoluble protein in inclusion bodies, then solubilized with 8 M urea and purified by Ni-affinity chromatography. The urea-solubilized rTs-ES-1 was then refolded using a protein refolding kit (Novagen, Germany) according to the manufacturer’s instructions. The concentration of the rTs-ES-1 was measured by the BCA method.
Generation of anti-rTs-ES-1 antibody
Antiserum against rTs-ES-1 was produced in mice immunized subcutaneously with 25 μg of purified rTs-ES-1 emulsified with an equal volume of the adjuvant ISA50v2 (Seppic, France), followed by two boost immunizations at 2-week intervals. One week after the last immunization, the mice were bled and the sera were collected and stored at -20°C.
Western blot analysis
Protein samples including the crude somatic extracts of adult worm and muscle larvae, AES, MES, and rTs-ES-1 were separated by SDS-PAGE with 12% polyacrylamide gel, then transferred onto an NC membrane (Millipore, USA). Another ES protein Ts87 was used as a loading control [18]. After being blocked with 5% (w/v) skimmed milk in PBS, the membrane was incubated with different T. spiralis-infected sera from swine (1:200), rabbit (1:500), mice (1:100) and human patients (1:200) or with mouse anti-rTs-ES-1 sera (1:10,000). The corresponding IRDye 800CW-conjugated secondary antibody was used to detect specific antibody binding and visualized with the Odyssey CLx Infrared Imaging System.
Real-time Quantitative PCR analysis of Ts-ES-1 gene transcription
To analyze the transcription of the Ts-ES-1 gene in different developmental stages of T. spiralis, total RNA was extracted from ML and adult worms with an RNA simple Total RNA Kit (TIANGEN, China) according to the manufacturer’s instructions. The cDNAs templates were reverse-transcribed from the same amount of total mRNA of ML and adult worms with an oligo dT primer using a Sensiscript Reverse Transcription Kit (Qiagen, Germany). A housekeeping Trichinella gene (GAPDH) was used as an internal control. Primers for detection of the Ts-ES-1 gene were designed as follows: 5’-gcgaaatcactggatgccgt-3’ (forward) and 5’-CTTGCACAGCAACGTTGCAT-3’ (reverse). Primers for GAPDH were 5’-TGCTTCTTGCACTACCAATGGCTTAG-3’ (forward) and 5’-ACCAGATGGACCATCGACTGTCTTTT-3’ (reverse). Real-time quantitative PCR was performed to determine gene transcription levels in ML and adult worms by using SYBR Premix Ex Taq (TaKaRa, Dalian China) in the DNA Engine Opticon 2 system (MJ Research, USA). All data were analyzed using the Opticon Monitor software, and threshold cycle (Ct) was calculated using the 2−ΔΔCt method. After being normalized by GAPDH, the fold-change of the Ts-ES-1 gene expression level in adult worms was calculated relative to that in ML.
Immunofluorescence assay (IFA)
T. spiralis muscle larvae collected from infected mice were fixed with 3% (v/v) paraformaldehyde and longitudinal sections were cut and Paraffin-embedded. After being blocked with normal goat serum for 1 h, the sections were incubated with the anti-rTs-ES-1 mouse sera (1:100) for 2 h. Dylight 488-conjugated goat-anti-mouse IgG was used as the secondary antibody at a dilution of 1:100 for 1 h. A larval section incubated with sera from normal mice under the same conditions served as a negative control. These sections were washed three times with PBS and then examined under a fluorescence microscope (Leica, Germany).
Immunization and challenge experiments
BALB/c mice were divided into three groups with 24 animals each. The mice in the first group were each vaccinated subcutaneously with 25 μg of rTs-ES-1 emulsified with ISA50v2, then boosted twice using the same method at intervals of 2 weeks. The second and third groups were inoculated with ISA50v2 emulsified with PBS alone, or with PBS only, as controls using the same immunization regimen as the first group. Four mice from each group were sacrificed one week after each vaccination, and the sera and spleens were collected for immunological tests. Two weeks after the final boost, the remaining 12 mice in each group were each challenged orally with 500 infective T. spiralis muscle larvae. At 5 dpi, six mice from each group were sacrificed to collect adult worms to evaluate the adult worm reduction. The muscle larvae were examined for another 6 remaining mice from each group using a routine digestion method (described previously) at 45 dpi. The reduction in the adult worm or muscle larvae burden was calculated compared with the worms collected from the PBS control group.
ELISA measurement of the antibody response
Mice sera were collected one week after each vaccination and measured for rTs-ES-1-specific IgG, IgG1, and IgG2a antibodies by using an indirect enzyme-linked immunosorbence assay (ELISA). Briefly, flat-bottom, 96-well microtiter plates were coated overnight at 4°C with 100 μl rTs-ES-1 at a concentration of 1.0 μg/ml in bicarbonate buffer (pH 9.6). After three washes with PBST, the microplates were blocked with 1% bovine serum albumin (BSA) in 100 μl of PBS for 1 h at 37°C. After another three washes with PBST, the microplates were probed with serial dilutions of immune sera for 1 h at 37°C. The plates were then washed and incubated with HRP-conjugated goat anti-mouse IgG, IgG1, or IgG2a for 1 h at 37°C. After the final wash, the substrate 3,3’,5,5’-tetramethylbenzidine (TMB) (BD, USA) was added to each well, and the reactions were stopped with 2 M H2SO4. Quantification of the reactions was determined by measuring the absorbance at 450 nm with an ELISA reader.
Cytokine analysis
One week after each immunization, four mice from each group were sacrificed. The spleens were ground through a sterile steel mesh into lymphocyte separation medium. After being centrifuged, the spleen cells were resuspended in complete RPMI-1640 containing 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin and adjusted to 1×107 cells/ml. For in vitro stimulation, a total of 1×106 splenocytes were incubated with 2 μg of rTs-ES-1 in 200 μl of complete RPMI-1640 in 96-well flat-bottomed cell culture plates for ELISPOT. After cell stimulation for 48 h at 37°C in a humidified atmosphere containing 5% CO2, the cytokines IFN-γ, IL-2, IL-4 and IL-5 were detected using an ELISPOT kit (BD, USA), according to the manufacturer's instructions.
