Summary
X-Linked severe combined immunodeficiency (SCID-X1) is a genetic disease that leaves newborns at high risk of serious infection and a predicted lifespan of less than one year in the absence of a matched bone marrow donor. The disease pathogenesis is due to mutations in the gene encoding the Interleukin-2 receptor gamma chain (IL-2Rγ) leading to a lack of functional lymphocytes. With the leukemogenic concerns of viral gene therapy there is a need to explore alternative therapeutic options. We have utilized induced pluripotent stem cell (iPSC) technology and genome editing mediated by TALENs to generate isogenic patient-specific mutant and gene corrected iPSC lines. While the patient-derived mutant iPSC have the capacity to generate hematopoietic precursors and myeloid cells, only wild-type and gene-corrected iPSC can additionally generate mature NK-cells and T-cell precursors expressing the correctly spliced IL-2Rγ. This study highlights the potential for the development of autologous cell therapy for SCID-X1 patients.
Keywords: TALEN, Lymphoid, Homologous Recombination, Myeloid, Differentiation
Primary immunodeficiencies (PIDs) constitute a large and heterogeneous group of rare heritable disorders, including X-linked Severe Combined Immunodeficiency (SCID-X1), Adenosine deaminase deficiency-SCID (ADA-SCID) and Wiskott-Aldrich syndrome (WAS), resulting in an underdeveloped and/or functionally compromised immune system. Allogeneic hematopoietic stem cell transplant (HSCT) from a matched donor confers significant therapeutic benefit with over 90% success rate, however, scarcity of HLA-matched donors makes this approach less universally viable (Mukherjee and Thrasher, 2013). Autologous transplantation of hematopoietic stem cells (HSC) genetically corrected by targeted genome editing has been shown to be efficacious in proof-of-concept studies (Genovese et al., 2014), and even clinically effective upon lentiviral gene therapy for patients with metachromatic leukodystrophy (Aiuti et al., 2013) and WAS (Biffi et al., 2013). However, these autologous HSCT-based approaches are limited by imperfect methodologies for the culture and expansion of HSCs ex vivo.
SCID-X1 is an inherited disorder affecting the immune system. It is caused by mutations in the common receptor gamma chain, a subunit of several cytokine receptors including interleukin-2, -4, -7, -9, -15 and -21 that commonly leads to a lack of T cells, functional B cells and an absence of NK cells, leaving these patients severely immune-compromised. SCID-X1 affects males with an estimated incidence of 1:50,000 newborns and these individuals are at high risk of infection due to defective generation of early lymphoid progenitors. Without treatment, life expectancy is less than one year with a poor quality of life. A proven cure is transplantation of HLA-matched bone marrow, however such patient matched donors are relatively limited.
Gene therapy trials on SCID-X1 patients were initiated in 1999 and promising initial results emerged showing T, B and NK cell function comparable to age matched controls (Cavazzana-Calvo et al., 2000). Unfortunately, after the development of T-cell acute lymphoblastic leukemia (T-ALL) as a consequence of vector-mediated genotoxicity, the trials were prematurely halted in 2003 and a new therapeutic approach is still sought (Hacein-Bey-Abina et al., 2008; Hacein-Bey-Abina et al., 2003b).
Transcription activator-like effectors (TALEs) from the plant pathogen Xanthomonas are sequence-specific DNA-binding proteins (Bogdanove et al., 2010; Kay and Bonas, 2009; Kay et al., 2007; Romer et al., 2007), which can be engineered to bind any desired target sequence (Boch et al., 2009; Moscou and Bogdanove, 2009). A pair of TALE nucleases (TALENs) can be used to generate a double-strand break (DSB) at a specific genomic locus and consequently to mediate HR (Bedell et al., 2012; Li et al., 2011). TALENs have been successfully employed to mediate site-specific genome modification by HR in human pluripotent stem cells (Ding et al., 2013; Hockemeyer et al., 2011), which offers the potential of an alternative gene/stem cell therapeutic approach.
Natural Killer (NK) cells are a key component of innate immunity and central to the host immune defense against pathogens and tumors (Biron et al., 1999; Vivier et al., 2011). NK cells have been successfully differentiated from CD34+ cord blood cells (Kao et al., 2007; Meek et al., 2010). Differentiation from pluripotent stem cells has proved somewhat harder to accomplish. Initial studies identified putative NK cells from the differentiation of hESC and iPSC; however these cells were characterized based solely on their expression of CD56 and lacked analysis of the functional receptors expressed on mature NK cells (Tabatabaei-Zavareh et al., 2007). As NK cells mature they start to express both activating and inhibitory receptors that regulate NK cell activity. Killer cell Ig-like receptors (KIRs) and CD94/NKG2 heterodimers are two major receptor types that interact with MHC on target cells. For iPSC, however, the yield is considerably lower than for ESC (Ni et al., 2011).
