Abstract
To begin to delineate the psychological characteristics associated with classic 7q11.23 duplication syndrome (duplication of the classic Williams syndrome region; hereafter classic Dup7), we tested 63 children with classic Dup7 aged 4–17 years. Sixteen toddlers aged 18–45 months with classic Dup7 and 12 adults identified by cascade testing also were assessed. For the child group, median General Conceptual Ability (similar to IQ) on the Differential Ability Scales-II was 85.0 (low average), with a range from severe disability to high average ability. Median reading and mathematics achievement standard scores were at the low average to average level, with a range from severe impairment to high average or superior ability. Adaptive behavior was considerably more limited; median Scales of Independent Behavior—Revised Broad Independence standard score was 62.0 (mild impairment), with a range from severe adaptive impairment to average adaptive ability. Anxiety disorders were common, with 50.0% of children diagnosed with Social Phobia, 29.0% with Selective Mutism, 12.9% with Separation Anxiety Disorder, and 53.2% with Specific Phobia. In addition, 35.5% were diagnosed with Attention Deficit/Hyperactivity Disorder and 24.2% with Oppositional Defiant Disorder or Disruptive Behavior Disorder-Not Otherwise Specified. 33.3% of the children screened positive for a possible Autism Spectrum Disorder and 82.3% were diagnosed with Speech Sound Disorder. We compare these findings to previously reported results for children with Williams syndrome and argue that genotype/phenotype studies involving the Williams syndrome region offer important opportunities to understand the contribution of genes in this region to common disorders affecting the general population.
Keywords: 7q11.23 duplication syndrome, Williams syndrome, intellectual ability, language, psychopathology, anxiety, autism spectrum disorder, speech sound disorder, adaptive behavior
INTRODUCTION
Ever since the Williams syndrome (WS) microdeletion of chromosome 7q11.23 was determined to be caused by a non-allelic homologous recombination [Dutly and Schinzel, 1996; Urbán et al., 1996], it had been expected that there would be a syndrome caused by microduplication of the same region. However, identification of individuals with this putative syndrome was hampered by a complete absence of information about the phenotype. The first case of 7q11.23 duplication syndrome (Dup7) was found by chance, when a boy who was being tested for velocardiofacial syndrome using real time PCR was found to have duplication of the elastin gene [Somerville et al., 2005]. Subsequent FISH testing indicated that this child’s duplication corresponded to the classic WS deletion region (hereafter, classic Dup7). In the ensuing nine years, 30 children with classic Dup7 aged 6 months to 17 years have been described as part of case series [5 in Berg et al., 2007; 14 in van der Aa et al., 2009; 5 in Dixit et al., 2013; 6 in Parrott et al., 2015] and mean performance levels on various psychological assessments were reported by Sanders et al. [2011] for four children with classic Dup7. In addition, 12 children with classic Dup7 were described in individual case reports [Somerville et al., 2005; Kriek et al., 2006; Depienne et al., 2007, 2009; Torniero et al., 2007; Merritt and Lindor, 2008; Orellana et al., 2008; Torniero et al., 2008; Malenfant et al., 2012; Değerliyurt et al., 2012; McGrew et al., 2012; Prontera et al., 2014; Zarate et al., 2014]. Hence, a total of 46 children with classic Dup7 has been described in the literature. Thirteen adults with classic Dup7 also were included in case series or individual case reports. Two individuals (aged 19 and 23 years) were probands [Berg et al., 2007; Kirchhoff et al., 2007]; the remaining 11 had one or more children with classic Dup7 and were identified primarily by cascade testing [2 in Berg et al., 2007; 1 in Merritt and Lindor, 2008; 1 in Torniero et al., 2008; 6 in van der Aa et al., 2009; 1 in Parrott et al., 2015]. Several additional individuals with larger or smaller duplications of 7q11.23 have been included in some of the case series. However, given that the phenotypes of individuals with shorter [e.g., Morris et al., 2003] or longer [e.g., Stock et al., 2003] deletions of the WS region differ in important ways from those of individuals with classic WS deletions, we expect that the phenotypes associated with shorter or longer duplications of the WS region also may differ from that of classic duplication of the WS region. Accordingly, in this manuscript we consider only individuals who have classic Dup7 and do not have any other reported copy number variant (CNV) that has been associated with intellectual disability.
Prior case reports have been instrumental in identifying characteristics that may be part of the classic Dup7 psychological phenotype [summarized in, e.g., Osborne and Mervis, 2007; van der Aa et al., 2009; Velleman and Mervis, 2011]. However, as shown in Table I, in most cases the specific information regarding performance on various types of psychological assessments needed to describe adequately both the typical presentation of the psychological phenotype and the variability among individuals with classic Dup7 is lacking, in part because information on the psychological phenotype often was derived from medical records. For intellectual ability/disability and language ability/disability, only a broad category (e.g., “normal,” “developmental/language delay,” “mild/moderate/severe disability”) was typically reported. Adaptive behavior was rarely mentioned for children and when described for adults was typically limited to employment information. Each of the behavioral characteristics considered for inclusion in the phenotype (presence or absence of anxiety, attention problems, and/or aggression/oppositional behavior) was mentioned for at most half the sample, with standardized assessment even in the form of a standardized questionnaire completed by a parent or teacher reported for fewer than 25%. The presence or absence of an autism spectrum disorder (ASD) or autistic features was mentioned for about half of the children and formally evaluated for about a third. The presence or absence of speech concerns (not addressed in Table I) was reported for only 20% of the children < 4 years old, 60% of children aged 4–17 years, and 31% of adults, with a formal diagnosis provided for only 11% of children aged 4–17 years (1 oromotor apraxia, 3 Childhood Apraxia of Speech) and 8% of adults (1 Dysarthria).
TABLE I.
Level of Information Provided Regarding Phenotypic Characteristics of Individuals With Classic Dup7 Reported in Prior Literature
| Level of Information Provideda |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 4 Years (N = 10b) | 4–17 Years (N = 35) | Adult (N = 13) | |||||||
|
|
|||||||||
| Characteristic | Not Addressed |
Class. or Description Only |
Standard. Assessmentc |
Not Addressed |
Class. or Description Only |
Standard. Assessmentc |
Not Addressed |
Class. or Description Only |
Standard. Assessmentc |
| Intellectual ability/disability | 30.0 | 60.0 | 10.0 | 5.7 | 62.9 | 31.4 | 53.8 | 46.2 | 0.0 |
| Language ability/disability | 10.0 | 90.0 | 0.0 | 20.0 | 62.9 | 17.1 | 53.8 | 46.2 | 0.0 |
| Adaptive behavior | 100.0 | 0.0 | 0.0 | 80.0 | 8.6 | 11.4 | 53.8 | 46.2 | 0.0 |
| Anxiety (present or absent) | 80.0 | 20.0 | 0.0 | 65.7 | 20.0 | 14.3 | 69.2 | 30.8 | 0.0 |
| Attention problems (present or absent) |
90.0 | 0.0 | 10.0 | 48.6 | 28.6 | 22.9 | 92.3 | 7.7 | 0.0 |
| Aggression/oppositional behavior (present or absent) |
90.0 | 0.0 | 10.0 | 62.9 | 20.0 | 17.1 | 84.6 | 15.4 | 0.0 |
| Autism spectrum disorder (present, absent, autistic features) |
50.0 | 40.0 | 10.0 | 42.9 | 25.7 | 31.4 | 69.2 | 30.8 | 0.0 |
Note: Class. = Classification, Standard. = Standardized
Cell values are percentages; slight deviations of the sum of percentages for a particular age group in a single row from 100 are due to rounding.
One additional participant (aged 6 mos.) was excluded because s/he was too young to be evaluated for most of these characteristics. S/he was described as having “global developmental delay.”
For intellectual ability/disability, language ability/disability, and adaptive behavior, a child was included in this category if at least one standard score from an appropriate assessment was provided. For anxiety, attention problems, and aggression/oppositional behavior, a child was included in this category if at least one T-score from an appropriate assessment (in all cases, a standardized questionnaire completed by a parent or a teacher) was provided or the authors indicated that the child’s classification as demonstrating/not demonstrating this characteristic was based on his/her T-score from such an assessment. For autism spectrum disorder, a child was included in this category if s/he completed the Autism Diagnostic Observation Schedule [Lord et al., 1999] or Autism Diagnostic Observation Schedule-2 [Lord et al., 2012] and/or a parent completed the Autism Diagnostic Interview – Revised [Lord et al., 1994] (11 of the 12 children) or if a parent or teacher completed a standardized questionnaire for autistic symptoms (1 child).
A specific, objective description of the psychological phenotype associated with classic Dup7, including both the typical presentation for each characteristic and the variability associated with the characteristic, is important for providing information both to professionals working with individuals who have this syndrome and to families. In addition, as individuals are identified who have duplications of 7q11.23 that include either a subset of the genes included in classic Dup7 or additional genes, this characterization of the classic Dup7 psychological phenotype will provide a basis for genotype/phenotype studies. The primary purpose of the present report is to provide a detailed description of the psychological phenotype of children with classic Dup7 based on direct assessment of a relatively large group of children aged 4–17 years with this syndrome. Assessment results for a smaller group of children aged 18–45 months and a small group of adults with classic Dup7 identified by cascade testing also are provided. The protocol for this study was prospectively reviewed and approved by the University of Louisville Institutional Review Board.
