Abstract
Objective
The Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT), a prospective double-blind randomized study, was designed to determine the effects of triple-drug lipid modification therapy versus mono-therapy over 24 months on the progression of atherosclerosis in the distal superficial femoral artery (SFA), as assessed by 3.0T magnetic resonance imaging (MRI).
Methods
A total of 102 patients were randomized to either mono-therapy with simvastatin (40 mg daily) or triple-therapy with simvastatin (40 mg daily), extended-release niacin (1500 mg daily), and ezetimibe (10 mg daily). MRI was performed at baseline and 6, 12, and 24 months. SFA wall, lumen, and total vessel volumes were quantified. MRI-derived SFA parameters and lipids were analyzed with multilevel models and nonparametric tests, respectively.
Results
Baseline characteristics did not differ between mono and triple-therapy groups, except for ethnicity (p= 0.02). SFA wall, lumen, and total vessel volumes increased non-significantly for both groups between baseline and 24-months. Non–high-density lipoprotein cholesterol was significantly reduced at 12 months with triple-therapy compared with mono-therapy (p= 0.01).
Conclusion
No significant differences were observed between mono-therapy using simvastatin and triple-therapy with simvastatin, extended-release niacin, and ezetimibe for 24-month changes in SFA wall, lumen, and total vessel volumes.
Keywords: peripheral artery disease, magnetic resonance imaging, lipids, atherosclerosis
INTRODUCTION
Peripheral artery disease (PAD) is associated with an increased risk of atherothrombotic and cardiovascular events and mortality.1-4 Despite the high prevalence of PAD the disease receives relatively little attention, and PAD patients are less likely to receive appropriate treatment for their atherosclerotic risk factors than patients with coronary artery disease.5,6 Several trials have determined clinical benefits of lowering plasma cholesterol concentrations.7-13 However, few studies have investigated the effects of lipid modification therapy on plaque burden in the arteries of the lower extremities.9 Previous placebo-controlled studies had shown that niacin increases high-density lipoprotein cholesterol (HDL-C) levels and lowers triglycerides and low-density lipoprotein cholesterol (LDL-C) levels, resulting in an improved atherogenic lipoprotein profile.14,15 Combination therapy with ezetimibe and statin had previously been shown to result in significantly greater reduction in LDL-C compared with statin alone.16 However, when ELIMIT was designed it was unknown whether ezetimibe and niacin were additive to standard statin lipid modification therapy in PAD patients. The goal of the Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT), a randomized control study in subjects with PAD, was to study the efficacy of intensive lipid-modifying triple-therapy (simvastatin, ezetimibe, and extended-release niacin) versus standard lipid-modifying mono-therapy (simvastatin) on the progression of atherosclerosis in the superficial femoral artery (SFA) as quantified by high resolution magnetic resonance imaging (MRI).
METHODS
Patients and Study Design
The ELIMIT study was approved by the Institutional Review Board and all subjects provided informed consent. Inclusion criteria were life-style-limiting claudication consistent with Fontaine Stage IIa/IIb or angiographically confirmed Trans-Atlantic Inter-Society Consensus A-C lesions in the SFA. PAD was confirmed by an ankle brachial index (ABI)<0.90 or by imaging including duplex ultrasound. Patients from the greater Houston area were screened and recruited at Ben Taub General Hospital, the Michael E. DeBakey Veterans Affairs Medical Center, and The Methodist Hospital between February 2005 and August 2008, and 102 patients were randomized (Figure 1). Initially, eligibility was restricted to patients who underwent revascularization for PAD within 3-months prior to enrollment. Subsequently, to improve recruitment this criterion was relaxed and approved by the Institutional Review Board on 03/01/2007 to also allow enrollment of patients who might undergo revascularization for PAD, had a revascularization for PAD >3-months prior to enrollment, or PAD patients who were medically managed.
Figure 1.
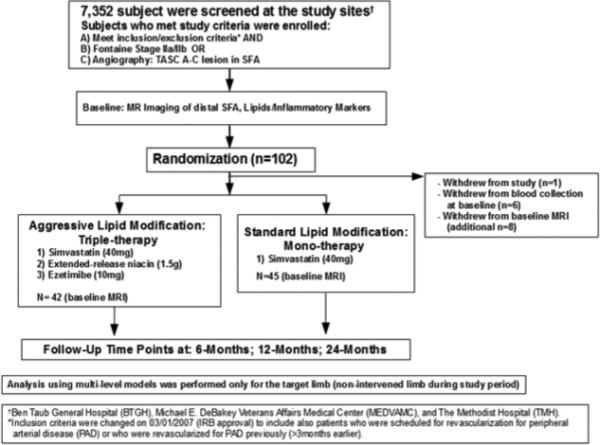
ELIMIT study design.