Statistical analysis
All data were compared by analysis of variance (one-way ANOVA) and Student’s t-test using SPSS 15.0 software. The data were expressed as the means ± standard error. p<0.05 was regarded as statistically significant.
Results
Cloning of the cDNA encoding Ts-ES-1
A T. spiralis adult cDNA library was immunoscreened with sera from swine experimentally infected with T. spiralis and a total of 43 positive clones encoding for 28 proteins were obtained (Table 1). Except for 8 enzymes involved in the intracellular processing, most of proteins identified are hypothetical Trichinella proteins with unknown function. Five of them contain signal peptide, 8 proteins contain apparent transmembrane domain, indicating these proteins could be the secreted proteins or surface antigens. Two of the positive clones strongly recognized by T. spiralis-infected swine sera encode the same open reading frame of a protein that shares 79% amino acid sequence identity with the 21 kDa excretory-secretory protein of T. pseudospiralis [19], hereby designated as Ts-ES-1 (excretory-secretory protein-1 of T. spiralis). The full-length DNA sequence of Ts-ES-1 contains 516-bp nucleotides that encode a protein of 172 amino acids, with the first 21 amino acids as a signal peptide (Fig 1). The molecular weight of full-length Ts-ES-1 is predicted to be 19.7 kDa, with a theoretical isoelectric point of 5.36.
Table 1. Identification of T. spiralis adult worm antigens recognized by pig infected sera.
| Clone# | Name | NCBI ID | MW(kDa) | SP* | TM** |
|---|---|---|---|---|---|
| 1-1-1 | 26S protease regulatory subunit 6B | XP_003371578.1 | 31.6 | Yes | No |
| 1-1-16 | hypothetical protein Tsp_04117 | XP_003374930.1 | 20.06 | No | No |
| 1-1-17 | hypothetical protein Tsp_04117 | XP_003374930.1 | 20.06 | No | No |
| 3-2-1 | 40S ribosomal protein S6 | XP_003377362.1 | 57.29 | No | No |
| 3-2-3 | putative fibronectin type III domain protein | XP_003374214.1 | 154.58 | Yes | Yes |
| 3-6-2 | putative transcription initiation factor TFIID subunit 5 | XP_003376268.1 | 106.06 | No | Yes |
| 4-1-1 | heparan sulfate glucosamine 3-O-sulfotransferase 3A1 | XP_003369975.1 | 39.93 | No | No |
| 5-1-1 | sodium/potassium-transporting ATPase subunit beta-1-interacting protein 3 | XP_003372552.1 | 81.04 | No | Yes |
| 6-1-6 | Pre-rRNA-processing protein TSR1-like protein | XP_003379828.1 | 87.08 | No | No |
| 6-2-1 | aspartate—tRNA ligase | XP_003373549.1 | 55.52 | No | No |
| 6-2-6 | aspartate—tRNA ligase | XP_003373549.1 | 55.52 | No | No |
| 6-2-9 | UDP N acetylglucosamine peptide | CDW56646.1 | 120.67 | No | No |
| 6-2-13 | aspartate—tRNA ligase | XP_003373549.1 | 55.52 | No | No |
| 6-2-21 | putative CBS domain pair | XP_003377393.1 | 128.1 | No | Yes |
| 6-2-23 | UDP N acetylglucosamine peptide | CDW56646.1 | 120.67 | No | No |
| 7-2-3 | 40S ribosomal protein S25 | XP_003370909.1 | 11.36 | No | No |
| 7-2-5 | putative regulator | XP_003379635.1 | 28.29 | No | Yes |
| 7-2-10 | conserved hypothetical protein | XP_003372742.1 | 93.77 | No | Yes |
| 7-2-14 | putative regulator | XP_003379635.1 | 28.29 | No | Yes |
| 7-2-15 | putative ATP synthase F1 delta subunit | AET09707.1 | 24.75 | No | No |
| 7-2-19 | 40S ribosomal protein S25 | XP_003370909.1 | 11.36 | No | No |
| 7-2-23 | putative ATP synthase F1 delta subunit | AET09707.1 | 24.75 | No | No |
| 8-3-1 | 5'-nucleotidase | XP_003374564.1 | 52.92 | No | No |
| 8-3-2 | conserved hypothetical protein | XP_003374833.1 | 155.86 | No | No |
| 8-3-4 | hypothetical protein | CBX25710.1 | 19.73 | Yes | No |
| 8-3-6 | conserved hypothetical protein | XP_003374833.1 | 155.86 | No | No |
| 8-3-8 | hypothetical protein | CBX25710.1 | 19.73 | Yes | no |
| 8-3-11 | conserved hypothetical protein | XP_003382240.1 | 102.19 | No | No |
| 8-3-13 | conserved hypothetical protein | XP_003374833.1 | 155.86 | No | No |
| 9-1-1 | lonCoA ligase 5 | XP_003380550.1 | 84.07 | No | Yes |
| 10-1-2 | adult-specific DNase II-5 | AAY32320.1 | 38.16` | Yes | No |
| 10-1-3 | adult-specific DNase II-5 | AAY32320.1 | 38.16 | Yes | No |
| 10-1-8 | 60S ribosomal protein L19 | XP_003374837.1 | 26.16 | Yes | No |
| 10-1-11 | adult-specific DNase II-5 | AAY32320.1 | 38.16 | Yes | No |
| 10-1-12 | adult-specific DNase II-5 | AAY32320.1 | 38.16 | Yes | No |
| 10-1-16 | THO complex subunit 4 | XP_003376451.1 | 21.71 | No | No |
| 10-2-1 | hypothetical protein Tsp_00979 | XP_003376752.1 | 26.07 | No | No |
| 10-2-2 | hypothetical protein Tsp_00979 | XP_003376752.1 | 26.07 | No | No |
| 10-2-5 | hypothetical protein Tsp_00979 | XP_003376752.1 | 26.07 | No | No |
| 10-2-8 | putative tetratricopeptide repeat-containing domain protein | XP_003377351.1 | 118.64 | No | No |
| 10-2-14 | actin-5C | XP_003373575.1 | 41.84 | No | No |
| 10-5-1 | choline-phosphate cytidylyltransferase B | XP_003374840.1 | 80.6 | No | No |
| 10-5-5 | hypothetical protein Tsp_12193 | XP_003380508.1 | 34.46 | No | Yes |
*SP: signal peptide
**TM: transmembrane domain.
Fig 1. Alignment of the deduced amino acids sequence of Ts-ES-1 with a homologue from T. pseudospiralis (GenBank accession no. AAF79206.1).

