Abstract
Background
Dodecapeptide SC4 is a broad-spectrum bactericidal agent that functions by disintegrating bacterial membranes and neutralizing endotoxins. For insight into which SC4 amino acids are functionally important, we assessed Gram-negative bactericidal effects in structure-activity relationships experiments. Subsequently, SC4 was tested in a murine bacteremia model to combine and compare the efficacy with Zosyn, a first-line antibiotic against Pseudomonas aeruginosa (P. aeruginosa).
Methods
SC4 alanine-scanning analogues and their activities on were tested on P. aeruginosa. Survival studies in P. aeruginosa challenged mice were executed to monitor overall efficacy of SC4 and Zosyn, as single modality and also as combination treatment. ELISAs were used to measure blood serum levels of selected inflammatory cytokines during treatment.
Results
Cationic residues were found to play a crucial role in terms of bactericidal activity against P. aeruginosa. In vivo, while only 9% (3/34) of control animals survived to day two and beyond, 44% (12/27) to 41% (14/34) of animals treated with SC4 or Zosyn, respectively, survived beyond one week. Combination treatment of SC4 and Zosyn demonstrated improved survival, i.e. 60% (12/20). The TNFα, IL-1, and IL-6 serum levels were attenuated in each treatment group compared to the control group.
Conclusions
These data show that combination treatment of SC4 and Zosyn is most effective at killing P. aeruginosa and attenuating inflammatory cytokines levels in vivo.
General Significance
Combination treatment of SC4 and Zosyn may be useful in the clinic as a more effective antibiotic therapy against Gram-negative infectious diseases.
Keywords: peptide, bactericidal, lipopolysaccharide, endotoxin neutralizing
1. Introduction
Standard antibiotics currently being used in the clinical setting often have therapeutic limitations, e.g. dose limiting toxicities or bacterial resistance [1, 2]. Therefore, new antibiotics are constantly needed, as well as combinations of antibiotics that will decrease mortality from bacterial infection in the clinic. In this regard, a relatively new class of antibiotics has been making their way into the clinical arena, so-called bacterial membrane disintegrating agents [3–5]. These compounds, commonly being both amphipathic and polycationic [6], generally kill bacteria by interacting with negatively charged groups on the surface of bacteria (e.g. lipopolysaccharide on Gram-negative bacteria), integrating into their membrane, and promoting leakiness and permeability [7–9].
Although these membrane disruptors have some advantages compared to standard antibiotics used in the clinic, e.g. broad-spectrum killing and reduced potential for bacterial resistance, most of these agents display the unwanted side effect of also disrupting eukaryotic cell membranes [10], which makes them less useful in the clinic. Dodecapeptide SC4, however, is one membrane disintegrator that disrupts bacterial cell membranes with no apparent lytic effect on eukaryotic cells [11, 12]. SC4 is highly effective against Gram-negative bacteria, and is exceptional in that it exhibits LD50 values in the nanomolar range [11]. Of the limited number of Gram-negative strains against which SC4 was tested, it appears to be most effective against Pseudomonas aeruginosa, displaying an LD50 value in the single digit nanomolar range [11]. SC4 is also effective at neutralizing the endotoxin lipopolysaccharide (LPS) released by disintegration of the Gram-negative bactericidal cell outer membrane [11]. LPS triggers the overproduction of various cytokines like TNFα and interleukin-1 (IL-1) in macrophages, which in turn can lead to septic shock and possibly death [13].
Developing treatments against P. aeruginosa is of particular interest, because this bacterium is the primary cause of Gram-negative infection in intensive care units [14]. In addition, many pathogens, like P. aeruginosa, acquire antibiotic resistance, and multiple drug resistant strains of P. aeruginosa have been reported worldwide [15]. For example, about 20 percent of P. aeruginosa strains show resistance to quinolone-based drugs, and 15 percent show resistance to the antibiotic imipenem [2]. In this regard, there is an urgent need for new antibiotics against P. aeruginosa, and other Gram-negative mediated infections [14]. However, for various reasons, there is an uneven supply of novel antibiotics and a reduction in the number of pharmaceutical companies engaged in the discovery and development of anti-infective agents [2]. Presently, a mixture of piperacillin (a semisynthetic penicillin) and tazobactam (a β-lactamase inhibitor), named Zosyn (marketed in the U.S. by Wyeth-Ayerst), is the most frequently employed antibiotic against Pseudomonas in the clinic [16].