Randomization
ELIMIT was a randomized, double-blind and double placebo-controlled study. Patients were randomized to receive either standard lipid-modifying mono-therapy with simvastatin 40 mg daily (or another statin if unable to tolerate simvastatin) -- or intensive lipid-modifying triple-therapy with simvastatin 40 mg daily (or another statin if unable to tolerate simvastatin), ezetimibe 10 mg daily, and extended-release niacin 1500 mg daily. Patients in the mono-therapy group also received placebo ezetimibe, and placebo niacin. The niacin placebo contained a nontherapeutic 50 mg dose of immediate release niacin to mimic the common physical symptoms associated with the use of the drug and to maintain the blinding of the drug for both the patient and study staff. The placebo was titrated to 3 pills per day as tolerated and did not exceed 150 mg. In addition, all patients continued to receive the standard of care including medical management and the option of lower-extremity revascularization, if indicated. Study staff and patients were blinded to treatment groups.
Study visits
Patients underwent MR imaging at baseline, 6-months, 12-months, and 24-months (Figure 1). Analysis was performed only for the target limb, which was defined as the non-intervened limb or the less symptomatic limb in patients who were not scheduled for revascularization. If the target limb was revascularized during the study, we used only MRI data up to and including the last imaging visit prior to the intervention.
Primary outcome
The primary outcome variable was the change in SFA wall volume over 24-months, as determined by MRI. The 24-month changes in SFA lumen and SFA total vessel volumes were also analyzed.
MRI Data and Analysis
MRI scans were acquired with a 3.0T system (Signa Excite, GE Healthcare, Milwaukee, Wisconsin) using an unilateral phased array coil with a field of view (FOV) of 8 cm (along z-axis) and 12 cm (in plane x and y axes; Pathway Biomedical, Inc.). The center of the coil was placed on the distal thigh, centered 8 cm above the patella and secured with a Velcro strap. Proton-density-weighted (PDW), T1-weighted (T1W), and T2-weighted (T2W) scans were acquired for both lower extremities. Typical acquisition time was approximately 60 minutes. PDW sequences were acquired with a repetition time of 2575 ms, echo time of 30 ms, number of slices= 40, flip angle of 90°, slice thickness of 2 mm, in-pla ne pixel spacing of 0.43x0.43 mm, number of excitations of 2, echo train length of 8, matrix size of 384×224, and a field of view of 22 cm. MRI PDW, T1W, and T2W scan quality was determined using a 4-point image quality scale (4 being best) using edge sharpness, amount of blurring, artifacts, and amount of noise. The MRI sequence with the best scan quality was selected for subsequent analysis.
MRI co-registration
Co-registration of MRI scans across follow-up visits was performed manually using anatomical landmarks (artery, vein, and muscle). The reader, blinded to patient data, identified naturally occurring anatomical landmarks unique within each patient. Co-registration was assessed by intra and inter-reader correlation.
Image analysis and quality control
Reading of the SFA measurements (wall, lumen, and total vessel volumes) was performed by 2 readers blinded to patient identifiers and scan dates using VesselMASS (University of Leiden, The Netherlands). Inter-reader variability was assessed for 2 observers using the PDW scans. To minimize variability, the readers performed an initial adjustment reading phase using 15 randomly assigned scans (read by both readers simultaneously; phase I). Following the initial adjustment, another 48 randomly assigned scans were analyzed (phases II-III). During phase II, 24 scans were read independently and observers discussed their analysis. For phase III another 24 scans were read independently and observers were blinded to reading results. All scans from phases I and II were reread for the main analysis. Inter-reader variability was determined by intra-class correlation (ICC) using a two-way model.17 Scans from 8 randomly selected patients were obtained from the ELIMT database for 3 imaging time-points (baseline, 12-, and 24-months). Lumen, wall and total vessel volumes were quantified for each scan.
Sample size estimation
Sample size estimates were calculated separately for SFA lumen and wall measurements. We assumed a between-patient standard deviation at baseline for lumen volume of 5 mm3, and 14 mm3 for wall volume. We estimated that each patient will have a maximum of 4 MRI exams from baseline to 24 months. We also assumed a follow-up difference between treatment groups for lumen of >1.5 mm3 or 9% and 6.7 mm3 or 10% for wall volume. The estimates were guided by SFA pilot data from our laboratory and intraclass correlation coefficients from carotid artery studies.18,19 These assumptions resulted in a probability (power) > 0.80 in a two-sided test at a significance level of 0.025 for each of the variables (lumen and wall volume), given an enrollment of 120 patients and a subsequent 10% lost-to-follow-up rate. Sample size methodology given in Murray20 and Snijders and Bosker21 were used for multilevel analyses.
Statistical Methods
Baseline characteristics of the drug therapy groups were compared using analysis of variance or chi-square tests for continuous or discrete variables, respectively, or nonparametric analogs when the assumptions of these tests were not met. Variables were expressed as mean ± standard deviation (SD) or standard error, medians and interquartile range (IQR), percentages, or frequencies, respectively. Equal variance was determined with the Bartlett's test. All tests were two-tailed and p-values < 0.05 were considered statistically significant.