Sequences were aligned using CLUSTALW and prepared for display using BOXSHADE. Identical amino acids are shaded in black and similar amino acids in gray. The signal peptide in the first 21 amino acids is marked with a red rectangle. The percentage of sequence identity to the T. pseudospiralis homologue is shown at the end of theTs-ES-1 sequence.
Recombinant Ts-ES-1 (rTs-ES-1) expression and recognition by T. spiralis-infected sera
Recombinant Ts-ES-1 (rTs-ES-1) without the signal peptide (approximately 17 kDa) but with His tags at both the N-terminus and the C-terminus was expressed in E. coli BL21 (DE3) cells as an insoluble inclusion body. After being solubilized with 8 M urea, rTs-ES-1 was purified with Ni-affinity chromatography and then refolded in 20 mM Tris, pH8.5. The molecular mass of the rTs-ES-1 (with His tags) was approximately 24 kDa (Fig 2A), consistent with the calculated molecular weight including the His tags. Western blotting confirmed that the expressed rTs-ES-1 could be recognized by mouse anti-Ts-ES-1 sera (Fig 2B) and anti-His antibody as well (Fig 2C).
Fig 2. The expression and purification of rTs-ES-1.
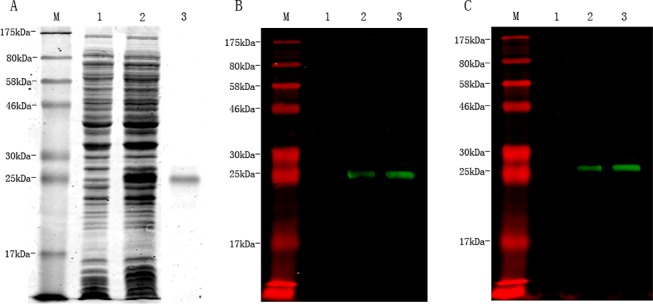
SDS—PAGE analysis showing rTs-ES-1 was highly expressed in IPTG-induced E. coli BL21 lysates (10 μl, Lane 2), not in the uninduced E. coli lysate(10 μl, Lane 1); the IMAC purified rTs-ES-1 was loaded in Lane 3 (1 μg) (A). Western blot analysis showing the specific recognition of expressed rTs-ES-1 in induced lysate (1 μl, Lane 2) or purified rTs-ES-1 (500 ng, Lane 3), but not in uninduced E. coli lysates (1 μl, Lane 1), by mouse anti-Ts-ES-1 sera (1:10,000) (B) or by mouse anti-His monoclonal antibody (1: 5,000) (C).
The purified rTs-ES-1 was used to evaluate its antigenicity by immunoblotting with different T. spiralis-infected animal or human sera. The results demonstrated that rTs-ES-1 was recognized not only by mouse anti-rTs-ES-1 antisera, but also by all T. spiralis-infected animal sera from swine, rabbits, mice, and human patients with trichinellosis (Fig 3A), whereas no reaction was detected with sera from healthy people or normal animals (Fig 3B). The results indicate that Ts-ES-1 is a highly immunogenic antigen and induces a strong antibody response in hosts during natural infection.
Fig 3. Recognition of recombinant Ts-ES-1 by anti-Ts-ES-1 antisera.
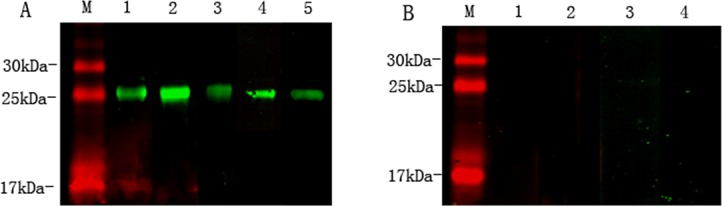
A. Western blot analysis showing the recognition of recombinant Ts-ES-1 (500 ng) by sera from T. spiralis-infected mice (Lane 1), swine (Lane 2), rabbits (Lane 3), human patients (Lane 4) and mouse anti-Ts-ES-1 sera (Lane 5). B. Western blot showing there is no recognition of the same amount of Ts-ES-1 (500 ng) by sera from normal mice (Lane 1), normal swine (Lane 2), normal rabbits (Lane 3) and healthy human (Lane 4).
Stages of Ts-ES-1 expression
Real-time quantitative PCR was performed to observe the transcription level of Ts-ES-1 gene at ML and adult worm life stages of T. spiralis. As shown in Fig 4A, After being normalized by GAPDH, the fold-change of the Ts-ES-1 gene expression level in adult worms was calculated relative to that in ML. We found that the gene expression of Ts-ES-1 increased significantly to 2.8-fold in the adult stage (p<0.05).
Fig 4. Stage expression of Ts-ES-1 expression in T. spiralis.
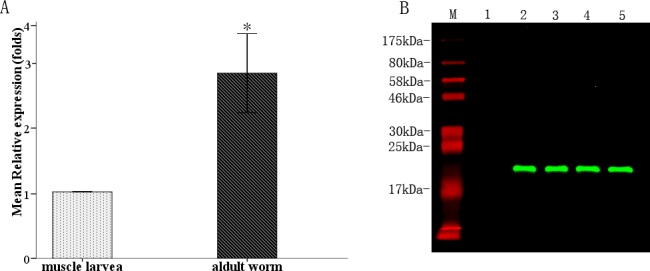
A. Real-time quantitative PCR analysis of stage expression levels of Ts-es-1 gene. After being normalized with GAPDH, the fold-change of the Ts-es-1 gene expression level in adult worms was calculated relative to that in ML. The fold values are presented as the mean of three experiments ± standard deviation. The asterisk (*) indicate significant difference (p < 0.05) between the transcription of Ts-es-1 gene in adult worm and in ML. B. Western blot analysis showing the native Ts-ES-1 protein recognized by mouse anti-Ts-ES-1 antisera in the somatic extracts of ML (Lane 2); somatic extracts of adult worms (Lane 3); ES products of ML (Lane 4) and ES products of adult worms (Lane 5). There was no reaction of mouse anti-Ts-ES-1 antisera to recombinant Ts-87 (Lane 1), another ES protein of T. spiralis.