2. Material and Methods
2.1. Peptide preparation
Dodecapeptide SC4 and related analogues were synthesized and purified as described earlier [11]. Purity and composition of SC4 and variants were verified by HPLC (Beckman Model 6300), amino acid analysis, and mass spectrometry.
2.2. Bactericidal assay
Pyrogen-free solutions were used throughout the assay. Pseudomonas aeruginosa type 1 is a clinical smooth strain isolate and was serotyped by the scheme of Homma [17], maintained in the lab by monthly transfer on blood agar plates, as described earlier [5, 11]. Log phase bacteria were obtained by transferring an overnight culture or scraping crystals off −85°C glycerol stocks of overnight cultures. Bacteria were washed and re-suspended in 0.9% sodium chloride with adjustment to an optical density at 650 nm which yields 3 × 108 CFU/ ml. Bacteria were then diluted 1:10 in 0.08 M citrate phosphate buffer, pH 7.0 (prepared by mixing 0.08 M citric acid with 0.08 M dibasic sodium phosphate). Bacteria (0.15 ml) were incubated with peptide in a final volume of 1.0 ml of buffer. The assay was done in 17×100 polypropylene tubes in a reciprocal water bath shaker at 37°C for 30 minutes. Following this 30 min. incubation, 10-fold dilutions were made in 0.9% sodium chloride. Dilutions were done to 10−4 and 20 µl of each dilution was streaked across a MacConkey agar plate (2%). Plates were incubated overnight at 37°C and counted the next morning. Peptide concentrations were converted to logarithm base ten and graphed. Bactericidal activity was determined by dose response where LD50 values, a lethal dose of killing 50% of the bacteria, were determined by best fits of a sigmoidal curve to the dose response data.
2.3. Bacteremia studies in mice
C57BL/6 male black mice (The Jackson Laboratory, Bar Harbor, ME) were injected intraperatoneally (i.p.) with 300 µl of a Klett 70, as determined by a Klett-Summerson photoelectric colorimeter (using a D10 and a D35 filter). This contained an approximate lethal dose of 1 × 108 CFU P. aeruginosa bacteria. Because the optimal dosing for Zosyn against P. aeruginosa in the clinic is via intravenous administration, either prolonged or continuous-infusion [16], Zosyn was administered subcutaneously (s.c.) to mice via Alzet mini-pumps. Mice were administered Zosyn (n=27) at a dose of 400 mg/kg/day dissolved in PBS, which is similar to the clinically recommended dose for humans of about 20 g/day [16]. For consistency, SC4 was also administered s.c. to mice (n=34) via Alzet mini-pumps at a dose of 10 mg/kg/day dissolved in PBS, and in mice treated with combination of these two agents (n=20), two pumps were implanted. Control mice (n=34) were treated with vehicle (PBS) alone by mini-pumps. Pumps were surgically implanted s.c. on the hind flank as described before [18, 19], one day prior to the bacterial inoculum to avoid undue stress on the animals on the day of inoculation. Mice were provided food and water ad libitum, and were monitored for two weeks post infection. The experimental protocol for these animal studies was approved by the University of Minnesota Research Animal Resources Ethical Committee. Data are plotted as the percentage of surviving mice versus time.