MATERIALS AND METHODS
Participants
The primary group of participants was composed of 63 children (27 girls, 36 boys) aged 4.01–17.76 years (mean : 8.80 years, SD : 3.68, median : 8.01). All children had classic Dup7 (duplication of the 25–27 protein coding genes in the classic WS region) with no additional known pathogenetic findings. Almost all of the children were diagnosed by genetic microarray; in all cases we used FISH or qPCR to confirm that the duplications were classic. The duplication origin was de novo for 38 children (60.3%), inherited from the mother for 10 (15.9%), inherited from the father for 5 (7.9%), and unknown for 10 (15.9%). Of the 63 participants, 58 were probands and 5 were siblings identified by cascade testing. Fifty-nine children lived with one or both biological parents, 3 lived with adoptive families, and 1 lived with a legal guardian. A few of the children participated in the research more than once; for these children, the data from the most recent assessment were used in the analyses.
A second group was composed of 16 toddlers and young preschoolers (11 girls, 5 boys) aged 18.33–45.57 months (mean : 28.93 months, SD : 8.35, median : 26.50). All children were confirmed to have classic Dup7 by either FISH or qPCR and did not have any other known pathogenetic findings. The duplication was de novo for 11 children (68.8%), inherited from the mother for 2 (12.5%), and inherited from the father for 3 (18.7%). Fourteen of the children were probands and 2 were identified by cascade testing. All children lived with one or both biological parents. A few children were tested more than once between ages 18 and 45 months; in these cases, the data from the initial assessment were included in the analyses. Eight of the 16 children also were included in the primary assessment group, at an older age.
The third group included 12 adults (7 females, 5 males) aged 27.47–61.05 years (mean : 36.39 years, SD : 9.13, median : 34.55) with classic Dup7, all of whom were identified by cascade testing. The duplication was confirmed in the same manner as for the other groups. The adult group included 6 mothers of probands, 5 fathers, and 1 grandmother. The origin of the duplication was de novo for 1 individual (8.3%), inherited from the mother for 2 (16.7%), inherited from the father for 1 (8.3%), and unknown for 8 (66.7%). Four additional adults with classic Dup7 who were identified by cascade testing were not included. One individual (grandfather) indicated that he was too busy to participate and three (1 father, 1 mother, 1 grandmother) had additional medical conditions that are known to affect intellectual ability (closed head injury, neurofibromatosis type 1, severe hypothyroidism).
Measures
Unless otherwise specified, the mean standard score (SS) for the general population for both the subtests and overall performance on the standardized assessments listed below is 100, with a SD of 15.
Intellectual Ability
The Differential Ability Scales 2nd edition [DAS-II; Elliott, 2007] was administered to children aged 4–17 years. This assessment provides specific information about strengths and weaknesses across a wide range of intellectual abilities. The Early Years form was administered to children aged 4–8 years and the School Age form to children aged 9–17 years. Both forms include six core subtests divided into three clusters of two subtests each: Verbal, Nonverbal Reasoning, and Spatial. The General Conceptual Ability (GCA; similar to IQ) is determined based on performance on the six core subtests. Children aged 7–17 years also completed the supplemental Working Memory and Processing Speed clusters.
The Mullen Scales of Early Learning [MSEL; Mullen, 1995] was administered to children aged 18–45 months. The MSEL includes four scales: Visual Reception (measuring primarily nonverbal reasoning), Fine Motor (measuring primarily visuospatial construction), Receptive Language, and Expressive Language. For each scale, mean T-score for the general population is 50 with a SD of 10. The Early Learning Composite (ELC; similar to DQ) is based on performance on the four scales.
The Wechsler Abbreviated Scale of Intelligence [WASI; Wechsler, 1999] was administered to the adults. The WASI provides a Verbal IQ, a Performance IQ, and a full-scale IQ.
Vocabulary Ability
The Peabody Picture Vocabulary Test 4th edition [PPVT-4; Dunn & Dunn, 2007] measures receptive single-word vocabulary. The Expressive Vocabulary Test 2nd edition [EVT-2; Williams, 2007], measures expressive single-word vocabulary.
Academic Achievement
Five subtests from the Wechsler Individual Achievement Test 3rd edition [WIAT-III; Wechsler, 2009] were used to assess reading and mathematics achievement.
Adaptive and Maladaptive Behavior
The parent interview form of the Scales of Independent Behavior-Revised [SIB-R; Bruininks et al., 1996] was used to measure the adaptive behavior of children aged 4–17 years. The SIB-R includes four clusters: Motor Skills, Social Interaction and Communication Skills, Personal Living Skills, and Community Living Skills. The Broad Independence SS is based on performance on all four clusters.
The SIB-R also assesses three types of maladaptive behavior: Internalized (behavior harmful to the child, unusual or repetitive behavior, withdrawal or inattentive behavior), Asocial (socially offensive behavior, uncooperative behavior), and Externalized (behavior that is hurtful to other people or to animals, behavior that damages or destroys property, disruptive behavior). The Generalized Maladaptive Index is based on performance on all three types of maladaptive behavior. For the Maladaptive indices, mean scaled score is 0, with a SD of 10 for clinical groups.
Psychopathology
Parents of children aged 4–17 years completed the Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule [ADIS-P; Silverman and Albano, 1996], a semi-structured interview designed to assess for current anxiety and related disorders (including externalizing disorders) in children and adolescents. Adults completed the Anxiety Disorders Interview Schedule Adult Version [ADIS-IV; Brown et al., 1996], a semi-structured interview designed to assess for current anxiety and related disorders in adults.
Autism Spectrum Disorder Screening
Parents of children aged 4–17 years completed the Social Communication Questionnaire [SCQ; Berument et al., 1999; Rutter et al., 2003], an ASD screening measure. The SCQ is based on the Autism Disorder Interview-Revised [ADI-R; Lord et al., 1994].
Speech Sound Disorder
Children aged 4–17 years were evaluated for Speech Sound Disorder, using DSM-5 criteria. The DSM-5 Speech Sound Disorder diagnosis includes both articulation disorders (motor speech disorders such as Childhood Apraxia of Speech or dysarthria) and phonological disorders (cognitive-linguistic disorders reflecting inaccurate or incomplete phonological representations or inappropriate phonological rules). Diagnostic decisions were made by a certified and licensed speech-language pathologist with extensive training and experience with children who have severe speech sound disorder. Decisions were based on video recordings of standardized speech assessments and expressive language sections of standardized language assessments and on narrow phonetic transcription of spontaneous speech samples. Diagnostic decisions for 47 participants also were made by another highly experienced certified and licensed speech-language pathologist based on the same materials; agreement on whether or not the participant had a speech sound disorder was 100%.
RESULTS
The distribution of SSs was non-normal for components of several of the assessments administered. For this reason, nonparametric statistics were used throughout the Results section.
Intellectual Abilities
Descriptive statistics for performance on the three measures of overall intellectual ability are provided in Table II and ability-level classifications for SSs and T-scores are presented in Table III. All 63 children completed the DAS-II core subtests, allowing for the computation of overall intellectual ability (GCA; similar to IQ) and Verbal, Nonverbal Reasoning, and Spatial cluster SSs. As indicated in Table II, median SSs were in the low average range for GCA and all three core clusters. As shown in Table III, score classifications varied from severe disability to high average with the majority of children classified in the low average or average range. Correlations among the core cluster SSs were large and significant: for Verbal SS and Nonverbal Reasoning SS, rs = 0.73 (P < 0.0001); for Verbal SS and Spatial SS, rs = 0.57 (P < 0.0001); and for Nonverbal Reasoning SS and Spatial SS, rs = 0.70 (P < 0.0001). At the group level, the distribution of SSs did not differ significantly for the three core cluster SSs (Friedman test, P = 0.21). At the individual level, the modal pattern of relations among the three core cluster SSs matched the finding at the group level. However, this pattern of no significant differences among a child’s Verbal, Nonverbal Reasoning, and Spatial SSs was shown by only 25 of the 63 children (39.7%). Each of the remaining children evidenced at least one significant difference in SSs for a pair of core clusters; 14 different patterns of relations among core cluster SSs were found with no single pattern evidenced by more than 5 children (7.9%). For some children there were large discrepancies among cluster SSs, with differences between the highest and lowest SSs of 30 or more points.
TABLE II.
Descriptive Statistics for Measures of Intellectual Ability
| Measure | N | Mean | Median | SD | Range |
|---|---|---|---|---|---|
| Differential Ability Scales-IIa | |||||
| General Conceptual Ability (GCA; similar to IQ) | 63 | 82.05 | 85.0 | 17.66 | 33 – 118 |
| Verbal Cluster SS | 63 | 84.14 | 88.0 | 20.42 | 30 – 120 |
| Nonverbal Reasoning Cluster SS | 63 | 86.64 | 87.0 | 15.38 | 39 – 127 |
| Spatial Cluster SS | 63 | 83.02 | 86.0 | 18.28 | 34 – 115 |
| Working Memory Cluster SS | 35 | 81.80 | 83.0 | 20.45 | 35 – 116 |
| Processing Speed Cluster SS | 35 | 82.40 | 86.0 | 14.38 | 46 – 105 |
| Mullen Scales of Early Learningb | |||||
| Early Learning Composite (ELC; similar to DQ) | 16 | 80.88 | 82.0 | 16.28 | 50 – 110 |
| Visual Reception T score | 16 | 44.81 | 46.5 | 9.12 | 25 – 60 |
| Fine Motor T score | 16 | 37.13 | 38.0 | 10.20 | 20 – 58 |
| Receptive Language T score | 16 | 44.94 | 47.0 | 12.74 | 20 – 63 |
| Expressive Language T score | 16 | 31.94 | 30.5 | 10.88 | 20 – 54 |
| Wechsler Abbreviated Scale of Intelligencec | |||||
| Full Scale IQ | 12 | 92.50 | 92.0 | 8.30 | 77 – 103 |
| Verbal IQ | 12 | 86.92 | 87.0 | 10.94 | 71 – 104 |
| Performance IQ | 12 | 100.92 | 101.0 | 9.83 | 89 – 115 |
For the general population, mean = 100 and SD = 15. Lowest possible SS is 30 for GCA, Verbal SS, and Processing Speed SS; 31 for Nonverbal SS; 32 for Spatial SS, and 33 for Working Memory SS.
