Abstract
Context
Bipolar disorder (BD) is a debilitating mental illness associated with high costs to diagnosed individuals and society. Within the past two decades, increasing numbers of children and adolescents have been diagnosed with BD. While functional magnetic resonance imaging (fMRI) studies have begun to investigate the neural mechanisms underlying BD, few have directly compared differences in BD-youths and BD-adults.
Objective
To address this gap, we conducted activation likelihood estimation (ALE) meta-analyses directly comparing the voxel-wise convergence of fMRI findings in BD-youths versus BD-adults, both relative to healthy control (HC) participants. We hypothesized that BD-youths (<18 years old) would show greater convergence of amygdala hyper-activation and prefrontal cortical hypo-activation versus BD-adults.
Data Sources
PubMed and PsycINFO databases were searched through July 2013 for original, task-related coordinate-based fMRI articles.
Study Selection
21 pediatric studies, 73 adult studies, and 2 studies containing distinct pediatric and adult groups within the same study met inclusion criteria for our ALE analyses.
Data Extraction and Synthesis
Coordinates of significant between-group differences were extracted from each published study. Recent improvements in GingerALE software were employed to perform direct comparisons of pediatric and adult fMRI findings.
Results
Analyses of emotional face recognition fMRI studies showed significantly greater convergence of amygdala hyper-activation among BD-youths than BD-adults. More broadly, analyses of fMRI studies employing emotional stimuli showed significantly greater convergence of hyper-activation among BD-youths than BD-adults in the inferior frontal gyrus and precuneus. In contrast, analyses of fMRI studies employing non-emotional cognitive tasks and also analyses aggregating emotional and non-emotional tasks showed significantly greater convergence of hypo-activation among BD-youths than BD-adults in the anterior cingulate cortex.
Conclusions
Our data suggest that amygdala, prefrontal, and visual system hyper-activation is important in the emotional dysfunction present in BD-youths, and that anterior cingulate cortex hypo-activation is relevant to the cognitive deficits in BD-youths. Future studies are required to determine if the developmental fMRI differences between BD-youths and BD-adults identified by our ALE meta-analyses are useful as brain-based diagnostic or treatment markers of BD, including either longitudinal neuroimaging studies of BD-youths as they become adults, or cross-sectional imaging studies directly comparing BD-youths to BD-adults.
INTRODUCTION
Bipolar disorder (BD) is among the most devastating psychiatric illnesses affecting adults worldwide, with an estimated prevalence of 1–4% 1,2. Despite our best psychopharmacological and psychotherapeutic treatments, BD is the most expensive mental health illness—it is twice as expensive as major depressive disorder including total healthcare costs and lost productivity 3,4. Ultimately, greater understanding of the neural dysfunction underlying BD is required to identify biologically-based targets to improve the specificity and efficacy of our diagnostic and treatment strategies for BD.
Relatedly, BD in children and adolescents has received increasing attention during the past two decades, given that 20–40% of BD-adults report their illness started during childhood or adolescence, rather than adulthood 5,6. BD is a burgeoning problem affecting youths, with approximately 20% of children and adolescents discharged from psychiatric hospitals in the United States diagnosed with BD in 2004 7. Not simply a psychiatric diagnostic trend, outpatient visits to practitioners of all specialties for pediatric BD diagnoses have also increased 40-fold during the same time period 8. This increase is international, as inpatient BD psychiatric diagnoses in German youths rose 68.5% from 2000–2007, outpacing the general rise in mental illness 9. Despite our best treatments, BD exacts a considerable toll on youths, including psychosocial and academic impairment 10–13, and high rates of psychiatric hospitalization 14 and suicide attempts 15. Greater understanding of the underlying neural alterations in BD-youths could lead to novel biologically-based diagnostic and treatment strategies.
These data raise an important question: are the neural alterations in BD-youths similar to those in BD-adults? To our knowledge, only four currently-published task-related functional magnetic resonance imaging (fMRI) studies have directly compared BD-youths and BD-adults in the same study 16–19. Specifically, Kim (2012) found that BD-youths had greater amygdala activation during emotional face recognition than BD-adults 16. Weathers (2012) found decreased anterior cingulate cortex (ACC) activation in BD-youths during failed response inhibition 17, while Weathers (2013) found increased prefrontal cortex (PFC) activation during successful response switches 18. Adelman (2013) found decreased fusiform activation in both BD-youths and BD-adults during successful emotional face encoding 19. More such studies are needed to elucidate developmentally-unique brain alterations among BD-youths versus BD-adults, but multi-group and longitudinal fMRI studies are inherently costly and the latter require time to image BD-youths as they age, a process that can not be expedited.
More broadly, fMRI studies in either BD-youths or BD-adults have demonstrated alterations in a PFC-amygdala-striatal circuit modulating numerous domains, including emotional face recognition, response inhibition, cognitive flexibility, and working memory 20–28. While important, these fMRI studies have inherent methodological limitations, including small sample sizes that limit their generalizability and statistical power. Second, variations in task design and implementation influence fMRI results even within singular psychological processes.
A novel approach to these limitations is an activation likelihood estimation (ALE) meta-analysis. ALE is a coordinate-based meta-analytic technique that uses the spatial coordinates and sample sizes from published studies to model the voxel-wise convergence in activation—that is, how likely that region was truly implicated in that illness or process 29–31. Leveraging large sample sizes across studies, ALE has greater power to detect convergence of brain regions implicated in a particular disorder and/or psychological process, with reduced susceptibility to false positives—estimated at 10–20% in smaller individual fMRI studies 29,30. ALE meta-analyses have evaluated BD-adult fMRI data 32–35, but none have included pediatric data. Moreover, to our knowledge, none have leveraged recent improvements in ALE methods enabling direct developmental comparisons of BD-youth to BD-adult data 36,37 as has been done in other neuropsychiatric disorders, including autism 38 and attention deficit hyperactivity disorder 39.