In the current study we were able to generate provirus free iPSC lines from a SCID-X1 patient, correct the genetic defect utilizing TALENs and differentiate these cells in vitro to NK cells expressing mature NK cell markers. Notably, while all tested lines were capable of generating myeloid and endothelial cells, only the wild type and gene corrected lines could differentiate into NK-cells and demonstrated the presence of a correctly spliced IL-2Rγ. This is the first evidence of genomic correction of SCID-X1 patient iPSC resulting in the regeneration of mature lymphoid cells in vitro and holds great promise for the development of novel therapeutic approaches for this incurable and terminal disease.
Eight iPSC lines were derived from bone marrow multipotent stem cells (BM-MSC) from an infant with SCID-X1, using a Cre-excisable lentiviral vector containing six reprogramming factors (Firth et al., 2014). Control iPSC were also generated from cord blood derived endothelial cells and dermal fibroblasts.
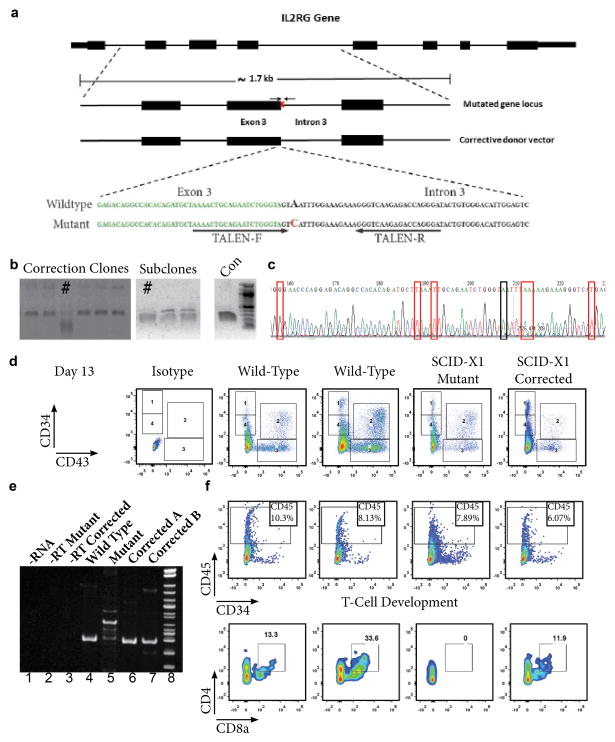
The donor patient harbored a novel splice-site mutation, c.468+3A>C of the IL2-Rγ. This specific mutation results in a lack of functional NK cells and T cells (Ginn et al., 2004). The mutation is an A to C substitution in the third base pair of intron 3 of the IL2Rγ gene, leading to aberrant splicing of the γc transcript. TALEN pairs were designed to target genomic sequences proximal to the described mutation (Figure 1a) and their functional activity at the desired target locus was validated (Figure S1a). The target SCID-X1 mutation was corrected by co-nucleofection of these TALENs in combination with a donor plasmid containing the corrective DNA sequence (Figure 1a). Corrected clones identified upon screening are shown in Figure 1b. Correction of the IL2Rγ gene in each clone was verified by sequencing an integration-specific PCR product of the target genomic DNA, where correction of the target mutation at the endogenous chromosomal locus was detected along with the presence of silent mutations introduced in the corrective sequence (Figure 1c). Integration of the corrective IL2Rγ sequence at its desired endogenous chromosomal locus was achieved at an overall efficiency of 2.6% without selection. The presence of the desired genetic correction and introduced silent mutations was confirmed by whole exome sequencing of the corrected and parental iPSC lines, which also verified the lack of significant off-target effects in any coding sequence due to gene correction with TALENs (Figure S1).
Figure 1. TALEN Mediated Gene Correction and Lymphoid Differentiation of SCID-X1 iPSC.