2.4. Cytokine levels in serum from mice
Serum cytokine levels were monitored by withdrawing blood from litter matched C57/BL6 mice, which were infected i.p. with log-phase growing P. aeruginosa inoculum, as mentioned above. Blood from mice treated with SC4, Zosyn, their combination, or only PBS (control group), was obtained by retro-orbital bleeding at four post-infection time points: 0, 60, 90, and 120 minutes. Due to the possibility of additional stress on animals during this procedure, these mice were different from those used in survival studies. Serum levels of cytokines TNFα, IL-1, and IL-6 were measured by ELISA according to the manufacturer’s instructions (R&D Systems; Minneapolis, MN).
2.5. Statistical Analysis
Data sets were analyzed using a commercially available software package (InStat 2.03, Graphpad Software, Inc.). A two-tailed Student’s t test was used to determine the validity of the differences between control and treatment data sets. A p value of 0.05 or less was considered significant.
3. Results and Discussion
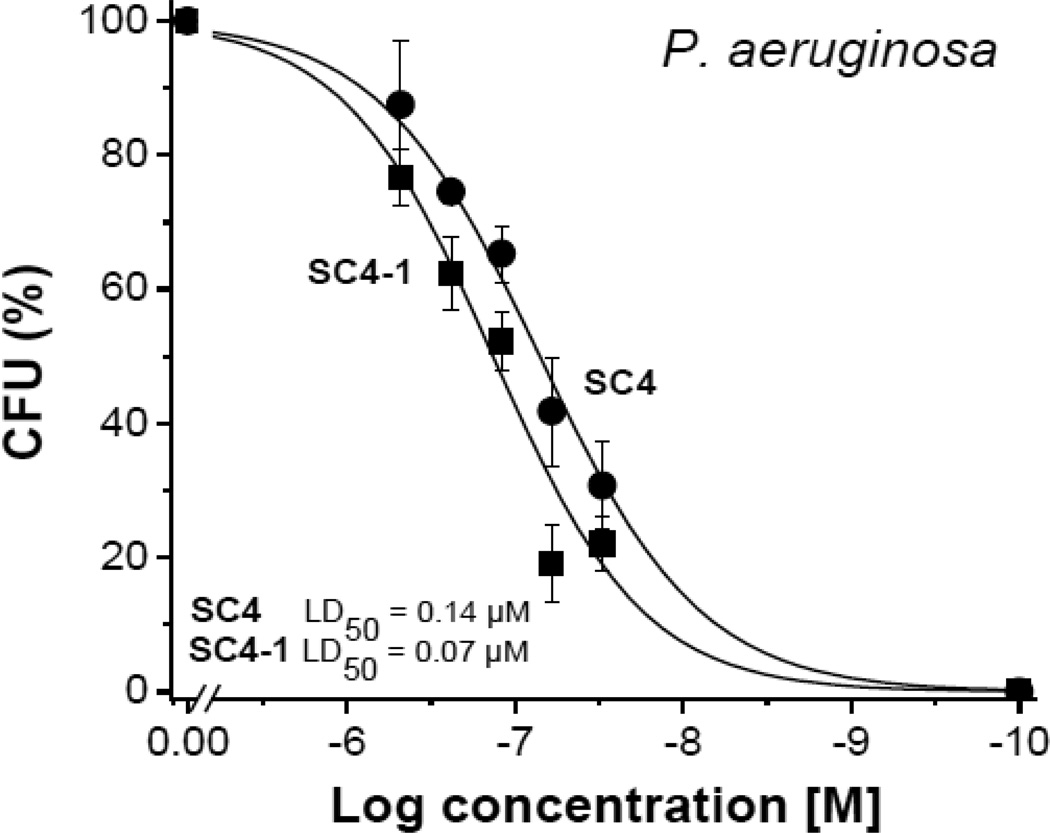
Previously, we reported that dodecapeptide SC4 was highly effective in vitro at killing P. aeruginosa [12]. Here, we initially investigated the ability of a set of alanine scanning variants of SC4 to kill P. aeruginosa. In this set of SC4 analogues, each residue in the dodecapeptide was replaced by an alanine through the sequence. Bactericidal activities were determined in vitro at different peptide concentrations as exemplified Figure 1. From these dose-response curves, we determined LD50 values of new analogues, as shown in Table 1. Only variant L2A (SC4-2) showed a significant decrease in activity, whereas variants K1A (SC4-1), R5A (SC4-5) and K8A (SC4-8) all demonstrated significant increases in activity by factors of 2 or 3 over that of parent SC4. Presumably, differences in activities are related to changes in peptide conformation and how the peptide interacts with the bacterial membrane. It appears that reducing the polycationic nature of SC4 increases its activity, whereas removal of L2 (SC4-2), with its branched alkyl side chain, decreases activity. Parent SC4 is reported to form helical conformation [11], and all three cationic residues are related to this helix conformation as residues i,i+3 or i,i+4 positioned on the same side of the helix. Apparently, reducing charge density improves the ability of SC4 to interact and to disrupt the bacterial membrane.
