Abstract
Significance: NOX2 is important for host defense, and yet is implicated in a large number of diseases in which inflammation plays a role in pathogenesis. These include acute and chronic lung inflammatory diseases, stroke, traumatic brain injury, and neurodegenerative diseases, including Alzheimer's and Parkinson's Diseases. Recent Advances: Recent drug development programs have targeted several NOX isoforms that are implicated in a variety of diseases. The focus has been primarily on NOX4 and NOX1 rather than on NOX2, due, in part, to concerns about possible immunosuppressive side effects. Nevertheless, NOX2 clearly contributes to the pathogenesis of many inflammatory diseases, and its inhibition is predicted to provide a novel therapeutic approach. Critical Issues: Possible side effects that might arise from targeting NOX2 are discussed, including the possibility that such inhibition will contribute to increased infections and/or autoimmune disorders. The state of the field with regard to existing NOX2 inhibitors and targeted development of novel inhibitors is also summarized. Future Directions: NOX2 inhibitors show particular promise for the treatment of inflammatory diseases, both acute and chronic. Theoretical side effects include pro-inflammatory and autoimmune complications and should be considered in any therapeutic program, but in our opinion, available data do not indicate that they are sufficiently likely to eliminate NOX2 as a drug target, particularly when weighed against the seriousness of many NOX2-related indications. Model studies demonstrating efficacy with minimal side effects are needed to encourage future development of NOX2 inhibitors as therapeutic agents. Antioxid. Redox Signal. 23, 375–405.
General Roles of Reactive Oxygen Species and Nicotinamide Adenine Dinucleotide Phosphate, Reduced Form Oxidase Enzymes
Reactive oxygen species (ROS) are produced by the partial reduction of oxygen to form superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). Other reactive molecules are also formed both enzymatically and non-enzymatically through the reaction of ROS with other species: peroxynitrite (ONOO−) is produced by the spontaneous reaction of O2•− with nitric oxide (NO), and hypochlorous acid (HOCl) is formed by the myeloperoxidase-catalyzed reaction of H2O2 with chloride. While O2•− is weakly reactive and H2O2 is a moderately potent oxidant, ONOO−, HOCl, and •OH are highly reactive and produce molecular damage in DNA, protein, and lipids, resulting, for example, in DNA strand breaks, chlorination of protein tyrosine residues, and loss of membrane integrity (79, 80).
Phagocytic cells have capitalized on this chemical reactivity, generating microbicidal ROS within the phagosome as a part of innate immune mechanisms. In addition to their microbicidal functions, ROS, especially H2O2, act as signaling molecules, impacting the function of signal transduction proteins, ion channels, and transcription factors (91, 327, 328). ROS are, thus, increasingly recognized as central players in a range of normal physiological processes. Early studies showed that H2O2 is produced under normal physiological conditions, for example, in response to the growth factors platelet-derived growth factor (PDGF) (291) and epidermal growth factor (12), and that it is overproduced in transformed cells expressing oncogenically activated Ras (115). Signaling pathways impacted by ROS include ERK1/2, JNK, nuclear factor-kappa B (NF-kappa B), focal adhesion kinase, AP-1, Akt, Ras, Rac, JAK-STAT, and many others (31).
The best characterized molecular mechanism by which ROS regulate signaling involves oxidation of low pKa cysteine residues that exist as thiolate anions (Cys-S−) at physiological pH, rendering them susceptible to oxidation by H2O2 (237, 328). This oxidation may occur directly or may require an additional protein such as a thioredoxin (312). Redox-sensitive thiols are often located in specialized protein environments such as active sites, where their oxidation typically inhibits enzymatic activity. Examples of such “oxidant-sensor” proteins include protein phosphatases (e.g., protein tyrosine phosphatases [PTPs], low-molecular-weight protein tyrosine phosphatases, and MAP kinase phosphatases), the lipid phosphatase PTEN, and regulatory enzymes of ubiquitin and ubiquitin-like proteins such as SUMO and Nedd8 (237, 250, 312). As one example, PTP is oxidized in response to growth factor activation of receptor tyrosine kinases, thus simultaneously triggering protein phosphorylation and inhibiting the means of removing tyrosine phosphates from target proteins. The net result is to markedly increase tyrosine phosphate levels over those seen in the absence of oxidative mechanisms (12). Physiological stimuli that increase H2O2 may also result in the oxidation of protein thiols that can be reversed by, for example, thioredoxin or glutathione. This serves as an “off/on” switch analogous to protein phosphorylation/dephosphorylation and enables rapid regulation of downstream signaling pathways.
In addition to their normal signaling roles, ROS are recognized as a double-edged sword, implicated by virtue of their reactivity and pro-inflammatory properties in the pathogenesis of a long list of diseases, many of them inflammatory and/or chronic in nature (17, 154). Due to this association, antioxidant therapy has been investigated both in animals and in a large number of human clinical trials. Unfortunately, this approach has been largely unsuccessful, probably as a result of the common use of vitamins and/or dietary compounds that are generally very weak antioxidants in vivo. In addition, efficient cellular enzymatic antioxidant systems (superoxide dismutase [SOD], catalase, peroxidases, etc.) probably render the added effect of exogenous antioxidants rather small (156). The disappointing results with antioxidant therapy clinical trials have turned attention in recent years to eliminating the production of ROS at its source.
Cellular sources of ROS
While cellular ROS have classically been described as arising from a variety of redox-active enzymes (xanthine oxidase, cyclooxygenases, lipoxygenases, myeloperoxidase, heme oxygenase, monoamine oxidases, aldehyde oxidase, nitric oxide synthases [NOS], and cytochrome P450) as well as from the mitochondrial respiratory chain, ROS production from these sources is largely an “accidental” byproduct of catalysis involving redox-active coenzymes that have a low but finite reactivity with molecular oxygen (225). Generally, these sources generate low amounts of ROS, but levels can increase under pathological conditions, as occurs, for example, in genetically mutated mitochondria (318) or in NOS that has been exposed to oxidants (the so-called “kindling reaction”) (157). NOX enzymes, on the other hand, efficiently produce O2•− or H2O2 as their primary catalytic function, thus earning the status of “professional” ROS-generating enzymes, and cellular mechanisms are in place to tightly regulate NOX activity (e.g., phosphorylation, transcription) for cells to regulate their ROS levels both acutely and chronically (17, 156).
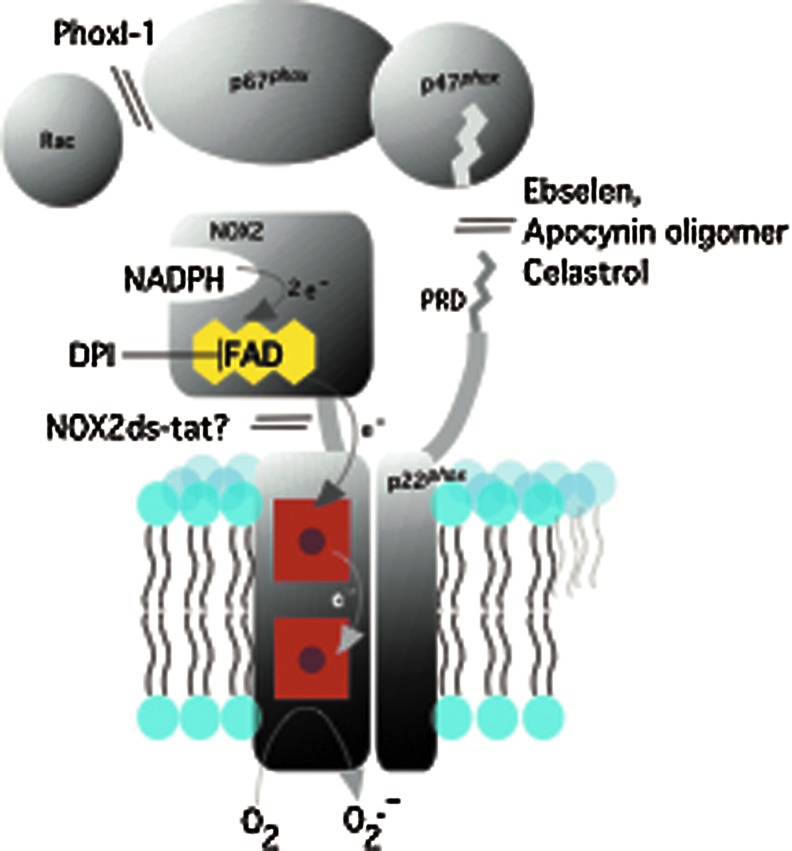
NOX enzymes are a family of NADPH-dependent oxygen reductases that are widely expressed in eukaryotes from plants to fungi to vertebrates. The catalytic NOX or dual oxidase (DUOX) subunit, represented in humans by seven paralogous genes (NOX1-5 and DUOX1 and 2), contains both flavin adenine dinucleotide (FAD) and two heme groups. The FAD, bound within a cytoplasm-facing flavoprotein dehydrogenase domain, oxidizes NADPH in a two-electron hydride transfer reaction; single electrons then pass in sequence from the FAD through the two non-identical heme groups located within a transmembrane domain, and, finally, to oxygen on the other side of the membrane, to form O2•− (Fig. 1). In some cases (e.g., NOX4, DUOX1 and 2), the major detectable product is H2O2 rather than O2•− (294). Except perhaps for NOX5, NOX family members require interactions with other membrane-associated partner proteins for stability and/or localization; these include p22phox for NOX1–4 (9, 62, 134, 178, 308), and DUOXA1 and DUOXA2 for DUOX1 and DUOX2, respectively (90, 188). NOX1–3 require assembly with regulatory subunits for full catalytic activity, while NOX4 is constitutively active.
FIG. 1.
Schematic diagram of NOX2 and NOX2 regulatory subunits, along with sites of inhibitor action. NOX2 and p22phox are shown in the membrane, along with NOX2 regulating cytosolic subunits. PRD refers to the proline-rich domain of p22phox. Shown also is the pathway of electron flow from FAD through the two heme groups (represented by red squares). FAD, flavin adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate, reduced form; NOX, NADPH oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
While NOX2 and its regulation were discovered and characterized first, the recognition of NOX enzymes as a family of ROS-generators has focused a great deal of attention on the other members of the family, especially with regard to their biochemistry, physiological, and pathophysiological roles. NOX4, for example, is frequently associated with fibrotic diseases, and has attracted a great deal of attention as a target for pharmaceutical development. Nevertheless, it has become apparent (vide infra) in recent years that the over-activity of NOX2 is also associated with a large number of diseases, particularly those with an inflammatory component. Here, we focus on NOX2 as a target for drug development, discussing its normal physiology and pathobiology, as well as possible complications that might arise from drug targeting of this enzyme.
Overview of the NOX2 enzyme
NOX2 and its regulatory partner proteins have been extensively characterized since their molecular cloning in the 1990s (4, 142, 163, 212, 243, 300, 317, 324). The catalytically dormant NOX2 in its membrane complex with p22phox becomes activated as a result of assembly with cytosolic regulatory partner proteins p40phox, p47phox, p67phox, and Rac1/2, a process triggered by phosphorylation of p47phox and probably other components, and by guanine nucleotide exchange on Rac. The structure and function of NOX enzymes has been extensively reviewed (17, 141, 153, 155, 287). For the present purpose, we point out that the presence of multiple specialized domains that mediate protein–protein interactions during the assembly process provide, in addition to the NADPH-binding site on NOX2, a number of candidate binding sites through which inhibitors might target the NOX2 system by disrupting assembly.
Physiological roles of NOX2
The known or proposed physiological roles and mechanisms of action of NOX2 are summarized in Table 1, as prologue to considering the possible complicating effects of drugs that target the NOX2 enzyme system. While levels of NOX2 are highest in phagocytes, NOX2 mRNA and/or protein have been detected at low levels in a large number of other tissues [(17), and Table 1]. In many cases, the co-expression and possible redundant function of other NOX isoforms complicates the interpretation of specific roles for NOX2. Likewise, the use of non-selective NOX inhibitors as tools (see next) also complicates interpretations. The use of genetic methods, including RNA interference and gene ablation, can be considered to be more definitive. Table 1 should, therefore, be considered in this context.
Table 1.
Physiological Roles of NOX2
| NOX2 tissue expression | Proposed function | Proposed mechanism | Evidence for NOX2 role | References |
|---|---|---|---|---|
| Neutrophil | Host defense | ROS damage to macromolecules | CGD, NOX2 KO mouse | (87) |
| ROS-dependent NET generation | CGD, NOX2 KO mouse | (74, 82) | ||
| ROS signaling | NOX2 KO mouse | (105, 149) | ||
| Macrophage | Host defense | ROS damage to macromolecules | CGD | (259) |
| ROS-dependent cytokine production | CGD | (13, 234) | ||
| ROS control of antigen processing | NOX2 KO mouse | (248) | ||
| Resolution of inflammation | ROS-dependent mediator production | CGD | (76) | |
| T-cell activation and proliferation | ROS oxidation of T-cell surface Cys thiols | p47phox mutant rat | (85) | |
| Dendritic cells | Host defense | ROS control of antigen processing | Ebselen; NOX2 KO mouse | (180, 256) |
| Microglial cells | Host defense | ROS damage to macromolecules | NOX2 KO mouse | (64) |
| ROS-regulated cytokine production, signaling, and transcription | NOX2 KO mouse | (136, 214) | ||
| Endothelial cells | Cell proliferation and survival | ROS-regulated transcription | NOX2 siRNA | (78, 221) |
| Endothelial permeability | ROS-regulated signaling and transcription | p47phox siRNA | (164) | |
| Vascular tone | O2•− depletion of NO | NOX2 KO mouse | (89) | |
| Vascular smooth muscle | Vascular tone, growth and development | ROS-regulated signaling and transcription | NOX2 siRNA | (29) |
| Skeletal muscle | Contractility | ROS regulation of Ca2+ channels, transcription | NOX2 KO mouse | (168) |
| Heart muscle | Contractility | ROS regulation of Ca2+ channels, transcription | NOX2 KO mouse | (231) |
| Neurons | Neuronal plasticity, memory | ROS regulated signaling, ion channels, and transcription | NOX2, p47phox KO mice | (139) |
| Neuronal development | ROS regulation of signaling and transcription | NOX2 KO mouse | (61) | |
| Pancreatic beta cells | Insulin secretion | ROS regulation of signaling and transcription | NOX2 siRNA and NOX2 KO mouse | (165, 337) |
| Hepatocytes | Apoptosis | ROS regulation of transcription | NOX2 expression | (191) |
| Hematopoeitic cells | Development, mitosis | ROS regulation of signaling and transcription | NOX2 KO mouse | (311) |
| Adipocytes | Differentiation | ROS regulation of signaling and transcription | Inhibitors, translocation of p47phox and p67phox | (255) |
| Pulmonary neuroepithelial bodies | O2 sensing | ROS activation of O2•− sensitive K+ channels | NOX2 siRNA | (34) |
| Lens epithelial cells | Cell proliferation | ROS regulation of signaling | p22phox siRNA, translocation of p47phox and p67phox | (320) |
CGD, chronic granulomatous disease; NADPH, nicotinamide adenine dinucleotide phosphate, reduced form; NO, nitric oxide; NOX, NADPH oxidase; NET, neutrophil extracellular trap; ROS, reactive oxygen species.
Professional phagocytes
Neutrophils and macrophages
NOX2 functions in neutrophils and macrophages in host defense against invading microorganisms (Table 1). Pursuing a chemical trail of microbial products (e.g., formylated peptides) and host-derived inflammatory factors (cytokines, lipid mediators), phagocytes locate and engulf invading microbes into phagosomes, in which a high concentration of NOX2-derived O2•− is generated. SODs form H2O2, which reacts with chloride in a myeloperoxidase-catalyzed reaction and forms the highly microbicidal HOCl (17, 152, 194, 195, 265). The central role of the NOX2 system in host defense is demonstrated by the inherited condition chronic granulomatous disease (CGD) in which phagocytes genetically defective in NOX2 or one of its interacting regulatory subunits fail to kill ingested microorganisms, despite normal phagocytosis, resulting in frequent and chronic infections in affected individuals. Definitive evidence for the microbicidal role of ROS was provided by experiments which showed that exogenous H2O2 could restore the ability of defective neutrophils from CGD neutrophils to kill microorganisms (87). Neutrophils from mice in which NOX2 or one of its regulating subunits is genetically deleted show identical microbicidal defects (119, 223).
Similar to neutrophils, some populations of monocytes and macrophages respond to chemotactic signals to gather at sites of inflammation. Monocytes take up residence in specific tissues, becoming macrophages that are specialized for their role as “first responders” within the local environment. Macrophages can be activated both by pathogens themselves and by damage-associated molecular patterns (58, 278), to which they respond by phagocytosis of the offending material, producing cytokines and other signaling molecules, and presenting antigens. Macrophages express relatively high levels of NOX2 components, although they are also reported to express other NOX isoforms (17, 160, 309). The NOX2-derived respiratory burst in microbicidal killing in macrophages is similar to, but less robust than, that of neutrophils (17, 58, 278).
In addition to direct damage to microbial molecules, NOX2-derived ROS promote the formation of neutrophil extracellular traps (NETs), which also participate in innate immunity (28). NETs are composed of chromatin strands to which antimicrobial proteins are attached. Neutrophils release NETS in novel cell-death pathways that are triggered by various stimuli; the NET response to some stimuli depends on ROS-initiated breakdown of the phagocyte nuclear envelope (82, 133, 152). Neutrophils from CGD patients and mouse NOX2 knockouts fail to make NETs under stimulation conditions that usually promote NET formation, and NET formation in neutrophils from various mouse strains correlates with the amount of ROS produced (74, 82). It is important to note that certain stimuli promote NETs without ROS involvement (35, 41) and that the role of NOX2-derived ROS in NETS in vivo is unclear (196, 211).