The protein expression level and distribution of native Ts-ES-1 was determined by Western blot with anti-Ts-ES-1 antisera raised in mice through immunization with rTs-ES-1. The results demonstrated that a tight band at approximately 20 kDa was detected by anti-Ts-ES-1 antisera not only in somatic extracts but also in ES products of both T. spiralis adult and muscle larval worms (Fig 4B), indicating that the native Ts-ES-1 is a secreted protein in both muscle larvae and adult stages. It is consistent with the finding that the same protein was identified in the pre-mature adult worm at 20 hours post-infection [20], and the homologue in T. pseudospiralis was found in the excretory-secretory proteins of muscle larvae [19]. The actual molecular mass (20 kDa) is greater than that predicted by the sequence for the mature protein without signal peptide (17 kDa) possibly due to post-translational modification of the natural protein. The mouse anti-Ts-ES-1 sera didn’t recognize recombinant Ts87, another ES protein of T. spiralis as a loading control [18].
Immunolocalization of Ts-ES-1
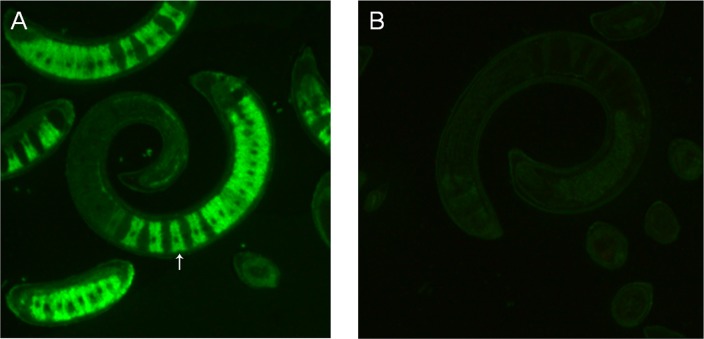
To localize the native Ts-ES-1 in the parasites, sections of T. spiralis muscle larvae were allowed to react with mouse anti-rTs-ES-1 sera and then incubated with Dylight488-conjugated goat-anti-mouse IgG. The results of IFA revealed that the native Ts-ES-1 was strongly and exclusively distributed in the stichocytes of T. spiralis muscle larvae stichosomes. In contrast, little reactivity was detected in sections of the muscle larvae when probed with normal mouse sera (Fig 5). The results clearly reveal that native Ts-ES-1 is highly expressed in stichocytes, consistent with the Western blot results showing that Ts-ES-1 is secreted and present in the ES products of larval and adult worms (Fig 4B).
Fig 5. Immunolocalization of Ts-ES-1 in sections of T. spiralis muscle larvae (400×).
Fluorescence was detected with Dylight 488-conjugated anti-mouse IgG secondary antibody. Longitudinal sections of T. spiralis ML were probed with mouse anti-Ts-ES-1 antisera (1:100) and native Ts-ES-1 was shown to be located intensively in the stichocytes at the posterior portion of T. spiralis ML stichosome (A). Nothing was detected when normal mouse serum at the same dilution was used as control (B).
Immunogenicity of rTs-ES-1
Recombinant Ts-ES-1 was used to immunize mice three times, and the mouse serum samples were collected from each immunized mouse one week after each immunization. The antibody titers of the serum samples against rTs-ES-1 were measured using ELISA. A high titer of specific IgG antibodies was elicited in all of the immunized mice one week after each immunization, and the highest IgG titer reached 1:256,000 after the third immunization (Fig 6A). The IgG subclass antibody levels were measured to further assess the efficacy of rTs-ES-1 in induction of the different IgG subclass responses in vivo. The results demonstrated that the predominant IgG subclass was IgG1, but there was also a significant level of IgG2a response especially after the first immunization boost (Fig 6B).
Fig 6. Antibody responses of vaccinated mice against rTs-ES-1 formulated with ISA50v2 adjuvant before challenge with T. spiralis ML.
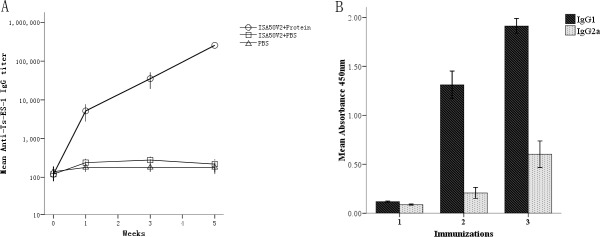
A. Specific IgG titer was detected one week after each immunization. B. Serum IgG subclass responses (OD at 1:100 dilutions) in mice upon vaccinations with rTs-ES-1 formulated with ISA50v2 were detected one week after each immunization. The values are presented as the arithmetic mean of four mice in the rTs-ES-1 group ± standard error.
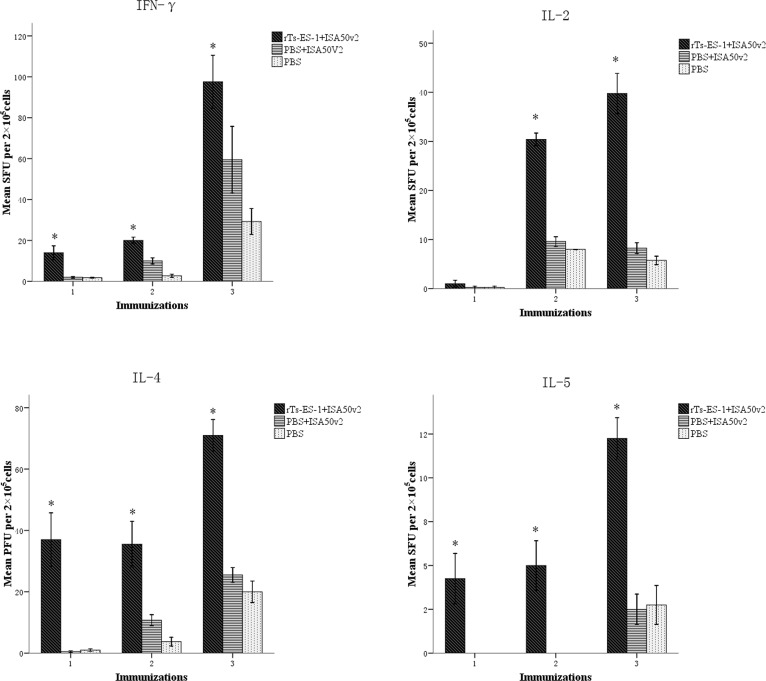
The cytokines secreted by immunized mouse splenocytes upon stimulation of rTs-ES-1 in vitro, including IFN-γ, IL-2, IL-4, and IL-5, were measured using ELISPOT. The levels of the typical Th1 cytokines (IFN-γ, IL-2) and Th2 cytokines (IL-4, IL-5) were significantly elevated in mice vaccinated with rTs-ES-1 compared to the adjuvant-alone control group (Fig 7), and the Th2 cytokines IL-4 and IL-5 were significantly increased even after the first immunization. Our results showed that rTs-ES-1 vaccination induced mixed Th1 and Th2 responses in mice in terms of the antibody response and cytokine production.