Therefore, we conducted the first developmental fMRI ALE meta-analysis in BD by directly comparing the convergence of neural alterations in BD-youths to BD-adults, while incorporating how both differed from healthy controls (HC). Our primary analysis focused on emotional face processing, the most well-researched function in BD 16,20–22,40–42. Based on a prior qualitative literature review, we predicted greater convergence of amygdala hyper-activation in BD-youths than BD-adults 42. Secondary analyses followed an emotional (e.g., theory-of-mind, reward tasks) or non-emotional (e.g., N-back, Go/NoGo) dichotomy from a prior BD-adult ALE study 32. This tested the hypothesis that on emotional tasks PFC hypo-activation would be more implicated in BD-youths than BD-adults 42, while for non-emotional tasks we hypothesized greater convergence of ACC hypo-activation in BD-youths than BD-adults 17. Tertiary analyses compared BD-youths to BD-adults across all fMRI studies 32,38,39.
METHODS
ALE Literature Search
As in prior developmental ALE meta-analyses 38,39, we conducted literature searches first in PubMed and then in PsycINFO on the same day (7/17/2013) using the terms “bipolar disorder” and “magnetic resonance imaging” 32,38,39,43–45. Study inclusion criteria were: original reports of task-related fMRI experiments and significant between-group differences between BD and HC participants reported in standard stereotactic coordinate space (Talairach or Montreal Neurological Institute [MNI]).
Study exclusion criteria were: reviews or meta-analyses; duplicate samples; other MRI data (e.g., structural MRI, diffusion tensor imaging, functional connectivity); “bipolar” used for high-risk samples or an unrelated context (e.g., “bipolar electrodes”); participants whose age overlapped our pediatric and adult criteria; no HC group or HCs were relatives of BD participants (e.g., discordant-twin studies); lacking between-group differences or stereotactic coordinates.
The first-level literature search yielded 1,181 unique published articles with 155 meeting initial inclusion criteria, including 3 four-group studies with separate BD-youth and BD-adult participant groups 16,17,19 (Figure 1).
Figure 1.
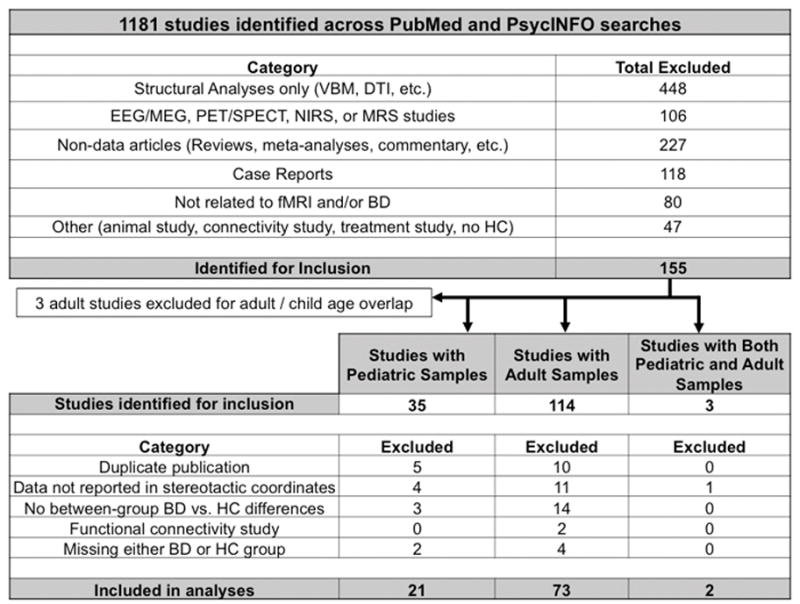
Flow diagram of literature search.
Note: BD = bipolar disorder; DTI = diffusion tensor imaging; EEG = electroencephalography; fMRI = functional magnetic resonance imaging; MEG = magnetoencephalography; MRS = magnetic resonance spectroscopy; NIRS = near-infrared spectroscopy; PET = positron emission tomography; SPECT = single-photon emission computed tomography; HC = healthy controls; VBM = voxel-based morphometry
Studies were categorized as having either pediatric (participant mean age plus one standard deviation <18 years) or adult (participant mean age minus one standard deviation ≥18 years) participants. This pediatric/adult cutoff is commonly used in research regulation, including institutional review boards, and has been used in prior developmental ALE meta-analyses where categorical, rather than continuous, comparisons of pediatric versus adult participants are required 38,39.
After second-level review, three adult studies were excluded because their participants’ age crossed into our definition of pediatric samples (i.e., two studies participants’ mean age minus one standard deviation was less than 18 years 46,47; one study’s age range of “16–50” crossed this definition 48). Articles reporting results from separate tasks (e.g., N-back and affective picture viewing 28) and articles reporting distinct comparisons between BD and HC samples in different mood states (e.g., euthymic BD versus HC; manic BD versus HC) 40,41,49 were entered as separate studies. Any ambiguities about whether studies met our criteria were resolved by a consensus decision between the first (EW) and last (DPD) authors with consultation from co-authors. Study data (e.g., coordinates, ages) were entered by the first author and checked by the second author (GKC).
