Abstract
Inhibition of acetylcholinesterase (AChE) after nerve agent exposure induces status epilepticus (SE), which causes brain damage or death. The development of countermeasures appropriate for the pediatric population requires testing of anticonvulsant treatments in immature animals. In the present study, exposure of 21-day-old (P21) rats to different doses of soman, followed by probit analysis, produced an LD50 of 62 μg/kg. The onset of behaviorally-observed SE was accompanied by a dramatic decrease in brain AChE activity; rats who did not develop SE had significantly less reduction of AChE activity in the basolateral amygdala than rats who developed SE. Atropine sulfate (ATS) at 2 mg/kg, administered 20 min after soman exposure (1.2XLD50), terminated seizures. ATS at 0.5 mg/kg, given along with an oxime within 1 min after exposure, allowed testing of anticonvulsants at delayed time-points. The AMPA/GluK1 receptor antagonist LY293558, or the specific GluK1 antagonist UBP302, administered 1 h post-exposure, terminated SE. There were no degenerating neurons in soman-exposed P21 rats, but both the amygdala and the hippocampus were smaller than in control rats at 30 and 90 days post-exposure; this pathology was not present in rats treated with LY293558. Behavioral deficits present at 30 days post-exposure, were also prevented by LY293558 treatment. Thus, in immature animals, a single injection of atropine is sufficient to halt nerve agent-induced seizures, if administered timely. Testing anticonvulsants at delayed time-points requires early administration of ATS at a low dose, sufficient to counteract only peripheral toxicity. LY293558 administered 1 h post-exposure, prevents brain pathology and behavioral deficits.
Keywords: immature rats, soman, seizures, acetylcholinesterase, atropine sulfate, GluK1 antagonists
Introduction
Nerve agents are potent, organophosphorus toxins that act primarily by inhibiting the activity of acetylcholinesterase (AChE). The resulting accumulation of acetylcholine at synaptic junctions produces peripheral cholinergic crisis (excessive salivation, lacrimation, rhinorrhea, bronchorrhea, cardiorespiratory suppression, eventual muscle paralysis, etc.), and, in the brain, induces seizures and status epilepticus (SE). Without timely pharmacological intervention, death will ensue, or if death is prevented but the SE is not controlled, brain damage will result, with long-term neurological and behavioral consequences (Apland et al., 2010, 2014; Coubard et al., 2008; Figueiredo et al., 2011a, 2011b; Filliat et al., 2007; Prager et al., 2014a, 2014b; Yanagisawa et al., 2006). The nerve agent sarin that was released during a terrorist attack in Syria, in August 2013, resulted in the death of over 1,400 civilians, 426 of which were children (Dolgin, 2013). Mass casualties during terrorist attacks that employ nerve agents are expected to disproportionately affect children due to their greater body surface area-to-body mass ratio, increased skin permeability, faster respiration rate, breathing at a level where nerve agent vapor density would be highest, and an increased susceptibility to seizures (American Academy of Pediatrics, 2000). The higher vulnerability of children necessitates the availability of effective countermeasures that will protect their lives.
Pharmacological treatments to counteract nerve agent toxicity exist for the adult population, and the scientific community is actively seeking to improve them by testing the efficacy of novel compounds in mitigating both the short-term and the long-term health consequences of nerve agent exposure. Specifically, for the control of the peripheral effects of nerve agent exposure, the Food and Drug Administration has approved the use of atropine, a muscarinic receptor antagonist, along with an oxime (pralidoxime), which reactivates the inhibited AChE (Bajgar, 2005, 2010; Marrs et al., 2006; Voicu et al., 2010). In addition, diazepam has been approved for the cessation of nerve agent-induced SE. Improvements to this regimen are likely to be made in the future, as more effective anticonvulsants are being discovered (Apland et al., 2013; Capacio et al., 2004; Figueiredo et al., 2011a; Figueiredo et al., 2011b; Gilat et al., 2005), and, as recent studies suggest, diazepam does not protect against brain damage and behavioral deficits associated with nerve agent exposure (Apland et al., 2014; Myhrer et al., 2005), while other anticonvulsant compounds have high neuroprotective efficacy (Apland et al., 2014). Due to the nature of this research, nerve agent studies can be carried out only in animal models. Do the findings from studies in adult animals apply to the young age as well, and only the dose has to be adjusted according to body weight? There is very limited information on immature animals, and although it is possible that adult and immature animals or humans would respond similarly to pharmacological countermeasures against nerve agents, the differences between a developing brain and an adult brain, and their implications, must be considered. For example, the cholinergic system– which plays a central role in the mechanisms of nerve agent action– is still at a developing stage in early postnatal life (Coyle and Yamamura, 1976). In addition, differences in the blood-brain barrier (BBB) permeability between developing and adult animals (Saunders et al., 2000; Vannucci and Simpson, 2003) could affect the pharmacokinetics of the injected drugs. Furthermore, there is evidence suggesting that immature animals differ from adult animals both in seizure susceptibility and in the extent and nature of neuropathology that seizures can induce (Ben-Ari and Holmes, 2006; Haut et al., 2004; Stafstrom and Holmes, 2002; Wasterlain et al., 2002); it is unclear what type of neuropathology nerve agents induce in immature animals, and, therefore, it is also unclear what is the nature of neuroprotection expected by drugs that terminate nerve agent-induced seizures in immature animals.
In the present study, we describe findings in postnatal-day-21 (P21) rats. It is difficult to precisely ascertain the corresponding age in humans. If we use the conversion factor for the early developmental phase (Andreollo et al., 2012; Sengupta, 2013), P21 would correspond to about a 6-month-old human; however, because the developmental stage of the brain in the two species must be taken into account (Andersen, 2003), and synaptogenesis– which is a basic parameter of brain development– is completed within the first 3 weeks of life in the rat and about 3.5 years in the human (Pressler and Auvin, 2013), a P21 rat may correspond to a human close to 4 years of age. In the P21 rats, we determined the LD50 of the nerve agent soman, measured AChE activity after soman exposure in brain regions that play an important role in seizure generation, and determined the effects of atropine administration on ongoing, behaviorally-monitored SE. In addition, because we have shown previously in young-adult rats that antagonists of kainate receptors containing the GluK1 subunit (GluK1Rs; formerly known as GluR5Rs, see Collingridge et al., 2009; Jane et al., 2009) are very effective anticonvulsants and neuroprotectants against soman exposure (Apland et al., 2013; Apland et al., 2014; Figueiredo et al., 2011b), we also examined the efficacy of these compounds in the immature, P21 rats.
Materials and Methods
Animals
Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). Male, immature rats (weighing 50-60g at the time of soman exposure on P21) were housed in groups of 5 with a surrogate mother and weaned on the day of experiments. Young-adult male rats (150-200g at the time of soman exposure) were housed in pairs until the day of experiments. All rats were provided with food and water ad libitum. Experiments were carried out with the approval of the Institutional Animal Care and Use Committees of the Uniformed Services University of the Health Sciences and the U.S. Army Institute of Chemical Defense.
Seizure Induction and assessment
Rats were exposed to soman (pinacoyl methylphosphonofluoridate; obtained from the Edgewood Chemical Biological Center, Aberdeen Proving Ground, Edgewood, MD) diluted in cold physiological saline, by subcutaneous injection over the right hind-quarter. The LD50 was determined for the immature rats (see next section), and the two age-groups (immature and young-adult rats) were administered a dose that corresponded to 1.2 X LD50. Seizures were monitored behaviorally and classified according to a minimally modified version of the Racine scale (Racine, 1972) as we have previously described (Figueiredo et al., 2011b): stage 0, no behavioral response; stage 1, behavioral arrest; stage 2, oral/facial movements, chewing, head nodding; stage 3, unilateral/bilateral forelimb clonus without rearing, Straub tail, extended body posture; stage 4, bilateral forelimb clonus plus rearing; stage 5, rearing and falling; and stage 6, full tonic seizures. Following soman injection, rats that went on to develop Stage 3 seizures or above were considered to have SE.
Median Lethal Dose Determination
Using the log-probit method (Litchfield and Wilcoxon, 1949), we determined the median lethal dose (LD50) of soman for the immature rats. P21 rats were randomly assigned into five groups of ten animals per group, and received five different doses ranging from 40 to 70 μg/kg soman (see results). Three groups were exposed in one day and the other two groups were exposed on the next day.
Groups and Drug treatments
P21 male rats were administered 1.2 X LD50 soman (74.4 μg/kg, s.c.). A group of the soman-exposed P21 rats was administered 2 mg/kg atropine sulfate (ATS; i.m.; Sigma Aldrich, St Louis, MO) at 20 min after soman injection. For comparison, we also injected young-adult rats with 1.2 X LD50 soman (132 μg/kg, s.c.) and administered 2 mg/kg ATS (i.m.) at 20 min after soman injection. Some animals from these groups were used for measurements of AChE activity (see below).
Another group of P21 male rats were administered 1.2 X LD50 soman (74.4 μg/kg, s.c.) followed by 0.5 mg/kg ATS (i.m) and 125 mg/kg HI-6 (i.p.) at 1 min post-soman exposure; HI-6 is a bispyridinium oxime that reactivates inhibited acetylcholinesterase, primarily in the periphery (Joosen et al., 2011; Mercey et al., 2012). At 60 min after soman injection, these rats were administered the GluK1/AMPA receptor antagonist LY293558 (20 mg/kg, i.m.; kindly provided by Raptor Pharmaceutical Corp., Novato, CA; Bleakman et al., 1996), or the GluK1 receptor antagonist UBP302 (250 mg/kg, i.p.; Tocris, Bristol, UK; More et al., 2004), or the drug vehicle.
