Abstract
Red blood cells (RBCs) have an important function in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids (EETs) in response to a low O2 environment such as encountered in the cardiac microcirculation during exercise. RBCs, in their role as sensors of low pO2, release ATP and critical lipid mediators, the EETs. Both cis- and trans-EETs are synthesized and stored in RBCs and are hydrolyzed by soluble epoxide hydrolases (sEH). The trans-EETs differ from cis-EETs in their higher vascular potencies and more rapid metabolism by sEH. Thus, inhibition of sEH results in greater trans-EET levels and increased positive vascular effects of trans-EETs vs cis-EETs. The trans-EETs are responsible for a significant decline in the elevated blood pressure in the spontaneously hypertensive rat on treatment with a sEH inhibitor to raise EET levels. We predict that trans-EETs and cis-EETs will occupy important therapeutic roles in a broad spectrum of diseases and abnormal physiological conditions such as that resulting from high salt intake and hypertension.
Keywords: epoxyeicosatrienoic acids, red blood cells
1. Introduction
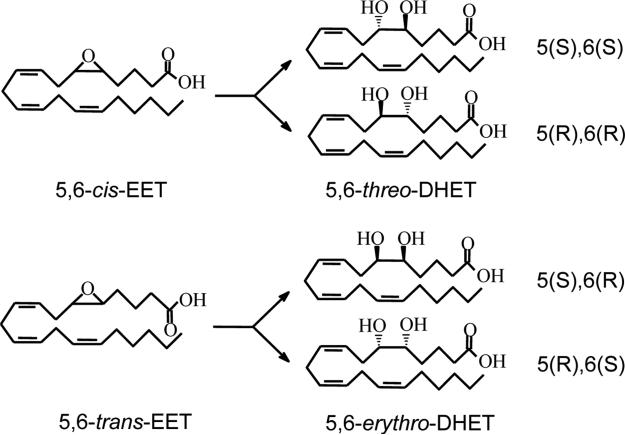
In 1981, Capdevila et al [1] reported that a hepatic microsomal monooxygenase metabolized arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs). A key paper by Nakamura, Bralton and Murphy [2] that facilitated our entry into studies on the rat and human red blood cell (RBC) as a reservoir for EETs raised the concept that EETs on release from RBCs act as vasoregulators of the microcirculation. Thus, erythrocytes can serve “as a circulating reservoir from which EETs can be released” [2]. EETs possess notable properties that impact beneficially on the vasculature and retard progression of vascular disease as they enhance blood flow, diminish mitogenesis and inhibit platelet aggregation. In addition, EETs are anti-inflammatory, inhibit cell migration, promote fibrinolysis and influence steroidogenesis [3-6]. Our initial study [7] on identification of trans-EETs recognized that all EETs produced by cytochrome P450 epoxygenases were of the cis-configuration [8,9]; however, formation of trans-EETs is possible through radical driven reactions as demonstrated for human RBCs by Nakamura et al and Jiang et al [2,7] (Fig. 1).
Fig. 1.
Structures of the 5,6-cis-EET, 5,6-trans-EET and their corresponding DHET enantiomers.
2. RBCs contain both cis- and trans-EETs
An additional key finding in our study of rat RBCs identified a 5,6-trans-EET with GC/MS and LC/MS/MS analyses [7]. This study produced direct evidence for the presence of 5,6-trans-EET in RBCs. As lipid peroxidation in RBCs can elevate esterified EETs by more than 30-fold [2], butylated hydroxytoluene and triphenylphosphine were used to quench free radical formation of EETs in blood samples to prevent free radical-mediated transformation of AA to EETs during analysis of RBC EET concentrations that was intended to reflect EET levels in circulating RBCs [7,10]. The trans-EETs occupied the sn-2 position of the RBC phospholipids from which they were released by phospholipase A2-mediated hydrolysis to achieve transient free levels in the RBC cytosol where they were released into the plasma by an ATP-dependent mechanism or subject to rapid hydrolysis forming erythro- dihydroxyeicosatrienoic acids (DHETs) [11]. Indeed, the rate of release of 14,15-EET from phosphatidylcholine and phosphatidylinositol surpassed that for AA [12].