For the general population, mean ELC = 100 and SD = 15. Lowest possible ELC is 49. Mean T-score is 50 and SD = 10; lowest possible T-score is 20.
For the general population, mean IQ = 100 and SD = 15. Lowest possible SS is 50 for Full-Scale IQ, 53 for Performance IQ, and 55 for Verbal IQ.
TABLE III.
Ability-Level Classification on Measures of Intellectual Ability
| Measure | Ability Classificationa,b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Severe Disability |
Moderate Disability |
Mild Disability |
Borderline | Low Average |
Average | High Average |
Superior | |
| Differential Ability Scales-II | ||||||||
| GCA | 4.8 | 3.2 | 9.5 | 20.6 | 27.0 | 31.7 | 3.2 | 0 |
| Verbal Cluster SS | 6.3 | 3.2 | 4.8 | 15.9 | 23.8 | 41.3 | 3.2 | 1.6 |
| Nonverbal Reasoning SS | 1.6 | 1.6 | 6.3 | 20.6 | 27.0 | 36.5 | 3.2 | 3.2 |
| Spatial SS | 6.3 | 3.2 | 11.1 | 6.3 | 31.7 | 39.7 | 1.6 | 0 |
| Working Memory SS | 2.9 | 8.6 | 14.3 | 16.7 | 14.3 | 37.1 | 5.7 | 0 |
| Processing Speed SS | 0 | 5.7 | 14.3 | 16.7 | 25.8 | 37.1 | 0 | 0 |
| Mullen Scales of Early Learningc | ||||||||
| ELC | – | 6.3 | 18.8 | 18.8 | 37.5 | 12.5 | 6.3 | 0 |
| Visual Reception T | – | 0 | 6.3 | 12.5 | 12.5 | 62.5 | 6.3 | 0 |
| Fine Motor T | – | 6.3 | 18.8 | 25.0 | 18.8 | 25.0 | 6.3 | 0 |
| Receptive Language T | – | 12.5 | 6.3 | 0 | 12.5 | 56.3 | 12.5 | 0 |
| Expressive Language T | – | 31.3 | 6.3 | 31.3 | 18.8 | 12.5 | 0 | 0 |
| Wechsler Abbreviated Scale of Intelligencec | ||||||||
| Full Scale IQ | – | 0 | 0 | 8.3 | 41.7 | 50.0 | 0 | 0 |
| Verbal IQ | – | 0 | 0 | 25.0 | 41.7 | 33.3 | 0 | 0 |
| Performance IQ | – | 0 | 0 | 0 | 25.0 | 50.0 | 25.0 | 0 |
Cell values are percentages; slight deviations of the sum of percentages in a single row from 100 are due to rounding.
SS and T ranges for ability classifications: Severe disability (SS < 40), moderate disability (SS : 40–54, T : 20), mild disability (SS : 55–69, T : 21–29), borderline (SS : 70–79, T : 30–36), low average (SS : 80–89, T : 37–43), average (SS : 90–109, T : 44–56), high average (SS : 110–119, T : 57–63), superior (SS : ≥ 120, T : ≥ 64).
The MSEL and the WASI are not normed low enough to differentiate between moderate and severe disability. Thus, some of the individuals in the “moderate disability” classification on these measures may actually have severe disability.
Thirty-five of the 37 children aged 7–17 years completed the subtests in the Working Memory and Processing Speed clusters. Median SS was in the low average range for both clusters (Table II). As indicated in Table III, score classification ranged from severe disability to high average for the Working Memory cluster and from moderate disability to average for the Processing Speed cluster. Modal classification was in the average range for both clusters. The correlation between Working Memory cluster SS and Processing Speed cluster SS was marginally significant (rs = 0.33, P = 0.052). Correlations between GCA and these cluster SSs were large and significant: for GCA and Working Memory, rs = 0.79 (P < 0.0001); for GCA and Processing Speed, rs = 0.51 (P = 0.002). At the group level, the distribution of SSs did not differ significantly among the GCA, Working Memory cluster, and Processing Speed cluster (Friedman test, P = 0.10). However, the pattern of no significant difference between the Working Memory cluster SS and Processing Speed cluster SS was shown by only 12 of the 35 children (34.3%); for 13 children (37.1%) Working Memory cluster SS was significantly lower than Processing Speed cluster SS and for 10 children (28.6%) Working Memory cluster SS was significantly higher than Processing Speed cluster SS. Working Memory cluster SS was within the range expected for GCA for 16 children (45.7%), significantly higher than expected for 8 (17.1%), and significantly lower than expected for 13 (37.1%). Processing Speed cluster SS was within the range expected for GCA for 19 children (54.3%), significantly higher than expected for 5 (14.3%), and significantly lower than expected for 11 (31.4%).
The 16 children aged 18–45 months all completed the MSEL. Median ELC was at the low average level (Table II), with a range from moderate disability (lowest possible classification for the MSEL) through high average (Table III). Median T scores were in the average range for the Visual Reception and Receptive Language scales, in the low average range for the Fine Motor scale, and in the borderline range for the Expressive Language scale. T-score distributions for each scale are indicated in Table III. A Friedman test indicated that the T-score distributions for the four scales differed significantly (P < 0.0001); post hoc comparisons indicated that the T-score distribution was significantly lower for the Expressive Language scale than for either the Receptive Language scale (adjusted P = 0.001) or the Visual Reception scale (adjusted P = 0.01). Correlations between T-scores were large and significant for Receptive Language and Fine Motor, rs = 0.69 (P = 0.003); Expressive Language and Fine Motor, rs = 0.64 (P = 0.007); Receptive Language and Visual Reception, rs = 0.59 (P = 0.017); and Receptive Language and Expressive Language, rs = 0.51 (P = 0.042). The correlation between T-scores for Expressive Language and Visual Reception was medium and not significant, rs = 0.45 (P = 0.08), as was the correlation between T-scores for Visual Reception and Fine Motor, rs = 0.39 (P = 0.13).
The 12 adults completed the WASI. As indicated in Table II, median SSs were in the average range for both Full Scale IQ and Performance IQ and in the low average range for Verbal IQ. Verbal IQs were relatively evenly divided among the borderline, low average, and average classifications. Performance IQs were in the low average through high average classifications (Table III). A Wilcoxon test indicated that at the group level, median Performance IQ was significantly higher than median Verbal IQ (P = 0.015). At the individual level, Performance IQ was significantly higher than Verbal IQ for 7 participants, Verbal IQ was significantly higher than Performance IQ for 1 participant, and Verbal IQ and Performance IQ were equivalent for 4 participants.
Vocabulary Ability
All 63 child participants and 11 of the 12 adult participants completed both the PPVT-4 and the EVT-2. Descriptive statistics for both measures are reported in Table IV, separately for children and adults. For the children, median SS was in the average range for both the PPVT-4 and the EVT-2. For the adults, median SS was in the average range for the PPVT-4 and the low average range for the EVT-2. The variability among SSs was much higher for the child group (severe disability through superior) than for the adult group (mild disability through average for receptive vocabulary, average for expressive vocabulary). Wilcoxon tests conducted to compare participants’ performance on the PPVT-4 and EVT-2 indicated that the median of the differences between the PPVT-4 and EVT-2 SSs was not significantly different from 0 for either the child group (P = 0.67) or the adult group (P = 0.96). At the individual level, PPVT-4 SS was equivalent to EVT-2 SS for 46 children (73.0%), significantly higher than EVT-2 SS for 12 (19.0%), and significantly lower than EVT-2 SS for 5 (8.0%). For the adults, PPVT-4 SS was equivalent to EVT-2 SS for 8 (72.7%), significantly higher than EVT-2 SS for 1 (9.1%), and significantly lower than EVT-2 SS for 2 (18.2%). PPVT-4 and EVT-2 SSs were strongly correlated for both children (rs = 0.74, P < 0.0001) and adults (rs = 0.61, P = 0.049).
TABLE IV.
Descriptive Statistics for Measures of Vocabulary Ability
| Age Group and Measure | N | Mean | Median | SD | Range |
|---|---|---|---|---|---|
| Children | |||||
| Peabody Picture Vocabulary Test-4a | 63 | 91.51 | 95.0 | 19.73 | 20 – 120 |
| Expressive Vocabulary Test-2a | 63 | 87.21 | 93.0 | 23.17 | 20 – 118 |
| Adults | |||||
| Peabody Picture Vocabulary Test-4a | 11 | 88.27 | 90.0 | 12.02 | 67 – 107 |
| Expressive Vocabulary Test-2a | 11 | 88.73 | 89.0 | 8.58 | 79 – 104 |
For the general population, mean = 100 and SD = 15. Lowest possible SS = 20.
