Abstract
Glutathione (GSH) is the most abundant intracellular thiol with diverse functions from redox signaling, xenobiotic detoxification, and apoptosis. The quantification of GSH is an important measure for redox capacity and oxidative stress. This protocol quantifies total GSH from Caenorhabditis elegans, an emerging model organism for toxicology studies. GSH is measured using the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) cycling method originally created for cell and tissue samples but optimized for whole worm extracts. DTNB reacts with GSH to from a 5′-thio-2-nitrobenzoic acid (TNB) chromophore with maximum absorbance of 412 nm. This method is both rapid and sensitive, making it ideal for studies involving a large number of transgenic nematode strains.
Introduction
Caenorhabditis elegans (C. elegans) is increasingly utilized in toxicology studies. This small nematode has a well-characterized physiology and fully sequenced genome. The ease of genetic mutability allow for the development of numerous transgenic strains with knock outs, over expression, functional null mutations, and fluorescently tagged proteins, which can be visualized due to the worm's transparency. Additionally RNAi technology is available. These factors allow for the performance of screening for toxicity to multiple compounds or screening multiple mutant strains with a toxicant of choice. The short lifespan of C. elegans is ideal for aging research and in studies involving multiple generations. C. elegans are highly homologous with humans, sharing 60-80% of genes (Kaletta and Hengartner, 2006; McDonald et al., 2006). Pathways for oxidative stress are highly conserved in the worm, and have been linked to lifespan, neuronal health and fertility (Gallo et al., 2011; Leiser et al., 2013; Park, 2013; Yang and Hekimi, 2010). While there are fluorescent transgenic reporter strains that can be used to investigate antioxidant gene expression (Choe et al., 2009; Dickinson et al., 2011; Link and Johnson, 2002), biochemical methods for analyzing oxidative stress are necessary.
Glutathione (GSH) is the major intracellular thiol utilized by cells for antioxidant protection, xenobiotic detoxification, cell signaling and apoptosis. GSH, the most abundant tripeptide, is composed of three amino acids, glutamate, cysteine and glycine, with a peptide linkage between the cysteine and glycine and a covalent bond between the gamma carboxyl group of glutamate and the amino group of cysteine. This unique tripeptide exists in the reduced form (GSH) or oxidized form as a disulfide (GSSG). Reactive oxygen species (ROS), reactive α,β-unsaturated aldehydes and ketones, as well as electrophilic xenobiotics, such as methylmercury, directly interact with GSH removing it from the available GSH pool. This can affect the GSH/GSSG ratio of the cell, altering the cell’s reducing capacity. Antioxidant enzymes glutathione peroxidases (GPx), glutathione S-transferases (GST), and glutathione reductase (GR) are important for cell survival, mitochondrial function, cell signaling, and regeneration of the GSH pool. Xenobiotic-induced alterations in oxidative stress and antioxidant signaling have been implicated in several diseases, including neurodegenerative diseases, metabolic syndrome, infertility, and inflammatory diseases (Balabanic et al., 2011; de Cock and van de Bor, 2014; Dusek et al., 2014; Morse and Rosas, 2014). For this reason levels of GSH are frequently measured as a biochemical marker of oxidative stress.
Basic Protocol 1: Quantification of Glutathione in Caenorhabditis elegans
Measurements of GSH in mammalian cell culture and tissues have traditionally been assessed by two methods, spectrophotometric using 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), also known as Ellman’s reagent, or with high performance liquid chromatography (HPLC). While the HPLC method can quantify GSH in the picomolar range and is capable of measuring several different types of thiols, there are several disadvantages for its use in C. elegans studies. Preparation of the samples for HPLC involves alkalization and derivitization, which can lead to loss of GSH in the sample (estimates of between 20-80% GSH recovery) (Reed et al., 1980). Processing and elution of the sample on the HPLC column additionally is timely, which does not lend it readily to experiments involving multiple worm strains or toxicant treatments. The spectrophotometric method described here is rapid and performed on a 96-well microtiter plate with minimal sample processing, allowing for both excellent recovery of GSH and sensitivity. This method is derived from the protocol described by Rahman et al. (Rahman et al., 2006), and has been optimized for the processing of C. elegans.
Materials
C. elegans samples – 30-50,000 L1 stage worms or 20,000 L4/adult worms per sample KPE buffer (recipe follows)
Extraction buffer (recipe follows)
GSH (reduced form) for standards
DTNB solution (recipe follows)
NADPH solution (recipe follows)
GR solution (recipe follows)
Sterile water
Liquid nitrogen
Water bath 96-well plate
Wand sonicator
Microplate (96-well plate) reader with filter to read absorbance at 412 nm Protein quantification assay kit
Steps
Extract GSH
- Harvest healthy synchronized worms by washing plates with room temperature sterile water, using 10 to 12 ml per plate. Transfer worm suspension to a 15 ml conical tube and centrifuge for 1 min at 400 × g and 23°C. Repeat washes until aqueous phase is clear (roughly 2 to 3 times).If starting from liquid culture, directly pellet worms in an eppendorf tube and remove supernatant to 50 μl.