Rat RBCs contained cis- and trans-EETs of ca 20 ng/109 RBCs, quantitated by LC/MS analysis, which indicated that cis- and trans-EETs are present in similar amounts in normal Sprague-Dawley rat RBCs [10]. Rat plasma EETs have been reported at ca 10 ng/ml, primarily esterified to phospholipids of circulating lipoproteins [13]. The incorporation of EETs into phospholipids of cells and their binding to fatty acid binding proteins [14] suggest long lasting effects of EETs on release by hormonal stimulation [15]. Levels of 14,15-trans-EET are higher than those of 11,12-trans- and 8,9-trans-EET which were similar to plasma concentrations of 5,6-trans-EET [10].
3. Biological activities of 5,6-EETs
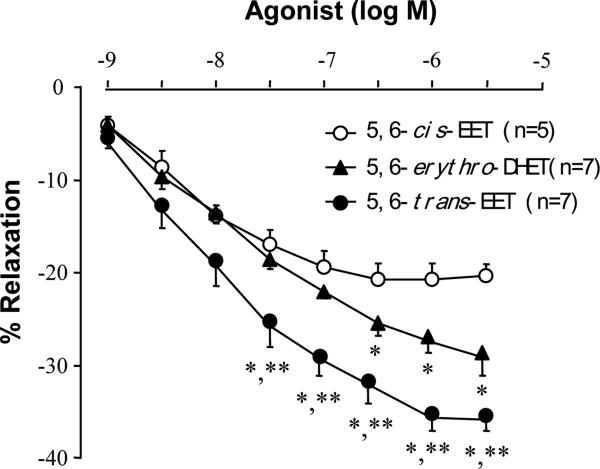
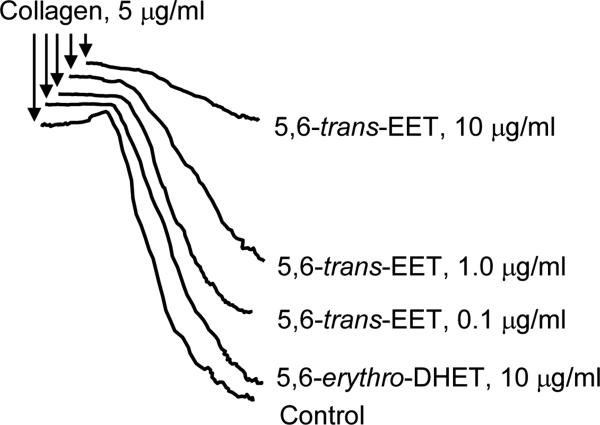
The trans-EET exhibited much greater vascular relaxation responses on rat renal interlobar arteries than those of 5,6-cis-EET (Fig 2). The 5,6-erythro-DHET metabolite of 5,6-trans-EET via the soluble epoxide hydrolase (sEH), also evoked vasodilation, an unexpected response as the DHET metabolite of EETs are usually inactive (Fig. 3). However, 5,6-cis-EET had similar effects to those of 5,6-trans-EET on collagen-induced platelet aggregation [9] (Fig 3).
Fig. 2.
Vasorelaxation effects of 5,6-trans-EET and 5,6-erythro-DHET compared to 5,6-cis-EET. Renal interlobar artery rings of Sprague-Dawley rats were preconstricted by phenylephrine (1 μM) and isometric tension was measured. (*P<0.05, comparing to 5,6-cis-EET; **P<0.05 comparing to 5,6-erythro-DHET.)
Fig. 3.
5,6-trans-EET inhibits Sprague-Dawley rat platelet aggregation. Platelets were preincubated with buffer (control), 5,6-erythro-DHET, or 5,6-trans-EET at the indicated concentrations for 2 min at 37°C. Collagen was added and platelet aggregation was allowed to proceed for 4 min, n=4-6.