Acetylcholinesterase Assay
Following soman exposure, rats that went on to develop SE were sacrificed immediately (at the onset of SE). Rats that did not develop SE were sacrificed 20 min after soman exposure. Total AChE activity was measured in the prelimbic cortex, basolateral amygdala (BLA), piriform cortex, and hippocampus, using an established spectrophotometric protocol (Ellman et al., 1961; Padilla et al., 1999). Rats were anesthetized with 3-5% isoflurane and rapidly decapitated. The brain was removed and placed in ice-cold phosphate buffer (0.1 M, pH 8.0). Coronal brain slices (500 μm-thick) containing the prelimbic cortex (Bregma 5.16 mm to 2.52 mm), BLA (Bregma −2.28 mm to −3.72 mm), piriform cortex (Bregma −1.72 mm to −3.00 mm), and hippocampus (Bregma −2.28 mm to −4.68 mm) were cut using a vibratome (series 1000; Technical Products International, St. Louis, MO). The subsequent procedure has been described previously (Prager et al., 2013; Prager et al., 2014a). Briefly, each structure was isolated by hand and placed in an Eppendorf tube. They were homogenized in phosphate buffer (0.1M, pH 8.0) + Triton 10%, centrifuged, and the supernatant was placed into a separate Eppendorf tube. Glutathione was used to construct a standard curve in the presence of DTNB [5.5'-dithio-bis(2-nitrobenzonic acid)]. Then, tissue homogenate supernatants (10 μL per well) were added to either 10 μL eserine or ethoprozine, in the presence of 5 μL acetylthiocholine (1 μM) and 175 μL DTNB (1 mM; all purchased from Sigma-Aldrich, St. Louis, MO). Samples were read by the Softmax Pro 5.2 kinetics every 20 sec for 4 min. The total butyrylcholinesterase inhibition was subtracted from the absorbance sample to provide a difference score, which was multiplied by the slope and intercept of the standard curve to provide the total AChE activity. AChE specific activity was calculated by dividing the total activity by the calculated protein concentration assayed by the Bradford method (Bradford, 1976) using a protein assay dye reagent (Biorad, Cat # 500-006).
Fixation and Tissue Processing
One day, 7, 30, or 90 days after soman administration, groups of rats were deeply anesthetized with pentobarbital (75–100 mg/kg, i.p.) and transcardially perfused with PBS (100 ml) followed by 4% paraformaldehyde (200 ml). The brains were removed and postfixed overnight at 4°C, then transferred to a solution of 30% sucrose in PBS for 72 h, and frozen with dry ice before storage at 80°C until sectioning. A 1-in-5 series of sections from the rostral extent of the amygdala to the caudal extent of the entorhinal cortex was cut at 40 μm on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus; Daigger, Vernon Hills, IL) in PBS for Nissl staining with cresyl violet. Two adjacent series of sections were also mounted on slides for Fluoro-Jade C (FJC) staining.
Fluoro-Jade C (FJC) Staining and analysis
FJC (Histo-Chem, Jefferson, AR) was used to identify irreversibly degenerating neurons (Schmued et al., 2005) in the brains of P21 soman-exposed rats, at 24 h and 7 days after exposure, as described previously (Apland et al., 2013; Figueiredo et al., 2011b). Mounted sections were air-dried overnight and then immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min. The slides were then rinsed for 2 min in 70% ethanol and 2 min in distilled water (dH20), and then incubated in 0.06% potassium permanganate solution for 10 min. After a 2 min rinse in dH20, the slides were transferred to a 0.0001% solution of FJC dissolved in 0.1% acetic acid for 10 minutes. Following three 1-minute rinses in dH20, the slides were dried on a slide warmer, cleared in xylene for at least 1 min, and coverslipped with DPX (Sigma-Aldrich). To assess the extent of neurodegeneration, we used a series of adjacent Nissl-stained sections to trace the regions of interest. The tracings from the Nissl-stained sections were superimposed on the FJC-stained sections, using the Stereo Investigator 9.0 (MicroBrightField, Williston, VT). The rating system used to assess the extent of neurodegeneration has been described previously (Apland et al., 2010; Figueiredo et al., 2011b).
Volumetric analysis
Volumetric analysis was performed 30 and 90 days after soman exposure. Nissl-stained sections containing the hippocampal formation (sections were 400 μm apart) or the amygdala (sections were 200 μm apart) were used to estimate stereogically the volume of these structures based on the previously described Cavalieri principle (Gundersen et al., 1988). Sections were viewed with a Zeiss Axioplan 2ie fluorescent microscope with a motorized stage (Oberkochen, Germany), interfaced with a computer, running StereoInvestigator 9.0 (MicroBrightField, Williston, VT). The hippocampus and the amygdala were identified on slide-mounted sections under a 2.5x objective, based on the atlas of Paxinos and Watson (Paxinos and Watson, 2005), and traced using the Stereo Investigator 9.0. The volume was calculated by using the stereological probe called Cavalieri Estimator. An overlay of a rectangular lattice with a grid size of 300 μm was placed over the brain region (amygdala or hippocampus) tracings, and each point marked was counted to estimate the volume. For each animal, the coefficient of error (CE) was calculated to assure sufficient accuracy of the estimate (CE < 0.05).
Context- and Cue-Dependent Fear Conditioning
Fear conditioning took place 30 or 90 days after soman exposure. Rats were placed in Context A, which was a rodent-conditioning chamber made of Plexiglas and metal grid floor (Coulbourn Instruments, Lehigh Valley, PA, USA). The chamber was dimly illuminated by a single house-light (2–3 lux) and enclosed within a sound-attenuating chamber (background dB = 55). The chamber was cleaned between testing runs with a 70 % EtOH solution and thoroughly dried. Prior to presentation of the stimuli, rats were left to explore the chamber for 5 min. Then, they were presented with three pairings of an auditory conditioning stimulus (CS; 5 kHz, 75 dB, 20 s) that co-terminated with a foot-shock unconditioned stimulus (US; 1.0 mA, 500 ms); the intervals between CS presentations were random. One day after the fear conditioning, rats were placed in Context A for 20 min, with no presentations of CS or US, in order to test for context-dependent fear acquisition. Three days after the fear conditioning, rats were placed into a novel context (Context B), and were presented with twenty auditory CS (5 kHz, 75 dB, 20 s) at random intervals and without electric shock, in order to test for cue-dependent fear acquisition. Context B consisted of plastic flooring covered with fresh bedding; it had a geometry different from that of context A, contained different spatial cues (red and black tape), and was cleaned with 1 % acetic acid solution. Freezing behavior during testing of context or cue-dependent fear acquisition was scored from digitized videos. Freezing was defined as the absence of all movements except those related to respiration (Fanselow, 1980). For the cue-dependent test, freezing was scored only during the CS presentations, while for the context-dependent test, freezing was scored throughout the session. Total freezing time during test sessions was used as a measurement of the extent of fear acquisition (the impact of the fear conditioning).
The Open Field Test
Thirty and 90 days after soman exposure, anxiety-like behavior was assessed in the open field apparatus (40X40X30 cm clear Plexiglas arena), as described previously (Aroniadou-Anderjaska et al., 2012; Prager et al., 2014a). One day prior to testing (on day 29 or 89 after soman exposure), animals were acclimated to the apparatus for 20 min; acclimation reduces the effects the novelty during testing, so that the time spent in the center measures the anxiety state of the animal, rather than the response to novelty (Faraday et al., 2001). On the test day, the rats were placed in the center of the open field, and activity was measured and recorded for 20 min, using an Accuscan Electronics infrared photocell system (Accuscan Instruments Inc., Columbus, OH). Data were automatically collected and transmitted to a computer equipped with “Fusion” software (from Accuscan Electronics). Locomotion (distance traveled in cm), total movement time, and time spent in the center of the open field were analyzed. Anxiety behavior was measured as the ratio of the time spent in the center over the total movement time, expressed as a percentage of the total movement time.
Statistical Analysis
All data are expressed as mean ± standard error. The Student's t-test was used to test for differences in latency to seizure onset between adult and P21 rats, and the differences in baseline AChE activity between these two groups. Results from the AChE assay were analyzed using either One-way ANOVA followed by Dunnett's post-hoc test, or one-way ANOVA followed by Tukey HSD post hoc test. Results from the behavioral seizures were analyzed using MANOVA followed by Bonferroni post-hoc test. One-way ANOVA with a Dunnett's post hoc test was used to analyze the results from the behavioral tests and from the volumetric analysis. Statistical analyses were made using the software package PAWS SPSS 22 (IBM, Armonk, NY, USA). Differences were considered significant with p < 0.05. Sample size “n” refers to the number of animals.
Results
Calculation of the median lethal dose (LD50) of soman in immature (P21) male rats
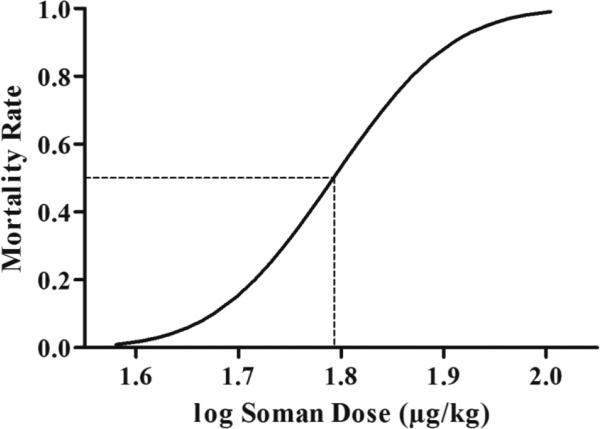
The doses of soman (10 rats/dose) were 40, 55, 57.5, 62.5 and 70 μg/kg, and produced response fractions (dead rats/total exposed) of 0/10, 4/10, 3/10, 5/10 and 7/10, respectively. These values were the input data for the log-probit method of calculating the LD50. Using the probit analysis function of the IBM SPSS Statistics 20 package, the estimated dose of soman expected to result in 50% mortality rate was calculated to be 62.02 μg/kg (95% confidence intervals: 56.63~72.15 μg/kg). The estimated soman doses and mortality rates were used to produce the log dose-response curve for soman, in P21 male rats (Fig. 1).
Figure 1. Determination of the Median Lethal Dose (LD50) of soman for P21 male rats.