Re-suspend pellet in 110 μl of extraction buffer and transfer to a chilled eppendorf tube. Vortex and freeze immediately with liquid nitrogen. Thaw samples in a water bath set to 37°C. Vortex for 5 sec. Freeze and thaw the samples two additional times to thoroughly lyse the worms.
Sonicate each sample using a wand sonicator (set to an output of 3 Watts) for 20 seconds.
Centrifuge for 10 min at 9200 × g, 4°C.
- Collect supernatant into a new chilled eppendorf tube.Pellet is fragile, leave behind 50 μl of extract to avoid taking up pellet debris. Samples should be placed on ice for immediate use or may be stored at -80°C for later analysis.
Measurement of Total GSH
Load the 96-well plate with 20 μl of KPE for blank, 20 μl of standards and 20 μl of sample into each well. Blank, standards and samples should be loaded in duplicate.
Mix DTNB and GR solutions together, add 120 μl to each well using a multichannel pipette.
Wait 30 sec and then add 60 μl of NADPH solution to each well using a multichannel pipette.
Immediately read absorbance at 412 nm every 30 sec for 2 min.
Calculate the rate of 2-nitro-5-thiobenoic acid formation (i.e. change in absorbance per min) for standards and samples.
Generate a standard curve by plotting standard concentration vs. rate of 2-nitro-5-thiobenoic acid formation (change in absorbance per minute).
Determine the GSH concentration in sample wells by using linear regression for the standard curve.
- Quantify protein content of the extract using the remainder of the extract. Divide GSH concentration by mg protein to normalize samples. Samples are expressed as nM GSH/mg protein.Any method of protein quantification may be used, such as Bradford or BCA assay.
Reagents and Solutions
KPE buffer
Solution A: 6.8 g KH2PO4 + 500 ml sterile H O, store at 4°C
Solution B: 11.4 g K2HPO4·3H2O to 500 ml sterile H2O, store at 4°C
Add 16 ml solution A to 84 ml solution B, pH to 7.5, add 0.327 g EDTA, store at 4°C
Extraction buffer
100 μl Triton X -100
60 mg sulfosalicylic acid
10 μl protease inhibitor
10 ml KPE
Make fresh prior to use, place on ice
GSH standards
Stock 2 mM GSH: 6.14 mg in 10 ml KPE, store as 1 ml aliquots in −20°C for up to one month
Serial dilute starting at 40 μM GSH (20 μl stock in 1 ml KPE) down to 0.625 μM
DTNB solution
2 mg DTNB
3 ml KPE
Prepare fresh each time, store on ice, wrapped in aluminum foil
NADPH solution
2 mg NADPH
3 ml KPE
Prepare fresh each time, store on ice, wrapped in aluminum foil
Glutathione Reductase (GR) solution
40 μl (250 units/ml) GR
3 ml KPE
Prepare fresh each time, store on ice
Commentary
Background Information
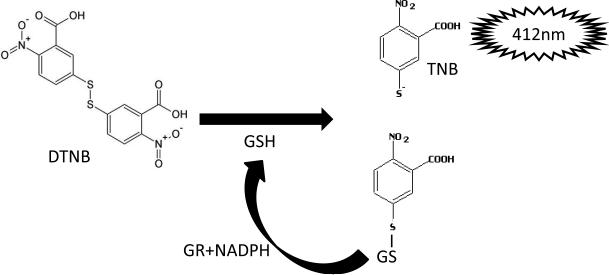
This protocol is a modification of the Tietze method for dertermination of GSH using DTNB, described previously (Rahman et al., 2006). DTNB directly reacts with GSH, cleaving its disulfide bond, yielding an oxidized GSH-3-thio-6 nitrobenzoate (TNB). TNB adducts produces a yellow chromophore, with maximal absorbance at 412 nm. The rate of formation of GSH-TNB is directly proportional to the concentration of GSH in the sample. The GSH-TNB is then reduced by GR in the presence of NADPH, recycling GSH back into the total GSH pool. The reaction of GSH with DTNB will continue, with signal amplification with each round of recycling of GSH by GR, until the DTNB is consumed. GR will also reduce GSSG present in the sample to form 2 GSH molecules, which will enter the total GSH pool and react with DTNB. Therefore, this protocol measures total GSH present in the sample (both oxidized and reduced forms of GSH). Known concentrations of GSH are used to generate a standard curve. The rate of change in absorbance at 412 nm is linear, allowing for linear regression analysis of the standard curve and quantification of total GSH content in the unknown samples.
Critical Parameters
Worm lysis
It is critical that the worms are thoroughly lysed before quantification of GSH. Repeated freezing the worms in liquid nitrogen and thawing in a water bath set to 37°C was found to be the most reliable method in our lab. Alternatively a dry ice-ethanol bath can be utilized if available. Freezing the worm pellet in a −80°C freezer is not recommended as it is a slow method and complete freezing will have to be allowed to occur. Sonication should be performed on ice to prevent degradation of proteins needed for normalization.