NOX2-mediated ROS generation in phagocytes, especially macrophages, leads to the elaboration of immune mediators from various cell types, including phagocytes: inflammatory cytokines (e.g., interleukin [IL]-1, IL-6 and tumor necrosis factor [TNF]-alpha), phagocyte-attracting chemokines (e.g., CXCL8, CCL3, and CCL4), and bioactive lipids (e.g., prostaglandins, leukotrienes, etc.) (58, 278). Such mediators are important in both innate and adaptive immune responses, recruiting inflammatory cells to sites of infection or inflammation. In macrophages of the vessel wall, low-density lipoprotein triggered TLR receptors to activate NOX2-dependent ROS generation, leading to the production of pro-inflammatory cytokines (13, 234) that are implicated in the development of atherosclerotic plaques; macrophages from CGD patients showed greatly reduced levels of pro-inflammatory cytokines (13, 234) and increased anti-inflammatory cytokine IL-10. To our knowledge, a decreased propensity to develop atherosclerosis has not been evaluated in CGD patients, but a protective effect has been noted in NOX2 gene-deleted mice (125).
Paradoxically, macrophages also participate in the resolution of inflammation by phagocytosis of apoptotic neutrophils, and in the regulation of the adaptive immune response via the elaboration of anti-inflammatory mediators. NOX2-deficient neutrophils and macrophages produce significantly lower levels of the anti-inflammatory mediators cyclopentenone prostaglandin D2 and transforming growth factor beta (32).
NOX2 also plays a role in non-canonical autophagy, such as that associated with phagocytosis in which the cell engulfs intracellular debris and helps prevent escape of a phagocytized organism. In neutrophils, Toll-like and Fcγ receptors activate NOX2, and the ROS produced participate in a signaling cascade that recruits the autophagy protein LC3 to phagosomes (105).
Microglia
Microglia are macrophage-like phagocytic cells that reside in the brain where they function in host defense and repair after tissue damage. As with other phagocytes, their effects may be mediated directly by ROS or indirectly via ROS-dependent inflammatory mediators. Spinal cord microglia from NOX2 knockout mice made less ROS and produced less pro-inflammatory cytokines in response to agonists (214) or injury (136) than did wild-type microglia, demonstrating an essential role for NOX2-derived ROS in up-regulating microglial pro-inflammatory cytokines.
Dendritic cells
Nox2-generated ROS also play a critical role in antigen processing before presentation by dendritic cells to naïve T lymphocytes [reviewed in ref. (145)]. Some of the evidence for a role of NOX2 in this area comes from studies which showed that the presentation of antigens such as ovalbumin by mouse bone marrow-derived dendritic cells to CD4+ T lymphocytes was decreased by the NOX2 inhibitor ebselen (180) and defective in dendritic cells isolated from NOX2 knockout mice (256). Observations that the pH within the phagosomes of dendritic cells isolated from wild-type mice, but not NOX2-deficient mice, rises slightly above pH 7 after internalization of beads or bacteria led to the model that NOX2-generated ROS consumes incoming protons, causing an increase in pH that is suboptimal for phagosomal (and also endosomal/lysosomal) proteases which prefer acidic environments. This process would serve to prevent complete proteolysis of antigens, enabling proper processing and presentation to occur. However, another study does not support this pH-based model and suggests a redox-based model instead (247). Besides the regulation of phagosomal pH, NOX2-generated ROS within phagosomes may directly oxidize and inactivate proteins of the phagosome, such as vacuolar H(+)-ATPase (75) and cysteine proteases (cathepsins B, L,S) (192), and also directly oxidize the antigens themselves to facilitate antigen presentation (44, 229).
Vascular cells
The physiological effects of NOX-generated ROS on various vascular cells, most notably endothelial and vascular smooth muscle cells (VSMCs), have been a focus of intense interest (27, 141, 159, 174, 260). Understanding specific roles for NOX2 is complicated by the presence of several NOX family members in vascular cell types, and different isoform expression in different anatomical regions of the vasculature (venous vs. arterial, lung vs. general circulation, microvasculature, etc.).
An important function of NOX enzymes in blood vessels is control of vascular tone, a process that involves both VSMC and endothelial cells. The endothelium produces NO, which potently mediates the relaxation of vascular smooth muscle (111). O2•− reacts rapidly with NO, eliminating its vasorelaxing effects. Angiotensin II (Ang II) also activates NOX-dependent ROS production in VSMC, signaling kinases and transcription pathways that increase vascular tone (159). While this is usually attributed to NOX1 (173) and/or NOX4 (264), siRNA depletion of NOX2 inhibited both basal and Ang II-induced ROS production in primary VSMC isolated from spontaneously hypertensive rats (29). NOX2 knockout mice displayed significantly less endothelial ROS production and significantly higher endothelium-dependent relaxation than wild-type mice (89). Human CGD patients manifest increased vascular NO and increased vasodilation, providing a direct link between NOX2 and endothelial function (314).
The endothelial NOX2 system also appears to play a role in regulating interactions between endothelial cells and neutrophils that lead to neutrophil migration through the endothelial layer. Coronary microvascular endothelial cells from p47phox−/− mice stimulated with TNF-alpha failed to initiate normal ROS-dependent kinase and transcription factor cascades, and, consequently, also failed to express ICAM-1, which is necessary for neutrophil adhesion (164). It should be cautioned, however, that p47phox and its homologue NOXO1, while usually assumed to be specific for NOX2 and NOX1, respectively, have the in vitro ability to activate the other NOX isoform (42); that is, p47phox can activate NOX1, and NOXO1 can activate NOX2. If such cross-activation occurs in vivo, this might lead to an incorrect interpretations of the role of NOX2 (153).
NOX-derived ROS also functions in vascular growth, proliferation, and apoptosis in both VSMCs and endothelial cells (78, 173). Various agonists differentially stimulate ROS production from various NOX isoforms through several receptor, kinase, and transcription pathways that lead to altered developmental programs (49, 78). In one study, siRNA against NOX2 inhibited both ROS production and p38-MAP kinase-dependent proliferation in an endothelial cell line and in primary endothelial cells, while overexpression of NOX2 increased both responses (221). Using the same methods, NOX4-derived ROS was also shown to contribute to both responses, indicating a degree of isoform functional redundancy.
Muscle cells
ROS modulate functions of both cardiac and skeletal muscle, notably calcium signaling and contractility (7, 118). In particular, ROS sensitize ryanodine receptors, increasing calcium release (98, 251). NOX2 is expressed in sarcolemma and t-tubule membranes (98, 186), and studies in knockout mice pinpointed the source of stretch-activated ROS in cardiac muscle cells as NOX2 (231). In addition, gp91ds-tat, a peptide inhibitor of NOX2, inhibited the normal ROS-induced intracellular calcium release. Skeletal muscle fibers from NOX2 knockout mice did not produce ROS in response to muscle activity, and they also failed to release specific histone deacetylases that usually control gene expression (168). Despite these associations, we are not aware of any reports of functional defects in cardiac or skeletal muscle function in CGD patients, suggesting that if such effects exist, they are subclinical or compensated.
Other cell types
Along with the cell types discussed earlier, ROS produced by NOX enzymes are implicated in signaling in a large number of tissues and cell types (17, 31, 225, 232), although little evidence specifically implicates NOX2. For example, NOX2 has been reported as a signal generator in hepatocytes (191) and adipocytes (255), based on tissue expression of NOX2 and the use of inhibitors and ROS scavenging. Over-expression of p22phox and membrane translocation of NOX2 cytosolic regulatory subunits correlates with ROS-mediated mitogenic signaling in lens epithelial cells (320). Evidence for the participation of NOX2-derived ROS in signaling pathways controlling beta cell insulin secretion has been obtained using siRNA (337) and NOX2-deficient mice (165). Neurons from NOX2-deficient mice showed impaired N-methyl-d-aspartate (NMDA) receptor-dependent long term potentiation (139) and also dysregulation of signaling pathways that are essential to proliferation (61). A study using NOX2-deficient mice also suggests that NOX2-derived ROS control release of the neurotransmitter glutamate in response to specific agonists (280). In hematopoietic cells, low oxygen tension in the bone marrow maintains quiescence and stem cell potential. The higher pO2 in the vasculature leads to ROS production that signals cell division and migration (72, 112); other signals also induce NOX2-derived ROS that promote hematopoietic cell differentiation, migration, and senescence (261, 268). Studies using knockout mice report that NOX2 is a major source of the ROS which control cell division in myeloid precursor populations (311), although the physiological significance is unclear as human CGD patients are not reported to have myeloid cell deficiencies. In addition to controlling the general types of physiological and developmental processes in the cell types discussed earlier, NOX2 is implicated as an oxygen sensor in pulmonary neuroepithelial bodies (34).
Diseases in Which NOX2 Is Implicated
Throughout the eukaryotic domain, NOX enzymes function to trigger adaptive mechanisms in response to stresses, environmental assaults, or other noxious stimuli (5). In this context, a role of NOX enzymes in causing direct damage to invading microbes can be considered to be one subclass of a stress response that exploits the ability of ROS to damage macromolecules of invading microbes. Other adaptive roles of ROS in innate immunity also conform to this paradigm; for example, ROS activate immune signaling pathways that trigger cytokine release, differentiation, apoptosis, etc. Paradoxically, NOX-generated ROS can damage host tissues directly, can initiate a hyper-inflammatory response that results in further tissue damage, and can cause longer-term changes, including alterations to cell apoptotic and differentiation programs, for example, differentiation of myofibroblasts which then deposit fibrotic material. While disease-triggering events are legion and may be unknown, a variety of diseases seem to have in common the central role of NOX-generated ROS in the pathogenic process. This section focuses on those conditions, summarized in Table 2, in which NOX2-derived ROS are considered to play an important role in pathogenesis, and which, therefore, represent candidate indications for NOX2-targeted drugs.
Table 2.
Diseases in Which NOX2 Is Implicated
| Disease | Type of evidence for NOX2 role | References |
|---|---|---|
| Hypertension | NOX2 siRNA in mice | (220) |
| Apocynin in mice | (303) | |
| Acute lung inflammation | NOX2 KO mice | (216, 217, 315, 316, 340) |
| p47phox KO mice | (114, 340) | |
| Chronic obstructive pulmonary disease | P47phox KO mice | (150) |
| Apocynin effects on H2O2 in patients | (283) | |
| Asthma | Steroids lower NOX2 in patients | (203) |
| Apocynin effects on H2O2 in patients | (284) | |
| Ebselen effects in guinea pigs | (338) | |
| Cystic fibrosis | Neutrophil infiltration seen in patients | (95) |
| Pulmonary hypertension | NOX2 KO mice | (198) |
| NOX2 activation and apocynin effects in lamb model | (302) | |
| Ischemia-reperfusion injury in lung | p47phox KO mice | (333) |
| Apocynin effects in mice, rats, and sheep | (43, 215, 333) | |
| Ischemic stroke | NOX2 KO mice | (116, 167, 297) |
| Apocynin effects in mice | (116, 295) | |
| VAS3870 effects in mice | (140) | |
| Traumatic brain injury | NOX2 KO mice | (64) |
| Apocynin effects in mice | (45, 279, 339) | |
| gp91(NOX2)ds-tat peptide in mice | (339) | |
| Alzheimer's disease | Aβ peptides activate ROS and cytokine production in vitro | (23) |
| gp91 (NOX2) activation in human AD brain | (10, 270) | |
| NOX2 KO mice | (209) | |
| gp91(NOX2)ds-tat inhibitor peptide in mice | (210) | |
| Parkinson's disease | NOX2 KO mice | (331) |
| NOX2 increased in human PD brain | (331) | |
| Amyotrophic lateral sclerosis | NOX2 increased in ALS patients | (330) |
| NOX2 KO mice | (175, 330) | |
| Apocynin effects in mice | (94) | |
| Schizophrenia | NOX2 increased in neurons in mouse models | (19, 280) |
| Apocynin effects in rodents | (19, 258, 280) | |
| NOX2 KO mouse | (280) | |
| p47phox mutated rat | (258) | |
| Muscle disorders | NOX2 activated by stretch in rodent in vitro models | (231) |
| NOX2 in muscle activated in mdx mice | (135) | |
| DPI inhibitor effects in vitro | (231) | |
| gp91(NOX2)ds-tat peptide inhibitor in vitro | (135, 231) | |
| NOX2 KO mice | (231) |
AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; DPI, diphenylene iodonium H2O2, hydrogen peroxide; PD, Parkinson's disease.
Vascular diseases
Hypertension
Hypertension is a major risk factor for stroke, heart failure, aneurysms, and peripheral artery disease, and it is a cause of chronic kidney disease. Even moderate elevation of arterial blood pressure predicts a shortened life. While multiple factors contribute to hypertension, oxidative stress is a unifying theme (264). In addition to vasculature, tissues involved in the pathogenesis of hypertension include the central nervous system (220, 342) and the kidney (249).
Evidence supports a role for ROS, particularly from NOX1, 2, and 4 in hypertension (264). In an acute model of Ang II-induced hypertension in mice, adenoviral-mediated delivery of either NOX2 siRNA or NOX4 siRNA to the brain subfornical organ prevented increases in mean arterial pressure and in heart rate, and simultaneous delivery of both siRNAs resulted in even greater suppression. The Ang II-induced increase in ROS was significantly inhibited in cultured forebrain neurons from NOX2- or NOX4-siRNA treated animals, and abolished in neurons from animals treated with both (220).
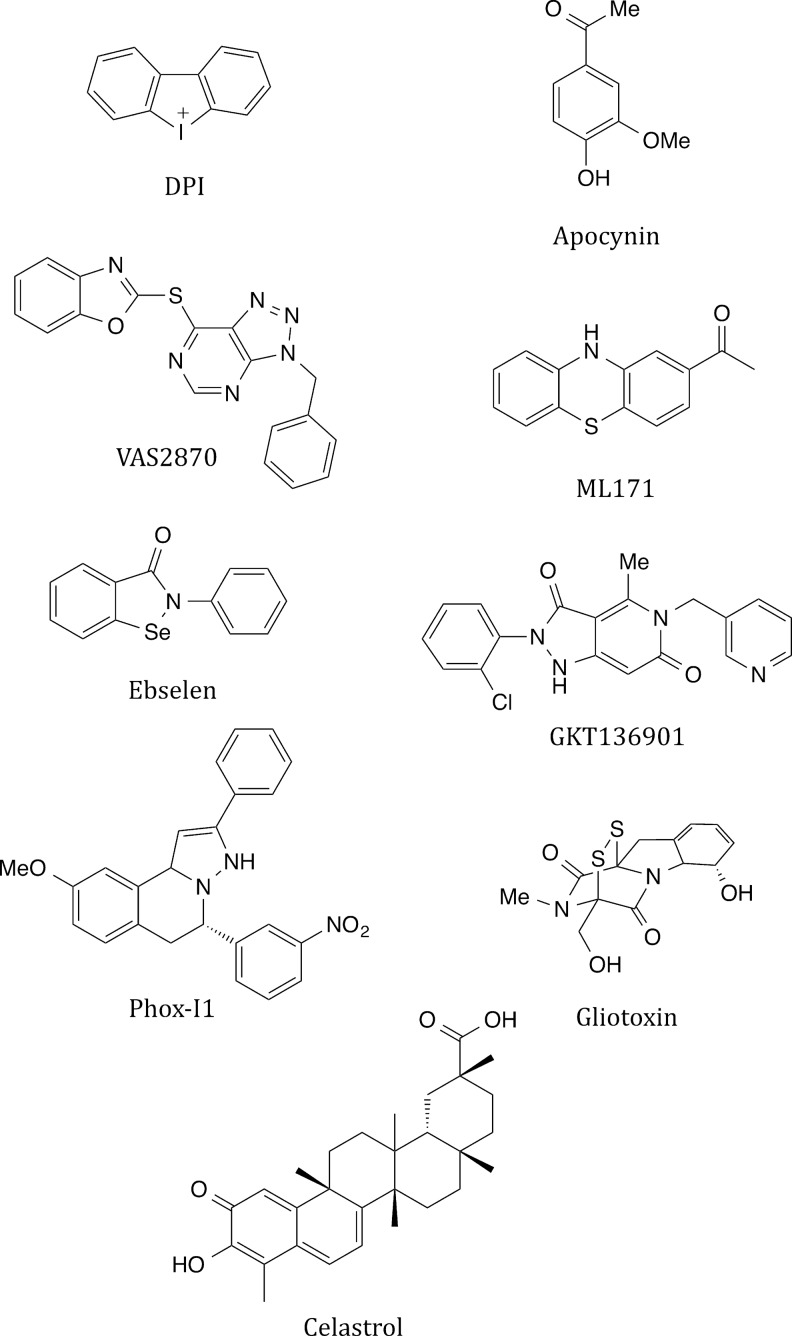
In another study (303), Dahl salt-sensitive rats were maintained on high-sodium drinking water, with or without the NOX inhibitor apocynin. By day 35, mRNA expression of renal cortical NOX2 and regulatory subunits markedly increased in high-salt rats but not in apocynin-treated rats. In apocynin-treated animals: (i) renal cortex showed a less oxidizing environment, based on reduced glutathione-to-oxidized glutathione (GSH:GSSG) ratios; (ii) renal cortical O2•− decreased; and (iii) renal glomerular and interstitial damage were markedly improved. Apocynin also decreased renal cortical monocyte/macrophage infiltration, improved renal hemodynamics, and decreased arterial pressure. Due to ambiguities about the mechanism of action of apocynin detailed next, definitive identification of NOX2 as the source of ROS in these studies should be confirmed using other approaches.
In contrast, eliminating NOX2-associated ROS production was ineffective in some chronic models of hypertension. In one study, transgenic mice overexpressing human renin (TTRhRen) exhibited hypertension and cardiac hypertrophy by age 10–12 weeks. Although TTRhRen/NOX2−/− mice had significantly lower ROS levels in heart and aorta, these mice still developed hypertension and cardiac hypertrophy (305). It is possible that other NOX isoforms might compensate for NOX2 loss in this model, or that other non-NOX mechanisms are involved. Consistent with roles for other NOX isoforms in hypertension, aortic media of spontaneously hypertensive rats showed ∼2.5-fold increased NOX4 mRNA and ∼10-fold increased NOX1 mRNA compared with control rats, whereas NOX2 and p22phox mRNA levels were similar (6).
Pulmonary hypertension
Pulmonary hypertension (PH) is a complex disease in which increased blood pressure develops in the lung vasculature, leading to extreme exertion symptoms during normal activity, exercise intolerance, and, in some cases, heart failure. PH has been classified into several types (273); this discussion is limited to hypoxia-related PH. Chronic hypoxia arising from obstructive sleep apnea, high altitude environment, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis can lead to PH. Currently, there is no known cure for PH, and current treatments aim at controlling symptoms and preventing additional lung damage.