Fig 7. Cytokines secreted by splenocytes from BALB/c mice immunized with rTs-ES-1 upon stimulation with 2 μg of rTs-ES-1 in vitro before challenge with T. spiralis ML.
Splenocytes secreting IFN-γ, IL-2, IL-4 and IL-5 were detected by ELISPOT one week after each immunization. The values are presented as the arithmetic mean of four mice in each group ± standard error. The asterisks (*) indicate significant differences (p < 0.01) between the rTs-ES-1 and control groups.
Partially protective immunity elicited by rTs-ES-1
Partially protective immunity against T. spiralis infection induced by rTs-ES-1 was observed in immunized BALB/c mice. The result of the challenge experiment showed that mice immunized with rTs-ES-1 formulated with the adjuvant ISA50v2 for three times, then challenged with 500 T. spiralis infective larvae, induced a 27% adult worm reduction (138 ± 20) and 42.1% muscle larvae (ML) reduction (3782 ± 766), which was significantly different from adjuvant-alone control group (adult 197 ± 20; ML 6780 ± 938) (p<0.01) (Table 2). There was no significant difference in the adult worm and muscle larvae burden between the adjuvant-alone (ISA50v2) and PBS-alone control groups. These results show that T. spiralis-secreted Ts-ES-1 enable the induction of partial protective immunity against T. spiralis infection in mice.
Table 2. Protective immunity elicited by immunizing rTs-ES-1 in mice against challenge with 500 T. spiralis larvae.
| Group | Mean adult worm burden ± SD (mouse#) | adult worm reduction | Mean muscle larvae per gram muscle ± SD (mouse#) | Muscle larvae burden reduction |
|---|---|---|---|---|
| PBS | 189 ± 19 (6) | 6532 ± 618 (6) | ||
| ISA50V2+PBS | 197 ± 20 (6) | 6780 ± 938 (6) | ||
| ISA50V2+ rTs-ES-1 | 138 ± 20 (6) | 27% * | 3782 ± 766 (6) | 42.1% * |
*p<0.01 compared with adjuvant control group.
Discussion
Generally, the lack of an effective vaccine and a reliable early diagnostic method for Trichinella infection gives rise to the establishment of mature and encapsulated Trichinella muscle larvae, which are usually resistant to treatment with anthelmintic drugs. With the purpose of discovering an effective vaccine or diagnostic antigen, the adult cDNA library of T. spiralis was immunoscreened with sera from pigs experimentally infected with 20,000 T. spiralis muscle larvae.
Infection with the Trichinella parasite results in the induction of immunity in hosts that is strong enough to trigger rapid defense against secondary infection [11–13,21]. The resistance to a secondary infection in pigs was infection dose-dependent. Infection with 25,000 muscle larvae induced almost complete resistance to re-infection [22]. In addition to activated mast cells [23], eosinophils [24], mucosal immunity [25] and Th1 cellular responses [26], which were determined to be involved in protective immunity against Trichinella infections, the most effective and consistent protective effects are attributed to specific anti-Trichinella antibodies [27]. Passive transfer of infected immune serum or parasite-specific monoclonal antibodies against a parasite-specific glycoprotein confers effective immunity on naive pups against Trichinella infections [21,28]. In this study, the sera or antibodies collected from protected pigs, which are elicited by infection with a high dose of infective muscle larvae (20,000), are supposed to recognize the antigens of T. spiralis that induce protective immunity.
Total 43 positive clones encoding for 28 proteins were identified by immunoscreening the adult cDNA expression library with T. spiralis-infected swine sera. One of the clones was identified as a Trichinella-secreted protein sharing 79% amino acid sequence identity with a previously identified secreted protein in T. pseudospiralis and was therefore designated as Ts-ES-1. There is no any homologue or functional domain found in other nematodes or species except for genetically related T. pseudospiralis, therefore, it is a Trichinella-specific protein with unknown function. Ts-ES-1 contains 172 amino acids with a typical signal peptide in the first 21 amino acids. The expression of Ts-ES-1 was detected in both the adult and the muscle larval stages of T. spiralis at the mRNA and protein levels. These results are consistent with the detection of the native Ts-ES-1 protein in the ES products of both the adult and the muscle larval worms identified in this study. Furthermore, secreted Ts-ES-1 was localized in the stichocytes of the muscle larvae using an IFA with specific antibodies. Similar to Trichuris nematodes, Trichinella stichocytes are glandular unicellular cells arranged in a row along the posterior portion of the esophagus that produce and secrete proteins into the lumen of the esophagus with different biological functions [29,30]. Nematode-released ES products are the primary interface between the parasites and the hosts, playing a wide range of roles crucial for their survival and reproduction. It has been determined that the proteins in ES products are essential for invading into host tissues [31], feeding [32], reproduction [33] and modulating the host immune system to evade host immune attack [34,35]. Therefore, nematode-secreted proteins have been targeted as major vaccine candidates [36]. Vaccines based on ES products have been reported for many parasites [15,37–39]. An ES product of adult Brugia malayi, Bm-iPGM, could induce 58.2% protection against larval challenge in BALB/c mice and 65–68% protection in M. coucha [40]. The 52.8-kDa protein from the ES products of S. japonicum could induce a 35.32% or 26.19% reduction in the worm burden and a 33.17% or 31.7% lower liver egg count in two experiments in vaccinated mice [41]. The ES antigens of T. spiralis are directly exposed to the host's immune system and are the main antigens that induce the immune responses in the host [42]. It has been reported that the ES products of T. spiralis could provide protective immunity [15], and some of the proteins identified within the ES products of T. spiralis have achieved some success in vaccines or immunodiagnosis [43,44]. For these reasons, we have further explored the Trichinella-secreted Ts-ES-1identified in this study for its potential as a vaccine against T. spiralis infection.