Fig. 1.
Examples of dose-response curves SC4 and alanine-scanning peptide analogues. Bactericidal activities of SC4 alanine-scanning peptide analogues against Pseudomonas aeruginosa (P. a.). Lines are sigmoidal curve fits used to determine LD50 values.
Table 1.
The LD50 values (µM) of SC4 and analogues on P. aeruginosa.
| Name | Variant | Sequence | P.a. LD50 (µM) |
|---|---|---|---|
| SC4 | parent | KLFKRHLKWKII | 0.14 |
| SC4-1 | K1A | ALFKRHLKWKII | 0.07 |
| SC4-2 | L2A | KAFKRHLKWKII | 0.31 |
| SC4-3 | F3A | KLAKRHLKWKII | 0.10 |
| SC4-4 | K4A | KLFARHLKWKII | 0.13 |
| SC4-5 | R5A | KLFKAHLKWKII | 0.06 |
| SC4-6 | H6A | KLFKRALKWKII | 0.10 |
| SC4-7 | L7A | KLFKRHAKWKII | 0.13 |
| SC4-8 | K8A | KLFKRHLAWKII | 0.04 |
| SC4-9 | W9A | KLFKRHLKAKII | 0.18 |
| SC4-10 | K10A | KLFKRHLKWAII | 0.12 |
| SC4-11 | I11A | KLFKRHLKWKAI | 0.17 |
| SC4-12 | I12A | KLFKRHLKWKIA | 0.14 |
To move into an in vivo model, we first performed bacteremia studies (5 to 7 mice per challenge) to establish the appropriate amount of log phase growing P. aeruginosa required to have 25% survival of untreated control mice. This was achieved with an infectious dose of P. aeruginosa of 7.1 log CFU/mouse (1 × 107 bacteria).
We then explored the effectiveness of SC4, as well as analogs K1A (SC4-), R5A (SC4-5), and K8A (SC4-8), against P. aeruginosa in this bacteremia model using 7.1 log CFU/mouse (1 × 107 bacteria) and 5 to 7 animals per group per study. For this study, we used a dose of the SC4 dodecapeptide (10 mg/Kg) that was essentially equivalent to ~200X the MIC value (~18 µM for SC4) against P. aeruginosa determined from the upper limit of the SC4 dose response curve. In this regard, a dose of 10 mg/Kg in a 20g mouse with 2 mL blood volume could have a maximal serum concentration of peptide of about 3.6 mM. However, because this dosage was not administered as a bolus, this maximum would not be reached. Rather administration of the drug was subcutaneous administered continuously via Alzet mini-pumps to a total dose of 10 mg/Kg over each 24 hour period. Nevertheless, administration of 10 mg/kg/day SC4 in this fashion to bacteria-challenged mice resulted in a survival rate of about 40% at the end of the study (Figure 2). We then used the same dosage with the SC4 analogues in this bacteremia model. However, although the three SC4 variants were more activity in vitro, there were no significant differences in in vivo activities compared to SC4 (data not shown).