While much of the evidence supports a role for NOX4 in the pathogenesis of PH (183, 184), evidence suggests that NOX2 is also involved. Obstructive sleep apnea, characterized by intermittent periods of hypoxia, is a risk factor for the development of PH. Experimentally, chronic intermittent hypoxia (CIH) induces PH, by increasing expression of NOX2 as well as NOX4, both of which may contribute to pulmonary vascular remodeling and hypertension (77, 198). Male mice exposed to CIH had increased right ventricular systolic pressure, right ventricle hypertrophy, and increased thickness of the right ventricular anterior wall and evidence of pulmonary vascular remodeling. Pathological changes were attenuated in NOX2 knockout mice that were subjected to CIH, consistent with a role for NOX2 in CIH-induced PH (198).
NOX2 may also contribute to PH indirectly via autophagy (302). In a study in which PH was induced surgically in fetal lambs, isolated pulmonary artery endothelial cells showed increased ROS production and translocation of p47phox, pointing to NOX2 activation. In addition, autophagy was increased compared with sham-operated fetal lambs. Inhibition of autophagy using 3-methyl adenine or chloroquine decreased NOX2 activation and O2•− generation, while administration of the antioxidant N-acetyl cysteine or the NOX2 inhibitor apocynin starting at birth improved lung oxygenation. Thus, autophagy may contribute to PH by increasing NOX2 activity. Collectively, these studies suggest that in addition to NOX4, NOX2 may be a target for drug development of PH.
Lung diseases
Acute lung injury and acute respiratory distress syndrome
NOX2-generated ROS are associated with a range of respiratory inflammatory diseases/injuries, including acute lung inflammation (ALI) and its more severe form, acute respiratory distress syndrome (ARDS). ALI/ARDS can result from an over-reaction of the host immune system to certain infections (certain influenza strains such as the 1918 flu, avian flu, sepsis, etc.) and also from physical trauma, blood loss, transfusion, hyperoxia, ventilator-induced lung injury, aspiration, and pancreatitis (181). Conventional anti-inflammatory drugs are ineffective in ARDS, which affects 200,000 U.S. patients yearly and is fatal in approximately 40% of cases (244). The NOX2 system and other NOX enzymes have been implicated in ALI/ARDS in several studies. NOX2 and other NOX isoforms are expressed in endothelial and epithelial cells of the lung, where they may participate in early signaling events preceding ALI/ARDS (39, 298). Neutrophil infiltration into the alveolar spaces of the lung during the acute phase of ALI/ARDs is clearly visible in postmortem lung sections from ARDS patients as well as in bronchoalveolar lavage fluid (181). In mouse models, the effects on inflammatory responses observed in NOX2 knockout mice or p47phox-deficient mice depend on the model system [reviewed in ref. (39)]. When TNF-alpha was used to induce ALI, mice deficient in NOX2 or p47phox exhibited markedly diminished inflammatory responses (340). However, in a sepsis-induced ALI mouse model, the inflammatory response in p47phox knockout mice was not significantly different compared with wild-type mice (143). The role of NOX2 in the development of hyperoxic lung injury is also unclear. In one set of studies (216, 217), NOX2-deficient mice exposed to acute hyperoxia had less severe pulmonary edema and neutrophil influx into the lung, but in another study, NOX1- but not NOX2-deficient mice were protected from lung injury (38).
Several studies have focused on the role of NOX2 in lung inflammation resulting from viral infection. When challenged with inactivated H5N1 influenza virus, mice deficient in p47phox showed less severe lung pathologies and decreased virus titers compared with control mice (114). NOX2-deleted mice infected with influenza A viruses displayed significant reductions in viral titers, peri-bronchial inflammation, BALF macrophages, BALF inflammatory cell O2•−, lung ONOO−, monocyte chemoattractant protein-1 (MCP-1), and alveolar epithelial cell apoptosis compared with wild-type mice. Lung levels of the anti-inflammatory factor IL-1beta were ∼3-fold higher in NOX2-deleted mice. In vivo administration of apocynin to infected wild-type mice decreased viral titer, airway inflammation, and inflammatory cell O2•− production after infection. These findings suggest that NOX2-selective inhibitors may have therapeutic potential for control of lung inflammation and damage in viral infections (315, 316).
Chronic obstructive pulmonary disease
COPD is projected to become the fourth leading cause of death worldwide by 2030 (170). COPD is characterized by progressive lung inflammation and irreversible narrowing of the airways. Three risk factors are associated with COPD: (i) cigarette smoking; (ii) heavy exposure to occupational and indoor air pollution; and (iii) alpha-1 antitrypsin deficiency (69, 110). Available therapies for COPD include long-acting bronchodilators and at late stages, glucocorticoids. These treatments are largely ineffective at attenuating the inflammation or reversing the airflow obstruction associated with the disease, highlighting the need for new therapies.
In patients with COPD, there is an accumulation of neutrophils and macrophages in the lungs of smokers versus non-smokers (228, 246, 322). Phagocyte-generated ROS can contribute to diminished enzymatic activity of proteinase inhibitor enzymes (24) such as secretory leukocyte proteinase inhibitor (40) and tissue inhibitors of matrix metalloproteinases (321), and also can increase the activity of proteinases such as matrix metalloproteinase (81). As in other lung inflammatory diseases, elevated ROS lead to increased pro-inflammatory cytokines. Oxidative stress may also damage the hypoxia response element within the vascular endothelial growth factor promoter of COPD patients (213), causing defective responses to hypoxia.
There are conflicting reports as to whether the deletion of p47phox reduces inflammation in the cigarette smoke-induced COPD mouse model. In one study (150), the number of macrophages and neutrophils and levels of IL-6, keratinocyte-derived chemokine (KC/CXCL1), and MCP1/CCL2 in BALF were lower in p47phox−/− mice exposed to cigarette smoke compared with mice exposed to air. However, in another study (334), while ROS production was decreased in BALF cells of p47phox−/− mice and NOX2−/− mice, the knockout mice showed increased lung inflammation with development of distal airspace enlargement and alveolar destruction. Inflammation was associated with activation of the TLR4-NF-kappa B pathway in the gene-deleted animals. The authors concluded that genetic ablation of components of NADPH-oxidase enhances susceptibility to the proinflammatory and lung-damaging effects of cigarette smoke. This finding may relate to pro-inflammatory effects seen in humans in which components of the NOX2 system are mutated, as discussed in the section “CGD, hyperinflammation, and autoimmune disease.” or may highlight the inadequacies of mouse models. While it is not clear from earlier descriptions as to whether NOX2 represents a promising target for human drug development, apocynin administered to COPD patients was effective in decreasing H2O2 levels in exhaled breath condensates (283).
Asthma
Asthma is a chronic inflammatory disorder of the airways that is characterized by episodic and reversible airflow obstruction and airway hyper-responsiveness (26). An estimated 300 million people worldwide suffer from asthma, with 250,000 annual deaths attributed to the disease (1). Emphasizing the need for new therapeutic approaches, current therapies are unsatisfactory in about half of the cases, and a subgroup of patients are refractory to current anti-inflammatory and bronchodilator therapies (100).
Oxidative stress and NOX enzymes appear to participate in the pathobiology of asthma, but it is not yet clear which isoform is likely to represent the best target for drug development. Blood of asthmatic children showed elevated biomarkers of oxidative stress (203). Consistent with the involvement of NOX2, inhaled corticosteroids that relieve symptoms also diminished NOX2 mRNA expression in circulating leukocytes from asthmatics. Administration of the NOX2 inhibitor apocynin to asthmatic patients led to decreased H2O2 levels in exhaled breath condensates (284). In guinea pigs, ebselen, a potent NOX2 inhibitor (276, 338), was able to improve the ovalbumin-induced asthmatic inflammatory responses, consistent with a role for NOX2 (276, 338). However, other evidence suggests that NOX2 may have a protective role in asthma, at least in mice. In the ovalbumin asthma model, NOX2 gene-deleted mice showed increased inflammatory responses and airway hyper-reactivity compared with wild-type mice. Based on co-culture experiments, the authors proposed that this resulted from increased interaction between Th2 cells and macrophages in the absence of NOX2 (14, 15).
Other NOX isoforms have also been associated with the pathobiology of asthma. In the ovalbumin asthma mouse model, increased expression of NOX1, 2, 3, and 4 was seen, and symptoms were improved using artesunate, an antimalarial drug with antioxidant properties (99). Primary airway smooth muscle cells isolated from biopsies from individuals with asthma versus healthy controls showed increased oxidative DNA damage along with increased ROS production that was attributed to increased NOX4 expression. Airway smooth muscle cells isolated from individuals with asthma exhibited increased bradykinin-induced contractility compared with non-asthmatic control cells. This was abrogated by NOX4 siRNA, diphenylene iodonium (DPI), or apocynin (93, 293). In addition to NOX4, epithelial DUOX1 was induced by the Th2 cytokines IL-4 and IL-13, which are commonly elevated within asthmatic airways (93). Thus, while oxidative stress appears to play a pathogenic role, a distinct role for NOX2 in asthma will require additional investigation.
Cystic fibrosis
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene resulting in misfolding of the CFTR protein and defective regulation of chloride transport by epithelial cells in several tissues; respiratory failure is the main cause of mortality and morbidity (95). CF, diagnosed in 1 out of 3000 births, is characterized by sustained neutrophil recruitment to lung and neutrophil-dominated inflammation from a very young age. Neutrophil NOX2-derived H2O2 can also fuel myeloperoxidase-dependent HOCl generation, which has been suggested to correlate with the severity of the disease (236, 329). DUOX1/2 may also contribute to CF (224). Thus, inhibitors of NOX2 and/or myeloperoxidase may decrease inflammation and diminish lung tissue damage in this condition.
Ischemic conditions
Ischemia-reperfusion injury after lung transplantation
Patients undergoing lung transplantation risk graft dysfunction secondary to reperfusion injury during which ROS are formed, leading to tissue destruction. In addition, patients with pulmonary artery blood clots, receiving cardiopulmonary bypass, or recovering from some form of pulmonary crisis are also likely to incur reperfusion injury. Currently, proven preventative or treatment drugs are unavailable, although clinical trials of promising therapies are under way (323).
ROS play an important role in lung ischemia-reperfusion injury (LIRI). In sheep subjected to LIRI, apocynin attenuated LIRI-induced increases in vascular permeability and pulmonary arterial hypertension (215). Apocynin also alleviated lung pathologies in a rat model of LIRI (43). In a mouse model of LIRI, wild-type mice, p47phox−/− mice, or chimeras created by bone marrow transplantation between p47phox−/− and wild-type mice were subjected to LIRI to investigate whether neutrophils deficient in p47phox would decrease the severity of LIRI (333). Both wild-type mice treated with apocynin and p47phox−/− mice displayed markedly decreased pulmonary dysfunction and injury (vascular permeability, edema, neutrophil infiltration, and lipid peroxidation) compared with untreated wild-type mice. In addition, in this study, pulmonary dysfunction and injury occurring after LIRI were significantly decreased in the p47phox−/−/wild-type (donor/recipient) chimeric mice, but not in wild-type/p47phox−/− donor/recipient chimeras. Moreover, the induction of TNF-alpha, IL-17, IL-6, RANTES (CCL5), KC (CXCL1), MIP-2 (CXCL2), and MCP-1 (CCL2) was significantly lower after LIRI in p47phox−/− mice and p47phox−/−/wild-type chimeras but not wild-type/p47phox−/− chimeras. These results suggest that NOX2 contributes to LIRI. Thus, NOX2 inhibitors may provide a novel therapeutic approach for ischemia-reperfusion in the lung.
Ischemic stroke
Ischemic stroke is a leading cause of death (171), and the repeated failure of promising experimental stroke treatments in human clinical trials (127) makes it likely that this situation will not change soon. Both NOX2 and NOX4 have been implicated in stroke pathogenesis (128, 140, 182). Most animal studies have used the transient middle cerebral artery occlusion model, measuring infarct volume and blood–brain barrier permeability as parameters that increase after occlusion and reperfusion. These two parameters were improved in ischemic NOX2 knockout mice, and apocynin also attenuated blood-brain barrier permeability in wild-type mice (130). Other studies report similar findings and provide further support for a role for NOX2 in the pathogenesis of ischemic stroke (167, 296, 341).
On the other hand, another study (140) showed that there was substantial protection from induced ischemic stroke in NOX4-deficient mice, but not in NOX1- or NOX2-deficient mice. Still other studies have shown that NOX1 may exert a protective effect in stroke (129). NOX1-deficient mice showed no difference in sub-cortical cerebral infarct volume, but a four-fold greater cortical infarct volume. Apocynin (116, 167, 296, 341) and the small molecule NOX inhibitor, VAS2870 (140) improved outcome in the mouse models of ischemic stroke. The reported discrepancies among studies may relate to the duration of ischemia before reperfusion, and, in general, suggest that NOX2 and/or NOX4 inhibition is likely to be most effective when administered early after stroke. These studies also emphasize the need for isoform-selective inhibitors.
Neuroinflammatory diseases
Traumatic brain injury
The number of traumatic brain injury (TBI)-associated deaths continues to increase worldwide. While acute care of head injuries has improved, extension of the primary lesion due to oxidative damage and inflammation, disruption of the blood–brain barrier, excessive release of the neurotransmitter glutamate, and other events leading to neuronal death can exacerbate the injuries of brain trauma patients, who, as a result, may die days or weeks later (210, 254).
Evidence for the contribution of NOX2-generated ROS to neuroinflammation and neuronal death comes from studies using the rodent cortical impact model of TBI. In one study, unilateral TBI was induced in NOX2 knockout and wild-type mice (64). After injury, NOX2 expression increased mainly in microglial cells of the ipsilateral hemisphere of the wild-type mice. The contusion area, number of TUNEL-positive cells, and amount of O2− and ONOO− metabolites produced were decreased in NOX2−/− mice. In other studies (45, 138, 279, 339), NOX activity in the cerebral cortex and hippocampal regions increased rapidly after impact, and pre- or post-treatment with apocynin or the NOX2 inhibitory peptide NOX2ds-tat markedly decreased O2•− levels in hippocampal neurons, oxidized lipid biomarker levels, blood–brain barrier disruption, microglial activation, and neuronal death. Thus, TBI may be an attractive indication for NOX2-directed drugs.
Alzheimer's disease
In 2010, 35.6 million people were estimated to be living with dementia, with an estimated 7.7 million new cases each year. The yearly cost of dementia health care in the United States is estimated at US$ 604 billion. Alzheimer's disease (AD) is the most common form of dementia and is estimated to account for 60–70% of cases. Current treatments fail to cure and only minimally impact the progression of the disease, although new approaches to treatment are being investigated in clinical trials (2).
Amyloid beta protein is found in plaques of brains from AD patients. Beta-amyloid peptides Aβ(1–40) and Aβ(1–42) are generated by proteolytic cleavage from amyloid precursor protein, a transmembrane protein that is important for neuron growth, survival, and repair (227, 267). Aβ(1–42) and Aβ(1–40) oligomers accumulate in fibrils in the extracellular spaces of the brain in AD patients (102, 202). Beta-amyloid fibrils directly activate NOX2 in primary rat microglial cells as well as in human neutrophils and monocytes (23), leading to the production of ROS and pro-inflammatory cytokines that participate in inflammatory tissue damage. Biochemical studies showed increased p47phox and p67phox in membrane fractions from human AD postmortem cortices as well as increased NOX2 activity in brain cortex homogenates compared with age-matched non-diseased brains (10, 270).
In addition to microglial NOX2, endothelial NOX2 may also contribute to the pathogenesis of AD due to effects on blood flow. In wild-type mice, agents that release NO or stimulate its in vivo production caused increased cerebral blood flow which was attenuated in Tg2576 transgenic mice that overexpress Aβ. This impairment was not observed, however, in Tg2576 mice lacking NOX2, implicating NOX2 in the vascular dysfunction induced by Aβ fibrils (209). Direct application of Aβ(1–40) onto the cortex increased ROS production in wild-type mice; this increase was abrogated by gp91(NOX2)ds-tat and also in the NOX2−/− mice. A NOS inhibitor prevented Aβ-induced modulation of blood flow, which was consistent with the idea that NOX2-generated O2•− scavenges NO, thus decreasing its bioavailability. While plaque load and brain Aβ levels did not differ between Tg2576 and Tg2576/NOX2−/− mice, Tg2576 mice lacking NOX2 were protected from behavioral dysfunction (210). These data point to AD as an indication for NOX2-targeted drugs.
Parkinson's disease
Parkinson's disease (PD) is the second most prevalent age-related neurodegenerative disease, with physiological manifestations that include tremor, rigidity, slowness of movement, and postural instability, along with impairments in speech, cognition, mood, and behavior. Pathologically, PD is characterized by the loss of dopaminergic neurons in the substantia nigra and the appearance in neurons of Lewy bodies composed of misfolded alpha-synuclein protein (277). Although the etiology of PD has been intensively pursued for decades, biochemical mechanisms, genetic and epigenetic factors leading to initiation and progression of the disease remain elusive. Only 10–15% of PD is due to known genetic mutations. Environmental exposure has been proposed to account for a subset of PD, and exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat, and rotenone increases the risk of PD in humans. To date, no drug therapy alters the progression of PD. Levodopa improves motor impairments, but dyskinesia can be an unsettling side effect. While a number of new therapeutics [reviewed in ref. (222)] are in the pipeline, whether they are able to alter disease progression remains to be determined. To date, drug candidates that have gone through clinical trials have proved disappointing, and new treatment approaches are being explored, which include gene transfer, cell-based therapies, and deep brain stimulation. Thus, disease-modifying drugs remain an unmet medical need.