Multilevel statistical models were used to describe changes over time in the MRI outcome variables and to compare the drug therapy groups. Multilevel models also known as mixed linear models are similar in principle to ordinary regression models, and are appropriate for modeling of longitudinal data because they take into account the correlations due to repeated measurements within subjects.21 The advantage of multilevel models as compared to more usual statistical methods, such as repeated measures analysis of variance, is the capability to use data with missing or irregularly timed observations, due to death or loss to follow-up, on the outcome variable. This was a useful feature for this study, because there were variations between patients in the timing of clinic visits and some patients did not return for all follow-up tests. Multilevel models were analyzed using xtmixed procedure in Stata Statistical Software, Release 12 (StataCorp LP, College Station, Texas). Residual analyses were used to assess the fit of the model and the need for data transformations. Graphical analyses were used to visualize the slopes and confidence intervals for each of the 2 groups.
An ICC <0.30 was considered as poor agreement, 0.30 to 0.70 as moderate to good agreement, and >0.70 as excellent.17,22-24 All statistical analyses were conducted with Stata Statistical Software, Release 12.
EXPERIMENTAL RESULTS
Patient information
A total of 102 patients were randomized and 87 patients completed baseline MRI. Between randomization and the baseline visit, 1 patient withdrew from the study, 8 patients opted out from baseline imaging, and 6 additional patients declined blood collection at baseline. Throughout the study, 8 patients died (4 in each group), and 10 patients underwent revascularization of the target limb (5 in each group) as clinically indicated for symptomatic PAD. There were no significant predictors among the baseline characteristics for the 10 patients whose target limb was intervened during the study. For the 2-year visit, we excluded the MRI measurements of 5 patients who returned for their last MRI after 27-months; however, all other imaging data of these 5 patients were included in the multilevel models. The multilevel models used all available imaging data, including patients who only completed baseline imaging (n= 20) or completed at least 2 imaging visits other than baseline (n= 4). The final multilevel model included a total of 246 MRI scans (131 in mono-therapy group and 115 in triple-therapy group) obtained from 91 patients (46 mono-therapy, and 45 triple-therapy) across all visits (baseline: n= 87, 6-months: n= 40, 12-months: n= 56, 24-months: n= 40, and 23 patients who returned for MRI one month after baseline). The average time from baseline to the 12-month imaging visit was 386.3± 52.9 days and from baseline to the 24 month imaging visit was 683.5± 62.9 days. On average, every patient completed 2.7 MRI visits during this 2-year study.
MRI co-registration
A randomly selected subset of 15 patients was used to co-register each of the landmark types (artery, vein and muscle). Offsets, measured relative to baseline using any landmark type were not significantly different (p > 0.05). The average offset between anatomical landmark types at baseline and 12-month was not significantly different (−1.10±12.6 mm; p= 0.36).
Co-registration offsets with respect to baseline were different for baseline-24 months (p <0.001) equal to 3.68 mm corresponding to the thickness of less than 2 image slices or 4.6% of the total extent of a scan (80 mm). Two readers co-registered MRI scans from 12 patients to assess reproducibility. Intra-reader correlation for co-registration was high for all landmark types (ICC artery= 0.97, ICC vein= 0.97, ICC muscle= 0.95). Inter-reader correlation was high for baseline-12 months (ICC= 0.81) and baseline-24 month (ICC= 0.81) comparisons, respectively.
MRI image quality
Quality scores were analyzed by 1 reader across 3 imaging visits in 12 patients for PDW, T1W and T2W sequences at baseline, 12- and 24-months. The quality scores of PDW scans of the SFA were significantly higher than those of T1W and T2W scans (3.10 versus 2.49 and 2.29); however, T1W and T2W quality scores were not significantly different. Inter-reader correlation of quality scoring was excellent (ICC= 0.77). Hence, PDW scans were used for the analysis.
MRI quality control
Inter-reader correlation was assessed prior to reading all MRIs and was excellent for the combined analysis of 48 PDW scans for lumen volume (ICC= 0.97) and total vessel volume (ICC= 0.85) and moderate to good for wall volume (ICC= 0.48). Subsequently, we performed an inter-reader variability analysis stratified by phase II and III readings (24 scans each). Phase II reading ICCs for lumen, wall, and total vessel volumes were 0.67, 0.32, and 0.39 (moderate to good). Conversely, phase III readings showed a markedly improved inter-reader agreement, with ICCs for lumen, wall, and total vessel volumes of 0.99, 0.6, and 0.93, respectively.
Baseline characteristics
Baseline characteristics did not differ between mono and triple-therapy groups, except for ethnicity (Table 1). There were no group differences in MRI parameters (wall volume, lumen volume, and total vessel volume) or lipids at baseline (Tables 2-3).
Table 1.