4. sEH of rat RBCs hydrolyzes cis- and trans-EETs
Our study on RBC-EETs also disclosed hydrolysis of cis-/trans-EETs by rat RBCs [11]. We found a typical sEH in rat RBC cytosol indistinguishable from that in hepatocytes that preferred hydrolysis of trans- over cis-EETs in a decreasing order for both cis- and trans-EETs from 14,15-, 11,12-, 8.9- to 5,6-EETs [11]. The VMAX of trans-EET hydrolysis by RBCs approaches 3 times that of the corresponding cis-EET. sEH activity is increased in diabetes [16] and in hypertension [17-20]. Increasing tissue and circulating EET levels are a therapeutic objective achieved by inhibition of sEH, which has been shown in experimental animals to control elevated blood pressure [18,19,21-24], reduce renal damage [25] and prevent strokes and moderate inflammation [26].
5. Inhibition of sEH lowers blood pressure in the spontaneously hypertensive rat (SHR)
As sEH inhibition should increase plasma trans-EETs to higher levels than cis-EETs based on preferential metabolism of trans-EETs, a study was designed in the SHR to characterize the relative effects of sEH inhibition on cis- and trans-EETs in lowering blood pressure in the SHR [24]. Control Wistar-Kyoto (WKY) rats demonstrated a manifestly higher total plasma EETs than the SHR (26 vs 16 ng/ml). However, sEH inhibition produced a significant elevation of plasma EETs in the SHR that involved only trans-EETs. Several factors have been shown to determine suppression of trans-EET activity in the SHR, the most important being the three-fold higher velocity of trans-EET hydrolysis by sEH compared to hydrolysis of cis-EETs, coupled to elevated sEH activity in the SHR [11]. Inhibition of sEH activity in the SHR produced elevated plasma EETs that lowered blood pressure via two-fold increase in plasma trans-EETs in contrast to the failure of sEH inhibition to increase total plasma cis-EET concentrations in either the WKY or the SHR [24]. Blood pressure was unaffected in control WKY rats. Additionally, the greater vasodilator potency of trans-EETs vis a vis cis-EETs also contributed to the blood pressure lowering response to sEH inhibition in the SHR.
6. The RBC secretes ATP that stimulates RBC EET release by activating P2 receptors
The production of ATP by RBCs has many ramifications as a critical determinant of microvascular function [27, 28]. RBCs exceed the ATP synthesizing capacity of most tissues as they produce millimolar amounts of ATP [29]. Indeed, an area of intensive studies, the regulation of coronary blood flow during exercise, has yielded to a mechanism centered in RBCs that secretes ATP which acts as an extracellular signaling molecule by interacting with specific P2 receptors to activate vasodilatation in the microcirculation [29]. Phospholipase stimulation of RBCs releases EETs stored in RBC membrane phospholipids [27]. AA also esterified to RBC glycerol phospholipids (10) are simultaneously cleaved by phospholipase activity and converted to EETs by monooxygenase-like activity [46] of hemoglobin (Hb). Then, EET release from rat RBCs follows in decreasing order: 14,15-, 11,12-, 8,9- and 5,6-EETs which exit from RBC via ATP transporters coupled to RBC membrane P2X7 receptors that respond, presumably, to both plasma and RBC cytosolic ATP to enhance EET release from RBCs [27].
ATP stimulation of erythrocyte P2X7 receptors releases both cis- and trans-EETs that are subject to hydrolysis by sEH in the erythrocyte cytosol, usually with loss of biological activity [27]. As noted, the sEH prefers hydrolysis of trans-EETs by a factor of three-fold [11]. Thus, inhibition of RBC sEH results in increased plasma trans-EETs, having greater vasodilator and anti-inflammatory potencies than cis-EETs and, thereby, offering therapeutic advantages superior to cis-EETs as demonstrated by Jiang et al [24]. Moreover, on passage of RBCs through the microcirculation, a positive synergistic effect of ATP and EETs on microvessels occurs by activating a retrograde dilator response in upstream “feeder” arteries [30] that increase blood flow to the microcirculatory sites exhibiting high O2 extraction. These findings are therefore critical to another aspect of the contributions of the RBC to the microcirculation in addition to O2 delivery; namely, allocation of blood flow distribution mediated by RBC production of ATP and EETs via a mechanism involving the RBC membrane P2X7 receptor [27].
Highlights.
Epoxyeicosatrienoic acids (EETs) are significant lipid mediators in circulatory regulation exerted by red blood cells (RBCs).
Both cis- and trans-EETs are synthesized, stored, released and hydrolyzed by RBCs.