Regardless of the initiating cause, oxidative stress remains a leading theory for explaining the progression of PD. Studies with cell and animal models reveal oxidative and inflammatory properties of PD-inducing toxins and their ability to activate glial cells. Activated microglia produce a host of factors that are toxic to neighboring dopaminergic neurons. In particular, the microglial NOX2 system exerts pathological effects both by direct ROS damage to neighboring neurons and also by triggering inflammatory cytokine signaling that results in a vicious cycle of sustained microglial activation and neuronal damage (292, 325). In a PD mouse model (331), MPTP induced overexpression of microglial NOX2, elevated ROS, and increased biomarkers of oxidative damage in the substantia nigra pars compacta. ROS production, oxidative damage, and neurodegeneration were substantially reduced in MPTP-treated NOX2-deleted mice. SOD infusion into the left striata attenuated the lesion on this side, but not on the contralateral, non-SOD infused side. NOX2 protein expression in six human PD midbrains was increased twofold in comparison to age-matched control brains, pointing to human relevance of the mouse model. Other NOX enzymes may also contribute to PD under some conditions or model systems; for example, similar approaches implicate neuronal NOX1 in PD pathogenesis in the rat 6-hydroxydopamine PD model (46) and rat paraquat PD model (51). Therefore, NOX2 and perhaps other NOX isoforms are attractive targets for slowing the progression of PD.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), a.k.a. Lou Gehrig's disease, results in loss of motor neurons, leading to progressive muscle paralysis (226). ALS is relatively rare, with a reported incidence of 1–2 per 100,000 per year. However, during the 1990s, clusters of cases were seen in Japan, Micronesia, and Indonesia with a local incidence at least 50 times higher than that seen worldwide (18). The average life expectancy of ALS patients ranges from 2 to 10 years after diagnosis. Mutations in SOD1 account for ∼20% of familial cases, which is about 2% of total cases (55, 59). The drug Riluzole (Rilutek) improves survival but does not reverse existing motor neuron damage, and patients should be monitored for liver damage, which occurs in ∼10% of treated individuals. Oxidative stress has been proposed to function in the progression of ALS, and several antioxidants, including vitamin E, N-acetyl cysteine, and selenium, have been investigated in clinical trials; none has made a significant impact on disease progression (207).
While the initiating causes and pathogenesis of ALS in humans is poorly understood, evidence indicates that NOX enzymes contribute to the neurodegenerative process (238). A transgenic mouse bearing the SOD1G93A mutation has been extensively used as a model of ALS; the mutation does not affect SOD enzymatic activity but rather affects its binding to some other proteins. NOX2 overexpression and increased biomarkers of oxidative stress have been observed in spinal cords of both ALS patients and SOD1G93A mice. In SOD1G93A mice in which NOX2 was deleted, neurodegeneration was delayed and survival was extended (330). The ability of NOX2 deletion to improve the life span of ALS mice was supported in another study in SOD1G93A mice (175) in which both NOX1 and NOX2 were induced; NOX2 deletion in these mice resulted in a dramatically increased life span. Deletion of NOX1 also improved life span, but less remarkably. Apocynin also dramatically increased the lifespan of SOD1G93A mice (94), but this result could not be repeated in two independent studies (166, 306). This may indicate either that the SOD1G93A mice provide an inadequate model human ALS, or that a more potent and/or selective NOX inhibitor may be needed.
Schizophrenia
Schizophrenia, affecting around 1% of the population worldwide (241), is a complex and disabling neuropsychiatric disorder. Despite a long history of antipsychotic drug development, approximately 30% of patients with severe schizophrenia are refractory to existing medications (200), emphasizing the need for new therapeutics and novel targets. The disease is marked by dysregulation of several neurotransmitter systems, including dopamine, glutamate, and gamma-aminobutyric acid. The behavioral and physiological characteristics of the disease can be mimicked by drugs that cause dopamine overproduction or block NMDA receptors. Oxidative stress increases in schizophrenia as evidenced by reports of changes in oxidative stress biomarkers such as increased oxidized-to-reduced glutathione ratios in the blood of patients (48, 233, 245) and decreased reduced glutathione levels in cerebrospinal fluid and postmortem prefrontal cortices of schizophrenic patients (63). Limited studies have also reported amelioration of symptoms after treatment of patients with the antioxidant N-acetyl cysteine (21, 33).
The ketamine rodent model mimics many of the cognitive, behavioral, and social deficits seen in human schizophrenia (190). Sub-anesthetic concentrations of ketamine produce psychotic-like symptoms in human volunteers, as well as impairments in memory and sustained attention performance that mimic the cognitive deficits observed in schizophrenia patients. In mice, the activation of NOX2 contributes to the dysfunction of GABAnergic interneurons after subchronic ketamine exposure (19, 20). Ketamine increases oxidative stress in rodent brain through the activation of neuronal NOX2, which initiates a cascade of events that, ultimately, leads to altered function in parvalbumin-expressing (PV+) neurons that are believed to control cognitive function impairments associated with schizophrenia. Significant increases in the expression of NOX2 and p22phox (but not NOX4) were observed in membrane preparations from brain cortex of ketamine-injected mice. This increase in NOX2 was accompanied by an increase in synaptosomal NADPH-oxidase activity and paralleled a loss of PV+ neurons (19). In another study (280), ketamine caused rapid behavioral alterations, release of neurotransmitters, and brain oxidative stress in wild-type mice; whereas NOX2-deficient mice did not display such alterations. Wild-type mice showed decreased expression of subunit 2A of the NMDA receptor after repeated ketamine exposure, which did not occur in NOX2-deficient mice, implicating NOX2 in down-regulation of NMDA receptor subunits.
Social isolation of rodents provides an alternative model of schizophrenia, leading to behavioral and histopathological alterations that are similar to those seen in the human disease (162). While NOX2 mRNA was not detected in the brains of control non-isolated animals, it was highly expressed in specific regions in the brains of socially isolated rats, which also showed increased brain biomarkers of oxidative stress. The treatment of isolated rats with apocynin prevented the behavioral and histopathological alterations. Moreover, rats with a functional mutation in p47phox (109) were protected from behavioral changes, loss of PV protein, and decrease in NMDA receptor subunit 2A (258). Thus, several lines of inquiry point to NOX2 as a novel and promising target for the treatment of schizophrenia.
Muscle disorders
The dysregulation of signal transduction from mechanical stretch to muscle contraction contributes to heart failure and muscle myopathies (230). In cardiac muscle cells, mechanical stretch depolarizes the plasma membrane as a result of a small influx of Ca2+ via l-type voltage-gated calcium channels. This influx stimulates opening of ryanodine receptors (RyR2 in the heart), which are calcium channels on the sarcoplasmic reticulum that release transient bursts of Ca2+ into the cytoplasm (30, 299). These “calcium sparks” induce shortening of myofibrils and contraction, which ends with relaxation when intracellular Ca2+ returns to resting levels and the RyR close. However, oxidative stress targets several cysteine residues on ryanodine receptors (11, 65, 98, 176), causing the receptor to become over-sensitized to Ca2+ levels, and to remain open longer than normal (231). Ultimately, the sarcoplasmic reticulum stores of Ca2+ fall to inadequate levels that cannot support contraction, leading to heart failure or skeletal muscle myopathies.
Recently, it was demonstrated that excessive Ca2+ release from over-sensitized RyR2 results from rapid and reversible ROS signaling originating in an intact microtubule network. These observations were made using single cardiomyocytes from mdx mice, which have a genetic mutation in the gene coding for the cytoskeletal protein, dystrophin. Myocytes from mdx mice had elevated ROS, and moderate stretching produced excessive Ca2+ release from over-sensitized RyR2 receptors (231). NOX2 and its subunits are localized in the membrane of the transverse tubules of rodent cardiomyocytes, and moderate stretching of isolated single myocytes activates ROS generation and translocation of NOX2 regulatory subunits to the membrane. Stretch-induced Ca2+ spark production was inhibited by gp91(NOX2)ds-tat, as well as by DPI. Moreover, in myocytes isolated from NOX2-deleted mice, stretch-induced ROS generation was absent (135, 231). Earlier observations (251) showed that NOX2 is present in skeletal muscle fibers, and its activation contributes to Ca2+ release from sarcoplasmic reticulum. ROS is also elevated in skeletal muscle from mdx mice or dysferlinopathy, another muscular dystrophy (230). It should be noted that NOX4-generated ROS and oxidation of RyR1 have also been implicated in muscular dystrophies (289).
Possible Complications Resulting from Inhibition of NOX2
When considering clinical drug development programs targeting NOX2, it is important to bear in mind possible complications that might arise from inhibiting the normal functions of this enzyme and to weigh these against the hypothetical benefits of treatment. The human genetic disorder CGD as well as animal models provide insights into this question.
CGD as a model for possible complications of NOX2 inhibition
CGD is a genetic disease that is characterized by severe bacterial and fungal infections and abscesses in the lung and liver (266). Before the advent of antibiotics, affected individuals frequently succumbed to infections during childhood, but with modern antibiotics and other therapies (131), individuals often survive into the fourth decade and beyond. Neutrophils from CGD patients are defective in the respiratory burst, the process by which molecular oxygen is reduced by the phagocyte NOX2 system to generate microbicidal ROS (see section “General roles of reactive oxygen species and NADPH oxidase enzymes” I, above). Genetically, the condition results from mutations in or deletion of any of the genes encoding subunits of the respiratory burst oxidase (97, 148) and is described in Table 3.
Table 3.
Genetic Origins of CGD
| Subunit | Function | Gene | % of cases | Inheritance |
|---|---|---|---|---|
| NOX2 | Catalytic | CYBB | 65 | X-linked |
| p22phox | Regulatory | CYBA | <5 | Autosomal recessive |
| p47phox | Regulatory | NCF1 | 30 | Autosomal recessive |
| p67phox | Regulatory | NCF2 | <5 | Autosomal recessive |
| p40phox | Regulatory | NCF4 | Single case | Autosomal recessive |
While targeting of NOX2 or its regulatory subunits with small-molecular-weight inhibitors raises concerns about the suppression of innate immunity resulting in increased infections, infection-related symptoms are seen only when the NADPH-oxidase activity is <15–20% of normal (97, 148); individuals with ROS-generating activity greater than this value are asymptomatic and are, therefore, never diagnosed with CGD. Moreover, an extensive analysis of deaths in a study population of 287 CGD patients followed for more than two decades (148) revealed that survival was independent of the particular gene affected, but depended solely on the extent of residual NADPH-oxidase activity. Significant mortality was manifested only when residual activity fell below ∼2%: a single death occurred among patients classified as having relatively high residual ROS generation, whereas around a third of those with severe ROS deficiency had died by age 40. It should be pointed out that today's patients are generally treated with prophylactic antibiotics, and that conclusions are likely to differ in naïve patients. Nevertheless, these studies point out that there is a considerable reservoir of excess NOX activity and that even a modest residual activity confers benefit. Dosing and scheduling of a NOX2-targeting drug can, in principle, be managed so as to avoid a high level of continuous inhibition, and the cyclic nature of dosing should result in intervals during which oxidase activity returns to levels that allow adequate microbicidal function. In addition, it should be noted that even in CGD patients with complete loss-of-oxidase function, the decreased survival is seen over the time scale of decades, and mortality from suppression of innate immunity is not likely to be an acute problem.
CGD, hyperinflammation, and autoimmune disease
In addition to impaired host defense, CGD is a disease of excessive inflammation and increased risk of autoimmune disorders (266). A hallmark of the disease is formation of granuloma, foci in which macrophages and other immune cells concentrate. Granuloma may occur in many organs, including the gastrointestinal tract, where they contribute to enteritis resembling Crohn's disease, and in the genitourinary tract, where they may result in blockages.
The pathogenesis of granulomatous lesions is not entirely clear, but it has been suggested to result, in part, from failure to kill and clear microbes after minor infections. However, recent studies indicate that this may be an over-simplification, and that excessive inflammation may be explained by an important role for the NOX2-derived ROS in regulating the inflammatory response through redox-sensitive signaling and transcription pathways (108, 266). Consistent with this interpretation, phagocytic cells in CGD are markedly perturbed in their gene expression, including increases in pro-inflammatory genes, decreases in anti-inflammatory genes, and alterations in apoptotic genes (144). The net result of the latter renders phagocytes less susceptible to apoptosis, perhaps accounting for their accumulation in granuloma. Another theory is that tryptophan catabolism via the O2•−-dependent enzyme indolamine 2,3-dioxygenase is defective in CGD, leading to a deficiency in production of kynurenine (240). The latter is thought to participate in regulating the immune response, and its absence may result in a hyperinflammatory state. Regardless of the explanation, it is obvious that phagocytes from CGD individuals are markedly abnormal, not only in their ability to kill certain microbes, but also in their significantly altered expression of inflammatory, apoptotic, and other genes, which is expected to result in altered function.
In addition to granuloma, there are a number of other clinical manifestations of an overly exuberant immune response in CGD. For example, fungal products result in a life-threatening “mulch pneumonitis” (271). In addition, CGD patients show an increased frequency of certain autoimmune diseases, including rheumatoid arthritis, a systemic lupus erythematosis (SLE)-like syndrome, and Guillain-Barré syndrome, an autoimmune demyelinating disease (86, 107, 108). Experiments in rodent models summarized next add further insights into mechanisms by which an absence of NOX2-dependent ROS generation in cells of the innate immune system may propagate effects through cell types of the adaptive immune system.
Because the majority of CGD patients have residual levels of ROS below 5% of normal, the extent to which drug targeting the NOX2 system may result in autoimmune side effects is not clear. In the study of 287 patients described earlier (148), the occurrence or severity of granulomatous complications did not correlate well with the extent of residual NOX activity, which might suggest that autoimmune dysfunction may still occur when residual ROS levels are 5–15% of normal. Additional models of partial loss of NADPH-oxidase activity are needed to better assess the risk of side effects, and some are presented next.
Autoimmune disease in mothers of CGD patients
Mothers of X-linked CGD patients show an increased incidence of autoimmune disorders, including an SLE-like syndrome and rheumatoid arthritis (177, 275). In kindreds with X-linked CGD, 9% have one individual diagnosed with lupus-like symptoms. A study of 19 CGD carrier mothers revealed a high incidence of lupus-like symptoms limited to cutaneous lesions, including 58% reporting photosensitive skin rashes and 42% with mouth ulcers. In addition, 37% showed joint pains (36). However, it is important to note that random X inactivation (a.k.a. Lyonization) implies that female carriers will have two populations of neutrophils, one that is entirely normal in its NADPH-oxidase activity, and a second which is CGD-like, lacking NOX2 activity (177). Depending on the developmental stage at which Lyonization occurs, the effect on the cell lineage can be skewed and can even give rise to mild CGD symptoms in female carriers. Skewed cell lineage has been verified in CGD carriers using a nitroblue tetrazolium (NBT) dye reduction test which stains only neutrophils that actively produce ROS (124); while normal individuals showed 98% NBT positive cells, female carriers showed a wide range (16–88%) of positive cells. Thus, while the ROS generation in CGD carriers is, on average, about half of normal, the average arises from normal ROS generation in some cells and absent ROS generation in others. Since CGD cells have grossly abnormal gene expression and immune function compared with normal cells, it seems likely that the same subpopulation of phagocytes which lacks ROS generation in the carrier state will closely resemble a CGD phagocyte in terms of immune function, apoptosis, etc., and that these severely compromised cells will predispose the carrier to autoimmune disorders. With regard to bacterial killing, neutrophils of CGD carriers, indeed, show a range of functional abilities, from near normal to inactivation almost as profound as that seen in CGD (235). While it also would be of great interest to investigate whether there is a correlation between the fraction of abnormal cells and the frequency of autoimmune disease, to our knowledge, this has not been done. Still, the presence of a severely compromised subpopulation of immune cells raises questions about the extent to which the CGD carrier state can be used as an appropriate model to predict the likelihood of autoimmune complications that might arise from partial NOX2 inhibition. Due to these considerations, in our opinion, the CGD carrier state is a poor model for predicting NOX2 drug side effects. In the next few sections, we consider inactivating (including partially inactivating) mutations in animals and humans as predictive models for possible complications of NOX2 inhibition.
Animal model association of autoimmune disease with compromised NOX2-dependent ROS generation
Studies in rats and mice have provided important insights into the physiological mechanisms by which a compromised NOX2 system may increase the risk or severity of autoimmune disorders. In genetic studies comparing inflammation-resistant E3 rats with Dark Agouti (DA) rats, which are predisposed to developing pristane-induced arthritis and other autoimmune conditions, the Pia4 region of the chromosome correlated with susceptibility to or severity of inflammatory diseases (101, 253). Positional cloning identified NCF1 (encoding p47phox) as the gene that was responsible (204, 313). Depending on the activating agonist used, neutrophils from affected animals showed between 25% and 50% of normal O2•− generation. Similarly, mice with a mutation in NCF1 had no detectable oxidative burst and showed enhanced collagen-induced arthritis as well as increased severity of experimental autoimmune encephalomyelitis (106, 107). Some of the female NCF1 mutated mice developed spontaneous severe arthritis without pristane. Other mouse strains mutated in other NOX/phox genes also show low or absent ROS generation, and are similarly susceptible to increased arthritis severity in various models (108).
Studies in rodents have provided possible insights into the mechanisms by which a compromised NOX2 system increases arthritis severity (108). In one study of arthritis-susceptible rats, a higher content of reduced thiols on the T-cell surface was seen and correlated with increased ability to induce arthritis in adoptive transfer experiments (85). T cells do not express the NOX2 system, so any oxidation of the T-cell surface most likely results from an interaction with phagocytes. It is difficult to understand, however, how the redox state of thiols on the cell surface would fail to equilibrate rapidly in a new host animal, and it seems unlikely that such changes alone would account for the observed results. Since it readily diffuses through membranes (197, 327), H2O2 can also, in theory, affect intracellular proteins in nearby cells, for example by oxidizing regulatory low pKa thiols in enzymes such as PTPs and in transcription factors (153), and this might lead to reprogramming of protein expression patterns and/or differentiation in target cells. In another study, cellular changes were tracked in NOX2 knockout mice that displayed aging-dependent spontaneous development of arthritis (161). The NOX2 deficiency was associated with changes in immune cell populations, with marked alterations in subpopulations of myeloid cells as well as lymphomegaly, splenomegaly, and increased levels of inflammatory cytokines, including interferon-γ and IL-17. Additional studies are needed to fully elucidate the mechanism by which decreased NOX2 activity is linked to the development of arthritis in rodents.