Baseline characteristics of ELIMIT.
| Variable | Mono-Therapy (n=48) | Triple-Therapy (n=47) | P-value |
|---|---|---|---|
| Age (years), (mean±SD) | 63.9 ± 7.1 | 62.1 ± 7.8 | 0.29 |
| Gender (% male) | 95.8 | 91.5 | 0.44 |
| Race (% black) | 8.3 | 27.7 | 0.02 |
| BMI (kg/m2), (mean±SD) | 31.4 ± 7.3 | 30.9 ± 8.0 | 0.73 |
| History of hypertension (%) | 85.4 | 80.9 | 0.59 |
| History of hyperlipidemia (%) | 95.7 | 97.7 | 0.99 |
| History of diabetes (%) | 39.6 | 42.6 | 0.84 |
| Current smoking (%) | 35.4 | 48.9 | 0.22 |
| Aspirin use (%) | 97.9 | 100.0 | 0.99 |
| Statin use (%) | 97.9 | 95.7 | 0.62 |
| Target limb ABI* (mean±SD) | 0.83 ± 0.04 | 0.76 ± 0.04 | 0.22 |
All values are proportions, means, standard deviations (STD). ABI=ankle–brachial index. P-values were calculated with the Kruskal-Wallis rank test, Fisher's exact test or t-test.
ABI mono-therapy group: n=34; ABI triple-therapy group: n=35.
Table 2.
MRI measurements of the superficial femoral artery (SFA) over time in ELIMIT by multilevel model prediction.
| Measurements | Mono-Therapy (n=46) | Triple-Therapy (n=45) | P-value |
|---|---|---|---|
| Baseline MRI Measures of the SFA | |||
| Total vessel volume [mm3] | 56.0 ± 3.33 | 55.2 ± 3.44 | 0.85 |
| Wall volume [mm3] | 39.3 ± 2.06 | 38.3 ± 2.16 | 0.68 |
| Lumen volume [mm3] | 16.5 ±1.91 | 17.3 ± 1.95 | 0.71 |
| MRI Measures of the SFA At 6-Months (change from baseline ± SE) | P-value* | ||
| Total vessel volume [mm3] | 57.1 ± 3.33 (1.1 ± 0.19) | 55.9 ± 3.41 (0.7 ± 0.11) | 0.79 |
| Wall volume [mm3] | 39.9 ± 1.74 (0.6 ± 0.05) | 39.0 ± 1.80 (0.7 ± 0.04) | 0.94 |
| Lumen volume [mm3] | 16.7 ± 1.94 (0.2 ± 0.09) | 17.4 ± 1.97 (0.1 ± 0.09) | 0.81 |
| MRI Measures of the SFA At 12-Months (change from baseline ± SE) | P-value* | ||
| Total vessel volume [mm3] | 58.2 ± 3.69 (2.2 ± 0.26) | 56.6 ± 3.82 (1.4 ± 0.24) | 0.79 |
| Wall volume [mm3] | 40.5 ± 1.85 (1.2 ± 0.06) | 39.7 ± 1.96 (1.4 ± 0.08) | 0.94 |
| Lumen volume [mm3] | 16.9 ± 2.05 (0.5 ± 0.12) | 17.5 ± 2.10 (0.2 ± 0.15) | 0.81 |
| MRI Measures of the SFA At 24-Months (change from baseline ± SE) | P-value* | ||
| Total vessel volume [mm3] | 60.5 ± 5.13 (4.5 ± 0.71) | 58.1 ± 5.50 (2.9 ± 0.55) | 0.79 |
| Wall volume [mm3] | 41.8 ± 3.03 (2.5 ± 0.19) | 41.1 ± 3.31 (2.8 ± 0.18) | 0.94 |
| Lumen volume [mm3] | 17.4 ± 2.49 (0.9 ± 0.33) | 17.6 ± 2.62 (0.3 ± 0.36) | 0.81 |
All values are means and or standard errors (SE).
P-values were obtained from multilevel models for the differences between groups with respect to change from baseline.
Table 3.
Lipids at baseline and 12-months.
| Variables | Mono-Therapy (n=48) | Triple-Therapy (n=47) | P-value |
|---|---|---|---|
| Baseline Lipids | |||
| Total cholesterol (mg/dl) | 173.5 (139, 198) | 162.0 (134, 202) | 0.50 |
| HDL cholesterol (mg/dl) | 41.5 (34, 48) | 39.0 (32, 46) | 0.54 |
| LDL cholesterol (mg/dl)⟂ | 96.0 (73, 113) | 88.0 (69, 120) | 0.85 |
| Non-HDL cholesterol (mg/dl) | 125.5 (98, 160) | 120.0 (95, 149) | 0.63 |
| Triglycerides (mg/dl) | 146.5 (99, 212) | 134 (95, 177) | 0.58 |
| hs-CRP (mg/L) | 2.97 (1.77, 6.17) | 3.31 (1.79, 8.08) | 0.59 |
| Lipid Data at 12-Months (change from baseline) | |||
| Variable | Mono-Therapy (n=36) | Triple-Therapy (n=31) | P-value* |
| Total cholesterol (mg/dl) | −7.50 (−30.50, 8.00) | −30.00 (−63.00, −1.00) | 0.040 |
| HDL cholesterol (mg/dl) | −0.50 (−4.00, 4.60) | 4.00 (−5.00, 9.00) | 0.13 |
| LDL cholesterol (mg/dl)‡ | −8.00 (−20.00, 4.00) | −28.00 (−48.00, −4.10) | 0.028 |
| Non-HDL cholesterol (mg/dl) | −5.50 (−30.00, 11.00) | −29.00 (−67.00, −12.00) | 0.012 |
| Triglycerides (mg/dl) | −1.50 (−68.50, 51.50) | −33.0 (−71.00, 10.00) | 0.12 |
| hs-CRP (mg/L)† | 0.26 (−1.35, 1.66) | −0.24 (−1.68, 0.51) | 0.17 |
All values are medians and interquartile range (IQR). P-values were calculated with the Kruskal-Wallis rank test. hs-CRP= high sensitive c-reactive protein.