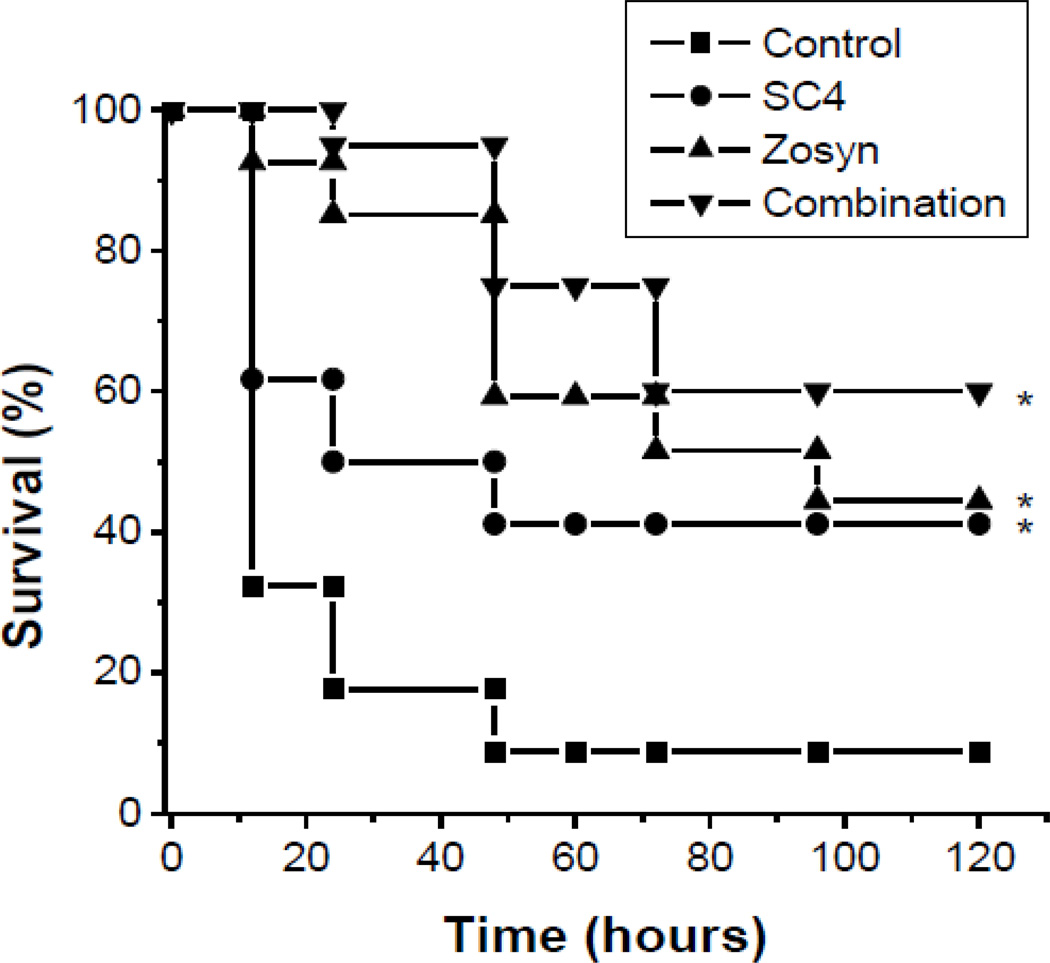
Fig. 2.
Combination of SC4 and Zosyn show enhanced protection against bacteria. SC4 and Zosyn were used in mouse bacteria model to assess in vivo efficacy against P.a.. The compounds were administered for 3 days by osmotic mini-pumps starting one day prior to i.p. injection of P.a. into C57/BL6 mice (n=20–34/group). Each mouse received a lethal dose of P.a. (300 µl of Klett 70). Survival of the mice were monitored for 14 days, however since no change in survival was noted after 120 hours, up to day 5 is plotted. *p < 0.03 (treatment group vs. control).