ALE Methods
GingerALE software (version 2.3.1) from the BrainMap Project was used to conduct ALE meta-analyses of eligible studies 50. Following prior ALE studies, our meta-analyses were conducted in Talairach space 31,32,35, with the Lancaster transformation applied to coordinates originally reported in MNI space 50,51. For uniformity, Talairach coordinates derived from the Brett transformation 52 were converted back to MNI space, and then re-converted to Talairach space with the Lancaster transform 32,35,50.
ALE is a coordinate-based meta-analysis technique that models the voxel-wise spatial convergence of activation foci gleaned from published studies after they are modeled in common stereotactic space. Initial pair-wise ALE analyses used random-effects methods to identify the convergence of hyper-activation in BD versus HC (i.e., BD-youths>HC-youths; BD-adults>HC-adults) and convergence of hypo-activation in BD versus HC (i.e., HC-youths>BD-youths; HC-adults>BD-adults). To account for inter-study differences in sample size and preprocessing methods (e.g., spatial normalization), for each pair-wise ALE comparison, GingerALE computed voxel-wise ALE values and smoothed them with a Gaussian kernel whose full-width-at-half-maximum (FWHM) was set by an algorithm modeling the probability distribution of “true” locations based on empirical estimates of spatial uncertainty in neuroimaging experiments 37. To be conservative, the smaller group size, rather than the total study size (i.e., BD+HC), determined the FWHM, as some BD adult studies contrasted one HC group to separate BD groups in different mood states 40,41,49. The Talairach daemon determined the anatomical locations for significant ALE clusters 53.
Then, developmental ALE analyses were conducted using subtraction contrasts of these pair-wise analyses to directly compare BD-youths to BD-adults 36. We determined regions with: (1) greater convergence of hyper-activations in BD-youths than BD-adults ([BD-youths>HC-youths] – [BD-adults>HC-adults]); (2) greater convergence of hyper-activations in BD-adults than BD-youths ([BD-adults>HC-adults] – [BD-youths>HC-youths]); (3) greater convergence of hypo-activations in BD-youths than BD-adults ([HC-youths>BD-youths] – [HC-adults>BD-adults]); and (4) greater convergence of hypo-activations in BD-adults than BD-youths ([HC-adults>BD-adults] - [HC-youths>BD-youths]).
To address multiple comparisons issues, all pair-wise and developmental ALE analyses were thresholded at p<0.05, whole-brain corrected, with a False Discovery Rate algorithm 54 using 10,000 p-value permutations and a minimum cluster size of 200 mm3.
Analytic Plan
Our primary analysis focused on emotional face perception because it is among the most well-researched neural processes in BD 16,20–24,40–42 (see eTable 1). To increase analytic power, as in prior ALE analyses with BD-adults 33,34, we excluded studies with emotional faces incorporated in another task (e.g., emotional Go/NoGo). Secondary ALE analyses grouped studies into either emotional or non-emotional categories 32. Emotional studies were defined as those involving any emotionally-valenced stimuli (e.g., faces, pictures, words), reward-based tasks (e.g., reversal learning), or mood inductions. Any other studies were defined as non-emotional. Tertiary exploratory ALE analyses aggregated all studies 32,38,39,43,45.
RESULTS
Study statistics
We identified 21 pediatric studies (N=452 BD-youths, N=421 HC-youths), 73 adult studies (N=1,495 BD-adults, N=1,528 HC-adults), and 2 studies with distinct pediatric and adult samples within the same study (N=45 BD-youths, N=55 HC-youths, N=45 BD-adults, N=64 HC-adults) meeting all inclusion criteria (Figure 1). Pediatric and adult studies did not differ in sample size [F(1,98)=0.16, p=0.69], percentage of female participants [F(1,96)=0.95, p=0.33], or percentage of euthymic BD participants [t(97)=0.71, p=0.48] (eTable 2). There was a significant difference in the percentage of medicated BD patients [t(31.6)=2.23, p=0.03; BD-youth: 58.6±38.3%, BD-adult: 77.4±28.8%] with more BD-adult studies having 100% of their sample medicated (N=4 BD-youth; N=26 BD-adult).
Primary ALE Meta-analyses: Emotional Face Perception
ALE analyses of emotional face perception included 6 pediatric (112 coordinates) and 24 adult (203 coordinates) studies.
Pair-wise analyses of pediatric studies showed that BD-youths had significant convergence of hyper-activation versus HC-youths in right amygdala/parahippocampal gyrus, left inferior frontal gyrus (IFG), and left putamen. BD-youths had significant convergence of hypo-activation versus HC-youths in left middle occipital gyrus and right IFG (Table 1).
Table 1.