In this study, mice vaccinated with rTs-ES-1 formulated with ISA50v2 adjuvant exhibited a significant reduction in the adult worms (27%, 138 ± 20 vs 197 ± 20) and muscle larvae (42.1%, 3782 ± 766 vs 6780 ± 938) after being challenged with T. spiralis compared to the adjuvant control group (p<0.01). The rTs-ES-1-induced protection was associated with a high level of anti-Ts-ES-1 antibody, including increased total IgG and the IgG1 and IgG2a subtypes, and the production of the splenocyte-secreted cytokines IFN-γ, IL-2, IL-4 and IL-5. It is commonly believed that the Th2 immune response is essential for protective immunity for helminth infections [45–47]. The humoral response contributes greatly to resistance against Trichinella infection by entrapping and expulsing infective larvae, reducing the fecundity of adult worms and eliminating newborn larvae [26]. In addition, it has also been demonstrated that the combined humoral and cellular immune responses are important for immunity against T. spiralis infection [48]. Some experimental results suggest that the cellular response as well as the humoral response may be involved in the mechanisms of protective immunity induced by recombinant protein immunization [49–51]. In this study, mice immunized with rTs-ES-1 produced not only a major Th2-associated immune response (IgG1 antibody, IL-4, and IL-5) but also a Th1-like response evidenced by high titers of IgG2a antibody, IFN-γ and IL-2. The results indicated that mice vaccinated with rTs-ES-1 produced a mixed humoral and cellular immune response that may contribute to the protective immunity observed in this study.
However, the worm reduction induced by immunization with rTs-ES-1 against T. spiralis larval challenge in this study was not high (adult worms reduction 27%; muscle larvae reduction 42.1%), similar to the level induced by other T. spiralis vaccine candidates identified so far [9,18,50–55]. The non-sterilizing immunity or low protection is a dilemma not only for vaccine development against Trichinella infection, but also for all other helminth infections. WHO admitted that the goal with consistent induction of 40% protection or better for Schistosomiasis was not reached with any of antigens in clinical trials [56]. The less than 50% protection was also seen in hookworm vaccines currently in clinical trials [57]. The low protection induced by single vaccine immunization for helminth infection may be caused by the complexity of the life cycle, diversity of stage-specific antigens, immune-evasion strategies and the modulatory effect of host responses [52]. However, the disease development by helminthic parasites usually depends on the intensity of infection [57]. Therefore, reducing the worm burden by vaccination, even not sterilizing, may significantly reduce the manifestation and seriousness of disease [57]. Nevertheless, new strategies are needed to improve the protection of vaccine against Trichinella infection. These strategies may include the multivalent vaccine with combination of more than one vaccine antigens or protective epitopes [58], vaccine that induces intestinal local immunity [18]. Glycoproteins induced strong immune response during infection and antibody against a tyvelose motif on several secreted and surface glycoproteins in T. spiralis L1 larvae effectively prevented niche establishment of the parasites in intestine epithelia, therefore a good vaccine target [21,28,59].
All results described in this study demonstrate that the newly identified Ts-ES-1 secreted by T. spiralis stichocytes plays an important role in the survival of T. spiralis in its host and therefore is a potential candidate for vaccine development against trichinellosis. The specific function of this Trichinella-secreted protein and the enhancement of immunogenicity and vaccine efficacy induced by this antigen are under further investigation.
Acknowledgments
We thank Jiajia Qin, Jingjing Huang, Xi Zhao, Xiaoqin Chen, Lei Fang and Fengyun Wang for their technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (81171598, 81371837, 81201313), the National Science and Technology Major Project (2012ZX10004220-012), and the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (IDHT20140212). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22(1): 127–145. 10.1128/CMR.00026-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dupouy-Camet J. Trichinella and trichinellosis—a never ending story. Vet Parasitol. 2009;159(3–4): 194–196. 10.1016/j.vetpar.2008.10.064 [DOI] [PubMed] [Google Scholar]
- 3. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149(1–2): 3–21. [DOI] [PubMed] [Google Scholar]
- 4. Murrell KD, Pozio E. Worldwide occurrence and impact of human trichinellosis. Emerg Infect Dis. 2011;17(12): 2194–2202. 10.3201/eid1712.110896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Verterinary Parasitology. 2009;163(3): 196–206. [DOI] [PubMed] [Google Scholar]
- 6. Dupouy-Camet J. Trichinellosis: a worldwide zoonosis. Vet Parasitol. 2000;93(3–4): 191–200. [DOI] [PubMed] [Google Scholar]
- 7. Cui J, Wang ZQ, Xu BL. The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop. 2011;118(1): 1–5. 10.1016/j.actatropica.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 8. Gajadhar AA, Pozio E, Gamble HR, Nöckler K, Maddox-Hyttel C, Forbes LB, et al. Trichinella diagnostics and control: mandatory and best practices for ensuring food safety. Vet Parasitol. 2009;159(3–4): 197–205. 10.1016/j.vetpar.2008.10.063 [DOI] [PubMed] [Google Scholar]
- 9. Fang L, Sun L, Yang J, Gu Y, Zhan B, Huang J, et al. Heat shock protein 70 from Trichinella spiralis induces protective immunity in BALBc mice by activating dendritic cells. Vaccine. 2014;32(35): 4412–4419. 10.1016/j.vaccine.2014.06.055 [DOI] [PubMed] [Google Scholar]