Fifty rats (10 rats per dose) were injected subcutaneously with soman at the following doses (μg/kg): 40, 55, 57.5, 62.5, and 70. Mortality rates were recorded at 24 hr following soman injection and used as the input data into the log-probit method of the IBM SPSS Statistics 20 package to determine the LD50. The plot shows the predicted mortality rates at different doses of soman at P21. The LD50 was 62.02 μg/kg (dashed line; p = 0.00414).
Latency to seizure onset and comparison with adults
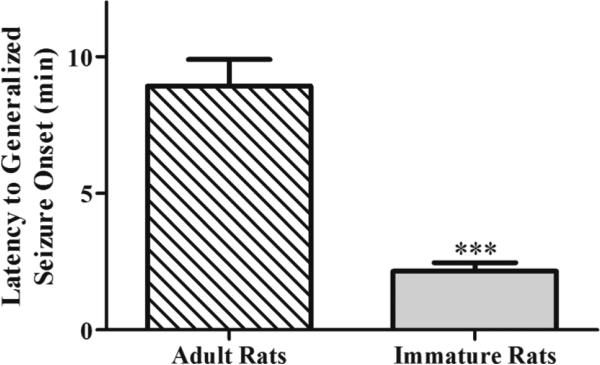
Soman, at 1.2 X LD50, was administered to 191 P21 rats (74.4 μg/kg), of whom 156 developed SE, as well as to 24 young-adult rats (132 μg/kg), of whom 16 developed SE. Mortality rates depended on the treatment and are reported below in the appropriate section. The latency to initiation of generalized seizures (stage 3 of the Racine scale) was significantly shorter in the P21 rats (2.15 ± 0.31 min, n = 20) compared to the young-adults (8.94 ± 0.25 min, n = 16, t(18.03)= −6.64, p < 0.001, Fig. 2).
Figure 2. The latency to SE onset after soman injection is shorter in P21 rats compared to adults.
P21 rats (n = 20) and young-adult rats (n = 16) were injected with the appropriate soman dose corresponding to 1.2 X LD50. ***p < 0.001 (Student's t-test).
Baseline AChE Activity in immature rats and comparison with adult rats
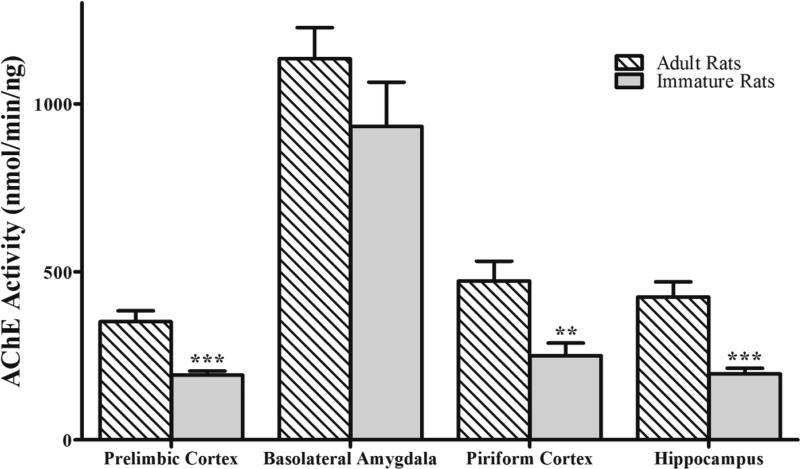
Baseline AChE activity (in nmol/min/ng) in the prelimbic cortex, BLA, piriform cortex, and hippocampus was measured in naïve P21 rats (n = 5) and compared with young-adult rats (n = 15). As in the young-adult rats (Prager et al., 2014a, and Fig. 3), AChE activity in the immature rats was significantly higher in the BLA (932.5 ± 132.2; F(3,16)=26.94; p < 0.001; Fig. 3) than in the prelimbic cortex (193.3 ± 11.8; p < 0.001), piriform cortex (250.8 ± 37.2; p < 0.001), and hippocampus (196.8 ± 16.7; p < 0.001). Between the two age groups, there was no statistically significant difference for the BLA (932.5 ± 132.2 for the P21 group and 1134.8 ± 92.1 for the adult group; t(8.27)= −1.25; p = 0.244), but in the prelimbic cortex (193.3 ± 11.8 in the P21 rats and 351.8 ± 32.4 in the adults; t(15.79)= −4.6; p < 0.001), piriform cortex (250.9 ± 37.2 in the P21 rats and 473.4 ± 58.6 in the adults; ; t(17.56) = −3.21; p = 0.005), and hippocampus (196.8 ± 16.7 in the P21 rats and 425.2 ± 45.0 in the adults; t(16.98) = −4.75; p < 0.001), AChE activity was significantly lower in the P21 rats (Fig. 3).
Figure 3. Compared to adult rats, baseline AChE activity in P21 rats is lower in the prefrontal cortex, piriform cortex, and hippocampus, but not in the basolateral amygdala.
For P21 rats, n = 5, and for the young-adult rats, n = 15. **p < 0.01, ***p < 0.001 (Student's t-test).
Inhibition of AChE activity in the basolateral amygdala plays a critical role in seizure induction following exposure of immature rats to soman
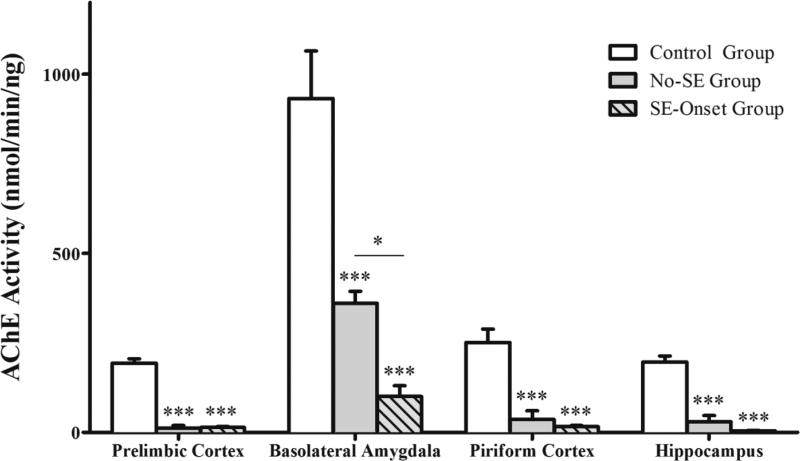
The AChE activity in the prelimbic cortex, BLA, piriform cortex, and hippocampus of naïve P21 rats (Control Group, n = 5) was compared to that of P21 rats that were exposed to soman but did not develop seizures (No-SE Group, n = 7; AChE activity was measured at 20 min after soman injection) and P21 rats that were exposed to soman and developed seizures (SE-Onset Group, n = 7; AChE activity was measured at the onset of stage 3 seizures). We found that, compared to the control group (for control values see baseline activity in the immature group in the previous section), AChE activity was significantly lower in all four brain regions in the rats that were exposed to soman (prelimbic cortex: F(2,16) = 176.93, p < 0.001; BLA: F(2,16) = 37.70, p < 0.001 ; piriform cortex: F(2,15) = 28.42, p < 0.001; hippocampus: F(2,16) = 52.68, p < 0.001; Fig. 4). In the No-SE group, AChE activity was 12.2 ± 7.2 nmol/min/ng in the prelimbic cortex (p < 0.001), 360.4 ± 33.7 in the basolateral amygdala (p < 0.001), 36.2 ± 24.2 in the piriform cortex (p < 0.001),, and 29.7 ± 17.4 in the hippocampus (p < 0.001). In the SE-Onset group, AChE activity was 13.4 ± 3.2 in the prelimbic cortex (p < 0.001), 100.1 ± 30.3 in the basolateral amygdala (p < 0.001), 15.9 ± 3.1 in the piriform cortex (p < 0.001), and 4.9 ± 0.8 in the hippocampus (p < 0.001). The only significant difference between the SE-Onset group and the No-SE group was the greater reduction of AChE activity in the basolateral amygdala of the former group (p = 0.024; Fig. 4). The differences between the SE-Onset group and the No-SE group in AChE activity in the prelimbic cortex (p = 0.992), piriform cortex (p = 0.789) and hippocampus (p = 0.381) were not statistically significant.
Figure 4. Reduction of AChE activity in P21 rats after injection of 1.2 X LD50 soman.
Soman-exposed rats that did not develop SE were sacrificed 20 min after soman injection (No-SE group, n = 7). Rats that developed SE were sacrificed at the onset of SE (SE-onset group, n = 7). The soman-exposed rats of both groups had significantly lower AChE activity in all brain regions, compared to the control group (n = 5). AChE activity in the basolateral amygdala of the SE-onset group was significantly lower than that in the No-SE group. ***p < 0.001 compared to the control group, and *p < 0.05 between the no-SE and the SE-onset groups (One-way ANOVA with Tukey HSD post-hoc).