P-values indicate group differences with respect to change from baseline. To convert from mg/dl to mmol/l, multiply by 0.026.To convert HDL-C or LDL-C from mg/dl to mmol/L, multiply by 0.0259. To convert triglycerides from mg/dl to mmol/l, multiply by 0.0113.
LDL-C: N=45 for each group. LDL-C was not calculated for 5 patients (n=3 in mono-therapy group, n=2 in triple-therapy group) with TG≥400 mg/dl. Estimation of LDL-C using the Friedewald formula is inaccurate when TG≥400 mg/dl.39
LDL-C: n=32 in mono-therapy group and n=30 in triple-therapy group.
CRP: n=29 in triple-therapy group.
MRI measurements of distal SFA
The primary endpoint (24-month change of SFA wall volume between mono-therapy and triple-therapy groups) was not significant (p= 0.94, Table 2, Supplemental Figure S1). There was no significant change over 24-months between groups for SFA total vessel volume (p= 0.79) or lumen volume (p= 0.81). Intra-group changes between imaging visits were also not significant for SFA wall volume (p= 0.94), lumen volume (p= 0.81), and total vessel volume (p= 0.79). Figure 2 shows cross-sectional MRI images of the SFA at baseline, 12-months, and 24-months for a patient from the mono-therapy group and from the triple-therapy group.
Figure 2.
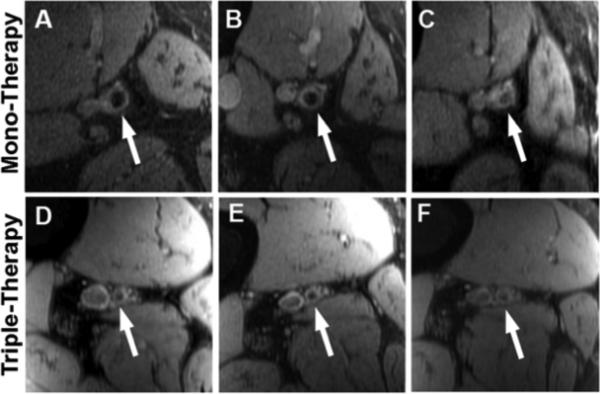
Cross-sectional MRI images of the superficial femoral artery (SFA). Panels A-C: SFA at baseline (A), 12-months (B) and 24-months (C) for a patient from the mono-therapy group. Panels D-F: SFA at baseline (D), 12-months (E) and 24-months (F) for a patient from the triple-therapy group.
Lipids at 12-months
Non-high density lipoprotein cholesterol (non-HDL-C) was significantly lower at 12-months in the triple-therapy group compared with the mono-therapy group (p= 0.01, Table 3). Similarly, total cholesterol and LDL-C were lower in the triple-therapy group at 12-months (p= 0.04 and p= 0.03, respectively). Conversely, the increase in high density lipoprotein cholesterol (HDL-C) in the triple-therapy group was not significant. Similarly, there were no significant differences between groups for the 12-month changes in triglycerides and high-sensitivity C-reactive protein (hs-CRP).
Major adverse cardiovascular events
During the study, a total of 19 major adverse cardiovascular events were recorded in 16 patients (6 mono-therapy, 10 triple-therapy), resulting in a total event rate of 15.7%. There was no difference between groups in major adverse cardiovascular events (p= 0.42) including all-cause death (4 in each group), myocardial infarction (1 in mono and 3 in triple therapy group), major stroke (2 in each group), and coronary revascularization (1 in mono and 2 in triple therapy group).
DISCUSSION
In the ELIMIT study, we have investigated the effects of lipid modification mono-therapy versus triple-therapy in PAD patients with life-style limiting claudication. The first major finding of the study was that the 24-month change in the distal SFA wall volume (primary endpoint) was not significantly different between the 2 treatment groups. Secondly, both, mono-therapy using simvastatin and triple-therapy using simvastatin, ezetimibe and extended-release niacin resulted in a modest but non-significant change in atherosclerosis in the wall of the distal SFA over a period of 2 years in PAD patients. The third finding was that non-HDL-C, total cholesterol, and LDL-C were reduced more significantly at 12-months in the triple-therapy group compared with the mono-therapy group, as expected.