DTNB reaction
All the reagents required for the DTNB reaction should be kept chilled on ice and the light sensitive reagents should be shielded from light. Leftover samples not directly plated should be stored on ice until the DTNB reaction is over. This will allow for quantification of proteins and if DTNB reaction has to be run a second time with a dilution of the sample.
Troubleshooting
Low concentrations of GSH and protein can occur if either not enough worms were used in the sample or from incomplete lysis of the worms. Increasing sonication or additional freeze-thaws can be added, however we have found the conditions described to be sufficient.
Concentrations of GSH above the standard curve result from samples that have higher than the recommended numbers of worms utilized. Samples may be diluted in KPE when loaded onto the 96-well plate.
Failure of samples or standards to develop can arise if NADH is used rather than NADPH.
Anticipated Results
Quantification of total GSH by this method yields concentrations in the same range as reported by HPLC protocols. The lowest GSH concentrations measured reliably by the DTNB method is 0.103 nM (Rahman et al., 2006). Total yield will vary across samples due to numbers of worms in each eppendorf and degree of lysis of the worm by the freeze-thaws and sonication. Results are normalized to protein content to account for this variability. GSH concentrations for N2 adults are in the range of 12 nmol/mg protein (Hartwig et al., 2009). It is common for GSH results to be normalized to N2 controls when comparing between strains and treatment conditions (Bornhorst et al., 2014; Caito et al., 2013). Alternatively, there are papers in the literature that normalize to worm number, with concentrations of GSH in N2 young adults of 0.8 nmol/120 worms (Liao and Yu, 2005).
Time Considerations
Extraction of GSH from worms can take up to 30 min but will vary with the number of samples in the study. Performing the GSH quantification, from making fresh solutions, diluting standards, plating the worm extracts and running the DTNB reaction will take around 1 h.
Figure 1.
DTNB reaction with GSH. The enzymatic reaction of GSH with DTNB creates the TNB chromophore. Reduction of GSH-TNB by GR in the presence of NADPH creates additional TNB and regenerates GSH for further reaction with DTNB. TNB is quantified at 412 nm.
Acknowledgments
Support was provided in part by grants NIH R01 ES07331, ES07331S1 and NIH R01 ES10563.
Literature Cited
- Balabanic D, Rupnik M, Klemencic AK. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod Fertil Dev. 2011;23:403–416. doi: 10.1071/RD09300. [DOI] [PubMed] [Google Scholar]
- Bornhorst J, Chakraborty S, Meyer S, Lohren H, Brinkhaus SG, Knight AL, Caldwell KA, Caldwell GA, Karst U, Schwerdtle T, Bowman A, Aschner M. The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of alpha-synuclein in C. elegans. Metallomics. 2014;6:476–490. doi: 10.1039/c3mt00325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, Valentine WM, Aschner M. Dopaminergic neurotoxicity of S-ethyl N,N-dipropylthiocarbamate (EPTC), molinate, and S-methyl-N,N-diethylthiocarbamate (MeDETC) in Caenorhabditis elegans. J Neurochem. 2013;127:837–851. doi: 10.1111/jnc.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock M, van de Bor M. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies? Environ Int. 2014;70:15–24. doi: 10.1016/j.envint.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Dickinson BC, Tang Y, Chang Z, Chang CJ. A nuclear-localized fluorescent hydrogen peroxide probe for monitoring sirtuin-mediated oxidative stress responses in vivo. Chem Biol. 2011;18:943–948. doi: 10.1016/j.chembiol.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J. The neurotoxicity of iron, copper and manganese in Parkinson's and Wilson's diseases. J Trace Elem Med Biol. 2014 doi: 10.1016/j.jtemb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Gallo M, Park D, Riddle DL. Increased longevity of some C. elegans mitochondrial mutants explained by activation of an alternative energy-producing pathway. Mech Ageing Dev. 2011;132:515–518. doi: 10.1016/j.mad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr. 2009;4:59–67. doi: 10.1007/s12263-009-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-span extension from hypoxia in Caenorhabditis elegans requires both HIF-1 and DAF-16 and is antagonized by SKN-1. J Gerontol A Biol Sci Med Sci. 2013;68:1135–1144. doi: 10.1093/gerona/glt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao VH, Yu CW. Caenorhabditis elegans gcs-1 confers resistance to arsenic-induced oxidative stress. Biometals. 2005;18:519–528. doi: 10.1007/s10534-005-2996-3. [DOI] [PubMed] [Google Scholar]
- Link CD, Johnson CJ. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 2002;353:497–505. doi: 10.1016/s0076-6879(02)53072-x. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;26:593–618. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D, Rosas IO. Tobacco smoke-induced lung fibrosis and emphysema. Annu Rev Physiol. 2014;76:493–513. doi: 10.1146/annurev-physiol-021113-170411. [DOI] [PubMed] [Google Scholar]
- Park SK. Electrolyzed-reduced water increases resistance to oxidative stress, fertility, and lifespan via insulin/IGF-1-like signal in C. elegans. Biol Res. 2013;46:147–152. doi: 10.4067/S0716-97602013000200005. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]