Academic Achievement
Of the 37 children aged 7–17 years, 33 were tested after the WIAT-III was released. For these children, median DAS-II GCA was 88.0 (mean = 86.70, SD = 14.23, range = 59–118). Descriptive statistics for their performance on three reading subtests and two mathematics subtests are presented in Table V. Performance was quite variable, with SDs well above the general population value of 15. Median SS was in the average range for each of the three reading subtests, with performance classification ranging from mild disability to superior for Word Reading and Pseudoword Decoding and from moderate disability (lowest possible classification on the WIAT-III) to superior for Reading Comprehension. Median SS for the two mathematics subtests was in the low average range, with performance ranging from moderate disability (lowest possible classification for this measure) to superior. Performance on all five achievement subtests was very strongly related to overall intellectual ability (DAS-II GCA) with rs ranging from 0.67 for both Reading Comprehension and Numerical Operations to 0.82 for Math Problem Solving (all Ps < 0.0001). Spearman correlations among achievement test SSs also were quite high, with rs ranging from 0.61 for the correlation between Reading Comprehension SS and Numerical Operations SS to 0.86 for the correlation between Word Reading SS and Math Problem Solving SS and 0.94 for the correlation between Word Reading SS and Pseudoword Decoding SS (all Ps < 0.0001).
TABLE V.
Descriptive Statistics for Wechsler Individual Achievement Test-III Subtests
| WIAT-III Subtesta | N | Mean | Median | SD | Range |
|---|---|---|---|---|---|
| Reading | |||||
| Word Reading SS | 32b | 90.91 | 91.0 | 19.31 | 57 – 132 |
| Pseudoword Decoding SS | 32b | 93.19 | 95.5 | 20.76 | 59 – 128 |
| Reading Comprehension SS | 33 | 89.91 | 90.0 | 17.67 | 40 – 128 |
| Mathematics | |||||
| Numerical Computation SS | 33 | 84.18 | 84.0 | 21.59 | 40 – 132 |
| Math Problem Solving SS | 33 | 82.03 | 82.0 | 20.02 | 40 – 127 |
For the general population, mean = 100, SD = 15. Lowest possible SS = 40.
One participant did not complete the Word Reading and Pseudoword Decoding subtests because she was selectively mute. She wrote her responses to the items on the Reading Comprehension, Numerical Computation, and Math Problem Solving subtests.
Adaptive and Maladaptive Behavior
The parents of all 63 children aged 4–17 years completed the SIB-R interview. Descriptive statistics for adaptive behavior SSs are presented in Table VI. Median Broad Independence SS was in the mild adaptive disability range, with median SSs in the borderline range for all four adaptive behavior clusters. The variability among children was quite high, with all SDs well above the general population value of 15. The distribution of adaptive-skill classifications is reported in Table VII. Despite identical or very similar median SSs, the distribution of SSs differed significantly among the adaptive behavior clusters (Friedman test, P < 0.0001). Post-hoc comparisons indicated that the Social Interaction & Communication Skills cluster SS distribution was significantly higher than the distribution for Community Living Skills (adjusted P < 0.0001) and also was higher than for Motor Skills (adjusted P = 0.052) and Personal Living Skills (adjusted P = 0.052). Descriptive statistics for the SIB-R maladaptive indices also are presented in Table VI. The median score for the General Maladaptive Index was in the mild disorder range as were the median scores for the Internalized and Asocial Maladaptive Indices. The median score for the Externalized Maladaptive Index was in the high-normal range. The distribution of maladaptive difficulty classifications is reported in Table VIII. For the General Maladaptive Index, the Internalized Maladaptive Index, and the Asocial Maladaptive Index, the performance of most children was classified in the moderate difficulty to high-normal categories. For the Externalized Maladaptive Index, the behavior of most children was classified in the mild difficulty to low-normal range. However, it is important to note that the behavior of 14% was classified in the severe to extreme difficulty range. The results of a Friedman test indicated that the distributions of scores on the maladaptive indices differed significantly (P < 0.001). Post-hoc comparisons indicated that the distribution of the Internalized Maladaptive Index scores differed significantly from the distribution of the Externalized Maladaptive Index scores (adjusted P < 0.001).
TABLE VI.
Descriptive Statistics for Scales of Independent Behavior-Revised
| Measure | N | Mean | Median | SD | Range |
|---|---|---|---|---|---|
| Adaptive Behaviora | |||||
| Broad Independence SS | 63 | 63.22 | 62.0 | 18.34 | 30 – 107 |
| Motor Skills SS | 63 | 68.63 | 72.0 | 18.87 | 30 – 115 |
| Social Interaction and Communication Skills SS | 63 | 72.62 | 72.0 | 19.21 | 30 – 110 |
| Personal Living Skills SS | 63 | 67.24 | 72.0 | 20.21 | 30 – 109 |
| Community Living Skills SS | 63 | 65.44 | 70.0 | 19.46 | 30 – 98 |
| Maladaptive Behaviorb | |||||
| General Maladaptive Index | 63 | −20.98 | −19.0 | 12.72 | −54 – −2 |
| Internalized Maladaptive Index | 63 | −19.70 | −20.0 | 12.15 | −46 – 4 |
| Asocial Maladaptive Index | 63 | −14.27 | −14.0 | 10.84 | −39 – 8 |
| Externalized Maladaptive Index | 63 | −10.84 | −6.0 | 14.53 | −50 – 10 |
For the general population, mean = 100, SD = 15. Lowest possible SS set at 30 to match lowest possible DAS-II GCA. (The SIB-R allows SSs of 0.)
For the general population, mean = 0. SD = 10 for clinical groups.
TABLE VII.
Adaptive-Behavior Skill Classification for Performance on the Scales of Independent Behavior-Revised (N = 63)
| Adaptive-Skill Classificationa,b |
|||||||
|---|---|---|---|---|---|---|---|
| Adaptive Behavior Component | Severe Disability |
Moderate Disability |
Mild Disability |
Borderline | Low Average | Average | High Average |
| Broad Independence | 11.1 | 25.4 | 22.2 | 25.4 | 7.9 | 7.9 | 0 |
| Motor Skills | 6.3 | 11.1 | 28.6 | 27.0 | 17.5 | 7.9 | 1.6 |
| Social lnteraction & Communication Skills | 4.8 | 12.7 | 23.8 | 17.5 | 20.6 | 19.0 | 1.6 |
| Personal Living Skills | 9.5 | 23.8 | 12.7 | 23.8 | 20.6 | 9.5 | 0 |
| Community Living Skills | 14.3 | 14.3 | 19.0 | 28.6 | 14.3 | 9.5 | 0 |
Cell values are percentages; slight deviations of the sum of percentages in a single row from 100 are due to rounding .
SS for adaptive behavior classifications: Severe disability (SS < 40), moderate disability (SS : 40–54), mild disability (SS : 55–69), borderline (SS : 70–79), low average (SS : 80–89), average (SS : 90–109), high average (SS : 110–119).
TABLE VIII.
Maladaptive Behavior Difficulty-Level Classification for Performance on the Scales of Independent Behavior-Revised (N = 63)
| Maladaptive Behavior Difficulty Levela,b |
||||||
|---|---|---|---|---|---|---|
| Maladaptive Behavior Component | Extreme | Severe | Moderate | Mild | High-normal | Low-normal |
| General Maladaptive Index | 9.5 | 9.5 | 27.0 | 33.3 | 20.6 | 0 |
| Internalized Maladaptive Index | 4.8 | 12.7 | 31.7 | 27.0 | 15.9 | 7.9 |
| Asocial Maladaptive Index | 0 | 6.3 | 22.2 | 30.2 | 30.2 | 11.1 |
| Externalized Maladaptive Index | 1.6 | 12.7 | 6.3 | 23.8 | 25.4 | 30.2 |
Cell values are percentages; slight deviations of the sum of percentages in a single row from 100 are due to rounding.
Maladaptive index scores for behavior difficulty classifications: Extreme (< −40), Severe −40 to −31), Moderate (−30 to −21), Mild (−20 to −11), High-normal (−10 to −1), Low-normal (0–10).
To assess relations among adaptive behavior SSs, maladaptive behavior index scores, and overall intellectual ability (DAS-II GCA), Spearman correlations were computed. SIB-R Broad Independence SS was strongly correlated with DAS-II GCA (rs = 0.64, P < 0.0001). SIB-R Broad Independence SS also was moderately correlated with SIB-R General Maladaptive Index (rs = 0.37, P = 0.003), indicating that better adaptive behavior was associated with less maladaptive behavior. SIB-R General Maladaptive Index was not significantly associated with DAS-II GCA (rs = 0.14, P = 0.29). An examination of the correlations among SIB-R cluster SSs indicated that all were strong and significant (all Ps < 0.0001), ranging from rs = 0.51 for Social Interaction & Communication Skills and Motor Skills to rs = 0.78 for Social Interaction & Communication Skills and Community Living Skills. Correlations with overall intellectual ability also were significant, ranging from rs = 0.37 (P = 0.003) for DAS-II GCA and Motor Skills SS to rs = 0.75 for DAS-II GCA and Social Interaction & Communication Skills SS (P < 0.0001). Examination of the correlations among the maladaptive indices indicated that only the correlation between Asocial Maladaptive Index score and Externalized Maladaptive Index score was significant (rs = 0.69, P < 0.0001).