Effects of mono- versus combination-drug therapy on circulating lipids
Several earlier studies have highlighted the potential benefit of niacin in addition to statin therapy. The HDL-Atherosclerosis Treatment Study (HATS) showed that in 160 men and women with clinical coronary disease, simvastatin plus niacin compared to placebo was associated with a significant regression in stenosis of the proximal coronary segments, as measured by angiography.25 The Coronary Drug Project showed a modest benefit in the reduction of nonfatal recurrent myocardial infarction with niacin treatment but no effect on total mortality.26
Recent large clinical trials have cast doubt on the benefits of niacin when added to statin compared to statin mono-therapy. The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial enrolled a total of 3,414 patients and found no incremental clinical benefit from the addition of extended-release niacin to simvastatin 40 mg daily.7 The Heart Protection Study 2: Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) was a large international trial that did not show any benefit in the reduction of major vascular events in more than 25,000 coronary heart disease patients randomized to either extended-release niacin/laropiprant plus statin therapy or statin therapy alone.27 Previous reports have demonstrated that ezetimibe changes LDL subclass composition and reduces LDL cholesterol levels.28,29 Although our study was not an outcomes trial, the combination of extended-release niacin with cholesterol lowering therapy using simvastatin and ezetimibe did not show any significant changes in SFA plaque volume when compared with simvastatin alone.
Lipid modification therapy and progression of atherosclerosis as measured by MRI
Several studies have assessed the impact of lipid modification therapy on imaging based surrogate markers of clinical end points.
West et al.9 studied the effects of ezetimibe on plaques in the SFA using MRI. In this 2-year study, atherosclerotic plaque volume was measured in the proximal SFA in 67 PAD patients. Statin naïve patients (n= 34) were randomized to simvastatin (40 mg) or simvastatin (40 mg) plus ezetimibe (10 mg), and patients on statins at baseline (n= 33) also received open-label ezetimibe. There was no significant change in plaque volume from baseline to year 2 in either randomized treatment group.
Although the study by West et al.9 had a design similar to ELIMIT, several details were different. In our study, 97% of patients were already on statin therapy prior to enrollment and participants had lower baseline LDL-C levels and there was no niacin arm in the study by West et al.9 ELIMIT evaluated change in wall volume in the distal SFA where West et al.9 studied the proximal segment of the SFA. However, both studies are among the first lipid modification trials describing the change of atherosclerosis disease burden of the SFA, as measured by MRI. Atherosclerosis plaque burden in the SFA did not change significantly over 2-years in either study, although significant plaque progression was reported in the study by West et al.9 in the group receiving open-label ezetimibe added to ongoing baseline statin.
In contrast to the SFA as in our study, the carotid artery has been used frequently as the imaging location of choice. The Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6: HDL and LDL Treatment Strategies in Atherosclerosis (ARBITER 6-HALTS) trial randomized 315 coronary heart disease patients on stable statin treatment to either ezetimibe 10 mg daily or to extended-release niacin 2,000 mg daily.30 The study showed a significant regression in carotid intima-media thickness at 7 or 14-months in the niacin plus statin group compared with the ezetimibe plus statin group.
The advent of rapid 3D isotropic black-blood MR imaging techniques which were not available when our study was designed, allow imaging of atherosclerotic plaque in the entire femoral artery. As demonstrated in this study, plaque imaging and analysis techniques developed and validated in other vascular beds are applicable to the femoral arteries with some optimization for local anatomy.31
Limitations
This study has several limitations. MR imaging was performed only in the distal SFA, and MRI measurements were utilized only from the target-limb. One of the challenges in MR imaging of SFA atherosclerosis is sampling a large enough field of view. When the ELIMIT study was conceived only an unilateral phased array coil was available. However, modern bilateral coils allow rapid acquisition of both SFA vessels simultaneously with an excellent signal to noise ratio.32 Novel, rapid 3D imaging techniques such as 3D motion sensitized driven equilibrium prepared rapid gradient echo (3D-MERGE) have been developed for imaging of the femoral artery with high isotropic resolution of 0.5 mm3 voxel size.33
Also, we assessed plaque burden but not plaque composition which has been associated with the risk of plaque rupture.34 However, during all imaging visits of this study, we acquired multi-contrast turbo spin echo sequences (T1W, T2W, and PDW) for all patients which will be used in a forthcoming study to determine SFA plaque composition.31
Inter-reader variability as determined by ICC was moderate to good for MRI wall volumes.17,22-24
Ethnicity, which was not part of the randomization protocol differed significantly between the mono- and triple-therapy groups. Also, the attrition rate was high in this study. However, the usage of multi level models maximized data utilization. A high rate of missing data and loss to follow-up due to death, comorbidity and inability to complete the study in excess of over 20% is not uncommon in PAD or niacin studies.9,35-37 It has been shown that ezetimibe changes LDL subclass composition.28,29
Finally, ELIMIT was not powered to assess the effect of lipid-modifying mono- versus triple-therapy on clinical outcomes in PAD patients, and study participants were followed for only 2 years.