Activation Likelihood Estimation (ALE) Meta-analyses Results for Emotional Face Perception Tasks Comparing Participants with Bipolar Disorder (BD) versus Healthy Controls (HC)
| Analysis | Side | Brain Region | BA | Talairach | Cluster Size (mm3) | Extrema Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Pair-wise Analyses: | ||||||||
| BD-youths > HC-youths | R | Amygdala | 26 | −4 | −12 | 1496 | 0.015 | |
| L | IFG | 47 | −30 | 18 | −8 | 824 | 0.016 | |
| R | PHG | 35 | 20 | −26 | −14 | 384 | 0.012 | |
| L | Putamen | −28 | −10 | −8 | 384 | 0.011 | ||
| HC-youths > BD-youths | L | Middle OG | 19 | −40 | −80 | 4 | 520 | 0.011 |
| R | IFG | 47 | 40 | 20 | −4 | 440 | 0.008 | |
| BD-adults > HC-adults | L | Amygdala | −22 | −4 | −12 | 2016 | 0.014 | |
| R | Pallidus | 18 | −8 | −8 | 1616 | 0.027 | ||
| R | dACC | 32 | 10 | 28 | 22 | 776 | 0.012 | |
| L | IFG | 46 | −32 | 30 | 18 | 760 | 0.012 | |
| R | Putamen | 20 | 2 | 8 | 496 | 0.017 | ||
| L | Caudate | −16 | 16 | 19 | 440 | 0.013 | ||
| HC-adults > BD-adults | R | Middle FG | 8 | 38 | 16 | 42 | 1680 | 0.014 |
| R | Amygdala | 22 | −4 | −12 | 1360 | 0.018 | ||
| L | pgACC | 24 | −2 | 36 | 0 | 1208 | 0.015 | |
| R | Insula | 13 | 32 | 24 | 10 | 984 | 0.021 | |
| L | Amygdala | −22 | 0 | −22 | 960 | 0.015 | ||
| R | STG | 38 | 32 | 12 | −22 | 880 | 0.021 | |
| R | IFG | 47 | 34 | 32 | −4 | 680 | 0.013 | |
| L | IFG | 47 | −46 | 32 | −8 | 624 | 0.022 | |
| R | Putamen | 24 | 4 | 2 | 616 | 0.013 | ||
| R | Caudate | 8 | 8 | −2 | 592 | 0.017 | ||
| L | Middle FG | 6 | −30 | 0 | 44 | 408 | 0.013 | |
| R | Mid Cingulate | 32 | 18 | 8 | 38 | 320 | 0.011 | |
| Developmental Contrasts: | ||||||||
| [BD-youths > HC-youths] – [BD-adult > HC-adult] | R | Amygdala | 28 | −6 | −10 | 512 | 2.968 | |
Note: L = left; R = right; dACC = dorsal anterior cingulate cortex; pgACC = pre-genual anterior cingulate cortex; BA = Brodmann Area; FG = frontal gyrus; IFG = inferior frontal gyrus; OG = occipital gyrus; PHG = parahippocampal gyrus; STG = superior temporal gyrus
Pair-wise analyses of adult studies showed that BD-adults had significant convergence of hyper-activation than HC-adults in regions including left amygdala, bilateral striatum, and left IFG. BD-adults had significant convergence of hypo-activation versus HC-adults in regions including bilateral amygdala, bilateral IFG, and left pre-genual ACC (pgACC).
Developmental ALE analyses directly comparing BD-youths to BD-adults showed greater convergence of hyper-activation in BD-youths than in BD-adults in right amygdala (Figure 2a). No areas showed significantly greater convergence of hyper-activation in BD-adults than in BD-youths, nor were there any significant between-group differences in convergence of hypo-activation.
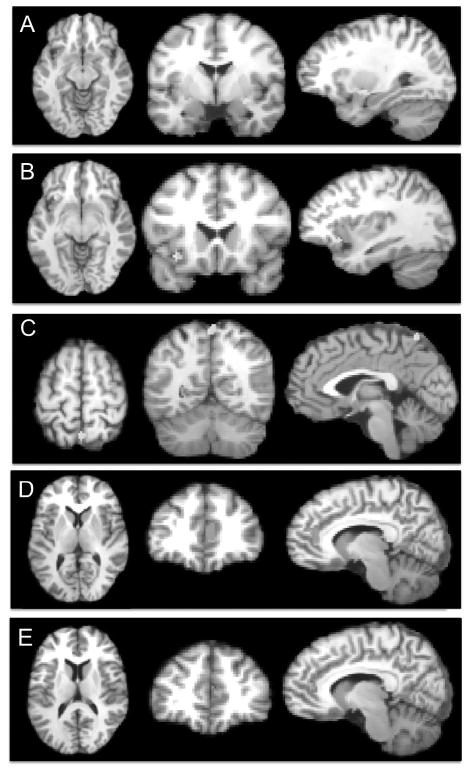
Figure 2.
Results from developmental contrasts of pair-wise activation likelihood estimation (ALE) analyses.
Note: (A) Greater convergence of hyper-activation in bipolar disorder (BD)-youths than BD-adults in the emotional face perception tasks contrast (X=28, Y=−6, Z=−10; right amygdala, cluster size = 512 mm3) (B) Greater convergence of hyper-activation in BD-youths than BD-adults in the emotional tasks contrast (X=−34, Y=16, Z=−8; left inferior frontal gyrus, cluster size = 392 mm3,) (C) Greater convergence of hyper-activation in BD-youths than BD-adults in the emotional tasks contrast (X=−31, Y=18, Z=−9; left precuneus, cluster size = 344 mm3) (D) Greater convergence of hypo-activation in BD-youths than BD-adults in the non-emotional tasks contrast (X=9, Y=36, Z=7; right pre-genual anterior cingulate cortex, cluster size = 728 mm3) (E) Greater convergence of hypo-activation in BD-youths than BD-adults in the contrast including data from all tasks (X=8, Y=40, Z=12; right pre-genual anterior cingulate cortex, cluster size = 664 mm3). The right side of all coronal and axial images corresponds to the right side of the brain. All images thresholded at p<0.05, whole-brain corrected.
Secondary Analyses: Emotional Paradigms
Analyses of emotional paradigms included 14 pediatric (194 coordinates) and 48 adult (356 coordinates) studies.