a) Schematic representation of the IL2Rγ gene, the endogenous target locus of our patient-specific mutation and the corrective donor vector used. The sequence of the patient-specific IL2Rγ splice-site target mutation and the corrective donor vector sequence are also shown below. The point mutation causing the alteration of this exon/intron consensus splice site is indicated in red. Exon 3 sequence immediately preceding the splice site is denoted by green, bold bases. TALEN binding sites are indicated by black arrows. b) Identification and isolation of corrected iPSCs through single-cell clonal amplification and screening of the PCR products with an XmaI restriction digest that is specific to the correction event. Corrected clones and subclones are identified by #. c) Chromatogram of the corrected iPSC clone indicated in Fig. 1b, as verified by sequencing. The red boxes indicate each of the silent mutations that were introduced to abolish TALEN activity on the corrective or corrected DNA sequences. Black box indicates the corrected disease-causing base. d) Comparative analysis of FACS data from wild type, SCID-X1 mutant and SCID-X1 corrected iPSC. Data shows CD34 and CD43 expression at Day 13 of differentiation. Isotype controls are included in the left panel. e) RT-PCR analysis of RNA extracted from T-cell precursors in the floating fraction generated from wild type, patient-derived SCID-X1 mutant iPSC or SCID-X1 corrected iPSC clones. f) FACS analysis of the floating fraction of cells co-cultured on OP9-DL feeders from two independent wild type, the SCID-X1 mutant and SCID-X1 gene corrected iPSC line. CD45+ and CD45+/CD4+/CD8a+ positive populations are indicated.
The interleukin signaling affected by mutations in the common gamma chain should only have a significant impact on lymphoid differentiation and not on other hematopoietic lineages. As expected, each iPSC line was capable of proficiently generating CD34+ hematopoietic progenitor cells and cells with cell surface markers of a hematopoietic stem cell; CD90+ CD34+, CD43+, CD38−, which acquired CD45 expression upon commitment to the myeloid lineage (Figure 1d, S2a). The mutant and corrected cell lines tested showed a similar capacity for generating mature myeloid colony forming units (CFU) in a methylcellulose based maturation assay (Figure S2b). After 14 days of myeloid maturation each line was equally capable of generating CD45+ CD14+ monocytes and CD45+ Glycophorin A+ erythroid progenitors (Figure S2c).
CD34+ progenitor cells derived from EBs were sorted at day 8 of differentiation and plated on OP9 cells overexpressing DL-1. After 14 days of differentiation on OP9-DL-1 in the presence of IL-7 we were able to generate CD45+ cells in the floating fraction that also co-expressed CD4 and CD8a. The mutant SCID-X1 line only generated CD45+ cells, no significant population of CD4+/CD8a+ cells was observed (Figure 1f). We were able to see the rescue of the aberrant splicing of the IL2Rγc transcript in CD45+ T-cell precursors derived from the gene corrected iPSC lines as compared to the patient iPSC-derived mutant CD45+ cells (Figure 1e, Compare lanes 5 with 4, 6 & 7).
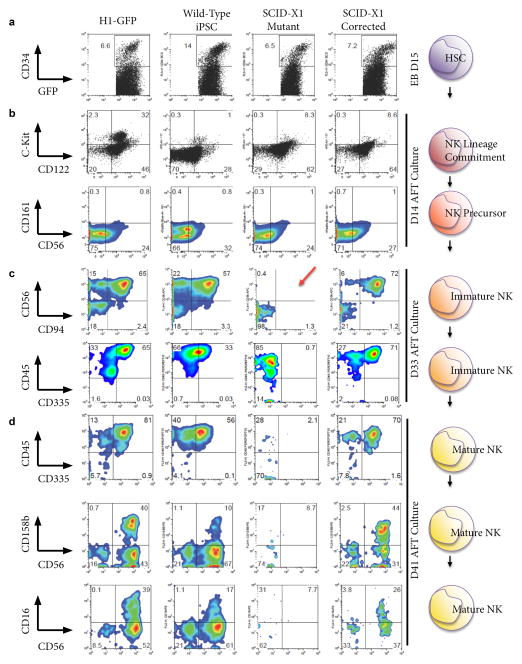
To determine whether lymphoid potential was restored in the corrected iPSC line, we first produced HSC from day 15 embryoid body (EB) cultures from each of the indicated GFP-transduced iPSC lines, purified them based on CD34 expression, then transferred these to AFT024 layer cultures (Ni et al., 2011). Hematopoietic precursors isolated from SCID-X1 mutant iPSC did not differ markedly from those of the various controls, generating similar percentages of CD34+ in EB culture by day 15 (Figure 2A). HSC derived from all lines generated cells which could progress to early immature NK cells as indicated by expression of CD122, and also by extremely low level expression of CD117 (c-kit), CD161 and CD56 (Figure 2B). Thus, in vitro, this patient’s defect in common γ-chain signaling does not prevent entry and early progression within the NK lineage.
Figure 2. Generation of Mature NK Cells.