Psychopathology
Parents of 62 of the 63 children aged 4–17 years completed the ADIS-P interview about their child; adults with classic Dup7 completed the ADIS-IV interview about themselves. The DSM-IV diagnoses that resulted from these interviews are indicated in Table IX. Anxiety disorders were very common. For the child group, 74.2% had at least one anxiety disorder diagnosis, with 59.7% having at least one anxiety disorder other than Specific Phobia. The most prevalent internalizing disorders were Specific Phobia, Social Phobia, and Selective Mutism. Of the 31 children who met criteria for Social Phobia, 14 (45.2%) also met criteria for Selective Mutism.
TABLE IX.
Percentage of Participants Diagnosed with DSM-IV Disorders Assessed by the ADIS-P (Children; N = 62) or ADIS-IV (Adults; N = 10)
| Internalizing Disordersa |
Externalizing Disorders |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Separation Anxiety | Social Phobia | Specific Phobia | GAD | Selective Mutism | ADHD | ODD | DBD |
| Children | 12.9 | 50.0 | 53.2 | 6.5 | 29.0 | 35.5 | 16.1 | 8.1 |
| Adults | – | 60.0 | 30.0 | 20.0 | – | – | – | – |
GAD (Generalized Anxiety Disorder), ADHD (Attention Deficit Hyperactivity Disorder), ODD (Oppositional Defiant Disorder), DBD (Disruptive Behavior Disorder – Not Otherwise Specified).
Note: – indicates that the disorder is not assessed by the ADIS-IV.
In addition, 2 children (3.2%) were diagnosed with Obsessive-Compulsive Disorder, 1 child (1.6%) was diagnosed with Major Depressive Disorder, and 1 child (1.6%) was diagnosed with Adjustment Disorder with Mixed Anxiety and Depressed Mood.
The most common externalizing disorder was Attention Deficit/Hyperactivity Disorder (ADHD). Of the 22 children who were diagnosed with ADHD, 11 (50.0%) had ADHD-Predominantly Inattentive Type, 5 (22.7%) had ADHD-Predominantly Hyperactive/Impulsive Type, and 6 (27.3%) had ADHD-Combined Type. Fifteen children (24.2%) were diagnosed with either Oppositional Defiant Disorder (ODD) or Disruptive Behavior Disorder—Not Otherwise Specified (DBD-NOS). The difference between children with the two diagnoses was that those diagnosed with ODD demonstrated significant and impairing oppositional-defiant behavior both at home and in one or more other settings whereas those diagnosed with DBD-NOS demonstrated significant and impairing oppositional-defiant behavior only at home. Note that all 15 children would be diagnosed with ODD based on DSM-5 criteria.
Six of the 10 adults (60%) had Social Phobia and half of these individuals also had at least one Specific Phobia. One-third of the individuals who had Social Phobia (including one of the individuals who had both Social Phobia and Specific Phobia) also had Generalized Anxiety Disorder. None of the adults was diagnosed with a mood disorder.
Screening for Autism Spectrum Disorder
Parents of the 42 most recent participants aged 4–17 years completed the SCQ. Mean SCQ raw score was 11.50 (SD : 7.17) with a median of 10.0 and a range from 0 to 30. Twenty eight children (66.7%) scored below the SCQ raw-score cutoffs recommended by Corsello et al. [2007] (12 for ages 4–7 years, 15 for ages 8 years and older) and were classified as nonspectrum. Fourteen (33.3%) exceeded the cutoff and screened positive for a possible ASD. As shown in Figure 1, the distribution of raw scores for the children classified Nonspectrum was approximately normal, with a mean of 7.29 (SD : 3.58, median : 7.0), well below the screening cutoff. The raw scores for the children who screened positive for a possible ASD were positively skewed, with a mean of 19.93 (SD : 4.55, median : 18.5), indicating that many participants who screened positive had raw scores well above the cutoff.
FIG. 1.
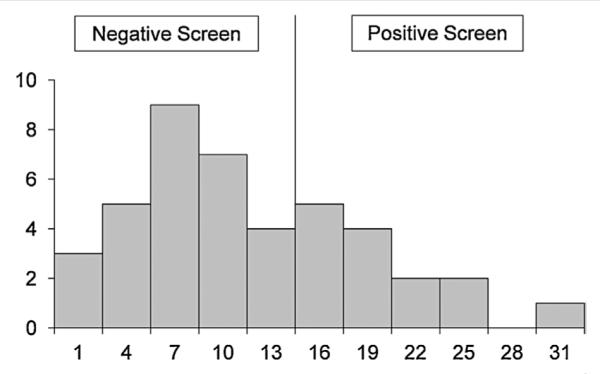
Distribution of raw scores on the Social Communication Questionnaire. Note that although the cut score for a positive screen for a possible autism spectrum disorder is 12 for children aged 4–7 years and 15 for children aged 8 years or older, no child aged 4–7 years had a raw score of 12, 13, or 14. Thus, in the Figure the division between a negative screen and a positive screen is shown at 15.
Speech Sound Disorder
All but one of the 63 participants aged 4–17 years was evaluated for DSM-5 Speech Sound Disorder. The remaining participant (aged 17 years) was selectively mute throughout her entire assessment. Of the 62 children, 51 (82.3%) were diagnosed with Speech Sound Disorder. Mean CA for children who had a speech sound disorder was 7.89 years (SD : 3.19, median : 7.01, range : 4.01–17.55 years); for children who did not have a speech sound disorder, mean CA was 12.20 years (SD : 2.78, median : 12.94, range : 6.98–17.70). The results of a Mann Whitney U test indicated that these CA distributions differed significantly (P < 0.0001). The 25 youngest participants (aged 4.01–6.78 years) all were diagnosed with Speech Sound Disorder. In contrast, of the 12 oldest participants (aged 12.18–17.70 years), only 5 (41.7%, aged 13.89–17.55 years) met criteria for this diagnosis.
DISCUSSION
The present research project is the first to systematically assess a relatively large group of individuals with classic Dup7. Results indicated a very broad range of ability levels—from severe disability to high average or superior—on standardized assessments of intelligence, vocabulary, and academic achievement, with adaptive behavior levels ranging from severe disability to average and maladaptive behavior levels ranging from extreme difficulty to normal. More than half of the participants were diagnosed with at least one anxiety disorder other than Specific Phobia and about one-quarter of the child participants were diagnosed with either ODD or DBD-NOS. More than three-quarters of the child participants were diagnosed with Speech Sound Disorder. One-third of the child participants screened positive for a possible ASD. In the remainder of the Discussion we briefly consider each of these findings and relate them to prior findings for children with WS. We then address limitations of this research and directions for future research.
Intellectual Ability, Vocabulary, and Academic Achievement
As indicated in Table I, when prior case studies of individuals with classic Dup7 mentioned intellectual abilities, typically only an overall classification was provided. The modal classification in prior research was intellectual disability (most commonly mild intellectual disability); the range of abilities reported was from severe intellectual disability to high average nonverbal intellectual ability. In the present study, we also identified a very wide range of intellectual abilities across individuals with classic Dup7. However, median overall intellectual ability SS was in the low average range for both the toddler group and the child group and at the bottom of the average range for the adults—considerably higher than suggested by the case-report classifications.
Although the case reports almost never addressed an individual’s intellectual strengths and weaknesses, the present study considered these for each participant. An examination of these patterns of relative strength and weakness indicated that the apparent consistency in level of overall performance across the three age groups masks clear differences in modal patterns of intellectual strength and weakness as a function of age group (It is important to keep in mind, as described in the Results, that these modal patterns are not characteristic of all individuals with classic Dup7.) For the toddlers, the modal pattern involved relative strengths in nonverbal reasoning and receptive language contrasted with relative weakness in expressive language. For the 4–17-year-olds, the modal pattern was a relatively flat profile, with no significant differences in verbal, nonverbal reasoning, and spatial abilities. In adulthood, the modal pattern involved nonverbal reasoning and spatial abilities (Performance IQ) that were significantly stronger than verbal abilities.
The average overall IQ or DQ for individuals with classic Dup7 contrasts with that for individuals with classic WS (deletion of the set of genes that is duplicated in classic Dup7), with the Dup7 group scoring on average considerably higher. In contrast to the mean MSEL ELC of 80.88 for toddlers with classic Dup7 and the mean DAS-II GCA of 82.05 for 4–17-year-olds with classic Dup7 that we reported, Mervis and John [2010] found a mean MSEL ELC of 61.45 for 144 toddlers and young preschoolers with classic WS and a mean DAS-II GCA of 64.56 for 120 4–17-year-olds with classic WS. In contrast to the mean WASI Full Scale IQ of 92.50 that we reported for adults with classic Dup7, Searcy et al. [2004] reported a mean Wechsler Adult Intelligence Scale-Revised [WAIS-R; Wechsler, 1981] Full Scale IQ of 67.4 for 80 adults with WS. These findings are consistent with previously-reported comparisons of groups of individuals with duplication of a given chromosomal region to groups of individuals with deletion of the same region [see, e.g., van der Aa et al., 2009]: The impact of a duplication on intellectual ability is typically milder than the impact of a deletion of the same set of genes.