Pair-wise ALE analyses showed that BD-youths had significant convergence of hyper-activation versus HC-youths in the right amygdala/parahippocampal gyrus, left IFG, right medial frontal gyrus, and precuneus. BD-youths had significant convergence of hypo-activation versus HC-youths in left pgACC, right IFG, and middle occipital gyrus (Table 2).
Table 2.
Activation Likelihood Estimation (ALE) Meta-analyses Results for Emotional Task Paradigms Comparing Participants with Bipolar Disorder (BD) versus Healthy Controls (HC)
| Analysis | Side | Brain Region | BA | Talairach | Cluster Size (mm3) | Extrema Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Pair-wise Analyses: | ||||||||
| BD-youths > HC-youths | R | Amygdala | 24 | −4 | −10 | 1768 | 0.018 | |
| L | IFG | 47 | −30 | 18 | −8 | 712 | 0.016 | |
| M | Precuneus | 7 | 0 | −62 | 58 | 448 | 0.013 | |
| R | pgACC | 32 | 10 | 40 | 10 | 416 | 0.014 | |
| R | Medial FG | 10 | 4 | 64 | 16 | 360 | 0.013 | |
| L | Putamen | −28 | −10 | −8 | 272 | 0.011 | ||
| R | PHG | 35 | 20 | −26 | −14 | 248 | 0.012 | |
| R | Cingulate | 24 | 12 | 12 | 30 | 224 | 0.011 | |
| HC-youths > BD-youths | L | pgACC | 24 | −4 | 28 | 10 | 536 | 0.011 |
| L | Middle OG | 19 | −40 | −80 | 4 | 432 | 0.011 | |
| R | IFG | 47 | 40 | 20 | −4 | 344 | 0.008 | |
| BD-adults > HC-adults | R | Pallidus | 18 | −8 | −8 | 5256 | 0.028 | |
| L | Uncus | 34 | −22 | 2 | −20 | 2896 | 0.016 | |
| L | Middle FG | 47 | −44 | 32 | −2 | 2296 | 0.030 | |
| R | dACC | 32 | 8 | 28 | 22 | 1072 | 0.013 | |
| R | Claustrum | 36 | −18 | 8 | 432 | 0.017 | ||
| R | Caudate | 8 | 8 | −2 | 416 | 0.015 | ||
| R | Insula | 13 | 38 | 8 | −6 | 408 | 0.017 | |
| L | IFG | 46 | −32 | 30 | 16 | 408 | 0.012 | |
| L | pgACC | 32 | −16 | 42 | 0 | 344 | 0.015 | |
| L | Caudate | −16 | 16 | 18 | 272 | 0.013 | ||
| HC-adults > BD-adults | R | Middle FG | 9 | 50 | 12 | 30 | 1792 | 0.018 |
| L | Amygdala | −24 | 0 | −22 | 1568 | 0.020 | ||
| R | Amygdala | 22 | −4 | −12 | 1408 | 0.018 | ||
| L | IFG | 47 | −46 | 32 | −8 | 1064 | 0.023 | |
| R | Putamen | 24 | 6 | 0 | 984 | 0.017 | ||
| R | STG | 38 | 32 | 12 | −22 | 928 | 0.021 | |
| L | pgACC | 24 | −2 | 36 | 0 | 912 | 0.015 | |
| R | Insula | 13 | 32 | 24 | 10 | 792 | 0.021 | |
| R | MTG | 21 | 56 | −10 | −6 | 760 | 0.016 | |
| R | Lingual Gyrus | 18 | 18 | −82 | −6 | 592 | 0.017 | |
| R | Caudate | 8 | 8 | −2 | 544 | 0.017 | ||
| R | IFG | 47 | 34 | 32 | −4 | 456 | 0.014 | |
| L | Putamen | −18 | 10 | 0 | 416 | 0.014 | ||
| L | Middle FG | 6 | −30 | 0 | 44 | 288 | 0.013 | |
| R | Mid Cingulate | 32 | 18 | 8 | 38 | 280 | 0.012 | |
| Developmental Contrasts: | ||||||||
| [BD-youths > HC- youths] – [BD-adult >HC-adult] | L | IFG | 47 | −34 | 16 | −8 | 392 | 3.011 |
| L | Precuneus | 7 | −1 | −59 | 59 | 344 | 3.011 | |
Note: L = left; M = Midline; R = right; dACC = dorsal anterior cingulate cortex; pgACC = pre-genual anterior cingulate cortex; BA = Brodmann Area; FG = frontal gyrus; IFG = inferior frontal gyrus; MTG = middle temporal gyrus; OG = occipital gyrus; PHG = parahippocampal gyrus; STG = superior temporal gyrus
Pair-wise analyses showed that BD-adults had significant convergence of hyper-activation versus HC-adults in regions including the left IFG, parahippocampal gyrus, and caudate. BD-adults had significant convergence of hypo-activation in regions including the bilateral amygdala, middle frontal gyrus, putamen, and right IFG.
Developmental ALE analyses showed greater convergence of hyper-activation in BD-youths than in BD-adults in left IFG (Figure 2b) and precuneus (Figure 2c). No areas showed significantly greater convergence of hyper-activation in BD-adults than in BD-youths, nor were there any significant between-group differences in convergence of hypo-activation.
Secondary Analyses: Non-emotional Paradigms
Analyses of non-emotional paradigms included 10 pediatric (47 coordinates) and 34 adult (219 coordinates) studies.
Pair-wise ALE analyses did not identify any regions where BD-youths had significant convergence of hyper-activation versus HC-youths. However, BD-youths had significant convergence of hypo-activation versus HC-youths in right caudate and pgACC.