More than 95% of ELIMIT participants were on statins prior to enrollment. We did not collect any information on statin use duration prior to randomization and therefore, cannot account for any plaque stabilization that may have occurred as a result of chronic statin use in our study population.38
CONCLUSION
No significant differences were observed between mono-therapy using simvastatin and triple-therapy with simvastatin, extended-release niacin, and ezetimibe for 24-month changes in SFA wall, SFA lumen, and SFA total vessel volumes. Mono-therapy and triple-therapy showed a modest non-significant increase of atherosclerosis in PAD patients, as quantified by the 24-month change in SFA wall volume.
Supplementary Material
Supplemental Figure S1. Results of the multilevel model analysis for the 2-year change in SFA wall volume for mono-therapy and triple-therapy. SFA wall volume estimates and 95% confidence intervals are shown for baseline, 6-, 12-, and 24-months.
Highlights (max of 5 highlights, each max. of 85 characters including spaces).
Mono and triple lipid modification therapy in peripheral artery disease patients
Imaged distal superficial femoral artery (SFA) by 3.0T magnetic resonance imaging
SFA plaque volume progressed non-significantly over 2-years for both groups
No difference between drug groups for the 2-year change of SFA wall volume
Non-high-density lipoprotein cholesterol was reduced more at 1-year in triple-therapy
Acknowledgements
This work was supported by NIH grants R01HL63090, R01HL075824, and R01 HL085769 and by funding from the Division of Atherosclerosis and Vascular Medicine, Department of Medicine, Baylor College of Medicine, Houston, Texas.
The authors wish to express their thanks to Kay Kimball for guidance with the statistical analysis, Alaina
Yawn and Lee Sanford for assistance in image acquisition and Shawna Johnson and Dr. Husam Athamneh for help with patient recruitment.
Study Funding: Merck & Co. and Abbott Laboratories supplied the study medication (simvastatin, ezetimibe, and niacin). Merck and Abbott had no input in the study design, analysis, or presentation of results.
Dr. Yang received grant support through the AHA South Central Affiliate Postdoctoral Fellowship Grant (ID: 2010POST4250013).
Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Service (HSR&D) Career Development Award (CDA-09-028).
Dr. Nambi was supported by an NIH Career Development Award (K23HL096893).
List of abbreviations
- PAD
peripheral artery disease
- SFA
superficial femoral artery
- MRI
magnetic resonance imaging
- ABI
ankle brachial index
- PDW
Proton-density-weighted
- T1W
T1-weighted
- T2W
T2-weighted
- ICC
intra-class correlation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Gerd Brunner, PhD: None.
Eric Y. Yang, MD: None.
Anirudh Kumar, BS: None.
Wensheng Sun, MS: None.
Salim S. Virani, MD/PhD: None.
Smita Negi, MD: None.
Tyler Murray, BA: None.
Peter H. Lin, MD: None.
Ron C. Hoogeveen, PhD: None.
Changyi Chen, MD/PhD: None.
Jing-Fei Dong, MD/PhD: None.
Panagiotis Kougias, MD: None.
Addison Taylor, MD/PhD: None.
Alan B. Lumsden, MD: None.
Vijay Nambi, MD/PhD: Research Grant, Modest: GE/Tomtec; Other Research Support, Modest: Roche. Christie M. Ballantyne, MD: Research Grant, Significant: Merck, Abbott; Speakers Bureau, Significant: Merck, Abbott; Consultant/Advisory Board, Significant: Merck, Abbott.
Joel D. Morrisett, PhD: None.