In contrast to the changing modal patterns of strength and weakness identified for the three age groups of individuals with Dup7, a consistent and quite different pattern of relative strengths and weaknesses has been found for individuals with classic WS [e.g., Mervis and John, 2010]. This pattern, which involves relative strength in language and in nonverbal reasoning and severe weakness in visuospatial construction, is apparent in toddlers as evidenced by the large difference between average T-score on the MSEL Fine Motor scale and average T-score on the other MSEL scales. The same pattern is shown by school-aged children with classic WS as evidenced by the ~20-point difference between mean SS on the DAS-II Spatial cluster and mean SSs on the Verbal cluster and Nonverbal Reasoning cluster. Furthermore, 86% of children with classic WS scored significantly higher on the DAS-II Verbal cluster and/or Nonverbal Reasoning cluster than on the Spatial cluster. This pattern of relative strengths and weaknesses is harder to detect on the WAIS-R. However, Searcy et al. [2004] found that mean Verbal IQ was significantly higher than mean Performance IQ for adults with WS.
Although receptive language was on average significantly stronger than expressive language for toddlers with classic Dup7, this difference was not apparent in the average SSs for school-age children and adults with classic Dup7 for receptive and expressive vocabulary abilities. Instead, average PPVT-4 and EVT-2 SSs were in the top of the low average to bottom of the average range for both groups, with about three-fourths of individuals in both age groups evidencing no significant difference between receptive and expressive vocabulary ability. Once again, the mean SSs for children with classic Dup7 found in this study (PPVT-4: 91.51, EVT-2: 87.21) were higher than the mean SSs reported by Mervis and John [2010] for 129 children with WS (PPVT-4: 81.84, EVT-2: 79.43).
Mean and median WIAT-III reading achievement scores also were at the bottom of the average range for children with classic Dup7, with median math achievement scores in the low average range. SDs were considerably larger than for the general population. WIAT-III findings have not been reported for children with WS. The mean WIAT-II SSs for reading achievement that Mervis and John [2010] reported for 44 9–17-year-olds with WS (Word Reading: 73.00, Pseudoword Decoding: 78.75, Reading Comprehension: 64.61) are considerably lower than the mean WIAT-III SSs for children with Dup7 in the present study (Word Reading: 90.91, Pseudoword Decoding: 93.19, Reading Comprehension: 89.91). SSs for performance on mathematics achievement tests have not been reported for children with WS, although a wide range of difficulties has been identified [e.g., O’Hearn and Luna, 2009].
Behavior
In contrast to the low-average to average performance levels that characterize the intellectual, vocabulary, reading, and math abilities of children with classic Dup7, adaptive behavior is considerably more limited. Mean levels of adaptive behavior are in the mild disability to borderline range, with the distribution of SIB-R SSs for Social Interaction and Communication skills higher than the SS distributions for Motor Skills, Personal Living skills, and Community Living skills. This average pattern of relative strengths and weaknesses in adaptive behavior is the same as the average pattern for 122 children with WS [Mervis and John, 2010], although the difference between Social Interaction and Communication skills and the three other types of adaptive skills is much larger for the WS group. Strikingly, despite considerable differences in overall intellectual ability favoring children with Dup7, mean SIB-R Social Interaction and Communication skills SS is almost identical for the two groups (Dup7: 72.62, WS: 73.16). In contrast, mean SSs for the three remaining types of adaptive skills assessed by the SIB-R average 6–11 points higher for the Dup7 group than the WS group.
We hypothesize that the considerably weaker adaptive behavior for children with Dup7 than would be expected given their intellectual abilities is due in large part to difficulties in maladaptive behavior with a likely role for executive functioning. There are no published data on executive functioning in individuals with classic Dup7. However, unpublished data collected by our research group for the Behavior Rating Inventory of Executive Function [BRIEF; Gioia et al., 2000] for children aged 6–17 years indicate median T-scores in the clinical range for both the Behavior Regulation Index and the Metacognition Index, suggesting that on average children with classic Dup7 have considerable difficulty with executive functioning in everyday contexts. Prior case studies of children with classic Dup7 addressed the possibility of behavior problems for about one-third (anxiety or aggression/oppositional behavior) to one-half (attention) of the children described (Table I), with standardized assessment provided in slightly less than half the reports in which the possibility of a problem was mentioned. Problems were identified often enough to suggest that systematic examination of behavior difficulties was important for children with classic Dup7.
In the present study, we measured behavior problems in children with classic Dup7 both using the continuous scales provided by the SIB-R maladaptive behavior indices and categorical DSM-IV diagnoses based on the ADIS-P. Categorical diagnoses based on the ADIS-IV were determined for adults with classic Dup7. SIB-R Maladaptive Behavior Indices were highly variable, especially for Externalized maladaptive behavior, for which the SD was almost 1.5 times as high as for the SIB-R clinical reference group. The median maladaptive index score was at the border between the mild and moderate categories for Internalized maladaptive behavior, and the distribution of Internalized index scores was significantly lower (worse) than for Externalized index scores and marginally significantly lower than for Asocial index scores. SIB-R maladaptive index scores have not been reported for children with WS.
The SIB-R Maladaptive Index scores were consistent with the possibility that many individuals with classic Dup7 would meet DSM-IV criteria for anxiety, attention, and/or oppositional disorders. The ADIS interviews confirmed this possibility. In particular, 50.0% of children were diagnosed with Social Phobia, 29.0% with Selective Mutism, 12.9% with Separation Anxiety Disorder, 6.5% for Generalized Anxiety Disorder, and 53.2% with Specific Phobia. Externalizing disorders also were common, with 35.5% diagnosed with ADHD and 24.2% diagnosed with either ODD or DBD-NOS. The rates for Social Phobia, Selective Mutism, Separation Anxiety Disorder, and ODD/DBD-NOS are all considerably higher than those previously reported for large samples of children with classic WS [e.g., Leyfer et al., 2006; Mervis et al., 2012]. The Specific Phobia and Generalized Anxiety Disorder rates are similar for children with classic Dup7 and children with classic WS, and the rate for ADHD is considerably higher for children with classic WS than for children with classic Dup7. Of the small sample of adults with classic Dup7 included in this study, 60% were diagnosed with Social Phobia, 20% with Generalized Anxiety Disorder, and 30% with Specific Phobia. All three of these rates are higher than those reported by Stinton et al. [2010] for 92 adults with WS. Relative to the rates for the sample of 20 adults with WS studied by Cherniske et al. [2004] the Social Phobia rate for classic Dup7 is considerably higher and the Specific Phobia rate for classic Dup7 is considerably lower. Cherniske et al. [2004] do not provide a rate for Generalized Anxiety Disorder but note that it is the second most common anxiety diagnosis, after Specific Phobia. While anxiety disorders are present for both individuals with classic Dup7 and classic WS, a particularly striking finding is that duplication and deletion of genes in this region appear to confer contrasting risk regarding both Social Phobia, which is very common for individuals with Dup7 but rare for individuals with WS, and Selective Mutism, which is common for individuals with Dup7 but has not been reported for individuals with WS.
Autism Spectrum Disorder Screen
The possibility of an ASD was mentioned in 57% of the prior case reports of children with classic Dup7, with results either of a formal assessment for autism (Autism Diagnostic Observation Schedule [ADOS, ADOS-2; Lord et al., 1999, 2012] and/or Autism Diagnostic Interview Schedule – Revised [ADI-R; Lord et al., 1994]) or a standardized autism screening questionnaire reported for 31% (11 children). Four of the 11 children were part of the Simons Simplex sample of children with autism that was screened for genetic disorders by Sanders et al. [2011]. Of the remaining seven children, three were described as meeting criteria for ASD (one based on assessment with both the ADOS and ADI-R, one based on assessment with the ADI-R, and one based on a standardized screening measure). These findings make clear the importance of assessing children with classic Dup7 for ASD using standardized instruments.
Formal diagnosis of ASD was beyond the scope of the present study. However, we did screen 42 children with classic Dup7 for ASD using the SCQ, with 33.3% meeting Corsello et al.’s [2007] criteria for a positive screen. For Corsello et al.’s sample, their cut score was associated with a specificity of .50 for children aged 5–7 years and .66 for children aged 11 years or older. Thus, while many of the children who screened positive likely would be found to have an ASD following a gold-standard formal assessment using the ADOS-2, ADI-R, and clinical judgment, a significant portion of the children who screened positive based on the SCQ likely would be classified nonspectrum following formal assessment. As there are no published autism screening findings for children with WS, a comparison of positive screening rates for children with classic Dup7 and children with classic WS is not possible. Lincoln et al. [2007] reported that 2 of 20 young children with WS met DSM criteria for autistic disorder. Klein-Tasman et al. [2007] found that 48% of young children with WS were classified autism or autism spectrum disorder on the ADOS (Module 1), but stress that their study was not designed to diagnose autism spectrum disorders and that many of these children would likely not meet DSM-IV criteria for autism spectrum disorder. At the same time, they also note that a co-morbid diagnosis of ASD likely is appropriate for some children with WS. More recently, Tordjman et al. [2012] reported on nine individuals with classic WS who met criteria for ASD based on gold-standard assessment. The comparative rate of ASD across children with WS and Dup7 warrants further study.