Pair-wise adult analyses showed that BD-adults had significant convergence of hyper-activation versus HC-adults in bilateral precuneus, left pgACC, and right putamen. BD-adults also had significant convergence of hypo-activation versus HC-adults in bilateral IFG, putamen, and posterior visual perception regions (eTable 3).
Developmental ALE analyses showed significantly greater convergence of hypo-activation in BD-youths than BD-adults in the right pgACC (Figure 2d). No areas showed significantly greater convergence of hypo-activation in BD-adults than in BD-youths, nor were there any significant between-group differences in convergence of hyper-activation.
Tertiary Analyses: All Paradigms
Analyses from all task paradigms included 24 pediatric (241 coordinates) and 82 adult (575 coordinates) studies.
Pair-wise ALE analyses showed that BD-youths had significant convergence of hyper-activation versus HC-youths in regions including the right amygdala, left PFC, and precuneus. BD-youths had significant convergence of hypo-activation versus HC-youths in regions including the right pgACC and caudate.
Pair-wise ALE analyses showed that BD-adults had significant convergence of hyper-activation than HC-adults in regions including the left pgACC, left IFG, and right pallidus. BD-adults had significant convergence of hypo-activation than HC-adults in regions including the bilateral putamen, bilateral IFG, and right lingual gyrus and inferior parietal lobe (eTable 4).
Developmental ALE analyses showed significantly greater convergence of hypo-activation in BD-youths than BD-adults in the right pgACC (Figure 2e). No areas showed significantly greater convergence of hypo-activation in BD-adults than in BD-youths, nor were there any significant between-group differences in convergence of hyper-activation.
DISCUSSION
Our study, the first developmental ALE meta-analysis to directly compare pediatric to adult BD fMRI studies, has several important findings. First, during emotional face perception, BD-youths showed greater convergence of right amygdala hyper-activation than BD-adults. Second, in response to emotional stimuli, BD-youths had greater convergence of hyper-activation in left IFG and left precuneus than BD-adults. Third, during non-emotional tasks and across all paradigms, BD-youths had greater convergence of right pgACC hypo-activation than BD-adults. Our findings suggest potentially unique neurodevelopmental alterations associated with BD in youths versus adults that require further study to determine their longitudinal progress, and if they may be utilized to improve diagnosis or treatment.
Greater convergence of amygdala hyper-activation in BD-youths than in BD-adults during emotional face perception aligns with several prior studies. Importantly, we independently replicated Kim’s (2012) finding of increased right amygdala activation to emotional faces among BD-youths versus both BD-adults and HC-youths, which was excluded from our present meta-analysis because their data was not reported in x/y/z coordinate space 16. Our result also aligns with a prior qualitative comparison of pediatric and adult BD fMRI studies, which found more amygdala hyper-activation in BD-youths than BD-adults during emotional face perception 42. Although other mental illnesses (e.g., depression, anxiety, schizophrenia) also exhibit amygdala alterations 55–58, we note that reduced amygdala size 58–62 and increased amygdala activation 16,20,21,28 in BD-youths are among the most replicated neuroimaging findings of any disorder or age group. In sum, these results suggest the need for greater study of amygdala hyper-activation in BD-youths in response to emotional faces, potentially as the target of biologically-based treatments including computer-assisted cognitive remediation 63,64.
Across all emotional paradigms, BD-youths showed greater convergence of IFG and precuneus hyper-activation than BD-adults. Both the IFG and precuneus are involved in emotion-cognition interaction paradigms as shown in a recent ALE meta-analysis in HCs 65. Moreover, the IFG facilitates inhibitory control of behavior, including emotional responding 66, while the precuneus is involved in emotional processing via its roles in attention, autobiographical memory, and social processing 67. The IFG exhibits prolonged neural pruning and myelination well into young adulthood 68,69 and a delayed maturational course in pediatric BD specifically 60,70,71. BD-youths show greater precuneus gray matter volume than HC-youths, suggesting insufficient pruning and underdevelopment in pediatric BD 71. Altogether, greater convergence of hyper-activation in the IFG and precuneus among BD-youths versus BD-adults during emotional tasks suggests that these regions play a more significant role in emotion-cognition interactions in BD-youths than in BD-adults. These findings warrant further study, potentially with ecologically-valid psychosocial interaction tasks (e.g., peer rejection 72) to explore these alterations’ developmental course in BD and their relationship to patients’ real-world emotional impairment (e.g., family functioning 73).
During non-emotional tasks, BD-youths had greater convergence of hypo-activation in right pgACC than BD-adults. A similar deficit was detected when all data were included, suggesting a common trait-like deficit present even during emotional tasks. The pgACC mediates activity between dorsal cognitive control prefrontal regions and ventral emotional regulatory regions 74, so this deficit may reflect an altered balance of cognitive and emotional activity in BD-youths. BD-youths show persistent cognitive difficulties even after several years of neural development and mood symptom abatement 13, so this pgACC activation deficit could underlie the cognitive control deficits that persist as BD-youths develop into adults. Further studies directly comparing youths and adults with BD on cognitively-challenging and ecologically-realistic paradigms (i.e., similar to school/work) could reveal whether these alterations represent a persistent marker of cognitive dysfunction in BD.