FACS analysis of dissociated cells from H1-Embryonic Stem Cells (ESC), Wild type iPSC, SCID-X1 Mutant iPSC and SCID-X1 Gene-corrected iPSC for the antigens indicated on the left at the following time points; a) Embryoid Bodies (EB) at Day 15; b) NK Lineage committed cells/NK precursors at Day 14 of AFT culture; c) Immature NK cells at Day 33 of AFT culture. The red arrow highlights the lack of CD56/CD94+ cells in the mutant iPSC and d) mature NK cells at Day 41 of AFT culture. Cell percentages are indicated at the corner of each gate.
By day 20, however, differences become evident when CD94 and CD56 emerge in control and corrected SCID-X1 cultures, but are absent in SCID-X1 cells (data not shown). The difference becomes much more pronounced as the cells progress; by day 33 of culture there is a clear defect in differentiation of the SCID-X1 cells. This suggests that these cells cannot pass into the early immature NK developmental stage as defined by expression of these two markers (Figure 2C). Similarly, while SCID-X1 derived cells in AFT culture are clearly hematopoietic, as they strongly express CD45, they are negative for CD335, a marker uniquely expressed on NK cells (Figure 2C). To rule out that the SCID-X1 cells were slower in developing than the wild type or gene-corrected cells, we continued a long term differentiation to day 41, at which point very few CD45+ SCID-X1 cells remain, and no evidence for their further development was observed. In contrast, the gene-corrected and control lines progressed to mature NK status, expressing both inhibitory and activating receptors, KIR (CD158b) and CD16, respectively, found on mature NK cells (Figure 2D). These cells continued to express CD335, which was never observed in the mutant cells. This demonstrates that correction of the IL2Rγ mutation in SCID-X1 iPSC results in restored developmental progression along the NK lineage, through to cells expressing multiple mature NK cell markers.
In this report, we have demonstrated that the precise correction of a novel splice-site mutation of the IL2Rγ gene, at its endogenous genomic locus in patient-derived iPSC, results in the correction of the splicing defect of the IL2Rγ transcript and thus rescues their ability to differentiate into NK cells. It is the first report, to our knowledge, to describe the derivation of iPSC from a SCID-X1 patient and the functional gene correction of a point mutation using genome editing technology using a selection-free approach. Upon gene correction, we generated mature NK cells and T-cell precursors that were otherwise abnormal or missing in the patient.
Improved viral approaches for gene therapy have been developed since the original SCID-X1 clinical trials (Hacein-Bey-Abina et al., 2002). Lentiviral gene therapy using autologous HSC has proven initially efficacious in the treatment of WAS (Aiuti et al., 2013; Verma, 2013) and metachromatic leukodystrophy (Biffi et al., 2013) patients. An ongoing clinical trial using a self-inactivating γ-retrovirus vector to transduce autologous CD34+ HSC with the IL2Rγ gene for transplantation in 9 SCID-X1 patients has also shown early clinical benefits (Hacein-Bey-Abina et al., 2014); these studies require long term follow-up to conclusively determine the safety of this approach. Leukemogenic outcomes were observed in the original trial as late as 68 months after gene therapy. All viral gene therapy vectors involve integration into the host genome and consequently bear the risk of insertional mutagenesis on a patient-to-patient basis.
Direct genome editing of patient derived CD34+ HSC using ZFNs was shown in a recent proof-of-concept study (Genovese et al., 2014). While this is an attractive alternative to viral gene therapy, these approaches are dependent on the hitherto elusive capability to efficiently culture and expand long-term repopulating HSC ex vivo. In addition, HSCT based approaches involve myeloablative pre-transplant conditioning, which poses a particularly high level of risk for these SCID-X1 patients, given their very young age and highly immune-compromised state. An iPSC-based approach provides an unlimited source of patient-derived, corrected cells from which immune cells can be derived continuously for transfusion, and could serve as a complementary approach to treat patients.
Adoptive transfer of NK cells is already being used to treat a number of malignancies (Geller et al., 2011; Ruggeri et al., 2002; Shi et al., 2008), thus creating an unlimited source of patient-specific NK cells in itself provides significant clinical benefit in the treatment and management of SCID-X1 and similar diseases. Further improvements and scaling up of our current protocols will be necessary to obtain sufficient cells for use in a clinical context. In summary, we have described here the precise genetic correction of a SCID-X1 patient derived iPSC line, and importantly, we provide evidence for recovery lymphoid differentiation from these isogenic gene-corrected iPSC, creating an ideal platform for improved modeling and therapy of SCID-X1 and similar PIDs.