Speech
The presence or absence of speech concerns was noted in 60% of prior case reports of children with classic Dup7, although formal assessment was reported for only 11%. In the present study, formal assessment for DSM-5 Speech Sound Disorder indicated that 82.3% met criteria for this disorder. The rate of Speech Sound Disorder was very high for the younger children and considerably lower for the older children, suggesting the possibility that for some individuals with classic Dup7, Speech Sound Disorder may resolve during childhood. However, it is notable that Speech Sound Disorder was evident for approximately 40% of the adolescents with classic Dup7. Note that all 63 children had received speech therapy at some point and that the majority was still receiving it at the time of participation in the present study. Although formal diagnosis of specific types of Speech Sound Disorder was beyond the scope of this study, this type of diagnosis has been completed for 33 of the children [Huffman et al., 2014]. Of these children, 52% met criteria for Childhood Apraxia of Speech with an additional 42% evidencing symptoms, and 21% met criteria for Dysarthria, with an additional 58% evidencing symptoms. Although many children with WS also evidence characteristics of motor speech disorders [Mervis and Velleman, 2011], in particular speech sound distortions, by school age most of their speech is intelligible.
Limitations and Future Directions
Although the present samples of 63 children aged 4–17 years and 16 children aged 18–45 months include many more children than all of the prior case reports combined, these samples are still relatively small given the large age span covered. In addition, although both probands with classic Dup7 and their siblings with classic Dup7 who were identified by cascade testing were included in the study, the number of non-proband siblings with Dup7 was too small to allow for the comparisons necessary to determine if there are systematic phenotypic differences between probands and non-probands with Dup7. It is possible that there is an ascertainment bias in favor of children with more significant disabilities. However, it is important to note that the current study yielded an estimate of cognitive functioning that is higher than would have been expected based on the extant literature. Future studies with larger samples will be critical for confirming and more precisely specifying the changes in patterns of relative strengths and weaknesses in intellectual abilities over time as well as for determining phenotypic similarities and differences between probands with Dup7 and their non-proband siblings who also have Dup7. Longitudinal studies would be especially valuable. Gold-standard assessment of large samples of children with classic Dup7 for ASD is critical as are systematic assessments for specific speech sound disorders (especially Childhood Apraxia of Speech and Dysarthria). It also will be important to examine relations among different aspects of the psychological phenotype of children with classic Dup7, for example, relations between intellectual abilities, the presence or absence of an ASD or specific symptoms of an ASD, and the presence or absence of Social Phobia or specific symptoms of Social Phobia. Similarly, examination of the relations of Childhood Apraxia of Speech to intellectual abilities, vocabulary abilities, grammatical abilities, reading achievement, Social Phobia or specific symptoms of Social Phobia, and Selective Mutism or specific symptoms of Selective Mutism would be valuable.
Comparisons between the children with classic Dup7 in this study and published findings for children with classic WS indicate that genotype/phenotype studies of this region are likely to be valuable for identifying genes that are involved, in transaction with other genes and the child’s environment, in the development and/or maintenance of characteristics common among individuals who have one of these syndromes. These genes also are likely to be important for the development or maintenance of these characteristics among individuals in the general population. Examples of characteristics for which genotype/phenotype studies of duplication/deletion of the classic WS region are likely to offer insight include general intellectual ability, visuospatial construction ability, characteristics associated with ASD, characteristics associated with Childhood Apraxia of Speech or Dysarthria, and characteristics associated with Social Phobia (and the contrasting phenotype of social disinhibition associated with classic WS), with Selective Mutism, and with Specific Phobia. Methodologically-rigorous studies of individuals with smaller duplications of the classic WS region have the potential to be particularly valuable.
CONCLUSIONS
The findings from the present study offer an initial documentation of the psychological phenotype of children with classic Dup7. Intellectual abilities are typically in the low average range. The modal pattern of relative strengths and weaknesses appears to change over time, with toddlers typically evidencing relative strengths in receptive language and nonverbal reasoning and relative weakness in expressive language. School-age children are more likely to show a relatively flat profile, and adults are more likely to show relative strengths in nonverbal reasoning and visuospatial construction and relative weakness in verbal abilities. At the same time, there is considerable variability both in overall level of intellectual abilities (from severe intellectual disability to high average ability) and in patterns of relative strengths and weaknesses. Intellectual abilities and academic skills are highly correlated. Adaptive behavior is on average considerably more limited than expected given overall intellectual ability. The majority of children has at least one anxiety disorder other than Specific Phobia, with Social Phobia and Selective Mutism most common; one-third has ADHD; and almost one-fourth has either ODD or DBD-NOS. More than three-fourths has Speech Sound Disorder, and one-third screened positive for a possible ASD. Although considerably more research is needed, the current findings in conjunction with findings from the literature on children with classic WS make clear that genotype/phenotype studies of this region are likely to yield insights into the contributions of genes in this region not only to characteristics associated with these syndromes but also to characteristics important within the general population.
ACKNOWLEDGMENTS
We are very grateful to the participants and their families. Without their commitment to furthering scientific knowledge of 7q11.23 duplication syndrome, progress in understanding this syndrome would not be possible. We thank the current and former members of the Neurodevelopmental Sciences Laboratory who conducted components of the psychological assessment, scored and entered data from these assessments, and/or videotaped the speech assessments.
Grant sponsor: Simons Foundation; Grant number: SFARI # 238896; Grant sponsor: National Institute of Child Health and Human Development; Grant number: R37 HD29957; Grant sponsor: National Institute of Neurological Disorders and Stroke; Grant number: R01 NS35102.
REFERENCES
- Berg JS, Brunetti-Pierri N, Peters SU, Kang S-HL, Fong CT, Salamone J, Freedenberg D, Hannig VL, Prock LA, Miller DT, Raffalli P, Harris DJ, Erickson RP, Cunniff C, Clark GD, Blazo MA, Peiffer DA, Gunderson KL, Sahoo T, Patel A, Lupski JR, Beaudet AL, Cheung SW. Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genet Med. 2007;9:427–441. doi: 10.1097/gim.0b013e3180986192. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism Screening Questionnaire: Diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo P, Barlow DH. Anxiety Disorder Interview Schedule Adult Version (ADIS-IV) Graywind Publications; San Antonio, TX: 1996. [Google Scholar]
- Bruininks RH, Woodcock R, Weatherman R, Hill B. Scales of Independent Behavior-Revised. Riverside; Chicago, IL: 1996. [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004;131:255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Jr, Leventhal BL, Lord C. Between a ROC and a hard place: Decision making and making decisions about using the SCQ. J Child Psychol Psychiatry. 2007;48:932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Değerliyurt A, Ceylaner S, Ozdağ H. A 7q11.23 microduplication patient with cerebral palsy and facial dysmorphism. Genet Couns. 2012;23:263–267. [PubMed] [Google Scholar]
- Depienne C, Heron D, Betancur C, Benyahia B, Trouillard O, Bouteiller D, Verloes A, Leguern E, Leboyer M, Brice A. Autism, language delay and mental retardation in a patient with 7q11 duplication. J Med Genet. 2007;44:452–458. doi: 10.1136/jmg.2006.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Heron D, Betancur C, Benyahia B, Trouillard O, Bouteiller D, Verloes A, Leguern E, Leboyer M, Brice A. Autism, language delay and mental retardation in a patient with 7q11 duplication. BMJ Case. 2009 doi: 10.1136/bcr.05.2009.1911. Rep bcr05.2009.1911 (adapted with permission from Depienne et al. 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A, McKee S, Mansour S, Mehta SG, Tanteles GA, Anastasiadou V, Patsalis PC, Martin K, McCullough S, Suri M, Sarkar A. 7q11.23 microduplication: A recognizable phenotype. Clin Genet. 2013;83:155–161. doi: 10.1111/j.1399-0004.2012.01862.x. [DOI] [PubMed] [Google Scholar]
- Dunn LE, Dunn DM. Peabody Picture Vocabulary Test. 4th edition Pearson Assessments; Minneapolis, MN: 2007. [Google Scholar]
- Dutly F, Schinzel A. Unequal interchromosomal rearrangements may result in elastin gene deletions causing the Williams-Beuren syndrome. Hum Mol Genet. 1996;5:1893–1898. doi: 10.1093/hmg/5.12.1893. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. 2nd edition Psychological Corporation; San Antonio, TX: 2007. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources, Inc; Lutz, FL: 2000. [Google Scholar]