Efficacy of atropine sulfate (ATS) against soman-induced seizures in immature versus adult rats
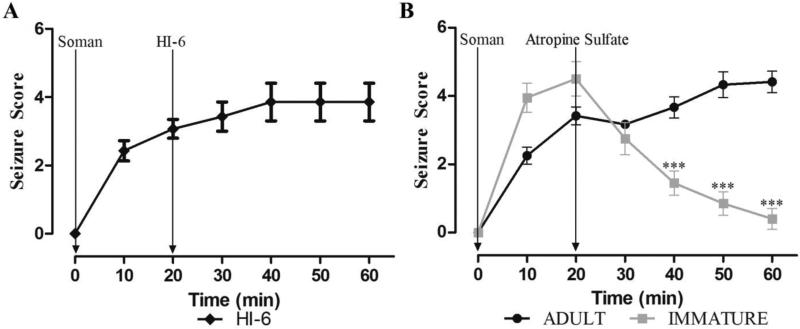
Testing of anticonvulsant treatments at different time points after nerve agent exposure requires control of the peripheral cholinergic crisis in order to prevent rapid death. For this purpose, most animal models employ administration of ATS within 1 min after exposure; HI-6 is often administered as well, either as a pretreatment or along with atropine. In order to mimic more closely a real-life scenario, where in the event of a terrorist attack it is unlikely that medical assistance will be administered within the first minute, we have employed administration of ATS and HI-6 at 20 min post-exposure in adult rats (Apland et al., 2013). We attempted to do the same in the immature rats, in the present study. For these experiments, we injected P21 rats (n = 100) with 1.2 X LD50 soman; all of the 100 rats developed seizures, but 67 rats died before the 20 min time-point. In the initial experiments, we injected surviving rats with 2 mg/kg ATS and 125 mg/kg HI-6 at 20 min after soman exposure; we observed that not only the peripheral effects of soman were controlled by ATS and HI-6, but seizures also were terminated. To determine if it was the ATS or the HI-6 that was responsible for the cessation of seizures, we administered 125 mg/kg HI-6 alone to 7 rats, at 20 min after soman injection; this treatment had no effect on seizures (Fig. 5A). However, when we administered 2 mg/kg ATS alone, SE was terminated in all of the P21 rats who received this treatment (n = 10; Fig. 5B). For comparison, twenty four young-adult rats were also exposed to 1.2 X LD50 soman. From these rats, 16 developed seizures and none of them died before the 20 min post-exposure time-point. Administration of 2 mg/kg ATS to the 16 rats did not suppress seizures (Fig. 5B), and 4 of these rats died soon after the injection of ATS. Comparisons between the adult and the immature rats showed that seizure severity in the immature rats was significantly reduced after ATS administration, at 40 min (F(1,20) = 22.43, p = 0.000126), 50 min (F(1,20) = 45.34 p = 0.000001) and 60 min (F(1,20) = 84.56 p = 0.000000015) minutes post-exposure (Fig. 5B).
Figure 5. Atropine sulfate (ATS) in immature but not in adult rats arrests generalized seizures induced by soman exposure.
Young-adult (n = 12) and P21 (n = 10) rats were exposed to a soman dose corresponding to 1.2 X LD50 (P21 rats: 74.4 μg/kg, young-adult rats: 132 μg/kg). A. Administration of HI-6 to P21 rats, at 20 min after soman injection, had no effect on seizures. B. Administration of 2.0 mg/kg ATS, at 20 min after soman injection, terminated seizures in the P21 rats, but not in the adult rats. ***p < 0.001 when seizure severity score is compared between P21 and adult rats at 40, 50, and 60 min after soman exposure (MANOVA, Bonferroni correction).
Efficacy of the GluK1KR antagonists LY293558 or UBP302 against soman-induced seizures in immature rats
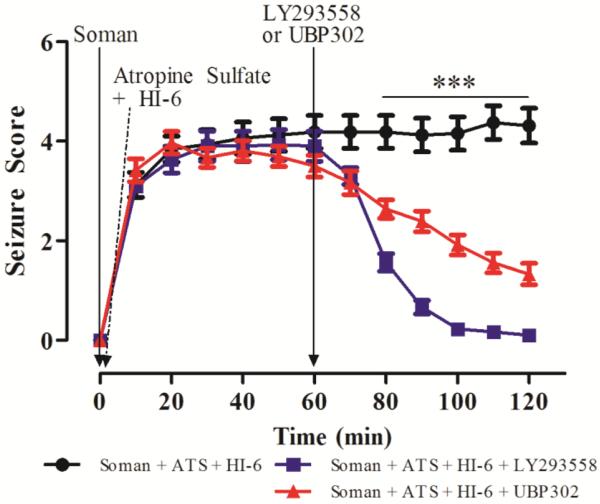
Next, we examined if the GluK1R antagonists which are effective in stopping seizures and preventing brain damage in adult rats, even when the anticonvulsants are administered at 1 h or longer after exposure (Apland et al., 2013; Figueiredo et al., 2011b), are also effective in the P21 rats. Since at this age, ATS alone at 2 mg/kg terminates soman-induced seizures, in this set of experiments we used a lower concentration of atropine, which was sufficient to control peripheral effects, but did not affect seizures. Thus, P21 rats were injected with 1.2 X LD50 soman (74.4 μg/kg) and treated with ATS (0.5 mg/kg) and HI-6 (125 mg/kg) at 1 min post-soman exposure; in this treatment paradigm, the survival rate was 59%. At 1 h post-exposure, there was no significant difference in seizure severity between the groups (F(2,48)= 1.55, p = 0.22). At this time point (1 h), rats were administered LY293558 (20 mg/kg; n = 15), or UBP302 (250 mg/kg; n = 18), or the drug vehicle (n = 16). Treatment with either drug significantly reduced seizure severity within 10 min (F(2,48)= 4.69, p = 0.014) and for the rest of the observation period (p < 0.001 for post-exposure times 80 to 120 min, Fig. 6; F values: F(2,48) = 28.41, 47.49, 66.89, 80.79, and 76.15 for the 80, 90, 100, 110, and 120 min post-exposure, respectively). In the rats who received only the vehicle, SE continued for at least the 2 h of observation.
Figure 6. Delayed post-treatment with the GluK1R antagonists LY293558 or UBP302 arrests soman-induced seizures in P21 rats.
Rats were exposed to 1.2 X LD50 soman (74.4 μg/kg) and treated with ATS (0.5 mg/kg) and HI-6 (125 mg/kg) at 1 min post-exposure. At 1 hr post-exposure, rats received LY293558 (20 mg/kg; n = 15), or UBP302 (250 mg/kg; n = 18), or the drug vehicle (n = 16). ***p < 0.001 for the difference in seizure score of the LY293558-treated and UBP302-treated groups compared to the vehicle-treated group (MANOVA, Bonferroni post-hoc test).
Immature rats do not undergo neuronal degeneration after soman-induced SE
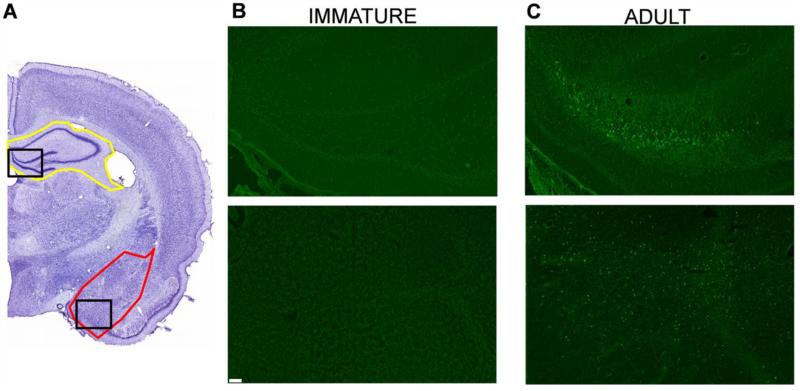
In adult rats, nerve agent-induced SE causes significant neuronal degeneration in many brain regions (Apland et al., 2010, 2013; Figueiredo et al., 2011a, 2011b) and particularly in the amygdala and the hippocampus (Apland et al., 2010). In the immature rats, the effects of the soman-induced SE on neuronal degeneration was examined at 24 h and 7 days after soman exposure in the group that received 0.5 mg/kg ATS and 125 mg/kg HI-6 at 1 min post-exposure, but no anticonvulsant treatment. There was no evidence for neuronal degeneration at either time point, in the amygdala or the hippocampus; we also inspected the rest of the brain on the FJC-stained sections, throughout the rostro-caudal extent, but, as in control rats, there were no FJC-stained cells in any brain region. To ensure that the staining procedure worked properly, we also processed sections from adult rats who had been exposed to 1.2XLD50 soman, receiving 2 mg/kg ATS and 125 mg/kg HI-6 at 1 min after exposure, but no anticonvulsant treatment; neurodegeneration was pronounced in these rats (Fig. 7).
Figure 7. Immature rats do not suffer neuronal degeneration, 1 day and 7 days after soman-induced SE.
A. Cresyl violet photomicrographs outline the brain regions (amygdala in red; hippocampus in yellow) from where the FJC photomicrographs (B and C) were taken (the specific areas shown in the photomicrographs are outlined with black rectangles). Immature and adult rats were exposed to the age-specific 1.2X LD50 of soman. In contrast to the adult rats (C), immature rats (B) did not display any degenerating cells. Magnification in A is 200x. Scale bar (for B and C) is 50 μm.