Supplementary Material
Acknowledgments
We would like to thank Nianxin Zhong, Hongying Chen and Dinithi Senadheera for excellent technical assistance. Research reported in this article made use of the Waitt Advanced Biophotonics Center Core Facility (supported by the Waitt Foundation, NCI P30 CA014195-40 and NINDS P30 NS072031-03), the Flow Cytometry and Functional Genomics Core Facilities (supported by NCI P30 CA014195-40) and the Stem Cell Core Facility. We would also like to acknowledge the UCLA JCCC and CFAR Flow Cytometry Core Facility (supported by NIH awards P30 CA016042 and 5P30 AI028697), the UCLA CFAR Gene and Cellular Therapy Core Facility (NIH 5P30 AI028697), and the Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA hESC Expansion Core. A collaboration with Jon Chestnut of Life Technologies made the TALENs used in this study. TM was supported by a Pioneer Fund Postdoctoral Scholar Award. ALF was supported by a CIRM Postdoctoral Training Fellowship #TG2-01158. WBG (2012), ARB (2010) and LA (2011) were supported by CIRM-Bridges Internships (#TB-01175). IMV is an American Cancer Society Professor of Molecular Biology, and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. This work was supported in part by grants from Ipsen/Biomeasure, Sanofi Aventis, the H.N. and Frances C. Berger Foundation, the Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002, and the California Institute for Regenerative Medicine (CIRM) CL1-00500-1.2.
Footnotes
Author Contributions
ALF, TM: Study conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of manuscript
DSA, ZG: Acquisition of data, Analysis and interpretation of data, Drafting of manuscript
SJQ, WBG, OS, LSA, ARB: Acquisition of data
EK: Analysis and interpretation of data
IEA: Provision of materials
JZ and IMV: Study conception and design, Drafting of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, Tan WF, Penheiter SG, Ma AC, Leung AYH, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–U133. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual review of immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bogdanove AJ, Schornack S, Lahaye T. TAL effectors: finding plant genes for disease and defense. Current Opinion in Plant Biology. 2010;13:394–401. doi: 10.1016/j.pbi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1723–1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese P, Schiroli G, Escobar G, Di Tomaso T, Firrito C, Calabria A, Moi D, Mazzieri R, Bonini C, Holmes MC, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Smyth C, Wong M, Bennetts B, Rowe PB, Alexander IE. A novel splice-site mutation in the common gamma chain (gammac) gene IL2RG results in X-linked severe combined immunodeficiency with an atypical NK+ phenotype. Human mutation. 2004;23:522–523. doi: 10.1002/humu.9235. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Fischer A, Cavazzana-Calvo M. Gene therapy of X-linked severe combined immunodeficiency. Int J Hematol. 2002;76:295–298. doi: 10.1007/BF02982686. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. The Journal of clinical investigation. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S, Bleesing J, Blondeau J, de Boer H, Buckland KF, et al. A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. The New England journal of medicine. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. The New England journal of medicine. 2003a;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003b;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hassan A, Lee P, Maggina P, Xu JH, Moreira D, Slatter M, Nademi Z, Worth A, Adams S, Jones A, et al. Host natural killer immunity is a key indicator of permissiveness for donor cell engraftment in patients with severe combined immunodeficiency. J Allergy Clin Immunol. 2014;133:1660–1666. doi: 10.1016/j.jaci.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao IT, Yao CL, Kong ZL, Wu ML, Chuang TL, Hwang SM. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007;16:1043–1051. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- Kay S, Bonas U. How Xanthomonas type III effectors manipulate the host plant. Current Opinion in Microbiology. 2009;12:37–43. doi: 10.1016/j.mib.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kay S, Hahn S, Marois E, Hause G, Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318:648–651. doi: 10.1126/science.1144956. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, Ando D, Urnov FD, Galli C, Gregory PD, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- Meek B, Cloosen S, Borsotti C, Van Elssen CH, Vanderlocht J, Schnijderberg MC, van der Poel MW, Leewis B, Hesselink R, Manz MG, et al. In vitro-differentiated T/natural killer-cell progenitors derived from human CD34+ cells mature in the thymus. Blood. 2010;115:261–264. doi: 10.1182/blood-2009-05-223990. [DOI] [PubMed] [Google Scholar]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Thrasher AJ. Gene therapy for PIDs: progress, pitfalls and prospects. Gene. 2013;525:174–181. doi: 10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, Park IH, Kaufman DS. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2011;85:43–50. doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer P, Hahn S, Jordan T, Strauss T, Bonas U, Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Tabatabaei-Zavareh N, Vlasova A, Greenwood CP, Takei F. Characterization of developmental pathway of natural killer cells from embryonic stem cells in vitro. PLoS One. 2007;2:e232. doi: 10.1371/journal.pone.0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM. Medicine. Gene therapy that works. Science. 2013;341:853–855. doi: 10.1126/science.1242551. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.