While rodent models have proved useful in dissecting cell types and pathways involved in NOX2-ROS suppression of hyperinflammation, they do not provide an adequate model for predicting whether therapeutic NOX2 inhibition is likely to cause side effects related to autoimmune disorders. In addition to the issue of species difference, most of the models described earlier (including the aging-dependent spontaneous arthritis model) used animals in which NOX2 activity was undetectable, providing a model for immunologic changes in CGD but not for a partially or intermittently inhibited state as would be seen with drug treatment. In the DA rat models of arthritis, a polymorphism in the NCF1 gene was associated with partial inhibition of NOX2-dependent O2•− generation, but the DA rat represents an animal that is already genetically predisposed to arthritis. In addition, strong arthritis-inducing stimuli such as pristane and collagen were used to cause disease in many of the studies, and it is not clear how relevant this will be to spontaneous arthritis in humans. Therefore, it is important to evaluate associations in humans between decreased (but not absent) NOX2-dependent ROS generation and autoimmune diseases.
Human disease associations with polymorphisms in NOX2 and phox components
In rheumatoid arthritis patients, a subset of male patients had a single nucleotide polymorphism in NCF4, the gene encoding p40phox, at a higher frequency than that of the general population (205). The polymorphism occurred in a non-coding region in the beginning of intron 4, but the effect on p40phox expression or NOX2 enzyme activity was not reported. The results were interpreted as consistent with the view that both multiple genes and also sex differences contribute to disease progression in different subsets of rheumatoid arthritis patients. A separate study by the same group reported a decreased risk of rheumatoid arthritis associated with increased copy number of NCF1 (p47phox) (206). NCF4 has also been associated with Crohn's disease (239). SLE patients (120) had a higher incidence of the 389 Q/Q polymorphism in NCF2 (encoding p67phox) than the control population, which has the more common 389 H/H allele. The Q mutation resulted in a weaker association of p67phox with the guanine nucleotide exchange factor Vav, resulting in 50% lower Fc receptor-activated O2•− generation. Similar to rheumatoid arthritis, multiple genes contribute to the development of SLE, and a change in a single gene is not sufficient to cause the disease (123). Since the disease-associated allele for both lupus and rheumatoid arthritis is rare in the general population, estimates regarding the increased risk associated with carrying the disease-associated allele are not reliable. However, it is safe to say that in the absence of additional predisposing genotypes, the likelihood of an individual with such an allele developing either disease is low. Indeed, in a study of the NCF2 polymorphism in China, no increased risk of SLE was seen (336). To our knowledge, no polymorphisms in the genes for NOX2 or p22phox have been reported to be associated with autoimmune or inflammatory diseases.
On the other hand, polymorphisms in the genes encoding NOX2 (CYBB) or phox proteins are associated with beneficial effects in some conditions. Williams–Beuren Syndrome (WBS) is a developmental disorder that is marked by arterial defects leading to hypertension. Deletion of one of the two functional copies of NCF1 protects a subset of WBS patients against hypertension, a finding that was recapitulated in a mouse model of the disease using the NOX inhibitor apocynin (37, 146). However, in another study of two patients with WBS and CGD who lacked any functional NCF1 gene, there was no protection against hypertension, suggesting that factors other (or in addition to) than NOX in vascular tissue may be involved in hypertension (281).
These studies imply that while decreased ROS generation from the NOX2 system correlates with certain autoimmune diseases, this association seems to be less pronounced in humans than it is in rodent models, and does not correspond to the increased frequency of autoimmune diseases seen in mothers of X-linked CGD patients. Therefore, in our opinion, in the absence of additional predisposing genotypes, existing evidence does not strongly support the hypothesis that partial inhibition of NOX2 poses a significant increased risk of autoimmune disease.
Conclusions regarding the likelihood of side effects from inhibiting the NOX2 system with small molecule drugs
Based on available literature, it seems probable that side effects from inhibiting the NOX2 system are not likely to be common or severe, although, undoubtedly, this will vary with the indication, duration of therapy, and drug dosage. The most serious concern surrounding NOX2 inhibition has been immunosuppression, resulting in life-threatening infections. However, the studies summarized here indicate that the NOX2 system has a large excess of microbicidal capacity, and we suggest that only under continuous, long-term inhibition of NOX2 would serious impairment of innate immunity occur. Co-administration of antibiotics could provide a further safeguard against life-threatening complications. The possibility of triggering an autoimmune disorder is another important consideration. While polymorphisms in human phox genes have shown an association between decreased NOX2 activity and autoimmune diseases and arthritis, these diseases are associated with multiple susceptibility genes as well as unknown environmental factors. Thus, the individual risk of developing an autoimmune disease as a result of inhibiting any single susceptibility gene product such as NOX2 seems to be low. As with many drug therapies, some genetically predisposed subpopulations will very likely be more susceptible to side effects, and therapeutic benefits should always be balanced against possible harmful effects.
Small-Molecule NOX Inhibitors: Progress and Prospects
Early attempts to inhibit the phagocyte NOX are summarized in Cross (52), and subsequent progress is summarized in Kim et al. (137). To date, however, only a limited number of NOX2 small-molecule inhibitors have been identified and very few of these appear to be promising for drug development. This section summarizes those commonly used for in vitro and in vivo studies, and describes recent progress toward the development of inhibitors that may show eventual clinical promise. Inhibitor structures are shown in Figure 2, and their proposed sites of action where information is available are mapped onto a diagram of the NOX2 system in Figure 1.
FIG. 2.
Structures of some commonly used NOX inhibitors.
NOX2 inhibitors commonly used as in vitro (and occasionally in vivo) tools
Diphenylene iodonium
DPI is a non-drug like molecule that was originally described as a general flavoprotein dehydrogenase inhibitor (179). Its mechanism of action involves abstraction of an electron from reduced flavin (FAD or flavin mononucleotide) to form a DPI radical, which then reacts to form a covalent adduct with the flavin, inactivating the coenzyme (201). Depending on its proximity to other groups, covalent adducts may also form with other cofactors such as the heme of NOX2 (68). This non-selective chemical mechanism causes DPI to inhibit a large number of flavin-dependent enzymes, including not only all of the NOX/DUOX enzymes, but also NOS, xanthine oxidase, and at higher concentrations mitochondrial respiration (8). NOX enzymes represent one of the more sensitive targets of DPI, which inhibits the neutrophil NOX2 system with an IC50 of 0.9 μM (92), while related analogs show IC50 values in the 0.5–0.75 μM range. Its relative potency against NOX enzymes has led to wide and extensive use of DPI as a tool to study the NOX enzymes in vitro (68). Toxicity limits the use of DPI in vivo; the LD50 of DPI is <10 mg/kg in rodents (83) and long-term administration at a lower dose (1.5 mg/kg/day, 4–5 weeks) led to cardiomyopathy (50). Low doses of DPI have been used, however, to support target validation of NOX-dependent pathologies in vivo (3). Although DPI can be considered a useful tool for in vitro NOX studies in situations where isoform selectivity is not an issue, its off-target effects, low solubility, and toxicity eliminate its development as a drug candidate.
Apocynin
Also known as acetovanillone, apocynin occurs naturally in certain plants (172) and was isolated from the root of Canadian hemp in 1883 and Picrorhiza kurroa in 1971 (73, 310). Plants containing the compound have been used as a traditional medicine, for example, for the treatment of jaundice, asthma, liver, and heart problems (219, 286). Since the early 1980s, apocynin has been extensively used as both an in vitro and in vivo NOX inhibitor (274). There is considerable debate about both its mechanism of action and the active form of the molecule. Reportedly, the metabolism of apocynin by myeloperoxidase generates the active dimeric and trimeric species (282). A trimeric form inhibited a NOX2 cell-free system with an IC50 of 30 nM, whereas apocynin itself was ineffective (187). Thus, the effectiveness of apocynin as an NOX2 inhibitor may be limited in vivo to inflamed regions harboring neutrophils or other myeloperoxidase (MPO)-expressing inflammatory cells; other cells and tissues such as vascular cells lack MPO and cannot generate the active oligomers, suggesting that apocynin may not inhibit NOX2 in such regions. The apocynin oligomer inhibits the NOX2 system by covalently modifying Cys196 of p47phox, thus preventing its assembly with p22phox, as in Figure 1 (187). Apocynin treatment of monocytes prevents the translocation of p47phox to the membrane, decreases NOX2-dependent ROS generation, and inhibits cyclooxygenase expression (16), which is proposed to account for protective effects of the compound in some experimental models of inflammation. While it blocked ROS-dependent signaling in vascular cells, apocynin failed directly to block O2− production by NOX1, NOX2, or NOX4 over-expressed in HEK293 cells. Rather, it interfered in assays detecting H2O2 or •OH, indicating that in this setting apocynin functions as an antioxidant (96).
Since it decreases markers of oxidative stress in vivo and is non-toxic, apocynin has been extensively used in both chronic and acute animal models of disease (including many of the experiments described in previous sections), including collagen-induced arthritis (106–108), in which apocynin was found to have remarkable preventative properties when administered before (but not after) collagen. Similarly, apocynin has been shown to prevent inflammation in models of inflammatory bowel disease and asthma (138). In a model of stroke-prone spontaneously hypertensive rats, apocynin significantly decreased the occurrence of stroke (332). In addition, apocynin has been investigated in a Phase 2 human clinical trial for asthma (284), where it showed anti-inflammatory properties, including a decrease in H2O2 in exhaled breath condensates. Thus, while apocynin shows promise as an anti-inflammatory molecule, it remains unclear as to whether its primary mode of action in vivo involves the inhibition of NOX2, its antioxidant properties, or both.
gp91(NOX2)ds-tat and other peptide inhibitors
Peptide-based inhibitors, by their nature, have the potential advantage of being more specific and having fewer off-target effects than small-molecule organic compounds, but they have numerous problems as drugs having to do with bioavailability, stability, delivery, and—over time—induction of neutralizing antibodies. Since the 1990s, a variety of NOX-targeted inhibitory peptides representing many regions of the NOX subunit have been developed (47, 56, 57, 70, 71) and employed in vitro, although to our knowledge, only one of these has been tested in vivo (47). gp91(NOX2)ds-tat is an 18-amino-acid peptide that includes a part of the B-loop of NOX2 that was designed to block the interaction between NOX2 and its regulatory subunit p47phox. However, effects on subunit assembly may be indirect, and we tentatively assign its effect to interrupting the interface between the dehydrogenase domain and the transmembrane domain (Fig. 1), based on structural considerations that place the B-loop at this interface (117). NOX2ds-tat inhibited NOX2 with an IC50 of 0.74 μM, but did not inhibit NOX1 or NOX4 in cell lines specifically expressing these isoforms (53). NOX2ds-tat also inhibited O2•− anion production from cultured endothelial cells (169), human resistance artery smooth muscle cells (304), and platelets (147). Moreover, the peptide showed protective effects in ex vivo and in vivo models. NOX2ds-tat improved acetylcholine-induced endothelium-dependent relaxation in aortic rings from mice with renovascular hypertension (126) and also suppressed angioplasty-induced neointimal proliferation in rat carotid artery (66).
Peptide-based inhibitors show a loss of bioactivity when administered orally, as most peptides are rapidly inactivated by gastrointestinal enzymes. The development of new technologies such as drug-encapsulating polymeric microparticles or nanoparticles may enhance the efficacy of peptides and other active pharmaceutical ingredients in the future (54). However, at this time, the development of non-peptide small-molecule NOX inhibitors seems more likely to result in clinically useful drugs.
Other reported NOX inhibitors
Other chemical compounds have been reported to inhibit NOX, but because of non-specific mechanisms of action or unresolved questions regarding effectiveness, these compounds are infrequently used as tools.
Phenylarsine oxide
This compound reacts covalently with vicinal cysteine residues, which is thought to be its mechanism of NOX2 inhibition. Specifically, phenylarsine oxide (PAO) at 50–100 μM reacted with NOX2 subunit and prevented its assembly with regulatory subunits; the compound failed to react with the NOX2 subunit after assembly had already been triggered (67). The administration of PAO (1 mg/kg intraperitoneal) provided an anti-inflammatory action on both hind paw edema induced by carrageenan and lung inflammation induced by lipopolysaccharide inhalation (242). However, given its mechanism of action, it is not clear whether beneficial effects were due to the inhibition of NOX2 or an unknown off-target effect.
Aminoethyl-benzenesulfono-fluoride
Aminoethyl-benzenesulfono-fluoride was originally characterized as an irreversible serine protease inhibitor, and it was subsequently discovered to inhibit NOX2 activity in a cell-free system when added before, but not after, enzyme activation. Specifically, the reagent inhibited the binding of p47phox to membranes containing NOX2/p22phox (60). The high concentration required for inhibition (nearly 1 mM) as well as off-target effects make this compound inappropriate for cell or in vivo applications.
Celastrol
This triterpenoid natural product isolated from the Chinese Thunder of God vine or Tripterygium wilfordii has been used in traditional Chinese medicine for the treatment of fever, chills, edema, and carbuncle (132). Recently, Jaquet et al. (121) tested celastrol on various NOX isoforms, and found that it acts as a general NOX inhibitor with some selectivity for NOX1 and NOX2 (IC50 values of 0.4 and 0.6 μM, respectively) compared with NOX4 and NOX5 (both ∼3 μM). For the NOX2 system, celastrol binds to p47phox, disrupting its interaction with p22phox, but its mode of action may differ between NOX1/2 and other NOX isoforms.
Fulvene-5
Long used as a dye, Fulvene-5 is an aromatic molecule with high water solubility. It was recently reported to inhibit NOX2 and NOX4 (218), and also blocked the growth of endothelial tumors in mice (22). However, Fulvene-5 inhibited these NOX isoforms by only ∼40–50% at 5 μM and assay controls were not reported. Thus, additional studies are needed to determine whether in vivo effects of this compound are a result of NOX inhibition or other mechanisms.
Gliotoxin
Gliotoxin is a disulfide-containing mycotoxin extracted from Aspergillus species that blocks NOX2 activity in neutrophils (IC50=8 μM) (335). In a cell-free system, pretreatment of membranes containing NOX2 prevents subsequent activation, but the compound is ineffective post-activation, suggesting modification of a site on NOX2 that is blocked once the active complex is assembled (199). In addition, the compound inhibits phosphorylation of p47phox by blocking the translocation of protein kinase C to the membrane, suggesting multiple or complex modes of action (199). The proposed chemical mechanism of the compound involves dithiol exchange at cysteine residues of target proteins; simultaneous addition of the reducing agent dithiothreitol abolished inhibition.
Other compounds
In addition to the compounds described earlier, other plant-derived natural products—many that have been used as traditional medicines—have been reported to inhibit NOX enzymes (122). These include Honokio (269, 307), Norathyriol (104), Abruquinone A (103), Magnolol (113, 319), and Prodigiosin (193, 208). However, little is known about their chemical or biochemical mechanisms of action, and some (e.g., Norathyriol, Abruquinone A, Magnolol) appear to target signaling pathways that lie upstream of NOX enzymes. Therefore, unless additional studies point to more specific effects, these compounds cannot currently be recommended as tools to study biological roles for NOX enzymes.
Systematic discovery of NOX inhibitors
While the NOX inhibitors described earlier were discovered fortuitously, the past decade has seen more systematic approaches in the search for NOX inhibitors. Such approaches involve cell-free or cell-based activity screens or screens involving disruption of protein–protein interactions between NOX regulatory subunits that are essential for activity. Practically speaking, cell-free activity assays are limited to the NOX2 system, due to the difficulty in obtaining sufficient amounts of material of other NOX isoforms as well as, in some cases, stability issues that make some isoforms insufficiently robust for robotic screening efforts. In some of these cases, binding assays can, in theory, be used as a surrogate for activity. Activity or binding assays are then applied to screening libraries of chemical compounds, which, depending on the size of the library can be considered medium-throughput or high-throughput screens, and that are carried out in an automated manner. Hits are then subjected to a series of counter-screens which are designed to establish the hits as true inhibitors and not artifacts, for example, compounds that interfere with the screening assay itself. Depending on the potency of the initial hit, compounds may then be subjected to an iterative process of chemical modification and re-testing in order to develop compounds with enhanced potency, improved isoform selectivity, improved solubility, decreased toxicity, and increased bioavailability. The goal of this process is to develop compounds not only as research tools but also with properties that are appropriate for drug development.
Screens using inhibition of NOX activity
VAS2870
This compound was found using a screen of small molecules to identify inhibitors of NOX2, carried out by the pharmaceutical company Vasopharm. The compound inhibited O2•− anion production with an IC50 of 11 μM in neutrophil cell lysates and 2 μM in whole neutrophil assays (301). The compound was subsequently characterized as an inhibitor of all NOX/DUOX isoforms (326). However, in a contradictory study, VAS2870 decreased ROS levels by an unidentified mechanism unrelated to direct NOX2 catalytic activity or subunit (84). The compound inhibited ROS generation and PDGF-mediated migration of VSMCs from rat thoracic aorta, and also blocked ROS generation induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells (285). In addition, VAS2870 decreased cell growth and promoted apoptosis in a rat liver tumor cell line that is dependent on NOX1 for growth (252). VAS2870 was effective in decreasing tissue damage in a stroke model, an effect that was attributed to the inhibition of NOX4 and not NOX2 (140). Despite its effectiveness, recent studies showed that VAS2870 given at the same doses that inhibit NOX enzymes have off-target effects based on alkylation of cysteines by the benzyltriazolopyrimidine moiety of VAS2870; one important off-target effect was shown to be alkylation of cysteine residues in the ryanodine receptor 1 (290). Therefore, at this time, while VAS2870 appears to be promising in the treatment of certain conditions, its in vivo mode of action is controversial, and additional studies are needed to clarify its mechanism. Caution is, therefore, advised in its use as a specific biochemical tool or in vivo probe.
ML171
ML171 and its phenothiazine analogues were identified in a medium-throughput (16,000 compounds) activity screen of NOX1-expressing HT-29 cells. ML171 and a related 2-(trifluoromethyl)-phenothiazine were identified as NOX1 inhibitors, and these and related compounds inhibited NOX1 with IC50's in the range of 0.2–1.0 μM, but showed little inhibition of NOX2. However, in our hands (Smith et al., Unpublished), ML171 also showed submicromolar inhibition of NOX2 and NOX4 in cell-free and whole cell L-012 assays, suggesting that the isoform selectivity of this compound requires additional evaluation. This inhibitor was used to elucidate the relevance of NOX1 in mechanisms of cancer invasion, where it blocked the formation of functional invadopodia in human colon cancer cells (88).