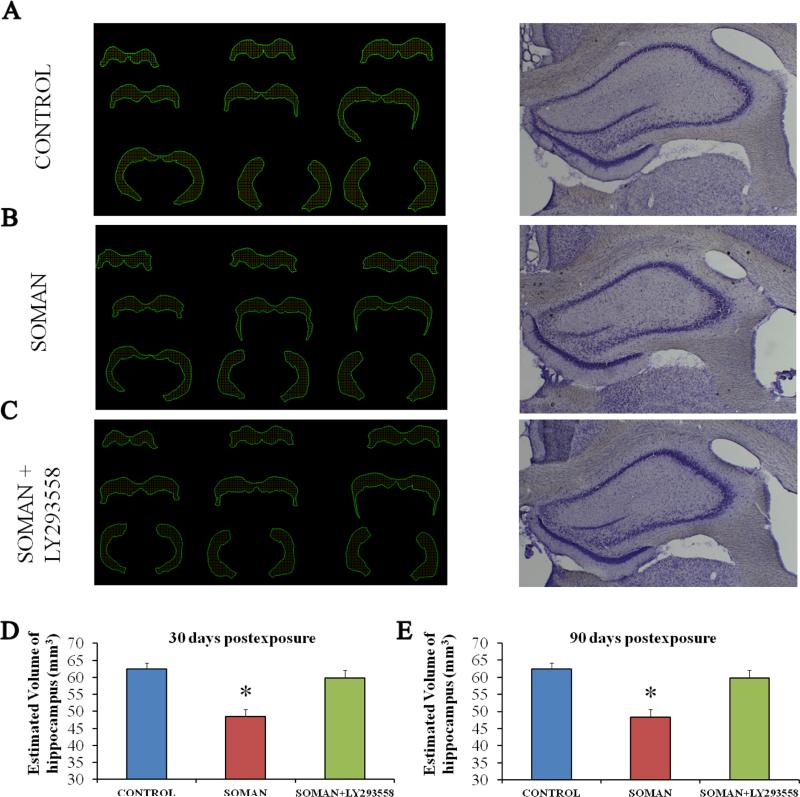
Amygdalar and hippocampal volume is significantly reduced 30 days and 90 days after exposure of immature rats to soman, and this is prevented by LY293558 treatment
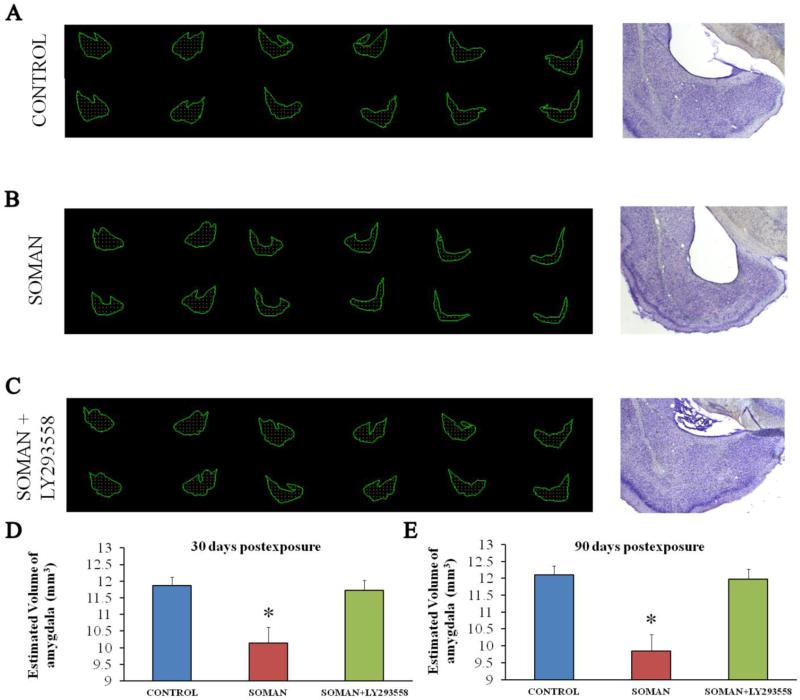
Despite the lack of neuronal degeneration after soman-induced SE, volumetric measurements of the amygdala and the hippocampus, 30 and 90 days after soman exposure, indicated that the size of these structures was reduced in the soman-exposed rats that received only ATS (0.5 mg/kg) and HI-6 at 1 min after exposure, but no anticonvulsant treatment. This type of pathology was not observed in the group that received LY293558 at 1 h after soman exposure (Figs. 8 and 9). Thus, at 30 days post-exposure, the size of the amygdala in the soman-exposed rats was 10.13 ± 0.49 μm3 (n = 8), while the control size was 11.86 ± 0.27 mm3 (n = 8, p = 0.008); in the soman-exposed rats treated with LY293558, the size of the amygdala (11.72 ± 0.3; n = 8; p = 0.82) did not differ from the control (Fig 8D; F(1,21) = 10.29; p = 0.001). At 90 days post-exposure, the size of the amygdala in the soman group was 12.10 ± 0.32 mm3 (n = 8), which was still significantly smaller than the control (p = 0.01); in the LY293558-treated group the size of the amygdala (11.98 ± 0.27, n = 8, p = 0.713) did not differ from the control (Fig. 8E; (F(1,21)=11.34; p < 0.001). The size of the hippocampus at 30 days post-exposure was 48.4 ± 2.2 mm3 in the soman-exposed group (n = 8) versus 62.5 ± 1.7 mm3 in the control group (n = 8, p = 0.01); in the soman-exposed rats treated with LY293558, the size of the hippocampus (59.8 ± 2.3, n = 8, p = 0.09) did not differ from the control (Fig. 9D; F(1,21)=12.53; p = 0.001). At 90 days post-exposure, the hippocampus was still significantly smaller in the soman group (41.2 ± 2.1, n = 8) compared to control (p = 0.01); in the LY293558-treated group the size of the hippocampus (62.4 ± 2.6, n = 8, p = 0.08) again did not differ from the control (Fig. 9E; F(1,21)=11.92; p < 0.001).
Figure 8. A reduction in amygdalar volume, 30 and 90 days after soman exposure, is prevented by LY293558 treatment.
A, B, C. Tracings of the amygdala in series of slices (left) and representative photomicrographs (right) from control animals (A, n = 8), soman-exposed animals that received only ATS (0.5 mg/kg) and HI-6 at 1 min post-exposure (B, n = 8), and soman-exposed animals that received LY293558 (20 mg/kg) at 1 h after soman injection (C, n = 8). D. Group data showing the estimated volume of the amygdala for all three groups, 30 days after the exposure. E. Group data showing the estimated volume of the amygdala for all three groups, 90 days after the exposure. *p < 0.05 (ANOVA, LSD post-hoc test).
Figure 9. A reduction in hippocampal volume, 30 and 90 days after soman exposure, is prevented by LY293558 treatment.
A, B, C: Tracings of the hippocampus in series of slices (left) and representative photomicrographs (right) from control animals (A, n = 8), soman-exposed animals that received only ATS (0.5 mg/kg) and HI-6 at 1 min post-exposure (B, n = 8), and soman-exposed animals that received LY293558 (20 mg/kg) at 1 h after soman injection (C, n = 8). D: Group data showing the estimated volume of the hippocampus for all three groups, 30 days after the exposure. E: Group data showing the estimated volume of the hippocampus for all three groups, 90 days after the exposure. *p < 0.05 (ANOVA, LSD post-hoc test).
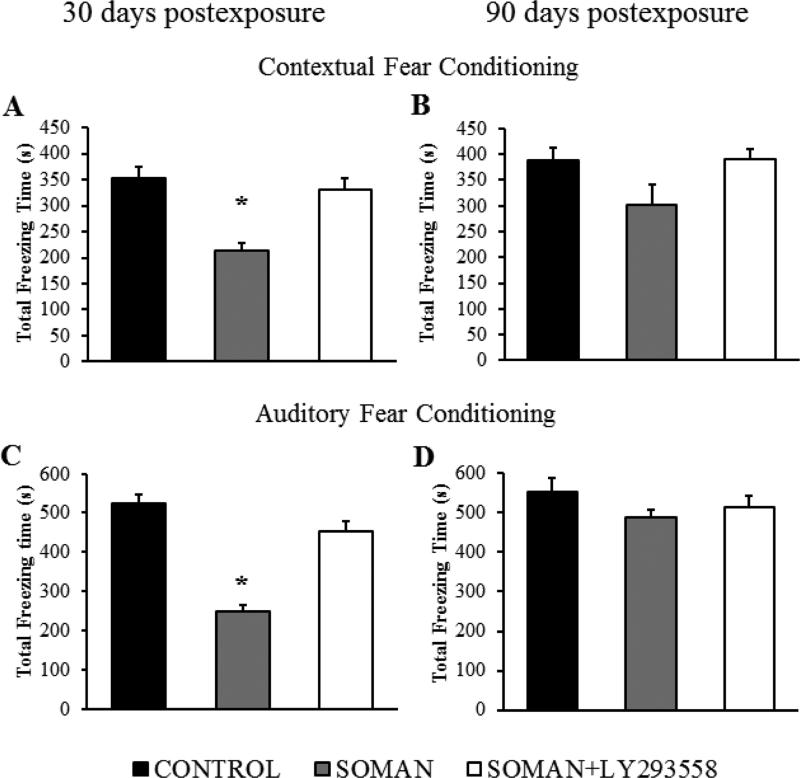
Soman-exposed immature rats display behavioral deficits 30 days post-exposure, which are prevented by LY293558 treatment
P21 rats that were exposed to soman and received only ATS (0.5 mg/kg) and HI-6 at 1 min after exposure, along with soman-exposed rats that were also treated with LY293558 at 1 h after exposure, as well as control rats, were tested for context- and cue-dependent fear conditioning. Fear conditioning took place at 30 days after exposure, and testing for context-dependent and cue-dependent acquisition of fear took place the next day and the third day after the fear-conditioning, respectively. In the context-dependent test, the total freezing time for the soman-exposed rats was 250 ± 15 s (n = 13), significantly lower than that of the control group (523 ± 24 s, n = 17, p = 0.002); for the soman-exposed rats treated with LY293558, the total freezing time (452 ± 28 s, n = 11, p = 0.07) did not differ from the control (Fig.10A; F(2,38) = 52.67; p < 0.001). In the cue-dependent fear acquisition test, the freezing time for the soman-exposed rats (214 ± 14 s) was significant lower compared to control (354 ± 20 s, p < 0.05), while for the soman-exposed rats that received LY293558, the freezing time (331 ± 21 s, p = 0.08) did not differ from the control (Fig. 10C; F(2,38)=50.98; p < 0.001). At 90 days post-exposure, there were no differences among the groups (Fig. 10B and D). Thus, the total freezing time in the context-dependent test was 489 ± 20 s for the soman-exposed group, 554 ± 35 s for the control group, and 513 ± 29 s for the soman-exposed rats treated with LY293558 (F(2,32)=2.86, p = 0.084). The total freezing time in the cue-dependent test was also similar among the groups (F(2,38)=2.90 p= 0.09). Thus, freezing time was 301 ± 40 s for the soman-exposed rats, 389 ± 25 s for the control rats, and 391 ± 19 s for the soman-exposed, LY293558-treated rats.
Figure 10. LY293558 treatment prevents impairment in fear conditioning, 30 days after soman exposure.