Of note, as in a previous BD fMRI meta-analysis 32, some regions (e.g., putamen) showed convergence in both hyper-activations and hypo-activations versus HC, particularly in BD-adults. While this may seem inconsistent, ALE evaluates the convergence of activations across studies, unlike individual fMRI studies whereby two-group contrasts should produce either hyper- or hypo-activation in each brain region. Thus, convergence of both hyper- and hypo-activation in the same region may result from sufficient numbers of published studies supporting both positions. Despite differences in the direction of activation, these regions reliably differed from HC, suggesting that they represent important targets for future longitudinal BD neuroimaging studies to examine their role in developmental change, illness progression, and treatment effects.
Limitations
Our study has several limitations including (1) publication bias, (2) participant factors including mood state, medication status, and psychiatric comorbidity, (3) a dichotomous categorization of pediatric versus adult studies, and (4) few pediatric BD studies evaluating language, memory, or social processing. First, ALE relies on published data and is subject to publication bias. Although unable to include unpublished studies, we maximized our yield of published studies by using PsycINFO to confirm our PubMed search, yielding 102 additional studies not found by PubMed. None met our inclusion criteria, suggesting a thorough search. Also, because ALE is a coordinate-based meta-analysis, we had to exclude studies not reporting results in three-dimensional coordinate space.
Second, several important factors, including mood state, medication status, and psychiatric comorbidity, are beyond the scope of our ALE meta-analysis because GingerALE currently does not allow covariates. To address this concern, pediatric and adult studies showed no significant differences in sample size, gender ratio, or mood state, although more adult than pediatric BD participants were medicated. With respect to BD-subtype, pediatric (16%) and adult studies (7%) did not significantly differ in their percentages of BD-II participants, t(84)=1.47, p=0.2). Six BD-NOS participants were included in the context of two pediatric BD fMRI studies, both aggregated with a predominately BD-I sample. Several other important factors germane to BD were also beyond the scope of our analyses as few fMRI studies report them, or address retrospective recall bias inherent in their assessment —e.g., BD onset/illness duration, severity/number of mood episodes, lifetime medication exposure, and/or substance use disorders.
Third, we used a dichotomous cut-off for pediatric versus adult studies, which is required for the subtraction contrasts employed by any ALE meta-analyses examining developmental alterations associated with neuropsychiatric illness. This cutoff is commonly used in IRB regulation of research, and thus bifurcates most published studies into pediatric versus adult regardless of technique (MRI, treatment, etc.). Nevertheless, pioneering longitudinal MRI studies (e.g., by Giedd and collaborators) have demonstrated that neural development continues throughout young adulthood 68,69.
One approach to address all three limitations would be to conduct a “mega-analysis”, whereby original fMRI data is pooled and re-analyzed. Our current ALE results could guide a priori hypotheses about particular brain regions. Age could be evaluated as both a dichotomous and also continuous factor. Individual participants’ MRI data could be excluded to address participant or study factors—e.g., the few BD-NOS participants, behavioral performance, or MR magnet strength. However, a mega-analysis would still be subject to publication bias and issues of data sharing and quality control 29. Longitudinal fMRI studies whereby BD participants are imaged annually from childhood through adulthood could also address these limitations, but would require time because aging can not be hastened.
Finally, we note the relative dearth of BD-youth studies assessing language, memory, and social processing that limited direct comparisons to BD-adults. This gap is fertile ground for future research 42.
Conclusions
Our results underscore important differences between pediatric and adult BD in amygdala, inferior PFC, and precuneus responses to emotional information and in pgACC responses to cognitive challenge. They also reveal numerous common regions exhibiting functional abnormalities in both age groups, including ventral PFC, amygdala, striatum, and posterior visual perception areas. Further cross-sectional fMRI studies involving groups of BD-youths and BD-adults compared to age-matched HC, as well as longitudinal neuroimaging studies following BD-youths as they become adults are needed to provide more information about the developmental progression of neural alterations associated with BD that may ultimately aid biologically-based approaches to diagnosis and treatment for BD.
Supplementary Material
Acknowledgments
Dr. Wegbreit is supported by NIH 5T32-MH019927-20.
Dr. Dickstein, Dr. Kim, Ms. Cushman, Ms. Puzia, and Ms. Weissman are supported by NIH 5R01-MH087513, 1R21-MH096850, 1R01-MH099703, and 5R01-MH092450, though the present study was not part of any of these grants.
Dr. Laird is supported by NIH R01-MH074457 and R01-MH084812.
Footnotes
All authors report no conflicts of interest.