In an attempt to improve survival, we next performed the same bacteremia model with SC4 in combination with Zosyn, the first-line treatment against P. aeruginosa in the clinic. Because patients in the clinical setting are normally administered approximately 4.5 grams of Zosyn every 6 hours [16], or about 250 mg/kg/day, we decided on administering Zosyn to mice at a dose of 400 mg/kg/day. Administration of this dosage of Zosyn alone resulted in a survival rate also of about 40% at the end of the study (Figure 2).
In bacteremia studies in mice (repeated 3 to 5 times), combination of SC4 and Zosyn improved survival of mice compared to administration of these drugs as single agents. In control animals, Figure 2 shows that only 32% (11/34) of the P. aeruginosa challenged mice survived up to 12 hours post administration of PBS alone (controls, n=34), and only 9% (3/34) of these control animals survived past 48 hours. In contrast, 62% (21/34) of the mice treated with single agent SC4 (n=34) survived past 12 hours, and 41% (14/34) remained alive by the 48 hour time point and survived at least to the close of the study. Treatment of mice with single agent Zosyn (n=27) initially demonstrated better results than with SC4, with 85% (23/27) of challenged mice surviving up to 48 hours. However, survival then dropped to 59% (16/27) at the 48 hour time point, and then to 44% (12/27) by the end of the study. In this regard, single agent treatment with SC4 or Zosyn demonstrated essentially the same overall survival percentage of 41% and 44%, respectively.
In contrast to single agent treatments, all mice in the combination group (20/20) survived past the first 12 hour time point, and 60% (12/20) of the mice survived to the end of the study, i.e. 14 days post infection. Although the reason for this remains unclear, it likely has to do with the fact that the mechanisms of action of these therapies are so different. Zosyn is a mixture of two antibiotics, piperacillin and tazobactam. Piperacillin is a semi-synthetic penicillin, which prevents bacterial growth through interference of the peptidoglycan layer synthesis in the bacterial wall, and tazobactam is a β-lactamase inhibitor that prevents inactivation of piperacillin by binding β-lactamases [16]. SC4 on the other hand is a bacterial membrane disintegrator, whose mechanism of action is not mediated through specific enzymes, such as β-lactamases, and should therefore be less prone to resistance [20].
Killing Gram-negative bacteria promotes the release of LPS into the blood stream, and this promotes macrophage-mediated cytokine production that generally triggers inflammation, septic shock, and can lead to mortality. Sepsis is the leading cause of intensive care unit mortality nationwide, accounting for >210,000 deaths in the United States annually [21]. Cytokines, especially pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6, have been shown to be critical early mediators of septic shock [22].