A and B show the total freezing time of the contextual fear-conditioned responses, 30 and 90 days after soman exposure, for the soman-exposed rats who received only ATS (0.5 mg/kg) and HI-6 at 1 min after exposure (n = 13), similarly treated rats who received also LY293558 at 1 h after soman exposure (n = 11), and controls (n = 17). C and D show the total freezing time for the auditory fear-conditioned responses, for the same groups, at 30 and 90 days after exposure. **p < 0.01, *p < 0.05 (ANOVA, Dunnett post-hoc test).
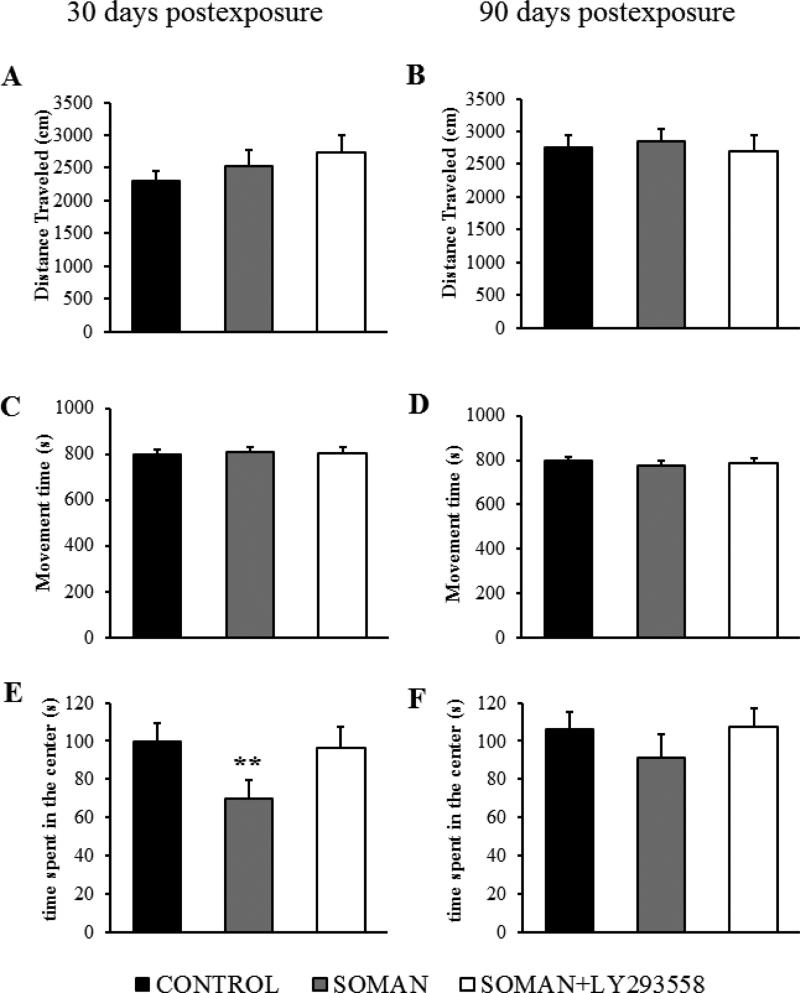
The same groups of rats were also tested in the open field. At 30 days after soman exposure the distance traveled in the open field was not significantly different (F(2,38)=1.57 p=0.221; Fig. 11A) between the soman-exposed rats (2522 ± 246 cm, n = 13), the soman-exposed rats that received LY293558 (2741 ± 254 cm, n = 11) and the controls (2293 ± 158 cm, n = 17) . However, the time spent in the center of the open field was significantly less (F(2,38)=51.57 p< 0.001) in the soman-exposed rats that did not receive anticonvulsant treatment (69.79 ± 9.87 s equivalent to 8.6 ± 1.06 % of the total movement time, n = 13, p = 0.003) compared to the time spent in the center by the control rats (99.53 ± 9.86 s equivalent to 12.51 ± 1.12 % of the total movement time, n = 17), or the soman-exposed that were treated with LY293558 (96.14 ± 11.18 s equivalent to 11.95 ± 1.08% of the total movement time, n = 11; Fig. 11E). At 90 days after exposure, the distance traveled in the open field was not significantly different (F(2,32)=1.39, p= 0.263; Fig. 11B) between the soman-exposed rats (2851 ± 193 cm, n= 11), the soman-exposed rats that received LY293558 (2698 ± 244 cm, n = 12) and the controls (2752 ± 201 cm, n = 12). The time spent in the center of the open field also did not differ significantly (F(2,32)=2.008, p= 0.151) between the soman-exposed rats that did not receive anticonvulsant treatment (91.02 ± 12.58 s equivalent to 11.7 ± 1.25 % of the total movement time, n = 11) and the control rats (106.34 ± 8.87 s equivalent to 13.36 ± 1.03% of the total movement time, n = 12), or the soman-exposed, LY293558-treated rats (107.38 ± 9.54 s equivalent to 13.68 ± 1.08% of the total movement time, n = 12, Fig. 11F).
Figure 11. LY293558 treatment prevents an increase in anxiety-like behavior, 30 days after soman exposure.
A and B show the distance travelled, C and D show the total movement time, and E and F show the time spent in the center of the open field, 30 and 90 days after exposure, for the soman-exposed rats who received only ATS (0.5 mg/kg) and HI-6 at 1 min after exposure (n = 13), similarly treated rats who received also LY293558 at 1 h after soman exposure (n = 11), and controls (n = 17). **p < 0.01 (ANOVA, Dunnett post-hoc test).
Discussion
The present study shows that 1) immature rats appear to be more susceptible to the toxicity of nerve agents, as suggested by the LD50 of soman for the P21 rats, which was lower than the LD50 for the adult rats; 2) immature rats are more prone to generating seizures after nerve agent exposure, as suggested by the time to seizure onset which was significantly shorter in the P21 rats compared to the adults; 3) as in adult rats, reduction of brain AChE activity appears to be the primary mechanism of seizure generation in the P21 rats, and the BLA plays a key role in that respect; 4) ATS, at a dose that does not affect nerve agent-induced seizures in adult rats, promptly terminates seizures in P21 rats; 5) the GluK1 antagonists LY293558 and UBP302 are effective in terminating soman-induced seizures in P21 rats, even when administered at 1 h after exposure; 6) soman-induced seizures do not cause neuronal degeneration in the brain of P21 rats, but they reduce the volume of the amygdala and the hippocampus, 30 and 90 days after exposure; 7) soman exposure results in fear-learning deficits and increased anxiety at 30 days after exposure; 8) both the brain pathology and the behavioral deficits are prevented if exposed rats are administered LY293558 at 1 h after exposure.
Since nerve agents induce seizures by inhibiting AChE, it seems reasonable to speculate that the faster onset of seizures upon exposure to soman in the P21 rats compared to adults is due to the lower concentration of the enzyme in some brain regions (Fig. 3), which may result in more rapid reduction of AChE activity in the brain. This is not a very plausible explanation, however, for two reasons: a) AChE activity was also dramatically reduced in the prelimbic cortex, the piriform cortex, and the hippocampus of the P21 rats that did not develop SE; the only difference of these rats from those who developed SE was that AChE in their BLA was reduced to a lesser extent (Fig. 4). b) AChE activity in the BLA, which appears to play a key role in the induction of seizures in the P21 rats, as in the adult rats (Prager et al., 2013), did not differ significantly between the two ages in the basal state (Fig. 3). Therefore, the shorter latency to seizure onset in the P21 rats is likely due to reasons that are not related to the baseline concentration of AChE in the brain. It is well recognized that the immature brain has a greater propensity for seizure generation regardless of the triggering stimulus, in both humans and animals (Hauser, 1994; Holmes, 2009). The reasons are not quite clear, but may include the high input resistance of neurons during development and the prevalence of gap junctions– which facilitate bursting and synchronous activity– and the immaturity of the GABAergic and glutamatergic system (Ben-Ari and Holmes, 2006; Holmes, 2009). Contribution of possible differences between P21 and adult rats in the pharmacokinetics of the injected soman must also be considered.
An unexpected finding in the present study was that ATS, at 2 mg/kg, a concentration that has no effect on nerve agent-induced seizures in adult animals (Fig. 5B and Apland et al., 2013; Figueiredo et al., 2011b; McDonough and Shih, 1997; Prager et al., 2014b; Shih and McDonough, 2000), terminated soman-induced seizures in the P21 rats. After nerve agent exposure, seizures are initiated by hyperstimulation of muscarinic receptors, as evidenced by the anti-seizure effects of muscarinic antagonists when administered shortly after exposure (Lallement et al., 1998; Shih and McDonough, 2000, 1999). In adult rats, ATS, a non-selective muscarinic antagonist, suppresses nerve agent-induced seizures, but with an ED50 of more than 50 mg/kg (Shih and McDonough, 1999). The BBB is weaker early in life (Betz and Goldstein, 1981; Matsuoka et al., 1999; Saunders et al., 2012) and appears to be more susceptible to increased capillary leakiness induced by organophosphorus agents (Song et al., 2004); this may explain the anti-seizure efficacy of relatively low concentrations of ATS in the immature rats. However, a weaker BBB in the P21 rats was not sufficient to confer efficacy to HI-6, which had no effect on seizures when administered at 20 min after exposure (Fig. 5A); it is likely though that the inefficacy of HI-6 had to do with the rapid ageing of soman-inhibited AChE (Marrs et al., 2006; Sirin et al., 2012; Talbot et al., 1988), which renders it non-reactivatable by oximes (Bajgar, 2005; Marrs et al., 2006). In the rat whole brain, the activity of choline acetyltransferase, the concentration of acetylcholine, and the number of muscarinic receptors do not mature until after the fourth postnatal week (Coyle and Yamamura, 1976). Therefore, in addition to a weaker BBB, differences between the P21 and the adult rats in the function of the muscarinic cholinergic system may be involved in the differential anticonvulsant response to ATS.