- 10. Bruschi F. The immune response to the parasitic nematode Trichinella and the ways to escape it. Current Drug Targets—Immune, Endocrine & Metabolic Disorders. 2002;2(3): 269–280. [DOI] [PubMed] [Google Scholar]
- 11. Auxiliadora DM, Bolas-Fernandez F. Dynamics of the IgG3 responses following immunisation of BALB/c mice with somatic and excretory/secretory antigens from various Trichinella species. Folia Parasitol (Praha). 2000;47(3): 172–180. [DOI] [PubMed] [Google Scholar]
- 12. Alizadeh H, Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982;12(1): 65–73. [DOI] [PubMed] [Google Scholar]
- 13. Russell DA, Castro GA. Immune rejection of Trichinella spiralis infective larvae in the guinea pig. J Parasitol. 1984;70(4): 593–595. [PubMed] [Google Scholar]
- 14. Chen X, Yang Y, Yang J, Zhang Z, Zhu X. RNAi-Mediated Silencing of Paramyosin Expression in Trichinella spiralis Results in Impaired Viability of the parasite. PloS One. 2012;7(11): e49913 10.1371/journal.pone.0049913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dea-Ayuela MA, Rama-Iñiguez S, Bolas-Fernández F. Vaccination of mice against intestinal Trichinella spiralis infections by oral administration of antigens microencapsulated in methacrilic acid copolymers. Vaccine. 2006;24(15): 2772–2780. [DOI] [PubMed] [Google Scholar]
- 16. Yang X, Yang Y, Wang Y, Zhan B, Gu Y, Cheng Y, et al. Excretory/Secretory Products from Trichinella spiralis Adult Worms Ameliorate DSS-Induced Colitis in Mice. PLoS One. 2014;9(5): e96454 10.1371/journal.pone.0096454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pecaut MJ, Smith AL, Jones TA, Gridley DS. Modification of immunologic and hematologic variables by method of CO2 euthanasia. Comp Med. 2000;50(6): 595–602. [PubMed] [Google Scholar]
- 18. Yang Y, Zhang Z, Yang J, Chen X, Cui S, Zhu X. Oral vaccination with Ts87 DNA vaccine delivered by attenuated Salmonella typhimurium elicits a protective immune response against Trichinella spiralis larval challenge. Vaccine. 2010;28(15): 2735–2742. 10.1016/j.vaccine.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 19. Nagano I, Wu Z, Nakada T, Matsuo A, Takahashi Y. Molecular cloning and characterization of a 21 kDa protein secreted from Trichinella pseudospiralis . J Helminthol. 2001;75(3): 273–278. [PubMed] [Google Scholar]
- 20. Zocevic A, Mace P, Vallee I, Blaga R, Liu M, Lacour SA, et al. Identification of Trichinella spiralis early antigens at the pre-adult and adult stages. Parasitology. 2011;138(4): 463–471. 10.1017/S0031182010001526 [DOI] [PubMed] [Google Scholar]
- 21. Appleton JA, Schain LR, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65(3): 487–492. [PMC free article] [PubMed] [Google Scholar]
- 22. Murrell KD. Trichinella spiralis: acquired immunity in swine. Exp Parasitol. 1985;59(3): 347–354. [DOI] [PubMed] [Google Scholar]
- 23. Blum LK, Thrasher SM, Gagliardo LF, Fabre V, Appleton JA. Expulsion of secondary Trichinella spiralis infection in rats occurs independently of mucosal mast cell release of mast cell protease II. J Immunol. 2009;183(9): 5816–5822. 10.4049/jimmunol.0900944 [DOI] [PubMed] [Google Scholar]
- 24. Watanabe N, Katakura K, Kobayashi A, Okumura K, Ovary Z. Protective immunity and eosinophilia in IgE-deficient SJA/9 mice infected with Nippostrongylus brasiliensis and Trichinella spiralis . Proc Natl Acad Sci U S A. 1988;85(12): 4460–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onah DN, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Korean J Parasitol. 2000;38(4): 209–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kołodziej-Sobocińska M, Dvoroznakova E, Dziemian E. Trichinella spiralis: macrophage activity and antibody response in chronic murine infection. Exp Parasitol. 2006;112(1): 52–62. [DOI] [PubMed] [Google Scholar]
- 27. Bell RG. The generation and expression of immunity to Trichinella spiralis in laboratory rodents. Adv Parasitol. 1998;41: 149–217. [DOI] [PubMed] [Google Scholar]
- 28. Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, et al. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis . Glycobiology. 1994;4(5): 593–603. [DOI] [PubMed] [Google Scholar]
- 29. Despommier DD, Müller M. The Stichosome and Its Secretion Granules in the Mature Muscle Larva of Trichinella spiralis . The Journal of Parasitology. 1976;62(5): 775–785. [PubMed] [Google Scholar]
- 30. Takahashi Y, Mizuno N, Shimazu K, Araki T. Ultrastructure Antigenicity and Histochemi try of Stichocyte Granules of Adult Trichinella spiralis . The Journal of Parasitology. 1992;78(3): 518–523. [PubMed] [Google Scholar]
- 31. Williamson AL, Lustigman S, Oksov Y, Deumic V, Plieskatt J, Mendez S, et al. Ancylostoma caninum MTP-1, an astacin-like metalloprotease secreted by infective hookworm larvae, is involved in tissue migration. Infect Immun. 2006;74(2): 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karanu FN, Rurangirwa FR, McGuire TC, Jasmer DP. Haemonchus contortus: identification of proteases with diverse characteristics in adult worm excretory-secretory products. Exp Parasitol. 1993;77(3): 362–371. [DOI] [PubMed] [Google Scholar]
- 33. Haffner A, Guilavogui AZ, Tischendorf FW, Brattig NW. Onchocerca volvulus: microfilariae secrete elastinolytic and males nonelastinolytic matrix-degrading serine and metalloproteases. Exp Parasitol. 1998;90(1): 26–33. [DOI] [PubMed] [Google Scholar]
- 34. Harnett W. Secretory products of helminth parasites as immunomodulators. Mol Biochem Parasit. 2014;195(2): 130–136. [DOI] [PubMed] [Google Scholar]