Dr. Wegbreit and Dr. Dickstein had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics. 2003;21(9):601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Keck PE, Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatr Pract. 2008;14(2):31–38. doi: 10.1097/01.pra.0000320124.91799.2a. [DOI] [PubMed] [Google Scholar]
- 5.Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7(2):111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 6.Scott EM, Hermens DF, Naismith SL, et al. Distinguishing young people with emerging bipolar disorders from those with unipolar depression. J Affect Disord. 2013;144(3):208–215. doi: 10.1016/j.jad.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62(2):107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 9.Holtmann M, Duketis E, Poustka L, Zepf FD, Poustka F, Bolte S. Bipolar disorder in children and adolescents in Germany: national trends in the rates of inpatients, 2000–2007. Bipolar Disord. 2010;12(2):155–163. doi: 10.1111/j.1399-5618.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 10.Escamilla I, Wozniak J, Soutullo CA, Gamazo-Garran P, Figueroa-Quintana A, Biederman J. Pediatric bipolar disorder in a Spanish sample: results after 2.6 years of follow-up. J Affect Disord. 2011;132(1–2):270–274. doi: 10.1016/j.jad.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JI, Case BG, Birmaher B, et al. Irritability and elation in a large bipolar youth sample: relative symptom severity and clinical outcomes over 4 years. J Clin Psychiatry. 2013;74(1):e110–117. doi: 10.4088/JCP.12m07874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan-Miller D, Peris T, Axelson D, Kowatch RA, Miklowitz DJ. Family functioning, social impairment, and symptoms among adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1085–1094. doi: 10.1016/j.jaac.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. J Am Acad Child Adolesc Psychiatry. 2009;48(3):299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo CJ, Esposito-Smythers C, Swenson L, et al. Factors associated with mental health service utilization among bipolar youth. Bipolar Disord. 2007;9(8):839–850. doi: 10.1111/j.1399-5618.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser M, Galling B, Correll CU. Suicidal ideation and suicide attempts in children and adolescents with bipolar disorder: a systematic review of prevalence and incidence rates, correlates, and targeted interventions. Bipolar Disord. 2013;15(5):507–523. doi: 10.1111/bdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim P, Thomas LA, Rosen BH, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am J Psychiatry. 2012;169(6):642–649. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weathers JD, Stringaris A, Deveney CM, et al. A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. Am J Psychiatry. 2012;169(6):633–641. doi: 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weathers J, Brotman MA, Deveney CM, et al. A developmental study on the neural circuitry mediating response flexibility in bipolar disorder. Psychiatry Res. 2013;214(1):56–65. doi: 10.1016/j.pscychresns.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adleman NE, Kayser RR, Olsavsky AK, et al. Abnormal fusiform activation during emotional-face encoding assessed with functional magnetic resonance imaging. Psychiatry Res. 2013;212(2):161–163. doi: 10.1016/j.pscychresns.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altshuler LL, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: A functional magnetic resonance imaging study. Bipolar Disord. 2008;10(6):708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keener MT, Fournier JC, Mullin BC, et al. Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychol Med. 2012;42(9):1913–1924. doi: 10.1017/S0033291711002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9(4):345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 25.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 26.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg HP, Leung H-C, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: State- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60(6):601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 28.Chang K, Adleman NE, Dienes K, Simeonova DJ, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61(8):781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 29.Costafreda SG. Pooling FMRI data: meta-analysis, mega-analysis and multi-center studies. Front Neuroinform. 2009;3:33. doi: 10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25(1):155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13(1):1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 33.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: A voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22(2):100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43(3):553–569. doi: 10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- 35.Houenou J, Frommberger J, Carde S, et al. Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. J Affect Disord. 2011;132(3):344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickstein DP, Pescosolido MF, Reidy BL, et al. Developmental meta-analysis of the functional neural correlates of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(3):279–289. e216. doi: 10.1016/j.jaac.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hulvershorn LA, Karne H, Gunn AD, et al. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry. 2012;71(7):603–610. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res. 2010;184(3):135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Wegbreit E, Pavuluri M. Mechanistic comparisons of functional domains across pediatric and adult bipolar disorder highlight similarities, as well as differences, influenced by the developing brain. Isr J Psychiatry Relat Sci. 2012;49(2):75–83. [PubMed] [Google Scholar]
- 43.Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res. 2011;193(2):71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 46.Strakowski SM, Adler CM, Cerullo MA, et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interv Psychiatry. 2008;2(4):225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29(9):1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 48.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: Evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69(4):381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, Anand A. Emotional response inhibition in bipolar disorder: A functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol Psychiatry. 2013;73(2):136–143. doi: 10.1016/j.biopsych.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laird AR, Eickhoff SB, Fox PM, et al. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3(3):243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 53.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage Apr. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 55.Beesdo K, Lau JYF, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57(9):961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 57.Whalley HC, Papmeyer M, Sprooten E, Lawrie SM, Sussmann JE, McIntosh AM. Review of functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disord. 2012;14(4):411–431. doi: 10.1111/j.1399-5618.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 58.Mana S, Paillere Martinot ML, Martinot JL. Brain imaging findings in children and adolescents with mental disorders: A cross-sectional review. Eur Psychiatry. 2010;25(6):345–354. doi: 10.1016/j.eurpsy.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: Results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 60.Caetano SC, Olvera RL, Glahn D, Fonseca M, Pliszka S, Soares JC. Fronto-limbic brain abnormalities in juvenile onset bipolar disorder. Biol Psychiatry. 2005;58(7):525–531. doi: 10.1016/j.biopsych.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 61.Usher J, Leucht S, Falkai P, Scherk H. Correlation between amygdala volume and age in bipolar disorder—A systematic review and meta-analysis of structural MRI studies. Psychiatry Res. 2010;182(1):1–8. doi: 10.1016/j.pscychresns.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Höschl C. Amygdala volumes in mood disorders—Meta-analysis of magnetic resonance volumetry studies. J Affect Disord. 2009;115(3):395–410. doi: 10.1016/j.jad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 63.McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am J Psychiatry. 2007;164(3):437–441. doi: 10.1176/ajp.2007.164.3.437. [DOI] [PubMed] [Google Scholar]
- 64.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 65.Cromheeke S, Mueller SC. Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0549-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 68.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 69.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalmar JH, Wang F, Spencer L, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009;15(3):476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adleman NE, Fromm SJ, Razdan V, et al. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. 2012;53(11):1149–1156. doi: 10.1111/j.1469-7610.2012.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young ME, Galvan T, Reidy BL, et al. Family functioning deficits in bipolar disorder and ADHD in youth. J Affect Disord. 2013;150(3):1096–1102. doi: 10.1016/j.jad.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.