Seizures did not return after the cessation of SE by 2 mg/kg ATS, at least for the two hours that we continued observing the rats. We did not examine, in the present study, whether ATS would also be effective if administered at 1 h after exposure or at more delayed time points. However, from studies in adult rats, it has become clear that muscarinic receptors are involved only in the initiation of seizures after exposure, while it is excessive glutamatergic activity that reinforces and sustains SE (McDonough and Shih, 1997). Accordingly, soman-induced SE can be terminated by certain glutamate receptor antagonists. Specifically, LY293558, an AMPA/GluK1R antagonist (Bleakman et al., 1996; Jane et al., 2009), or UBP302, a GluK1R antagonist (More et al., 2004), terminate soman-induced seizures even when administerd at 1 h or 2 h after exposure (Apland et al., 2014; Figueiredo et al., 2011b). The efficacy of these two compounds may lie on the fact that GluK1Rs play an important role in the regulation of neuronal excitability of at least two highly seizure-prone regions, the amygdala (Apland et al., 2009; Aroniadou-Anderjaska et al., 2007, 2008; Braga et al., 2003; Rogawski et al., 2003) and the hippocampus (Carta et al., 2014; Gisabella et al., 2012; Pinheiro et al., 2013; Salmen et al., 2012); both GABAergic and glutamatergic synaptic transmission is modulated by GluK1Rs in different brain regions (Jane et al., 2009), but the net effect of GluK1R activation appears to be an increase in excitability in both the BLA (Aroniadou-Anderjaska et al., 2012) and the hippocampus (Smolders et al., 2002). During development, there are changes taking place in the distribution (Bahn et al., 1994) and the function of GluK1Rs (Segerstrale et al., 2010). Although it is unclear how the fuction of GluK1Rs may differ in the brain of a P21 rat from an adult rat, the present study shows that in P21 rats, LY293558 and UBP302 are effective in stopping soman-induced seizures when administered at 1 h after exposure, with UBP302 having a slower time course of action, as we had observed in the adult rats (Apland et al., 2014).
It is becoming increasingly understood that prolonged seizures early in life may not cause neuronal death/loss, yet, they produce long-term pathological alterations, inhibiting brain growth, modifying neuronal circuits, and leading to lasting behavioral deficits (Holmes, 2005; Holmes and Ben-Ari, 1998; Stafstrom, 2002). The present study shows that this is also the case when prolonged seizures are induced by soman exposure in P21 rats. There was no irreversible neuronal degeneration at either 24 h or 7 days post-exposure, suggesting that there was no neuronal loss. This is in contrast to the severe neuronal degeneration that is seen in young-adult rats at 24 h and 7 days after soman exposure (Apland et al., 2010, 2013, 2014; Figueiredo et al., 2011a, 2011b; Prager et al., 2013, 2014a). The resistance of immature neurons to seizure-induced degeneration and death is attributed in part to their lower vulnerability to oxidative stress (Patel and Li, 2003) and glutamate toxicity (Bickler et al., 1993; Liu et al., 1996; Marks et al., 1996). However, despite the absence of neuronal degeneration, the volumes of the amygdala and the hippocampus were significantly smaller in the P21 rats than in control rats, at 30 days and 90 days post-exposure. Considering that prolonged seizures induce neurogenesis in the hippocampus (Holmes and Ben-Ari, 1998; Parent et al., 1997) and, at least in some models, in the amygdala as well (Park et al., 2006), it may seem surprising that the volume of these structures decreased. However, significant atrophy of temporal lobe structures has also been observed after P12 and P25 rats were subjected to lithium/pilocarpine-induced SE (Kubova and Mares, 2013). Furthermore, amygdalar and hippocampal atrophy is a common pathology in temporal lobe epilepsy (Aroniadou-Anderjaska et al., 2008; Cascino et al., 1991; Cendes et al., 1993), and a smaller amygdala volume was also found in a significant number of the victims of the sarin attack in Tokyo, in 1995 (Rogers et al., 2009). It appears, therefore, that prolonged seizures or chronic recurrent seizures can result in atrophy of temporal lobe structures, and when prolonged seizures occur early in life, atrophy may ensue despite the absence of neuronal loss; in this latter case, perhaps a smaller number or thickness of synaptic connections contribute to the reduction in the total volume, and/or death of glia cells.
Abnormalities in the amygdala and the hippocampus may also explain the behavioral deficits seen at 30 days after exposure. The amygdala plays a key role in fear-conditioning (Johansen et al., 2011), and the ability for “fear-learning”, as reflected in the total freezing time, was impaired in the rats exposed to soman at P21. The amygdala, but the hippocampus as well, also play a central role in anxiety (Adhikari, 2014; Engin and Treit, 2007; Kwon and Park, 2014). A substantial number of the victims of the sarin attack in Tokyo, in 1995, exhibit symptoms of anxiety disorders, years later (Hoffman et al., 2007; Ohtani et al., 2004; Rogers et al., 2009; Yanagisawa et al., 2006), along with atrophy of the amygdala (Rogers et al., 2009). In the present study, anxiety-like behavior was increased 30 days after exposure of P21 rats to soman. These behavioral deficits were absent 2 months later, despite that there was no reversal of the amygdalar and hippocampal atrophy. It is possible that during the 2-month-period, which corresponds to more than 8 human years (Andreollo et al., 2012; Sengupta, 2013), synaptic or biochemical adjustments rectified these behavioral deficits. Others have found persistent cognitive deficits, as revealed by the Morris water-maze test, 3 months after P12 rats and P25 rats experienced lithium/pilocarpine-induced SE (Kubova and Mares, 2013). Thus, whether or not recovery will occur may depend, in addition to age, on other factors such as the nature of the behavioral deficit. For example, in adult mice exposed to soman-induced SE, increased anxiety-like behavior was still present 90 days after the exposure, but deficits in auditory and contextual fear-conditioned responses recovered during the 30 to 90 day post-exposure period (Coubard et al., 2008).
The present study provides support to the increasing evidence that prolonged seizures early in life may not be benign in long-term, even when they have not produced irreversible neuronal degeneration. The data suggest that children are likely to be more prone to developing seizures upon exposure to nerve agents, but a single injection of atropine will be sufficient to halt seizures, if administered timely. Control of seizures is necessary in order to prevent long-term brain pathologies and behavioral deficits, and administration of LY293558 is an effective anticonvulsant and neuroprotectant even when administered at 1 h after exposure. Testing of anticonvulsants at delayed time-points after nerve agent exposure in immature animal models requires timely administration of ATS at a low dose, which will alleviate peripheral toxicity and reduce mortality, without preventing or halting seizures.
Highlights.
The LD50 of soman was determined in postnatal-day-21 rats.
Rats with no seizures after 1.2XLD50 soman had less reduction of AChE in the amygdala
Atropine sulfate (ATS) at 2 mg/kg, given at 20 min after soman, blocked seizures
With ATS at 0.5 mg/kg, LY293558 or UBP302 at 1 h after exposure terminated seizures
LY293558 prevented brain pathology and behavioral deficits
Acknowledgments
We thank Dr. Cara H. Olsen for her expert advice on the procedure for the estimation of LD50. This work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant Number 5U01NS058162-07].
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests.
Contributor Information
Steven L. Miller, Email: stevenmiller17@gmail.com.
Vassiliki Aroniadou-Anderjaska, Email: vanderjaska@usuhs.edu.
Taiza H. Figueiredo, Email: taiza.figueiredo.ctr@usuhs.edu.
Eric M. Prager, Email: eric.prager683@gmail.com.
Camila P. Almeida-Suhett, Email: camilapalmeida@gmail.com.
James P. Apland, Email: james.p.apland.civ@mail.mil.