REFERENCES
- 1.Belch JJ, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003 Apr 28;163(8):884–892. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005 Oct 25;112(17):2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Murphy TP, Lovell MB, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116(18):2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101. [DOI] [PubMed] [Google Scholar]
- 4.Lumsden AB, Rice TW, Chen C, et al. Peripheral arterial occlusive disease: magnetic resonance imaging and the role of aggressive medical management. World J Surg. 2007 Apr;31(4):695–704. doi: 10.1007/s00268-006-0732-y. [DOI] [PubMed] [Google Scholar]
- 5.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012 Oct 24;308(16):1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker GJ, McClenny TE, Kovacs ME, Raabe RD, Katzen BT. The importance of increasing public and physician awareness of peripheral arterial disease. J Vasc Interv Radiol. 2002 Jan;13(1):7–11. doi: 10.1016/s1051-0443(07)60002-5. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011 Dec 15;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West AM, Anderson JD, Meyer CH, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. 2011 Apr 16;218(1):156–162. doi: 10.1016/j.atherosclerosis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppola G, Novo S. Statins and peripheral arterial disease: effects on claudication, disease progression, and prevention of cardiovascular events. Arch Med Res. 2007 Jul;38(5):479–488. doi: 10.1016/j.arcmed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003 Sep 23;108(12):1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 12.Schillinger M, Exner M, Mlekusch W, et al. Statin therapy improves cardiovascular outcome of patients with peripheral artery disease. Eur Heart J. 2004 May;25(9):742–748. doi: 10.1016/j.ehj.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Berneis K, Rizzo M, Spinas GA, et al. The predictive role of atherogenic dyslipidemia in subjects with non-coronary atherosclerosis. Clin Chim Acta. 2009 Aug;406(1-2):36–40. doi: 10.1016/j.cca.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Vega GL, McGovern ME, et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002 Jul 22;162(14):1568–1576. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- 15.Blankenhorn DH, Azen SP, Crawford DW, et al. Effects of colestipol-niacin therapy on human femoral atherosclerosis. Circulation. 1991 Feb;83(2):438–447. doi: 10.1161/01.cir.83.2.438. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003 May 20;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979 Mar;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 18.Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002 Dec 3;106(23):2884–2887. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 19.Adams GJ, Simoni DM, Bordelon CB, Jr., et al. Bilateral symmetry of human carotid artery atherosclerosis. Stroke. 2002 Nov;33(11):2575–2580. doi: 10.1161/01.str.0000035736.30488.7a. [DOI] [PubMed] [Google Scholar]
- 20.Murray D. Design and Analysis of GroupRandomized Trials. Oxford University Press Inc; New York, NY: 1998. [Google Scholar]
- 21.Snijders TAB, Bosker RRJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. SAGE; 1999. [Google Scholar]
- 22.Nunnally JC, Bernstein IH. Psychometric theory. McGraw-Hill Inc; New York: 1994. [Google Scholar]
- 23.Carod-Artal FJ, Ferreira Coral L, Stieven Trizotto D, Menezes Moreira C. Self- and proxy-report agreement on the Stroke Impact Scale. Stroke. 2009 Oct;40(10):3308–3314. doi: 10.1161/STROKEAHA.109.558031. [DOI] [PubMed] [Google Scholar]
- 24.Etherington J, Innes G, Christenson J, et al. Development, implementation and reliability assessment of an emergency physician performance evaluation tool. CJEM. 2000 Oct;2(4):237–245. doi: 10.1017/s1481803500007260. [DOI] [PubMed] [Google Scholar]
- 25.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001 Nov 29;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 26.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986 Dec;8(6):1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 27.Haynes R, Jiang L, Hopewell JC, et al. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013 May;34(17):1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo M, Rini GB, Spinas GA, Berneis K. The effects of ezetimibe on LDL-cholesterol: quantitative or qualitative changes? Atherosclerosis. 2009 Jun;204(2):330–333. doi: 10.1016/j.atherosclerosis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Berneis K, Rizzo M, Berthold HK, Spinas GA, Krone W, Gouni-Berthold I. Ezetimibe alone or in combination with simvastatin increases small dense low-density lipoproteins in healthy men: a randomized trial. Eur Heart J. 2010 Jul;31(13):1633–1639. doi: 10.1093/eurheartj/ehq181. [DOI] [PubMed] [Google Scholar]
- 30.Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010 Jun 15;55(24):2721–2726. doi: 10.1016/j.jacc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Karmonik C, Basto P, Vickers K, et al. Quantitative segmentation of principal carotid atherosclerotic lesion components by feature space analysis based on multicontrast MRI at 1.5 T. IEEE Trans Biomed Eng. 2009 Feb;56(2):352–360. doi: 10.1109/TBME.2008.2003100. [DOI] [PubMed] [Google Scholar]
- 32.Brown R, Karmonik C, Brunner G, et al. Simultaneous Bilateral Magnetic Resonance Imaging of the Femoral Arteries in Peripheral Arterial Disease Patients. J. Mag Reson. Imag. 2011;34(1):150–156. doi: 10.1002/jmri.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balu N, Wang J, Zhao X, Hatsukami T, C Y. Targeted Multi-contrast Vessel Wall Imaging of Bilateral Peripheral Artery Disease. Proceedings of the 18th Annual Meeting of ISMRM Stockholm. 2010 [Google Scholar]
- 34.Hellings WE, Moll FL, De Vries JP, et al. Atherosclerotic plaque composition and occurrence of restenosis after carotid endarterectomy. JAMA. 2008 Feb 6;299(5):547–554. doi: 10.1001/jama.299.5.547. [DOI] [PubMed] [Google Scholar]
- 35.Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011 Sep;32(18):2274–2281. doi: 10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 36.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013 Jul 3;310(1):57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009 Nov 26;361(22):2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 38.Zhao XQ, Dong L, Hatsukami T, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging. 2011 Sep;4(9):977–986. doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Results of the multilevel model analysis for the 2-year change in SFA wall volume for mono-therapy and triple-therapy. SFA wall volume estimates and 95% confidence intervals are shown for baseline, 6-, 12-, and 24-months.