The trans-EETs exhibit more potent vascular activities and are metabolized more rapidly by soluble epoxide hydrolase (sEH) than cis-EETs.
Inhibition of sEH results in greater trans-EET levels and vascular effects of trans- vs cis-EETs.
The trans- and cis-EETs have important therapeutic effects in hypertension and cardiovascular diseases.
Acknowledgements
This investigation was supported by National Institutes of Health Grants HL34300.
Abbreviations
- RBC
red blood cell
- EET
epoxyeicosatrienoic acid
- DHET
dihydroxyeicosatrienoic acid
- SHR
spontaneously hypertensive rat
- WKY
Wistar-Kyoto rat
- sEH
soluble epoxide hydrolase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981;78(9):5362–6. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura T, Bratton DL, Murphy RC. Analysis of epoxyeicosatrienoic and monohydroxyeicosatetraenoic acids esterified to phospholipids in human red blood cells by electrospray tandem mass spectrometry. J Mass Spectrom. 1997;32(8):888–96. doi: 10.1002/(SICI)1096-9888(199708)32:8<888::AID-JMS548>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49(3):590–6. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 4.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 5.Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010:6027–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nithipatikom K, Gross GJ. Review article: epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 2010;15(2):112–9. doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, McGiff JC, Quilley J, et al. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem. 2004;279(35):36412–8. doi: 10.1074/jbc.M403962200. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila JH, Karara A, Waxman DJ, et al. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990;265(19):10865–71. [PubMed] [Google Scholar]
- 9.Fitzpatrick FA, Ennis MD, Baze ME, et al. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem. 1986;261(32):15334–8. [PubMed] [Google Scholar]
- 10.Jiang H, Quilley J, Reddy LM, et al. Red blood cells: reservoirs of cis- and trans epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2005;75(1-4):65–78. doi: 10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Zhu AG, Mamczur M, et al. Hydrolysis of cis- and trans epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther. 2008;326(1):330–7. doi: 10.1124/jpet.107.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267(6):3686–90. [PubMed] [Google Scholar]
- 13.Karara A, Wei S, Spady D, et al. Arachidonic acid epoxygenase: structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem Biophys Res Commun. 1992;182(3):1320–5. doi: 10.1016/0006-291x(92)91877-s. [DOI] [PubMed] [Google Scholar]
- 14.Widstrom RL, Norris AW, Spector AA. Binding of cytochrome P450 monooxygenase and lipoxygenase pathway products by heart fatty acid-binding protein. Biochemistry. 2001;40(4):1070–6. doi: 10.1021/bi001602y. [DOI] [PubMed] [Google Scholar]
- 15.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279(6):F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez M, Clare-Salzler M. Eicosanoid imbalance in the NOD mouse is related to a dysregulation in soluble epoxide hydrolase and 15-PGDH expression. Ann N Y Acad Sci. 2006:1079130–4. doi: 10.1196/annals.1375.019. [DOI] [PubMed] [Google Scholar]
- 17.Sellers KW, Sun C, Diez-Freire C, et al. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19(6):626–8. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, Xu F, Huse LM, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 19.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39(2 Pt 2):690–4. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 20.Ai D, Fu Y, Guo D, et al. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104(21):9018–23. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-Salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys. 2007;47(1):87–98. doi: 10.1385/cbb:47:1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh S, Chiang PC, Wahlstrom JL, et al. Oral delivery of 1,3-dicyclohexylurea nanosuspension enhances exposure and lowers blood pressure in hypertensive rats. Basic Clin Pharmacol Toxicol. 2008;102(5):453–8. doi: 10.1111/j.1742-7843.2008.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Carroll MA, Chander PN, et al. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008:133480–7. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Quilley J, Doumad AB, et al. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300(6):H1990–H1996. doi: 10.1152/ajpheart.01267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289(3):F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 26.Schmelzer KR, Kubala L, Newman JW, et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102(28):9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Zhu AG, Mamczur M, et al. Stimulation of rat erythrocyte P2X(7) receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151(7):1033–40. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18(9):350–5. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Farias M, III, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288(4):H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- 30.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234(4778):868–70. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]