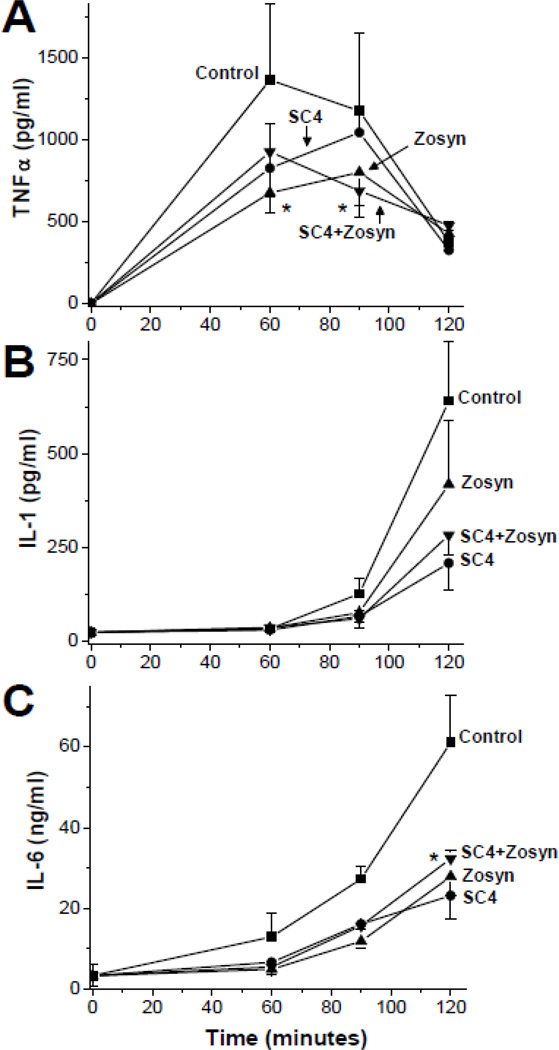
To assess whether SC4 and Zosyn can suppress cytokine release and potentially reduce the possibility for septic shock, we monitored the blood serum cytokine profile of TNF-α, IL-1, and IL-6 during the initial or acute phase of the infection up to 2 hours. For all three cytokines, serum levels were highest in control mice, and were attenuated in treated mice for any of the treatment regimes (Figure 3). For example, in control mice, TNF-α peaked at 60 minutes with 1363 ± 463 pg/ml, and subsequently dropped to 375 ± 125 pg/ml by 120 minutes into range with levels in treated mice (Figure 3A). On the other hand, IL-1 and IL-6 increased exponential-like by 120 min. At this time point, IL-1 levels in control mice were elevated to 640 ± 160 pg/ml, whereas the average level was 420 ± 169 pg/ml in Zosyn treated mice, 209 ± 71 pg/ml in SC4 treated mice, and 284 ± 54 pg/ml in the combination treatment group, a more than 2-fold suppression compared to control (Figure 3B). After 2 hours, IL-6 serum levels in control mice reached 61 ± 12 ng/ml, whereas levels were reduced to about half that in treated groups: 23 ± 6 ng/ml for SC4, 28 ± 5 ng/ml for Zosyn, and 32 ± 2 ng/ml for the combination (Figure 3C). At this point, it is unclear whether treatment-related attenuated cytokine levels result directly from LPS neutralization following bacterial lysis, or indirectly from bacterial growth inhibition (bacteria titers in serum were also reduced in treated groups, data not shown), or even some combination of both.
Fig. 3.
Serum cytokine levels upon treatment with SC4, Zosyn and combination after P.a. bacteria challenge. SC4 (10 mg/kg) and Zosyn (400 mg/kg) were used in mouse bacteria model to assess cytokine serum levels of TNF-α (A), IL-1 (B) and IL-6 (C) after P.a. challenge. The compounds were administered by osmotic mini-pumps one day prior to i.p. injection of P.a. into C57/BL6 mice. Cytokines levels were assessed by ELISA (R&D systems, Minneapolis, MN). Data points shown are means of 2 independent experiments (n=2–4) ± SEM. *p < 0.03 (combination vs. control).
In conclusion, our data demonstrate that the bacterial membrane disintegrating dodecapeptide SC4 is as effective as Zosyn at killing P. aeruginosa in vivo, and that the combination of the two significantly improves survival outcome. In addition, we showed that all treatment regimes attenuated LPS-induced serum cytokine levels in mice challenged with log phase P. aeruginosa. As new strains of antibiotic resistant bacteria emerge, such novel bactericidal agents and combination strategies should be particularly useful in the clinical setting.
Highlights.
Dodecapeptide SC4 is highly effective against Pseudomonas aeruginosa (P.a.).
SC4 attenuates inflammatory cytokine levels in serum.
Combination treatment of SC4 and Zosyn is most effective at killing P.a. in vivo.