GKT136901 and related compounds
The pharmaceutical company Genkyotex used an NOX4-expressing cell line in a high-throughput screen (HTS) of 136,000 compounds to discover moderate potency pyrazolopyridine dione hits that were then optimized using medicinal chemistry, resulting in GKT136901 as a lead compound (151). In cell-free systems, GTK136901 demonstrated high potency for both NOX4 (Ki=165 nM) and NOX1 (Ki=160 nM) and weaker inhibition of NOX2 (Ki=1.5 μM) (262), making this compound a dual inhibitor of NOX1/4. Counterscreening against panels of biomedically important enzymes revealed no major off-target effects. In addition, the compound showed excellent pharmacokinetic properties and good oral bioavailability.
GKT136901 and related molecules were highly effective in in vitro assays of human fibroblast differentiation, epithelial cell apoptosis, and epithelial-mesenchymal transition (262). In addition, the compound was effective in preventive and curative murine models of bleomycin-induced pulmonary fibrosis, and in protection against diabetic nephropathy (263). A related compound, GKT137831, has completed Phase I clinical trials, where it has shown excellent safety and pharmacokinetic properties. To our knowledge, this compound is currently the most advanced NOX inhibitor in the drug development pipeline. While showing great promise for NOX4- and NOX1-related diseases, its lack of significant NOX2 activity makes it inappropriate for the treatment of most disease indications listed in Table 2.
Screens for disruption of subunit interactions
Ebselen and congeners
NOX2 activation in vivo depends on the binding of the C-terminal proline-rich domain (PRD) of its heterodimeric partner p22phox to the regulatory subunit p47phox (288). A HTS was designed to monitor this interaction via fluorescence polarization. In this screen (276), the binding of a synthetic, rhodamine-labeled peptide corresponding to the PRD of p22phox (rho-PRD) to recombinant glutathione-S-transferase-p47-bis-SH3 showed increased fluorescence polarization, while displacement of rho-PRD by the unlabeled PRD caused decreased signals. Using this principle, 230,000 compounds were screened; dose dependencies were carried out among initial hits; and 55 compounds were identified for confirmation in activity assays, resulting in the identification of 3–5 bona fide inhibitors. Among these was ebselen (272), a selenium-containing compound that had previously been identified as a glutathione peroxidase mimetic. Ebselen showed inhibition of cell-free NOX2 activity with an IC50 value of 0.6 μM. Another hit compound, Thr101, an analog of ebselen with sulfur in place of selenium, was also inhibitory. Systematic modifications of the structure identified many other potent (sub-micromolar IC50 values) analogs containing either Se or S. These compounds, in general, showed excellent selectivity for NOX2 and NOX1 compared with NOX4 and NOX5, and some showed marked selectivity for NOX2 compared with NOX1. For the NOX2 system, the compounds also inhibited the translocation of p47phox to the membrane, consistent with its targeting of the bis-SH3 domain of p47phox. While the selenium-containing compounds have been shown to have a number of off-target effects (257), the sulfur-containing compounds show promise for selectively targeting NOX2 and possibly NOX1, and may prove useful as in vitro probes.
Phox-I1
The binding of Rac-GTP to p67phox is thought to result in a conformational change in p67phox that allows an interaction with and activation of NOX2 (185). A recent study (25) used an in silico screening approach to identify potential inhibitors, which were then tested in an NOX2 activity assay. A crystal structure of the p67phox-Rac1 complex (158) was used to build a model of the Rac binding site on p67phox. Docking simulations (189) were then used to virtually screen 340,000 compounds for potential binding to the model binding site, and PhoxI1 was identified among the top hits. Although the compound bound tightly to recombinant p67phox with a Kd of ∼ 100 μM, it inhibited NOX2-dependent ROS generation in intact neutrophils with an IC50 of ∼10 μM, suggesting that Rac2 competition for the binding site inside the cell significantly decreases its in vivo potency. While this level of potency is unlikely to be sufficient for drug development, improved analogs may prove useful and are likely to show significant isoform selectivity.
Future prospects
NOX2 is a potentially important and novel target for the development of therapeutic agents for a range of serious diseases shown in Table 2, but additional approaches are needed to identify new isoform-selective small-molecule inhibitors. The high degree of structural and catalytic homology within the catalytic core of various NOX isoforms makes finding isoform-selective inhibitors a challenge. For example, the NADPH binding site is highly conserved among the NOX isoforms, suggesting that inhibitors targeting this site may be non-selective for NOX isoforms. Other regions of NOX subunits and their regulatory proteins, however, are structurally unique among the isoforms. Most screening approaches to date have not taken advantage of isoform-specific structural features, and, in some cases, have identified non-selective inhibitors. Fortuitously, some general screens have already identified promising inhibitors, some of which show significant isoform selectivity and potential for drug development. GKT136901, for example, shows selectivity for NOX4 and NOX1, and has completed Phase 1 clinical trials. While probing new chemical libraries using existing screening methods will, undoubtedly, result in new hits, future breakthroughs are likely to result from a combination of techniques relying on ultra high-throughput and/or virtual screening, while simultaneously taking advantage of isoform-specific structural features. Figure 1 shows a summary of the locus of action, where known, of various inhibitors on the NOX2 system. An inspection of Figure 1 reveals that there are specific protein–protein interactions which could, in principle, be targeted with new high-throughput assays. For example, neither the binding of p67phox to p47phox nor the interaction of p40phox (not shown) with its targets has been exploited. Similarly, targeting the binding of p47phox or NOXO1 to its phospholipid in the membrane is another possible approach that could be used to block assembly of the active complex. Since some of these interactions require structural interactions that differ significantly among different NOX isoform systems; such an approach may be selective, compared for example with targeting the NADPH binding site.
In addition, improved ROS assay methods may help identify new inhibitors. For example, currently, many of the reagents used for screening ROS are non-selective and detect a variety of other reactive molecules (e.g., NO, ONOO−, and HOCl); while others show high background signals. This results in a high false-positive rate, and is likely to miss weaker but valid inhibitors that could provide the foundation for novel chemical series.
NOX2 inhibitors show particular promise for the treatment of inflammatory diseases, both acute and chronic. Theoretical side effects include pro-inflammatory and autoimmune complications, and while these should be considered in any therapeutic program, in our opinion they do not appear to be serious enough to eliminate NOX2 as a drug target, particularly when weighed against the seriousness of many of the indications listed in Table 2. As with any first-in-class drug, proof of concept for efficacy with minimal side effects is needed for the acceptance of NOX2 inhibitors as therapeutic agents.
Abbreviations Used
- AD
Alzheimer's disease
- ALI
acute lung inflammation
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- ARDS
acute respiratory distress syndrome
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- CGD
chronic granulomatous disease
- CIH
chronic intermittent hypoxia
- COPD
chronic obstructive pulmonary disease
- CYBA
gene name for p22phox
- CYBB
gene name for NOX2
- DA
Dark Agouti
- DPI
diphenylene iodonium
- DUOX
dual oxidase
- FAD
flavin adenine dinucleotide
- GSH
glutathione, reduced
- GSSG
glutathione, oxidized
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- HTS
high-throughput screen
- IL
interleukin
- KC
keratinocyte-derived chemokine
- LIRI
lung ischemia-reperfusion injury
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- NBT
nitroblue tetrazolium
- NCF1
gene name for p47phox
- NCF2
gene name for p67phox
- NCF4
gene name for p40phox
- NET
neutrophil extracellular trap
- NF-kappa B
nuclear factor-kappa B
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NOS
Nitric oxide synthase
- NOX
NADPH oxidase
- O2•−
superoxide
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- PAO
phenylarsine oxide
- PD
Parkinson's disease
- PDGF
platelet-derived growth factor
- PH
pulmonary hypertension
- phox
phagocytic oxidase
- PRD
proline-rich domain
- PTP
protein tyrosine phosphatase
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosis
- SOD
superoxide dismutase
- TBI
traumatic brain injury
- TNF
tumor necrosis factor
- TTRhRen
transgenic mice overexpressing human renin
- VSMC
vascular smooth muscle cell
- WBS
Williams-Beuren syndrome
Acknowledgments
This work was supported by NIH grant RO1 CA105116 to J.D.L. and an American Heart Association SDG grant 344025 to B.A.D.
Author Disclosure Statement
Dr. Lambeth is co-founder of Genkyotex (Geneva, Switzerland), a pharmaceutical company that develops drugs targeting NADPH oxidases and serves on its scientific advisory board. None of the other authors have anything to disclose.
References
- 1.Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach, edited by WHO. Geneva: World Health Organization Press, 2007 [Google Scholar]
- 2.Dementia: A Public Health Priority. Geneva: World Health Organization, 2012 [Google Scholar]
- 3.Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol J-P, Patel NSA, Cuzzocrea S, and Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock 23: 107–114, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Abo A, Pick E, Hall A, Totty N, Teahan CG, and Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353: 668–670, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Aguirre J. and Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic Biol Med 49: 1342–1353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, and Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res 29: 813–820, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Akki A, Zhang M, Murdoch C, Brewer A, and Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol 47: 15–22, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, and Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Ansari MA. and Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med 51: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, and Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281: 40354–40368, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, and Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272: 217–221, 1997 [PubMed] [Google Scholar]
- 13.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, and Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104: 210–218, 21p following 218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee ER. and Henderson WR., Jr. Characterization of lung stem cell niches in a mouse model of bleomycin-induced fibrosis. Stem Cell Res Ther 3: 21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee ER. and Henderson WR., Jr. Role of T cells in a gp91phox knockout murine model of acute allergic asthma. Allergy Asthma Clin Immunol 9: 6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, and Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med 37: 156–165, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Beghi E, Chio A, Couratier P, Esteban J, Hardiman O, Logroscino G, Millul A, Mitchell D, Preux PM, Pupillo E, Stevic Z, Swingler R, Traynor BJ, Van den Berg LH, Veldink JH, and Zoccolella S. The epidemiology and treatment of ALS: focus on the heterogeneity of the disease and critical appraisal of therapeutic trials. Amyotroph Lateral Scler 12: 1–10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, and Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318: 1645–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Behrens MM, Ali SS, and Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28: 13957–13966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, and Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64: 361–368, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, and Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest 119: 2359–2365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianca VD, Dusi S, Bianchini E, Dal Pra I, and Rossi F. Beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J Biol Chem 274: 15493–15499, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Bodas M, Tran I, and Vij N. Therapeutic strategies to correct proteostasis-imbalance in chronic obstructive lung diseases. Curr Mol Med 12: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, Meller J, Seibel W, Mizrahi A, Pick E, Filippi MD, and Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem Biol 19: 228–242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, Casale TB, Chan-Yeung M, Chen R, Chowdhury B, Chung KF, Dahl R, Drazen JM, Fabbri LM, Holgate ST, Kauffmann F, Haahtela T, Khaltaev N, Kiley JP, Masjedi MR, Mohammad Y, O'Byrne P, Partridge MR, Rabe KF, Togias A, van Weel C, Wenzel S, Zhong N, and Zuberbier T. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on severe asthma. J Allergy Clin Immunol 126: 926–938, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Brandes RP. and Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med 18: 15–19, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, and Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Brochet DX, Yang D, Cheng H, and Lederer WJ. Elementary calcium release events from the sarcoplasmic reticulum in the heart. Adv Exp Med Biol 740: 499–509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DI. and Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JR, Goldblatt D, Buddle J, Morton L, and Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J Leukoc Biol 73: 591–599, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Bulut M, Savas HA, Altindag A, Virit O, and Dalkilic A. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry 10: 626–628, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Buttigieg J, Pan J, Yeger H, and Cutz E. NOX2 (gp91phox) is a predominant O2 sensor in a human airway chemoreceptor cell line: biochemical, molecular, and electrophysiological evidence. Am J Physiol Lung Cell Mol Physiol 303: L598–L607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, and Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 190: 4136–4148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cale CM, Morton L, and Goldblatt D. Cutaneous and other lupus-like symptoms in carriers of X-linked chronic granulomatous disease: incidence and autoimmune serology. Clin Exp Immunol 148: 79–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campuzano V, Segura-Puimedon M, Terrado V, Sanchez-Rodriguez C, Coustets M, Menacho-Marquez M, Nevado J, Bustelo XR, Francke U, and Perez-Jurado LA. Reduction of NADPH-oxidase activity ameliorates the cardiovascular phenotype in a mouse model of Williams-Beuren syndrome. PLoS Genet 8: e1002458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, and Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnesecchi S, Pache JC, and Barazzone-Argiroffo C. NOX enzymes: potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavarra E, Lucattelli M, Gambelli F, Bartalesi B, Fineschi S, Szarka A, Giannerini F, Martorana PA, and Lungarella G. Human SLPI inactivation after cigarette smoke exposure in a new in vivo model of pulmonary oxidative stress. Am J Physiol Lung Cell Mol Physiol 281: L412–L417, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, Stan R, Croce K, and Mayadas TN. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood 120: 4421–4431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng G, Ritsick D, and Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem 279: 34250–34255, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Chiang CH, Chuang CH, and Liu SL. Apocynin attenuates ischemia-reperfusion lung injury in an isolated and perfused rat lung model. Transl Res 158: 17–29, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Chiang CL, Ledermann JA, Aitkens E, Benjamin E, Katz DR, and Chain BM. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin Cancer Res 14: 4898–4907, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, and Suh SW. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res 1481: 49–58, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Aid S, Kim HW, Jackson SH, and Bosetti F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem 120: 292–301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciobica A, Padurariu M, Dobrin I, Stefanescu C, and Dobrin R. Oxidative stress in schizophrenia - focusing on the main markers. Psychiatr Danub 23: 237–245, 2011 [PubMed] [Google Scholar]
- 49.Clempus RE. and Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res 71: 216–225, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper JM, Petty RK, Hayes DJ, Morgan-Hughes JA, and Clark JB. Chronic administration of the oral hypoglycaemic agent diphenyleneiodonium to rats. An animal model of impaired oxidative phosphorylation (mitochondrial myopathy). Biochem Pharmacol 37: 687–694, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Cristovao AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, and Kim YS. NADPH oxidase 1 mediates alpha-synucleinopathy in Parkinson's disease. J Neurosci 32: 14465–14477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cross AR. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med 8: 71–93, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Addio SM. and Prud'homme RK. Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev 63: 417–426, 2011 [DOI] [PubMed] [Google Scholar]
- 55.D'Alessandro A. and Zolla L. The SODyssey: superoxide dismutases from biochemistry, through proteomics, to oxidative stress, aging and nutraceuticals. Expert Rev Proteomics 8: 405–421, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Dahan I, Molshanski-Mor S, and Pick E. Inhibition of NADPH oxidase activation by peptides mapping within the dehydrogenase region of Nox2-A “peptide walking” study. J Leukoc Biol 91: 501–515, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Dahan I. and Pick E. Strategies for identifying synthetic peptides to act as inhibitors of NADPH oxidases, or “all that you did and did not want to know about Nox inhibitory peptides”. Cell Mol Life Sci 69: 2283–2305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dale DC, Boxer L, and Liles WC. The phagocytes: neutrophils and monocytes. Blood 112: 935–945, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261: 1047–1051, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, and Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Dickinson BC, Peltier J, Stone D, Schaffer DV, and Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 7: 106–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, and Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 86: 1729–1737, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, and Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 12: 3721–3728, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, and Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflamm 7: 41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donoso P, Sanchez G, Bull R, and Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci 16: 553–567, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, and Pagano PJ. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol 288: H946–H953, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Doussiere J, Bouzidi F, Poinas A, Gaillard J, and Vignais PV. Kinetic study of the activation of the neutrophil NADPH oxidase by arachidonic acid. Antagonistic effects of arachidonic acid and phenylarsine oxide. Biochemistry 38: 16394–16406, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Doussiere J, Gaillard J, and Vignais PV. The heme component of the neutrophil NADPH oxidase complex is a target for aryliodonium compounds. Biochemistry 38: 3694–3703, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Ekeowa UI, Gooptu B, Belorgey D, Hagglof P, Karlsson-Li S, Miranda E, Perez J, MacLeod I, Kroger H, Marciniak SJ, Crowther DC, and Lomas DA. Alpha1-antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clin Sci (Lond) 116: 837–850, 2009 [DOI] [PubMed] [Google Scholar]
- 70.El-Benna J, Dang PM, and Perianin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem Pharmacol 80: 778–785, 2010 [DOI] [PubMed] [Google Scholar]
- 71.El-Benna J, Dang PM, and Perianin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell Mol Life Sci 69: 2307–2314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eliades A, Matsuura S, and Ravid K. Oxidases and reactive oxygen species during hematopoiesis: a focus on megakaryocytes. J Cell Physiol 227: 3355–3362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engels F, Renirie BF, t Hart BA, Labadie RP, and Nijkamp FP. Effects of apocynin, a drug isolated from the roots of Picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett 305: 254–256, 1992 [DOI] [PubMed] [Google Scholar]
- 74.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, and Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 1: 181–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Y. and Forgac M. Inhibition of vacuolar H(+)-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J Biol Chem 269: 13224–13230, 1994 [PubMed] [Google Scholar]
- 76.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, and Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113: 2047–2055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, and Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frey RS, Ushio-Fukai M, and Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fridovich I. Superoxide anion radical (O2•−), superoxide dismutases, and related matters. J Biol Chem 272: 18515–18517, 1997 [DOI] [PubMed] [Google Scholar]