- Huffman MJ, Velleman SL, Mervis CB. 7q11.23 duplication syndrome and Childhood Apraxia of Speech. 10th Annual National Conference on Childhood Apraxia of Speech; Nashville, TN. Jul, 2014. [Google Scholar]
- Kirchhoff M, Bisgaard AM, Bryndorf T, Gerdes T. MLPA analysis for a panel of syndromes with mental retardation reveals imbalances in 5.8% of patients with mental retardation and dysmorphic features, including duplications of the Sotos syndrome and Williams-Beuren syndrome regions. Eur J Med Genet. 2007;50:33–42. doi: 10.1016/j.ejmg.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB, Lord C, Phillips KD. Socio-communicative deficits in young children with Williams syndrome: Performance on the Autism Diagnostic Observation Schedule. Child Neuropsychol. 2007;13:444–467. doi: 10.1080/09297040601033680. [DOI] [PubMed] [Google Scholar]
- Kriek M, White SJ, Szuhai K, Knijnenburg J, van Ommen GJ, den Dunnen JT, Breuning MH. Copy number variation in regions flanked (or unflanked) by duplicons among patients with developmental delay and/or congenital malformations; detection of reciprocal and partial Williams-Beuren duplications. Eur J Hum Genet. 2006;14:180–189. doi: 10.1038/sj.ejhg.5201540. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Woodruff-Borden J, Klein-Tasman BP, Fricke JS, Mervis CB. Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am J Med Genet B. 2006;141B:615–622. doi: 10.1002/ajmg.b.30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AJ, Searcy YM, Jones W, Lord C. Social interaction behaviors discriminate young children with autism and Williams syndrome. J Am Acad Child Adolesc Psychiatry. 2007;46:323–331. doi: 10.1097/chi.0b013e31802b9522. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Western Psychological Services; Los Angeles, CA: 1999. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, BishopS L. Autism Diagnostic Observation Schedule. 2nd edition Western Psychological Services; Los Angeles, CA: 2012. [Google Scholar]
- Malenfant P, Liu X, Hudson ML, Qiao Y, Hrynchak M, Riendeau N, Hildebrand MJ, Cohen IL, Chudley AE, Forster-Gibson C, Mickelson EC, Rajcan-Separovic E, Lewis ME, Holden JJ. Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. J Autism Dev Disord. 2012;42:1459–1469. doi: 10.1007/s10803-011-1389-4. [DOI] [PubMed] [Google Scholar]
- McGrew SG, Peters BR, Crittendon JA, Veenstra-Vanderweele J. Diagnostic yield of chromosomal microarray analysis in an autism primary care practice: Which guidelines to implement? J Autism Dev Disord. 2012;42:1582–1591. doi: 10.1007/s10803-011-1398-3. [DOI] [PubMed] [Google Scholar]
- Merritt JL, Lindor NM. Further clinical description of duplication of Williams-Beuren region presenting with congenital glaucoma and brachycephaly. Am J Med Genet A. 2008;146A:1055–1058. doi: 10.1002/ajmg.a.32235. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: Implications for intervention approaches. Am J Med Genet C. 2010;154C:229–248. doi: 10.1002/ajmg.c.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Velleman SL. Children with Williams syndrome: Language, cognitive, and behavioral characteristics and their implications for intervention. Perspect Lang Learn Educ. 2011;18:98–107. doi: 10.1044/lle18.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Dida J, Lam E, Crawford-Zelli NA, Young EJ, Henderson DR, Onay T, Morris CA, Woodruff-Borden J, Yeomans J, Osborne LR. Duplication of GFT2I results in separation anxiety in mice and humans. Am J Hum Genet. 2012;90:1064–1070. doi: 10.1016/j.ajhg.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, Sommer A, Moore CA, Hopkin RJ, Spallone PA, Keating MT, Osborne L. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: Genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A. 2003;123A:45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- O’Hearn K, Luna B. Mathematical skills in Williams syndrome: Insight into the importance of underlying representations. Dev Disabil Res Rev. 2009;15:11–20. doi: 10.1002/ddrr.47. [DOI] [PubMed] [Google Scholar]
- Orellana C, Bernabeu J, Monfort S, Roselló M, Oltra S, Ferrer I, Quiroga R, Martínez-Garay I, Martínez F. Duplication of the Williams-Beuren critical region: Case report and further delineation of the phenotypic spectrum. J Med Genet. 2008;45:187–189. doi: 10.1136/jmg.2007.054064. [DOI] [PubMed] [Google Scholar]
- Osborne LR, Mervis CB. Rearrangements of the Williams-Beuren syndrome locus: Molecular basis and implications for speech and language development. Expert Rev Mol Med. 2007;9:1–16. doi: 10.1017/S146239940700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott A, James J, Goldenberg P, Hinton RB, Miller E, Shikany A, Aylsworth AS, Kaiser-Rogers K, Ferns SJ, Lalani SR, Ware SM. Aortopathy in the 7q11.23 microduplication syndrome. Am J Med Genet A. 2015;167A:363–370. doi: 10.1002/ajmg.a.36859. [DOI] [PubMed] [Google Scholar]
- Prontera P, Serino D, Caldini B, Scarponi L, Merla G, Testa G, Muti M, Napolioni V, Mazzotta G, Piccirilli M, Donti E. Brief report: Functional MRI of a patient with 7q11.23 duplication syndrome and autism. J Autism Dev Disord. 2014;44:2608–2613. doi: 10.1007/s10803-014-2117-7. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, Mason CE, Bilguvar K, Celestino-Soper PB, Choi M, Crawford EL, Davis L, Wright NR, Dhodapkar RM, DiCola M, DiLullo NM, Fernandez TV, Fielding-Singh V, Fishman DO, Frahm S, Garagaloyan R, Goh GS, Kammela S, Klei L, Lowe JK, Lund SC, McGrew AD, Meyer KA, Moffat WJ, Murdoch JD, O’Roak BJ, Ober GT, Pottenger RS, Raubeson MJ, Song Y, Wang Q, Yaspan BL, Yu TW, Yurkiewicz IR, Beaudet AL, Cantor RM, Curland M, Grice DE, Günel M, Lifton RP, Mane SM, Martin DM, Shaw CA, Sheldon M, Tischfield JA, Walsh CA, Morrow EM, Ledbetter DH, Fombonne E, Lord C, Martin CL, Brooks AI, Sutcliffe JS, Cook EH, Jr, Geschwind D, Roeder K, Devlin B, State MW. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy YM, Lincoln A, Rose F, Klima E, Bavar N, Korenberg JR. The relationship between age and IQ in adults with Williams syndrome. Am J Ment Retard. 2004;109:231–236. doi: 10.1352/0895-8017(2004)109<231:TRBAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule. Graywind Publications; San Antonio, TX: 1996. [Google Scholar]
- Somerville MJ, Mervis CB, Young EJ, Seo E-J, del Campo M, Bamforth S, Peregrine E, Loo W, Lilley M, Perez-Jurado LA, Morris CA, Scherer SW, Osborne LR. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med. 2005;353:1694–1701. doi: 10.1056/NEJMoa051962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinton C, Elison S, Howlin P. Mental health problems in adults with Williams syndrome. Am J Intellect Dev Disabil. 2010;115:3–18. doi: 10.1352/1944-7558-115.1.3. [DOI] [PubMed] [Google Scholar]
- Stock AD, Spallone PA, Dennis TR, Netski D, Morris CA, Mervis CB, Hobart HH. Heat shock protein 27 gene: chromosomal and molecular location and relationship to Williams syndrome. Am J Med Genet A. 2003;120A:320–325. doi: 10.1002/ajmg.a.20055. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Toutain A, Sarda P, Carlier M, Saugier-Veber P, Baumann C, Cohen D, Lagneaux C, Tabet AC, Verloes A. Autistic disorder in patients with Williams-Beuren syndrome: A reconsideration of the Williams-Beuren syndrome phenotype. PLoS One. 2012;27:e30778. doi: 10.1371/journal.pone.0030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torniero C, dalla Bernardina B, Novara F, Vetro A, Ricca I, Darra F, Pramparo T, Guerrini R, Zuffardi O. Cortical dysplasia of the left temporal lobe might explain severe expressive-language delay in patients with duplication of the Williams-Beuren locus. Eur J Hum Genet. 2007;15:62–67. doi: 10.1038/sj.ejhg.5201730. [DOI] [PubMed] [Google Scholar]
- Torniero C, dalla Bernardina B, Novara F, Cerini R, Bonaglia C, Pramparo T, Ciccone R, Guerrini R, Zuffardi O. Dysmorphic features, simplified gyral pattern and 7q11.23 duplication reciprocal to the Williams-Beuren deletion. Eur J Hum Genet. 2008;16:880–887. doi: 10.1038/ejhg.2008.42. [DOI] [PubMed] [Google Scholar]
- Urbán Z, Helms C, Fekete G, Csiszár K, Bonnet D, Munnich A, Donis-Keller H, Boyd CD. 7q11.23 deletions in William syndrome arise as a consequence of unequal meiotic crossover. Am J Hum Genet. 1996;59:958–962. [PMC free article] [PubMed] [Google Scholar]
- Van der Aa N, Rooms L, Vandeweyer G, van den Ende J, Reyniers E, Fichera M, Romano C, Delle Chiaie B, Mortier G, Menten B, Destrée A, Maystadt I, Männik K, Kurg A, Reimand T, McMullan D, Oley C, Brueton L, Bongers EM, van Bon BW, Pfund R, Jacquemont S, Ferrarini A, Martinet D, Schrander-Stumpel C, Stegmann AP, Frints SG, de Vries BB, Ceulemans B, Kooy RF. Fourteen new cases contribute to the characterization of the 7q11.23 microduplication syndrome. Eur J Med Genet. 2009;52:94–100. doi: 10.1016/j.ejmg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Velleman SL, Mervis CB. Children with 7q11.23 duplication syndrome: Speech, language, cognitive, and behavioral characteristics and their implications for intervention. Perspect Lang Learn Educ. 2011;18:108–116. doi: 10.1044/lle18.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test-III. Harcourt Assessment; San Antonio, TX: 2009. [Google Scholar]
- Williams KT. Expressive Vocabulary Test. 2nd edition Pearson Assessments; Minneapolis, MN: 2007. [Google Scholar]
- Zarate YA, Lepard T, Sellars E, Kaylor JA, Alfaro MP, Sailey C, Schaefer GB, Collins RT., 2nd Cardiovascular and genitourinary anomalies in patients with duplications within the Williams syndrome critical region: Phenotypic expansion and review of the literature. Am J Med Genet A. 2014;164A:1998–2002. doi: 10.1002/ajmg.a.36601. [DOI] [PubMed] [Google Scholar]