- 35. White RR, Artavanis-Tsakonas K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence. 2012;3(7): 668–677. 10.4161/viru.22832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum . Mol Cell Proteomics. 2009;8(1): 109–121. 10.1074/mcp.M800206-MCP200 [DOI] [PubMed] [Google Scholar]
- 37. Ridi RE, Tallima H. Vaccine-induced protection against murine schistosomiasis mansoni with larval excretory-secretory antigens and papain or type-2 cytokines. The Journal of Parasitology. 2013;99(2): 194–202. 10.1645/GE-3186.1 [DOI] [PubMed] [Google Scholar]
- 38. Dixon H, Johnston CE, Else KJ. Antigen selection for future anti-Trichuris vaccines: a comparison of cytokine and antibody responses to larval and adult antigen in a primary infection. Parasite Immunol. 2008;30(9): 454–461. 10.1111/j.1365-3024.2008.01035.x [DOI] [PubMed] [Google Scholar]
- 39. Gour JK, Kumar V, Singh N, Bajpai S, Pandey HP, Singh RK. Identification of Th1-responsive leishmanial excretory-secretory antigens (LESAs). Exp Parasitol. 2012;132(3): 355–361. 10.1016/j.exppara.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 40. Singh PK, Kushwaha S, Rana AK, Misra-Bhattacharya S. Cofactor independent phosphoglycerate mutase of Brugia malayi induces a mixed Th1/Th2 type immune response and inhibits. BioMed Research International. 2014;2014(2014): 590281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao X, Hong Y, Zhang M, Han Y, Wu M, Wang X, et al. Cloning, expression and characterization of protein disulfide isomerase of Schistosoma japonicum . Exp Parasitol. 2014;146: 43–51. [DOI] [PubMed] [Google Scholar]
- 42. Bai X, Wu X, Wang X, Liu X, Song Y, Gao F, et al. Inhibition of mammalian muscle differentiation by excretory/secretory products of muscle larvae of Trichinella spiralis in vitro. Parasitol Res. 2012;110(6): 2481–2490. 10.1007/s00436-011-2789-2 [DOI] [PubMed] [Google Scholar]
- 43. Cui J, Wang L, Sun GG, Liu LN, Zhang SB, Liu RD, et al. Characterization of a Trichinella spiralis 31kDa protein and its potential application for the serodiagnosis of trichinellosis. Acta Trop. 2014;142: 57–63. 10.1016/j.actatropica.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 44. Wang B, Wang Z, Jin J, Ren H, Liu L, Cui J. Cloning, expression and characterization of a Trichinella spiralis serine protease gene encoding a 35.5 kDa protein. Exp Parasitol. 2013;134(2): 148–154. 10.1016/j.exppara.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 45. Scales HE, Ierna MX, Lawrence CE. The role of IL-4, IL-13 and IL-4Ralpha in the development of protective and pathological responses to Trichinella spiralis . Parasite Immunol. 2007;29(2): 81–91. [DOI] [PubMed] [Google Scholar]
- 46. Hussaarts L, Yazdanbakhsh M, Guigas B. Priming dendritic cells for Th2 polarization: lessons learned from helminths and implications for metabolic disorders. Frontiers in Immunology. 2014;5: 499 10.3389/fimmu.2014.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunology. 2014; 10.1038/mi.2014.101 [DOI] [PubMed] [Google Scholar]
- 48. Devillea S, de Pooter A, Aucouturier J, Laine-Prade V, Cote M, Boireau P, et al. Influence of adjuvant formulation on the induced protection of mice immunized with total soluble antigen of Trichinella spiralis . Vet Parasitol. 2005;132(1–2): 75–80. [DOI] [PubMed] [Google Scholar]
- 49. Yang J, Yang Y, Gu Y, Li Q, Wei J, Wang S, et al. Identification and characterization of a full-length cDNA encoding paramyosin of Trichinella spiralis . Biochem Bioph Res Co. 2008;365(3): 528–533. [DOI] [PubMed] [Google Scholar]
- 50. Feng S, Wu X, Wang X, Bai X, Haining S, Tang B et al. Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection. The Journal of parasitology. 2013;99(3): 426–432. 10.1645/12-46.1 [DOI] [PubMed] [Google Scholar]
- 51. Li X, Yao J, Pan A, Liu W, Hu X, Wu Z, et al. An antigenic recombinant serine protease from Trichinella spiralis induces protective immunity in BALB/c mice. Parasitol Res. 2013;112(9): 3229–3238. 10.1007/s00436-013-3500-6 [DOI] [PubMed] [Google Scholar]
- 52. Ortega-Pierres G, Vaquero-Vera A, Fonseca-Linan R, Bermudez-Cruz RM, Arguello-Garcia R. Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review. J Helminthol. 2015: 1–14. [DOI] [PubMed] [Google Scholar]
- 53. Li LG, Wang ZQ, Liu RD, Yang X, Liu LN, Sun GG, et al. Trichinella spiralis: Low vaccine potential of glutathione S-transferase against infections in mice. Acta Trop. 2015;146: 25–32. 10.1016/j.actatropica.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Wang Z, Li L, Cui J. Molecular characterization of Trichinella spiralis aminopeptidase and its potential as a novel vaccine candidate antigen against trichinellosis in BALB/c mice. Parasit Vectors. 2013;6: 246 10.1186/1756-3305-6-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei J, Gu Y, Jing Yang, Yang Y, Wang S, Cui S, et al. Identification and characterization of protective epitope of Trichinella spiralis paramyosin. Vaccine. 2011;29(17): 3162–3168. 10.1016/j.vaccine.2011.02.072 [DOI] [PubMed] [Google Scholar]
- 56. McManus DP, Loukas A. Current Status of Vaccines for Schistosomiasis. Clin Microbiol Rev. 2008;21(1): 225–242. 10.1128/CMR.00046-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6(11): 733–741. [DOI] [PubMed] [Google Scholar]
- 58. Gu Y, Wei J, Yang J, Huang J, Yang X, Zhu X. Protective immunity against Trichinella spiralis infection induced by a multi-epitope vaccine in a murine model. PLoS One. 2013;8(10): e77238 10.1371/journal.pone.0077238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McVay CS, Bracken P, Gagliardo LF, Appleton J. Antibodies to tyvelose exhibit multiple modes of interference with the epithelial niche of Trichinella spiralis . Infect Immun. 2000;68(4): 1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.