Acknowledgements
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V 'Great Lakes' RCE (NIH award U54-AI-057153).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE., Jr Trends in antimicrobial drug development: implications for the future. Clin Infect Dis. 2004;38:1279–1286. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RP. The antibiotic pipeline--challenges, costs, and values. N Engl J Med. 2004;351:523–526. doi: 10.1056/NEJMp048093. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Dings RP, Nesmelova I, Debbert S, Haseman JR, Maxwell J, Hoye TR, Mayo KH. Topomimetics of amphipathic beta-sheet and helix-forming bactericidal peptides neutralize lipopolysaccharide endotoxins. J Med Chem. 2006;49:7754–7765. doi: 10.1021/jm0610447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dings RP, Mayo KH. A journey in structure-based drug discovery: from designed peptides to protein surface topomimetics as antibiotic and antiangiogenic agents. Acc Chem Res. 2007;40:1057–1065. doi: 10.1021/ar700086k. [DOI] [PubMed] [Google Scholar]
- 5.Dings RP, Haseman JR, Mayo KH. Probing structure-activity relationships in bactericidal peptide betapep-25. Biochem J. 2008;414:143–150. doi: 10.1042/BJ20080506. [DOI] [PubMed] [Google Scholar]
- 6.Maloy WL, Kari UP. Structure-activity studies on magainins and other host defense peptides. Biopolymers. 1995;37:105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki K, Yoneyama S, Fujii N, Miyajima K, Yamada K, Kirino Y, Anzai K. Membrane permeabilization mechanisms of a cyclic antimicrobial peptide, tachyplesin I, and its linear analog. Biochemistry. 1997;36:9799–9806. doi: 10.1021/bi970588v. [DOI] [PubMed] [Google Scholar]
- 8.Andreu D, Merrifield RB, Steiner H, Boman HG. N-terminal analogues of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry. 1985;24:1683–1688. doi: 10.1021/bi00328a017. [DOI] [PubMed] [Google Scholar]
- 9.Mayo KH, Haseman J, Ilyina E, Gray B. Designed beta-sheet-forming peptide 33mers with potent human bactericidal/permeability increasing protein-like bactericidal and endotoxin neutralizing activities. Biochim Biophys Acta. 1998;1425:81–92. doi: 10.1016/s0304-4165(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 10.Hwang PM, Vogel HJ. Structure-function relationships of antimicrobial peptides. Biochem Cell Biol. 1998;76:235–246. doi: 10.1139/bcb-76-2-3-235. [DOI] [PubMed] [Google Scholar]
- 11.Mayo KH, Haseman J, Young HC, Mayo JW. Structure-function relationships in novel peptide dodecamers with broad-spectrum bactericidal and endotoxin-neutralizing activities. Biochem J. 2000;349:717–728. doi: 10.1042/bj3490717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockwood NA, Haseman JR, Tirrell MV, Mayo KH. Acylation of SC4 dodecapeptide increases bactericidal potency against Gram-positive bacteria, including drug-resistant strains. Biochem J. 2004;378:93–103. doi: 10.1042/BJ20031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 14.NNIS. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 15.Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S146–S155. doi: 10.1086/320186. [DOI] [PubMed] [Google Scholar]
- 16.Kim A, Sutherland CA, Kuti JL, Nicolau DP. Optimal dosing of piperacillin-tazobactam for the treatment of Pseudomonas aeruginosa infections: prolonged or continuous infusion? . Pharmacotherapy. 2007;27:1490–1497. doi: 10.1592/phco.27.11.1490. [DOI] [PubMed] [Google Scholar]
- 17.Homma JY. A new antigenic schema and live-cell slide agglutination procedure for the infrasubspecific serologic classification of Pseudomonas aeruginosa . Jpn J Exp Med. 1976;46:329–336. [Google Scholar]
- 18.Dings RP, Chen X, Hellebrekers DM, van Eijk LI, Zhang Y, Hoye TR, Griffioen AW, Mayo KH. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J Natl Cancer Inst. 2006;98:932–936. doi: 10.1093/jnci/djj247. [DOI] [PubMed] [Google Scholar]
- 19.Dings RP, Yokoyama Y, Ramakrishnan S, Griffioen AW, Mayo KH. The designed angiostatic peptide anginex synergistically improves chemotherapy and antiangiogenesis therapy with angiostatin. Cancer Res. 2003;63:382–385. [PubMed] [Google Scholar]
- 20.Lockwood NA, Mayo KH. The future of antibiotics: bacterial membrane disintegrators. Drugs of the Future. 2003;28:911–923. [Google Scholar]
- 21.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]