- 80.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci 893: 13–18, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Fu X, Kassim SY, Parks WC, and Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem 276: 41279–41287, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, and Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gatley SJ. and Martin JL. Some aspects of the pharmacology of diphenyleneiodonium, a bivalent iodine compound. Xenobiotica 9: 539–546, 1979 [DOI] [PubMed] [Google Scholar]
- 84.Gatto GJ, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhibit Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, and Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A 103: 12831–12836, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gelderman KA, Hultqvist M, Olsson LM, Bauer K, Pizzolla A, Olofsson P, and Holmdahl R. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal 9: 1541–1567, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Gerber CE, Bruchelt G, Falk UB, Kimpfler A, Hauschild O, Kuci S, Bachi T, Niethammer D, and Schubert R. Reconstitution of bactericidal activity in chronic granulomatous disease cells by glucose-oxidase-containing liposomes. Blood 98: 3097–3105, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, and Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, and Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87: 26–32, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Grasberger H. and Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281: 18269–18272, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Hancock JT. The role of redox mechanisms in cell signalling. Mol Biotechnol 43: 162–166, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Hancock JT. and Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J 242: 103–107, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, and Wu R. Differential regulation of dual NADPH oxidases/peroxidases, DUOX1 and DUOX2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, and Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayes E, Pohl K, McElvaney NG, and Reeves EP. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch Immunol Ther Exp (Warsz) 59: 97–112, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Heyworth PG, Cross AR, and Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 15: 578–584, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Hidalgo C, Sanchez G, Barrientos G, and Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem 281: 26473–26482, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Ho WE, Cheng C, Peh HY, Xu F, Tannenbaum SR, Ong CN, and Wong WS. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free Radic Biol Med 53: 498–507, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Holgate ST. Stratified approaches to the treatment of asthma. Br J Clin Pharmacol 76: 277–291, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holmdahl R, Sareila O, Pizzolla A, Winter S, Hagert C, Jaakkola N, Kelkka T, Olsson LM, Wing K, and Backdahl L. Hydrogen peroxide as an immunological transmitter regulating autoreactive T cells. Antioxid Redox Signal 18: 1463–1474, 2013 [DOI] [PubMed] [Google Scholar]
- 102.Hooper NM. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans 33: 335–338, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Hsu MF, Raung SL, Tsao LT, Kuo SC, and Wang JP. Cellular localization of the inhibitory action of abruquinone A against respiratory burst in rat neutrophils. Br J Pharmacol 120: 917–925, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hsu MF, Raung SL, Tsao LT, Lin CN, and Wang JP. Examination of the inhibitory effect of norathyriol in formylmethionyl-leucyl-phenylalanine-induced respiratory burst in rat neutrophils. Free Radic Biol Med 23: 1035–1045, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, and Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A 106: 6226–6231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hultqvist M. and Holmdahl R. Ncf1 (p47phox) polymorphism determines oxidative burst and the severity of arthritis in rats and mice. Cell Immunol 233: 97–101, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, and Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A 101: 12646–12651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hultqvist M, Olsson LM, Gelderman KA, and Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol 30: 201–208, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Hultqvist M, Sareila O, Vilhardt F, Norin U, Olsson LM, Olofsson P, Hellman U, and Holmdahl R. Positioning of a polymorphic quantitative trait nucleotide in the Ncf1 gene controlling oxidative burst response and arthritis severity in rats. Antioxid Redox Signal 14: 2373–2383, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Hunt JM. and Tuder R. Alpha 1 anti-trypsin: one protein, many functions. Curr Mol Med 12: 827–835, 2012 [DOI] [PubMed] [Google Scholar]
- 111.Ignarro LJ, Buga GM, Wood KS, Byrns RE, and Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iiyama M, Kakihana K, Kurosu T, and Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal 18: 174–182, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Ikai T, Akao Y, Nakagawa Y, Ohguchi K, Sakai Y, and Nozawa Y. Magnolol-induced apoptosis is mediated via the intrinsic pathway with release of AIF from mitochondria in U937 cells. Biol Pharm Bull 29: 2498–2501, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, and Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, and Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652, 1997 [DOI] [PubMed] [Google Scholar]
- 116.Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, and Sobey CG. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol 156: 680–688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jackson HM, Kawahara T, Nisimoto Y, Smith SM, and Lambeth JD. Nox4 B-loop creates an interface between the transmembrane and dehydrogenase domains. J Biol Chem 285: 10281–10290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal 15: 2477–2486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jackson SH, Gallin JI, and Holland SM. The p47-phox mouse knock-out model of chronic granulomatous disease. J Exp Med 182: 751–758, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FP, Jr., Reiff A, Myones BL, Kaufman KM, McCurdy D, Harley JB, Silverman E, Kimberly RP, Vyse TJ, Gaffney PM, Moser KL, Klein-Gitelman M, Wagner-Weiner L, Langefeld CD, Armstrong DL, and Zidovetzki R. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A 109: E59–E67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaquet V, Marcoux J, Forest E, Leidal KG, McCormick S, Westermaier Y, Perozzo R, Plastre O, Fioraso-Cartier L, Diebold B, Scapozza L, Nauseef WM, Fieschi F, Krause K-H, and Bedard K. NADPH oxidase (NOX) isoforms are inhibited by celastrol with a dual mode of action. Br J Pharmacol 164: 507–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaquet V, Scapozza L, Clark R, Krause KH, and Lambeth JD. Small molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Johannesson M, Hultqvist M, and Holmdahl R. Genetics of autoimmune diseases: a multistep process. Curr Top Microbiol Immunol 305: 259–276, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Johansen KS. Nitroblue tetrazolium slide test. Use of the phorbol-myristate-acetate-stimulated NBT-reduction slide test for routine and prenatal detection of chronic granulomatous disease and diagnosis of heterozygous carriers. Acta Pathol Microbiol Immunol Scand C 91: 349–354, 1983 [PubMed] [Google Scholar]
- 125.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, and Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 298: H24–H32, 2009 [DOI] [PubMed] [Google Scholar]
- 126.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Kahle MP. and Bix GJ. Successfully climbing the “STAIRs”: surmounting failed translation of experimental ischemic stroke treatments. Stroke Res Treat 2012: 374098, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kahles T. and Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for Nox 2. Antioxid Redox Signal 18: 1400–1417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, and Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis 40: 185–192, 2010 [DOI] [PubMed] [Google Scholar]
- 130.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, and Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38: 3000–3006, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Kang EM, Choi U, Theobald N, Linton G, Long Priel DA, Kuhns D, and Malech HL. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood 115: 783–791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kannaiyan R, Shanmugam MK, and Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303: 9–20, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Kaplan MJ. and Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189: 2689–2695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawahara T, Ritsick D, Cheng G, and Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem 280: 31859–31869, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, and Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal 5: ra56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim D, You B, Jo EK, Han SK, Simon MI, and Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 107: 14851–14856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, and Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 21: 1147–1158, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, and Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol 90: 441–448, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, and Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol 26: 5908–5920, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: pii:, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kleniewska P, Piechota A, Skibska B, and Goraca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 60: 277–294, 2012 [DOI] [PubMed] [Google Scholar]
- 142.Knaus UG, Heyworth PG, Evans T, Curnutte JT, and Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 254: 1512–1515, 1991 [DOI] [PubMed] [Google Scholar]
- 143.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, and Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect Immun 69: 5991–5996, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kobayashi SD, Voyich JM, Braughton KR, Whitney AR, Nauseef WM, Malech HL, and DeLeo FR. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol 172: 636–643, 2004 [DOI] [PubMed] [Google Scholar]
- 145.Kotsias F, Hoffmann E, Amigorena S, and Savina A. Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid Redox Signal 18: 714–729, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Kozel BA, Knutsen RH, Ye L, Ciliberto CH, Broekelmann TJ, and Mecham RP. Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation. J Biol Chem 286: 44926–44936, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, and Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 100: 917–924, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, and Gallin JI. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 363: 2600–2610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuiper JW, Sun C, Magalhaes MA, and Glogauer M. Rac regulates PtdInsP(3) signaling and the chemotactic compass through a redox-mediated feedback loop. Blood 118: 6164–6171, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Lagente V, Planquois JM, Leclerc O, Schmidlin F, and Bertrand CP. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin Exp Pharmacol Physiol 35: 601–605, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 152.Lam GY, Huang J, and Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol 32: 415–430, 2010 [DOI] [PubMed] [Google Scholar]
- 153.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 154.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43: 332–347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lambeth JD, Kawahara T, and Diebold B. Regulation of Nox and DUOX enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lambeth JD, Krause KH, and Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol 30: 339–363, 2008 [DOI] [PubMed] [Google Scholar]
- 157.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, and Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, and Rittinger K. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol Cell 6: 899–907, 2000 [DOI] [PubMed] [Google Scholar]
- 159.Lassegue B. and Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee CF, Qiao M, Schroder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee K, Won HY, Bae MA, Hong JH, and Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A 108: 9548–9553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lei M, Luo L, Ma SQ, Zhang Y, Wu XH, and Li L. [Behavioral and neurobiological abnormalities induced by social isolation as a useful animal model of schizophrenia]. Sheng Li Xue Bao 65: 101–108, 2013. [Article in Chinese] [PubMed] [Google Scholar]
- 163.Leto TL, Lomax KJ, Volpp BD, Nunoi H, Sechler JMG, Nauseef WM, Clark RA, Gallin JI, and Malech HL. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science 248: 727–730, 1990 [DOI] [PubMed] [Google Scholar]
- 164.Li JM, Fan LM, Christie MR, and Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol 25: 2320–2330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li N, Li B, Brun T, Deffert-Delbouille C, Mahiout Z, Daali Y, Ma XJ, Krause KH, and Maechler P. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes 61: 2842–2850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J, Moreno A, Sanchez R, Carrion IJ, Levine BA, Al-Nakhala BM, Sullivan SM, Gill A, and Perrin S. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet 42: 392–399, 2010 [DOI] [PubMed] [Google Scholar]
- 167.Liu W, Chen Q, Liu J, and Liu KJ. Normobaric hyperoxia protects the blood brain barrier through inhibiting Nox2 containing NADPH oxidase in ischemic stroke. Med Gas Res 1: 22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu Y, Hernandez-Ochoa EO, Randall WR, and Schneider MF. NOX2-dependent ROS is required for HDAC5 nuclear efflux and contributes to HDAC4 nuclear efflux during intense repetitive activity of fast skeletal muscle fibers. Am J Physiol Cell Physiol 303: C334–C347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lopes NH, Vasudevan SS, Gregg D, Selvakumar B, Pagano PJ, Kovacic H, and Goldschmidt-Clermont PJ. Rac-dependent monocyte chemoattractant protein-1 production is induced by nutrient deprivation. Circ Res 91: 798–805, 2002 [DOI] [PubMed] [Google Scholar]
- 170.Lopez AD. and Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol 100: 481–499, 2006 [DOI] [PubMed] [Google Scholar]
- 171.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, and Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu X, Wan S, Jiang J, Jiang X, Yang W, Yu P, Xu L, Zhang Z, Zhang G, Shan L, and Wang Y. Synthesis and biological evaluations of novel apocynin analogues. Eur J Med Chem 46: 2691–2698, 2011 [DOI] [PubMed] [Google Scholar]
- 173.Lyle AN. and Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 21: 269–280, 2006 [DOI] [PubMed] [Google Scholar]
- 174.Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 342: 325–339, 2010 [DOI] [PubMed] [Google Scholar]
- 175.Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, and Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest 117: 2913–2919, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marengo JJ, Hidalgo C, and Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J 74: 1263–1277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martin-Villa JM, Corell A, Ramos-Amador JT, Ruiz-Contreras J, and Arnaiz-Villena A. Higher incidence of autoantibodies in X-linked chronic granulomatous disease carriers: random X-chromosome inactivation may be related to autoimmunity. Autoimmunity 31: 261–264, 1999 [DOI] [PubMed] [Google Scholar]
- 178.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 179.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem 269: 22459–22462, 1994 [PubMed] [Google Scholar]
- 180.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, and Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol 171: 3010–3018, 2003 [DOI] [PubMed] [Google Scholar]
- 181.Matthay MA. and Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McCann SK, Dusting GJ, and Roulston CL. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J Neurosci Res 86: 2524–2534, 2008 [DOI] [PubMed] [Google Scholar]
- 183.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, and Weissmann N. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 52: 1033–1042, 2012 [DOI] [PubMed] [Google Scholar]
- 184.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 185.Mizrahi A, Berdichevsky Y, Casey PJ, and Pick E. A Prenylated p47phox-p67phox-Rac1 chimera is a quintessential NADPH oxidase activator: membrane association and functional capacity. J Biol Chem 285: 25485–25499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, and Hussain SN. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal 10: 559–574, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Mora-Pale M, Joon Kwon S, Linhardt RJ, and Dordick JS. Trimer hydroxylated quinone derived from apocynin targets cysteine residues of p47phox preventing the activation of human vascular NADPH oxidase. Free Radic Biol Med 52: 962–969, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, and Leto TL. DUOX maturation factors form cell surface complexes with DUOX affecting the specificity of reactive oxygen species generation. FASEB J 23: 1205–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, and Olson AJ. AutoDock4 and autodocktools4: automated docking with selective receptor flexibility. J Comput Chem 30: 2785–2791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mouri A, Nagai T, Ibi D, and Yamada K. Animal models of schizophrenia for molecular and pharmacological intervention and potential candidate molecules. Neurobiol Dis 53: 61–74, 2013 [DOI] [PubMed] [Google Scholar]
- 191.Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sanchez A, Fernandez M, and Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J 405: 251–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nagaoka Y, Otsu K, Okada F, Sato K, Ohba Y, Kotani N, and Fujii J. Specific inactivation of cysteine protease-type cathepsin by singlet oxygen generated from naphthalene endoperoxides. Biochem Biophys Res Commun 331: 215–223, 2005 [DOI] [PubMed] [Google Scholar]
- 193.Nakashima T, Iwashita T, Fujita T, Sato E, Niwano Y, Kohno M, Kuwahara S, Harada N, Takeshita S, and Oda T. A prodigiosin analogue inactivates NADPH oxidase in macrophage cells by inhibiting assembly of p47phox and Rac. J Biochem 143: 107–115, 2008 [DOI] [PubMed] [Google Scholar]
- 194.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219: 88–102, 2007 [DOI] [PubMed] [Google Scholar]
- 195.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol 30: 195–208, 2008 [DOI] [PubMed] [Google Scholar]
- 196.Nauseef WM. Editorial: nyet to NETs? A pause for healthy skepticism. J Leukoc Biol 91: 353–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Niethammer P, Grabher C, Look AT, and Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, and Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nishida S, Yoshida LS, Shimoyama T, Nunoi H, Kobayashi T, and Tsunawaki S. Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase. Infect Immun 73: 235–244, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nose M, Accordini S, Artioli P, Barale F, Barbui C, Beneduce R, Berardi D, Bertolazzi G, Biancosino B, Bisogno A, Bivi R, Bogetto F, Boso M, Bozzani A, Bucolo P, Casale M, Cascone L, Ciammella L, Cicolini A, Cipresso G, Cipriani A, Colombo P, Dal Santo B, De Francesco M, Di Lorenzo G, Di Munzio W, Ducci G, Erlicher A, Esposito E, Ferrannini L, Ferrato F, Ferro A, Fragomeno N, Parise VF, Frova M, Gardellin F, Garzotto N, Giambartolomei A, Giupponi G, Grassi L, Grazian N, Grecu L, Guerrini G, Laddomada F, Lazzarin E, Lintas C, Malchiodi F, Malvini L, Marchiaro L, Marsilio A, Mauri MC, Mautone A, Menchetti M, Migliorini G, Mollica M, Moretti D, Mule S, Nicholau S, Nose F, Occhionero G, Pacilli AM, Pecchioli S, Percudani M, Piantato E, Piazza C, Pontarollo F, Pycha R, Quartesan R, Rillosi L, Risso F, Rizzo R, Rocca P, Roma S, Rossattini M, Rossi G, Sala A, Santilli C, Sarao G, Sarnicola A, Sartore F, Scarone S, Sciarma T, Siracusano A, Strizzolo S, Tansella M, Targa G, Tasser A, Tomasi R, Travaglini R, Veronese A, and Ziero S. Rationale and design of an independent randomised controlled trial evaluating the effectiveness of aripiprazole or haloperidol in combination with clozapine for treatment-resistant schizophrenia. Trials 10: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.O'Donnell BV, Tew DG, Jones OT, and England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290 (Pt 1): 41–49, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ohnishi S. and Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci 61: 511–524, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Okros Z, Endreffy E, Novak Z, Maroti Z, Monostori P, Varga IS, Kiraly A, and Turi S. Changes in NADPH oxidase mRNA level can be detected in blood at inhaled corticosteroid treated asthmatic children. Life Sci 91: 907–911, 2012 [DOI] [PubMed] [Google Scholar]
- 204.Olofsson P. and Holmdahl R. Positional cloning of Ncf1—a piece in the puzzle of arthritis genetics. Scand J Immunol 58: 155–164, 2003 [DOI] [PubMed] [Google Scholar]
- 205.Olsson LM, Lindqvist AK, Kallberg H, Padyukov L, Burkhardt H, Alfredsson L, Klareskog L, and Holmdahl R. A case-control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res Ther 9: R98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Olsson LM, Nerstedt A, Lindqvist AK, Johansson SC, Medstrand P, Olofsson P, and Holmdahl R. Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxid Redox Signal 16: 71–78, 2011 [DOI] [PubMed] [Google Scholar]
- 207.Orrell RW. Motor neuron disease: systematic reviews of treatment for ALS and SMA. Br Med Bull 93: 145–159, 2010 [DOI] [PubMed] [Google Scholar]
- 208.Pandey R, Chander R, and Sainis KB. Prodigiosins: a novel family of immunosuppressants with anti-cancer activity. Indian J Biochem Biophys 44: 295–302, 2007 [PubMed] [Google Scholar]