References
- Adhikari A. Distributed circuits underlying anxiety. Front. Behav. Neurosci. 2014;8:1–6. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Chemical-biological terrorism and its impact on children: a subject review. Committee on Environmental Health and Committee on Infectious Diseases. Pediatrics. 2000;105:662–670. doi: 10.1542/peds.105.3.662. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andreollo NA, Santos EF, Araujo MR, Lopes LR. Rat's age versus human's age: what is the relationship? Arg. Bras. Cir. Dig. 2012;25:49–51. doi: 10.1590/s0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MF. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience. 2009;159:380–389. doi: 10.1016/j.neuroscience.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, Qashu F, Braga MF. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J. Pharmacol. Exp. Ther. 2013;344:133–140. doi: 10.1124/jpet.112.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302. J. Pharmacol. Exp. Ther. 2014;351:359–372. doi: 10.1124/jpet.114.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology. 2010;31:485–492. doi: 10.1016/j.neuro.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience. 2012;221:157–169. doi: 10.1016/j.neuroscience.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–315. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J. Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica. 2005;48:3–21. [PubMed] [Google Scholar]
- Bajgar J. Optimal choice of acetylcholinesterase reactivators for antidotal treatment of nerve agent intoxication. Acta Medica. 2010;53:207–211. doi: 10.14712/18059694.2016.78. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- Betz AL, Goldstein GW. Developmental changes in metabolism and transport properties of capillaries isolated from rat brain. J. Physiol. 1981;312:365–376. doi: 10.1113/jphysiol.1981.sp013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Gallego SM, Hansen BM. Developmental changes in intracellular calcium regulation in rat cerebral cortex during hypoxia. J. Cereb. Blood Flow Metab. 1993;13:811–819. doi: 10.1038/jcbfm.1993.103. [DOI] [PubMed] [Google Scholar]
- Bleakman R, Schoepp DD, Ballyk B, Bufton H, Sharpe EF, Thomas K, Ornstein PL, Kamboj RK. Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahyd roisdoquinoline-3 carboxylic-acid. Mol. Pharmacol. 1996;49:581–585. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J. Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capacio BR, Byers CE, Merk KA, Smith JR, McDonough JH. Pharmacokinetic studies of intramuscular midazolam in guinea pigs challenged with soman. Drug Chem. Toxicol. 2004;27:95–110. doi: 10.1081/dct-120030727. [DOI] [PubMed] [Google Scholar]
- Carta M, Fievre S, Gorlewicz A, Mulle C. Kainate receptors in the hippocampus. Eur. J. Neurosci. 2014;39:1835–1844. doi: 10.1111/ejn.12590. [DOI] [PubMed] [Google Scholar]
- Cascino GD, Jack CR, Jr., Parisi JE, Sharbrough FW, Hirschorn KA, Meyer FB, Marsh WR, O'Brien PC. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann. Neurol. 1991;30:31–36. doi: 10.1002/ana.410300107. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coubard S, Beracochea D, Collombet JM, Philippin JN, Krazem A, Liscia P, Lallement G, Pierard C. Long-term consequences of soman poisoning in mice: part 2. Emotional behavior. Behav. Brain Res. 2008;191:95–103. doi: 10.1016/j.bbr.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Yamamura HI. Neurochemical aspects of the ontogenesis of cholinergic neurons in the rat brain. Brain Res. 1976;118:429–440. doi: 10.1016/0006-8993(76)90310-3. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav. Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol. Biochem. Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Aroniadou-Anderjaska V, Qashu F, Apland JP, Pidoplichko V, Stevens D, Ferrara TM, Braga MF. Neuroprotective efficacy of caramiphen against soman and mechanisms of its action. Br. J. Pharmacol. 2011a;164:1495–1505. doi: 10.1111/j.1476-5381.2011.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. The GluK1 (GluR5) Kainate/{alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J. Pharmacol. Exp. Ther. 2011b;336:303–312. doi: 10.1124/jpet.110.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–519. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gilat E, Kadar T, Levy A, Rabinovitz I, Cohen G, Kapon Y, Sahar R, Brandeis R. Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol. Appl. Pharmacol. 2005;209:74–85. doi: 10.1016/j.taap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Gisabella B, Bolshakov VY, Benes FM. Kainate receptor-mediated modulation of hippocampal fast spiking interneurons in a rat model of schizophrenia. PloS One. 2012;7:e32483. doi: 10.1371/journal.pone.0032483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia 35 Suppl. 1994;2:S1–6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–617. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Eisenkraft A, Finkelstein A, Schein O, Rotman E, Dushnitsky T. A decade after the Tokyo sarin attack: a review of neurological follow-up of the victims. Mil Med. 2007;172:607–610. doi: 10.7205/milmed.172.6.607. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr. Neurol. 2005;33:1–11. doi: 10.1016/j.pediatrneurol.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Holmes GL. The 2008 Judith Hoyer lecture: epilepsy in children: listening to mothers. Epilepsy Behav. 2009;16:193–202. doi: 10.1016/j.yebeh.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Ben-Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron. 1998;21:1231–1234. doi: 10.1016/s0896-6273(00)80642-x. [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP. Increasing oxime efficacy by blood-brain barrier modulation. Toxicol. Lett. 2011;206:67–71. doi: 10.1016/j.toxlet.2011.05.231. [DOI] [PubMed] [Google Scholar]
- Kubova H, Mares P. Are morphologic and functional consequences of status epilepticus in infant rats progressive? Neuroscience. 2013;235:232–249. doi: 10.1016/j.neuroscience.2012.12.055. [DOI] [PubMed] [Google Scholar]
- Kwon OY, Park SP. Depression and anxiety in people with epilepsy. J. Clin. Neurol. 2014;10:175–188. doi: 10.3988/jcn.2014.10.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Clarencon D, Masqueliez C, Baubichon D, Galonnier M, Burckhart MF, Peoc'h M, Mestries JC. Nerve agent poisoning in primates: antilethal, anti-epileptic and neuroprotective effects of GK-11. Arch. Toxicol. 1998;72:84–92. doi: 10.1007/s002040050472. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr., Wilcoxon F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian M, Tandon P, Yang Y, Hori A, Holmes GL. Age-dependent effects of glutamate toxicity in the hippocampus. Brain Res. Dev. Brain Res. 1996;97:178–184. doi: 10.1016/s0165-3806(96)00141-1. [DOI] [PubMed] [Google Scholar]
- Marks JD, Friedman JE, Haddad GG. Vulnerability of CA1 neurons to glutamate is developmentally regulated. Brain Res. Dev. Brain Res. 1996;97:194–206. doi: 10.1016/s0165-3806(96)00149-6. [DOI] [PubMed] [Google Scholar]
- Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol. Rev. 2006;25:297–323. doi: 10.2165/00139709-200625040-00009. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Okazaki M, Kitamura Y, Taniguchi T. Developmental expression of P-glycoprotein (multidrug resistance gene product) in the rat brain. J. Neurobiol. 1999;39:383–392. doi: 10.1002/(sici)1097-4695(19990605)39:3<383::aid-neu5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr., Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci. Biobehav. Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, et al. Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology. 2004;47:46–64. doi: 10.1016/j.neuropharm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Andersen JM, Nguyen NH, Aas P. Soman-induced convulsions in rats terminated with pharmacological agents after 45 min: neuropathology and cognitive performance. Neurotoxicology. 2005;26:39–48. doi: 10.1016/j.neuro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Iwanami A, Kasai K, Yamasue H, Kato T, Sasaki T, Kato N. Post-traumatic stress disorder symptoms in victims of Tokyo subway attack: a 5-year follow-up study. Psychiatry Clin Neurosci. 2004;58:624–629. doi: 10.1111/j.1440-1819.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- Padilla S, Lassiter TL, Hunter D. Biochemical measurement of cholinesterase activity. Methods Mol. Med. 1999;22:237–245. doi: 10.1385/0-89603-612-X:237. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Cho H, Kim H, Kim K. Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience. 2006;140:673–684. doi: 10.1016/j.neuroscience.2006.02.076. [DOI] [PubMed] [Google Scholar]
- Patel M, Li QY. Age dependence of seizure-induced oxidative stress. Neuroscience. 2003;118:431–437. doi: 10.1016/s0306-4522(02)00979-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Ed. 4th. Elsevier; New York, NY.: 2005. [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crepel V, Perrais D, Mulle C. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex. 2013;23:323–331. doi: 10.1093/cercor/bhs022. [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Rossetti F, Olsen CH, Braga MF. The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: relation to anxiety-like behavior. Neuropharmacology. 2014a;81:64–74. doi: 10.1016/j.neuropharm.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Figueiredo TH, Long RP, 2nd, Aroniadou-Anderjaska V, Apland JP, Braga MF. LY293558 prevents soman-induced pathophysiological alterations in the basolateral amygdala and the development of anxiety. Neuropharmacology. 2014b;89C:11–18. doi: 10.1016/j.neuropharm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler R, Auvin S. Comparison of Brain Maturation among Species: An Example in Translational Research Suggesting the Possible Use of Bumetanide in Newborn. Front. Neurol. 2013;4:36. doi: 10.3389/fneur.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Gryder D, Castaneda D, Yonekawa W, Banks MK, Lia H. GluR5 kainate receptors, seizures, and the amygdala. Ann. N. Y. Acad. Sci. 2003;985:150–162. doi: 10.1111/j.1749-6632.2003.tb07079.x. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, Kato N, Kasai K. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174:210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Salmen B, Beed PS, Ozdogan T, Maier N, Johenning FW, Winterer J, Breustedt J, Schmitz D. GluK1 inhibits calcium dependent and independent transmitter release at associational/commissural synapses in area CA3 of the hippocampus. Hippocampus. 2012;22:57–68. doi: 10.1002/hipo.20846. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell. Mol. Neurobiol. 2000;20:29–40. doi: 10.1023/A:1006991809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Segerstrale M, Juuri J, Lanore F, Piepponen P, Lauri SE, Mulle C, Taira T. High firing rate of neonatal hippocampal interneurons is caused by attenuation of afterhyperpolarizing potassium currents by tonically active kainate receptors. J. Neurosci. 2010;30:6507–6514. doi: 10.1523/JNEUROSCI.4856-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. The Laboratory Rat: Relating Its Age With Human's. Int. J. Prev. Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Efficacy of biperiden and atropine as anticonvulsant treatment for organophosphorus nerve agent intoxication. Arch. Toxicol. 2000;74:165–172. doi: 10.1007/s002040050670. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr. Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol. Biochem. Behav. 1999;64:147–153. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Sirin GS, Zhou Y, Lior-Hoffmann L, Wang S, Zhang Y. Aging mechanism of soman inhibited acetylcholinesterase. J. Phys. Chem. B. 2012;116:12199–12207. doi: 10.1021/jp307790v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O'Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, et al. Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat. Neurosci. 2002;5:796–804. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- Song X, Pope C, Murthy R, Shaikh J, Lal B, Bressler JP. Interactive effects of paraoxon and pyridostigmine on blood-brain barrier integrity and cholinergic toxicity. Toxicol. Sci. 2004;78:241–247. doi: 10.1093/toxsci/kfh076. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Assessing the behavioral and cognitive effects of seizures on the developing brain. Prog. Brain Res. 2002;135:377–390. doi: 10.1016/S0079-6123(02)35034-9. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Holmes GL. Effects of uncontrolled seizures. Neural changes in animal models. Adv. Exp. Med. Biol. 2002;497:171–194. [PubMed] [Google Scholar]
- Talbot BG, Anderson DR, Harris LW, Yarbrough LW, Lennox WJ. A comparison of in vivo and in vitro rates of aging of soman-inhibited erythrocyte acetylcholinesterase in different animal species. Drug Chem. Toxicol. 1988;11:289–305. doi: 10.3109/01480548809017884. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1127–1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Voicu VA, Bajgar J, Medvedovici A, Radulescu FS, Miron DS. Pharmacokinetics and pharmacodynamics of some oximes and associated therapeutic consequences: a critical review. J. Appl. Toxicol. 2010;30:719–729. doi: 10.1002/jat.1561. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Niquet J, Thompson KW, Baldwin R, Liu H, Sankar R, Mazarati AM, Naylor D, Katsumori H, Suchomelova L, et al. Seizure-induced neuronal death in the immature brain. Prog. Brain Res. 2002;135:335–353. doi: 10.1016/S0079-6123(02)35031-3. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J. Neurol. Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]