- 209.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, and Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci 25: 1769–1777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, and Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A 105: 1347–1352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Parker H. and Winterbourn CC. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol 3: 424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Parkos CA, Dinauer MC, Walker LE, Rodger AA, Jesaitis AJ, and Orkin SH. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome. Proc Natl Acad Sci U S A 85: 3319–3323, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, and Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis 6: 209–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pawate S, Shen Q, Fan F, and Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77: 540–551, 2004 [DOI] [PubMed] [Google Scholar]
- 215.Pearse DB. and Dodd JM. Ischemia-reperfusion lung injury is prevented by apocynin, a novel inhibitor of leukocyte NADPH oxidase. Chest 116: 55S–56S, 1999 [DOI] [PubMed] [Google Scholar]
- 216.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, and Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pendyala S. and Natarajan V. Redox regulation of Nox proteins. Respir Physiol Neurobiol 174: 265–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, Valo M, Sturk C, Carrillo CO, Sohn A, Cerimele F, Dumont D, Losken A, Williams J, Brown LF, Tan X, Ioffe E, Yancopoulos GD, and Arbiser JL. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol 126: 2316–2322, 2006 [DOI] [PubMed] [Google Scholar]
- 219.Peters EA, Hiltermann JTN, and Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31: 1442–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 220.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, and Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, and Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 222.Poewe W, Mahlknecht P, and Jankovic J. Emerging therapies for Parkinson's disease. Curr Opin Neurol 25: 448–459, 2012 [DOI] [PubMed] [Google Scholar]
- 223.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, and Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202–209, 1995 [DOI] [PubMed] [Google Scholar]
- 224.Pongnimitprasert N, El-Benna J, Foglietti MJ, Gougerot-Pocidalo MA, Bernard M, and Braut-Boucher F. Potential role of the “NADPH oxidases” (NOX/DUOX) family in cystic fibrosis. Ann Biol Clin (Paris) 66: 621–629, 2008 [DOI] [PubMed] [Google Scholar]
- 225.Pourova J, Kottova M, Voprsalova M, and Pour M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol (Oxf) 198: 15–35, 2010 [DOI] [PubMed] [Google Scholar]
- 226.Pratt AJ, Getzoff ED, and Perry JJ. Amyotrophic lateral sclerosis: update and new developments. Degener Neurol Neuromuscul Dis 2012: 1–14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, and Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26: 7212–7221, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Profita M, Sala A, Bonanno A, Riccobono L, Ferraro M, La Grutta S, Albano GD, Montalbano AM, and Gjomarkaj M. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol 298: L261–L269, 2010 [DOI] [PubMed] [Google Scholar]
- 229.Prokopowicz ZM, Arce F, Biedron R, Chiang CL, Ciszek M, Katz DR, Nowakowska M, Zapotoczny S, Marcinkiewicz J, and Chain BM. Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J Immunol 184: 824–835, 2010 [DOI] [PubMed] [Google Scholar]
- 230.Prosser BL, Khairallah RJ, Ziman AP, Ward CW, and Lederer WJ. X-ROS signaling in the heart and skeletal muscle: stretch-dependent local ROS regulates [Ca(2+)](i). J Mol Cell Cardiol 58: 172–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Prosser BL, Ward CW, and Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011 [DOI] [PubMed] [Google Scholar]
- 232.Quinn MT, Ammons MC, and Deleo FR. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin Sci (Lond) 111: 1–20, 2006 [DOI] [PubMed] [Google Scholar]
- 233.Raffa M, Atig F, Mhalla A, Kerkeni A, and Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 11: 124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rahman FZ, Hayee B, Chee R, Segal AW, and Smith AM. Impaired macrophage function following bacterial stimulation in chronic granulomatous disease. Immunology 128: 253–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Repine JE, Clawson CC, White JG, and Holmes B. Spectrum of function of neutrophils from carriers of sex-linked chronic granulomatous disease. J Pediatr 87: 901–907, 1975 [DOI] [PubMed] [Google Scholar]
- 236.Reynolds WF, Sermet-Gaudelus I, Gausson V, Feuillet MN, Bonnefont JP, Lenoir G, Descamps-Latscha B, and Witko-Sarsat V. Myeloperoxidase promoter polymorphism −463G is associated with more severe clinical expression of cystic fibrosis pulmonary disease. Mediators Inflamm 2006: 36735, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rhee SG, Chang TS, Bae YS, Lee SR, and Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14: S211–S215, 2003 [DOI] [PubMed] [Google Scholar]
- 238.Riboldi G, Nizzardo M, Simone C, Falcone M, Bresolin N, Comi GP, and Corti S. ALS genetic modifiers that increase survival of SOD1 mice and are suitable for therapeutic development. Prog Neurobiol 95: 133–148, 2011 [DOI] [PubMed] [Google Scholar]
- 239.Roberts RL, Hollis-Moffatt JE, Gearry RB, Kennedy MA, Barclay ML, and Merriman TR. Confirmation of association of IRGM and NCF4 with ileal Crohn's disease in a population-based cohort. Genes Immun 9: 561–565, 2008 [DOI] [PubMed] [Google Scholar]
- 240.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, and Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451: 211–215, 2008 [DOI] [PubMed] [Google Scholar]
- 241.Rossler W, Salize HJ, van Os J, and Riecher-Rossler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 15: 399–409, 2005 [DOI] [PubMed] [Google Scholar]
- 242.Roussin A, Le Cabec V, Lonchampt M, De Nadai J, Canet E, and Maridonneau-Parini I. Neutrophil-associated inflammatory responses in rats are inhibited by phenylarsine oxide. Eur J Pharmacol 322: 91–96, 1997 [DOI] [PubMed] [Google Scholar]
- 243.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, and Orkin SH. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature 322: 32–38, 1986 [DOI] [PubMed] [Google Scholar]
- 244.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, and Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 245.Ruiz-Litago F, Seco J, Echevarria E, Martinez-Cengotitabengoa M, Gil J, Irazusta J, and Gonzalez-Pinto AM. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: a 12-months follow-up study. Psychiatry Res 200: 218–222, 2012 [DOI] [PubMed] [Google Scholar]
- 246.Ruparelia P, Szczepura KR, Summers C, Solanki CK, Balan K, Newbold P, Bilton D, Peters AM, and Chilvers ER. Quantification of neutrophil migration into the lungs of patients with chronic obstructive pulmonary disease. Eur J Nucl Med Mol Imaging 38: 911–919, 2011 [DOI] [PubMed] [Google Scholar]
- 247.Rybicka JM, Balce DR, Chaudhuri S, Allan ER, and Yates RM. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J 31: 932–944, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rybicka JM, Balce DR, Khan MF, Krohn RM, and Yates RM. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc Natl Acad Sci U S A 107: 10496–10501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salguero G, Akin E, Templin C, Kotlarz D, Doerries C, Landmesser U, Grote K, and Schieffer B. Renovascular hypertension by two-kidney one-clip enhances endothelial progenitor cell mobilization in a p47phox-dependent manner. J Hypertens 26: 257–268, 2008 [DOI] [PubMed] [Google Scholar]
- 250.Salmeen A. and Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal 7: 560–577, 2005 [DOI] [PubMed] [Google Scholar]
- 251.Sanchez G, Pedrozo Z, Domenech RJ, Hidalgo C, and Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol 39: 982–991, 2005 [DOI] [PubMed] [Google Scholar]
- 252.Sancho P. and Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-β-induced apoptosis of liver tumor cells. Biochem Pharmacol 81: 917–924, 2011 [DOI] [PubMed] [Google Scholar]
- 253.Sareila O, Kelkka T, Pizzolla A, Hultqvist M, and Holmdahl R. NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15: 2197–2208, 2010 [DOI] [PubMed] [Google Scholar]
- 254.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, and Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185–193, 1995 [DOI] [PubMed] [Google Scholar]
- 255.Sautin YY, Nakagawa T, Zharikov S, and Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293: C584–C596, 2007 [DOI] [PubMed] [Google Scholar]
- 256.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, and Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126: 205–218, 2006 [DOI] [PubMed] [Google Scholar]
- 257.Schewe T. Molecular actions of ebselen—an antiinflammatory antioxidant. Gen Pharmacol 26: 1153–1169, 1995 [DOI] [PubMed] [Google Scholar]
- 258.Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, and Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66: 384–392, 2009 [DOI] [PubMed] [Google Scholar]
- 259.Schneider SD, Rusconi S, Seger RA, and Hossle JP. Adenovirus-mediated gene transfer into monocyte-derived macrophages of patients with X-linked chronic granulomatous disease: ex vivo correction of deficient respiratory burst. Gene Ther 4: 524–532, 1997 [DOI] [PubMed] [Google Scholar]
- 260.Schroder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol 10: 122–126, 2010 [DOI] [PubMed] [Google Scholar]
- 261.Schroder K, Schutz S, Schloffel I, Batz S, Takac I, Weissmann N, Michaelis UR, Koyanagi M, and Brandes RP. Hepatocyte growth factor induces a proangiogenic phenotype and mobilizes endothelial progenitor cells by activating Nox2. Antioxid Redox Signal 15: 915–923, 2011 [DOI] [PubMed] [Google Scholar]
- 262.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 263.Sedeek M, Gutsol A, Montezano AC, Burger D, Nguyen Dinh Cat A, Kennedy CRJ, Burns KD, Cooper ME, Jandeleit-Dahm K, Page P, Szyndralewiez C, Heitz F, Hebert RL, and Touyz RM. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci 124: 191–202, 2013 [DOI] [PubMed] [Google Scholar]
- 264.Sedeek M, Hebert RL, Kennedy CR, Burns KD, and Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009 [DOI] [PubMed] [Google Scholar]
- 265.Segal AW. How neutrophils kill microbes. Annu Rev Immunol 23: 197–223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Segal BH, Veys P, Malech H, and Cowan MJ. Chronic granulomatous disease: lessons from a rare disorder. Biol Blood Marrow Transplant 17: S123–S131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect Biol 3: pii: , 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shao L, Li H, Pazhanisamy SK, Meng A, Wang Y, and Zhou D. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol 94: 24–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sheu ML, Chiang CK, Tsai KS, Ho FM, Weng TI, Wu HY, and Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med 44: 2043–2050, 2008 [DOI] [PubMed] [Google Scholar]
- 270.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, and Fujimoto S. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun 273: 5–9, 2000 [DOI] [PubMed] [Google Scholar]
- 271.Siddiqui S, Anderson VL, Hilligoss DM, Abinun M, Kuijpers TW, Masur H, Witebsky FG, Shea YR, Gallin JI, Malech HL, and Holland SM. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis 45: 673–681, 2007 [DOI] [PubMed] [Google Scholar]
- 272.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14: 313–323, 1993 [DOI] [PubMed] [Google Scholar]
- 273.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, and Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 274.Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, and Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med 8: 251–258, 1990 [DOI] [PubMed] [Google Scholar]
- 275.Sleasman JW. The association between immunodeficiency and the development of autoimmune disease. Adv Dent Res 10: 57–61, 1996 [DOI] [PubMed] [Google Scholar]
- 276.Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, and Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol 19: 752–763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Smith Y, Wichmann T, Factor SA, and DeLong MR. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37: 213–246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Soehnlein O. and Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10: 427–439, 2010 [DOI] [PubMed] [Google Scholar]
- 279.Song SX, Gao JL, Wang KJ, Li R, Tian YX, Wei JQ, and Cui JZ. Attenuation of brain edema and spatial learning de fi cits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol Med Report 7: 327–331, 2013 [DOI] [PubMed] [Google Scholar]
- 280.Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, and Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 30: 11317–11325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Stasia MJ, Mollin M, Martel C, Satre V, Coutton C, Amblard F, Vieville G, van Montfrans JM, Boelens JJ, Veenstra-Knol HE, van Leeuwen K, de Boer M, Brion JP, and Roos D. Functional and genetic characterization of two extremely rare cases of Williams-Beuren syndrome associated with chronic granulomatous disease. Eur J Hum Genet 21: 1079–1084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Stefanska J. and Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008: 106507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Doniec Z, Bialasiewicz P, Nowak D, and Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm Pharmacol Ther 25: 343–348, 2012 [DOI] [PubMed] [Google Scholar]
- 284.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, and Pawliczak R. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res 38: 90–99, 2012 [DOI] [PubMed] [Google Scholar]
- 285.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, and Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006 [DOI] [PubMed] [Google Scholar]
- 286.Stolk J, Hiltermann T, Dijkman J, and Verhoeven A. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95, 1994 [DOI] [PubMed] [Google Scholar]
- 287.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008 [DOI] [PubMed] [Google Scholar]
- 288.Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, and Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci U S A 91: 5345–5349, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, and Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A 108: 16098–16103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sun QA, Hess DT, Wang B, Miyagi M, and Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, and Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 292.Surace MJ. and Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci 69: 2409–2427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, and Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 185: 267–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tang XN, Cairns B, Cairns N, and Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 154: 556–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tang XN, Cairns B, Kim JY, and Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res 34: 338–345, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tang XN, Zheng Z, Giffard RG, and Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol 70: 606–615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tasaka S, Amaya F, Hashimoto S, and Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10: 739–753, 2008 [DOI] [PubMed] [Google Scholar]
- 299.Taur Y. and Frishman WH. The cardiac ryanodine receptor (RyR2) and its role in heart disease. Cardiol Rev 13: 142–146, 2005 [DOI] [PubMed] [Google Scholar]
- 300.Teahan C, Rowe P, Parker P, Totty N, and Segal AW. The X-linked chronic granulomatous disease gene codes for the β-chain of cytochrome b-245. Nature 327: 720–721, 1987 [DOI] [PubMed] [Google Scholar]
- 301.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 302.Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, and Konduri GG. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L651–L663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, and Manning RD., Jr. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 295: R1858–R1865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 305.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, and Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 45: 530–537, 2005 [DOI] [PubMed] [Google Scholar]
- 306.Trumbull KA, McAllister D, Gandelman MM, Fung WY, Lew T, Brennan L, Lopez N, Morre J, Kalyanaraman B, and Beckman JS. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol Dis 45: 137–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tsai SK, Huang SS, and Hong CY. Myocardial protective effect of honokiol: an active component in Magnolia officinalis. Planta Med 62: 503–506, 1996 [DOI] [PubMed] [Google Scholar]
- 308.Ueno N, Takeya R, Miyano K, Kikuchi H, and Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem 280: 23328–23339, 2005 [DOI] [PubMed] [Google Scholar]
- 309.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, and Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia role of tumor necrosis factor-α, NAD (P) H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 23: 418–424, 2003 [DOI] [PubMed] [Google Scholar]
- 311.Urao N, McKinney RD, Fukai T, and Ushio-Fukai M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: role in reparative mobilization of progenitor cells. Stem Cells 30: 923–934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Veal EA, Day AM, and Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14, 2007 [DOI] [PubMed] [Google Scholar]
- 313.Vingsbo-Lundberg C, Nordquist N, Olofsson P, Sundvall M, Saxne T, Pettersson U, and Holmdahl R. Genetic control of arthritis onset, severity and chronicity in a model for rheumatoid arthritis in rats. Nat Genet 20: 401–404, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, and Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 120: 1616–1622, 2009 [DOI] [PubMed] [Google Scholar]
- 315.Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, and Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog 7: e1001271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Vlahos R, Stambas J, and Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol Sci 33: 3–8, 2012 [DOI] [PubMed] [Google Scholar]
- 317.Volpp BD, Nauseef WM, Donelson JE, Moser DR, and Clark RA. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci U S A 86: 7195–7199, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wallace DC. Mitochondrial diseases in man and mouse. Science 283: 1482–1488, 1999 [DOI] [PubMed] [Google Scholar]
- 319.Wang JP, Hsu EF, Raung SL, Chang LC, Tsao LT, Lin PL, and Chen CC. Inhibition by magnolol of formylmethionyl-leucyl-phenyl alanine-induced respiratory burst in rat neutrophils. J Pharm Pharmacol 51: 285–294, 1999 [DOI] [PubMed] [Google Scholar]
- 320.Wang Y. and Lou MF. The regulation of NADPH oxidase and its association with cell proliferation in human lens epithelial cells. Invest Ophthalmol Vis Sci 50: 2291–2300, 2009 [DOI] [PubMed] [Google Scholar]
- 321.Wang Y, Rosen H, Madtes DK, Shao B, Martin TR, Heinecke JW, and Fu X. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J Biol Chem 282: 31826–31834, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Watt AP, Schock BC, and Ennis M. Neutrophils and eosinophils: clinical implications of their appearance, presence and disappearance in asthma and COPD. Curr Drug Targets Inflamm Allergy 4: 415–423, 2005 [DOI] [PubMed] [Google Scholar]
- 323.Weyker PD, Webb CA, Kiamanesh D, and Flynn BC. Lung ischemia reperfusion injury: a bench-to-bedside review. Semin Cardiothorac Vasc Anesth 17: 28–43, 2013 [DOI] [PubMed] [Google Scholar]
- 324.Wientjes FB, Hsuan JJ, Totty NF, and Segal AW. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J 296 (Pt 3): 557–561, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wilkinson BL. and Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation 3: 30, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wingler K, Altenhoefer S, Kleikers PM, Radermacher K, Kleinschnitz C, and Schmidt HHW. VAS2870 is a pan-NADPH oxidase inhibitor. Cell Mol Life Sci 69: 3159–3160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]
- 328.Winterbourn CC. and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 329.Witko-Sarsat V, Allen RC, Paulais M, Nguyen AT, Bessou G, Lenoir G, and Descamps-Latscha B. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol 157: 2728–2735, 1996 [PubMed] [Google Scholar]
- 330.Wu DC, Re DB, Nagai M, Ischiropoulos H, and Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A 103: 12132–12137, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, and Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A 100: 6145–6150, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H, and Kim-Mitsuyama S. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke 39: 3049–3056, 2008 [DOI] [PubMed] [Google Scholar]
- 333.Yang Z, Sharma AK, Marshall M, Kron IL, and Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol 40: 375–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, and Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yoshida LS, Abe S, and Tsunawaki S. Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem Biophys Res Commun 268: 716–723, 2000 [DOI] [PubMed] [Google Scholar]
- 336.Yu B, Chen Y, Wu Q, Li P, Shao Y, Zhang J, Zhong Q, Peng X, Yang H, Hu X, Chen B, Guan M, Wan J, and Zhang W. The association between single-nucleotide polymorphisms of NCF2 and systemic lupus erythematosus in Chinese mainland population. Clin Rheumatol 30: 521–527, 2010 [DOI] [PubMed] [Google Scholar]
- 337.Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y, Wang S, Xi J, and Li J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of beta-cells via JNK, p38 MAPK and p53 pathways. PLoS One 5: e15726, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhang M, Nomura A, Uchida Y, Iijima H, Sakamoto T, Iishii Y, Morishima Y, Mochizuki M, Masuyama K, Hirano K, and Sekizawa K. Ebselen suppresses late airway responses and airway inflammation in guinea pigs. Free Radic Biol Med 32: 454–464, 2002 [DOI] [PubMed] [Google Scholar]
- 339.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, and Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7: e34504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhang WJ, Wei H, Tien YT, and Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic Biol Med 50: 1517–1525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhao H, Mayhan WG, Arrick DM, Xiong W, and Sun H. Alcohol-induced exacerbation of ischemic brain injury: role of NAD(P)H oxidase. Alcohol Clin Exp Res 34: 1948–1955, 2010 [DOI] [PubMed] [Google Scholar]
- 342.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, and Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]