Abstract
Persistent itch is a common symptom of allergic contact dermatitis (ACD) and represents a significant health burden. The chemokine CXCL10 is predominantly produced by epithelial cells during ACD. Although the chemokine CXCL10 and its receptor CXCR3 are implicated in the pathophysiology of ACD, it is largely unexplored for the itch and pain accompanying this disorder. Here, we showed that CXCL10 and CXCR3 mRNA, protein and signaling activity were upregulated in the dorsal root ganglion (DRG) after contact hypersensitivity (CHS), a murine model of ACD, induced by squaric acid dibutylester. CXCL10 directly activated a subset of cutaneous DRG neurons innervating the area of CHS through neuronal CXCR3. In behavioral tests, a CXCR3 antagonist attenuated spontaneous itch- but not pain-like behaviors directed to the site of CHS. Injection of CXCL10 into the site of CHS elicited site-directed itch- but not pain-like behaviors, but neither type of CXCL10-evoked behaviors was observed in control mice. These results suggest that CXCL10/CXCR3 signaling mediates allergic itch but not inflammatory pain in the context of skin inflammation. Thus, the upregulation of CXCL10/CXCR3 signaling in sensory neurons may contribute to itch associated with ACD. Targeting the CXCL10/CXCR3 signaling might be beneficial for the treatment of allergic itch.
Keywords: CXCR3, CXCL10, chemokine, itch, pain, primary sensory neurons
1. Introduction
Spontaneous itch and pain are the most common symptoms in various skin diseases, including allergic contact dermatitis (ACD) [2]. Although the pathophysiology of ACD is well studied [16], little is known of the cellular mechanisms underlying pruritus accompanying ACD. That the itch is resistant to treatment with antihistamines [13], suggests its mediation by histamine-independent pruritic pathways and the need to develop alternative antipruritic therapies..
Cytokines have been implicated in the development of ACD inflammation [7; 18; 21; 42]. Of these cytokines, CXCL10, a chemokine attracting T cells and dendritic cells, is predominantly produced by epidermal cells in the challenged skin of contact hypersensitivity (CHS) [9; 15; 46]. Moreover, the level of CXCL10 is specifically upregulated in the skin of allergic but not irritant contact dermatitis [8; 9]. During the elicitation phase of ACD, CXCL10 specifically attracts activated T cells bearing its receptor CXCR3 to the site of allergen reaction. CXCL10 knockout mice displayed an impaired CHS response with reduced inflammatory cell infiltrates into the skin, indicating the involvement of CXCL10 in the amplification of the inflammatory process [7]. Although CXCL10 is regarded as a key proinflammatory mediator in the pathogenesis of ACD, a possible contribution of CXCL10 to itch and pain that accompany ACD has not been explored.
Itch and pain are normally initiated and mediated by nociceptive primary afferents with their cell bodies in dorsal root ganglia or trigeminal ganglia. Although many studies have shown that CXCL10 acts on immune cells to regulate immunity through CXCR3 [19; 45], little is known of the role of CXCL10/CXCR3 signaling in sensory neurons. Increasing evidence indicates that both CXCL10 and CXCR3 are expressed in the nervous tissues [20; 31], including primary sensory neurons [1]. Moreover, CXCL10/CXCR3 signaling in sensory neurons was upregulated under inflammatory or neuropathic pain conditions and was implicated in the maintenance of a chronic pain state [1; 10; 43]. Our recent study showed that cutaneous primary sensory neurons innervating the CHS skin became more excitable [34]. Particularly, these neurons, or a subset, are implicated in the encoding of itch or pain [4; 17; 27; 39]. However, the mechanisms underlying neuronal hyperexcitability in the context of skin inflammation remain unknown. The nerve terminals of these neurons in the skin of CHS are readily exposed to a cytokine milieu containing CXCL10 [6; 22; 48]. Thus, CXCL10 may exert its pruritic or nociceptive effects directly, by exciting sensory neurons through CXCR3, indirectly, by activating immune cells to induce the release of inflammatory mediators that target sensory neurons, or both. Although the action of CXCL10 on immune cells is well studied, its effects on primary sensory neurons, and its contribution to the genesis of pruritus and pain associated with CHS, have not been explored. Our purpose was to investigate whether CXCL10 directly activated cutaneous sensory neurons through CXCR3, and mediated allergic itch and inflammatory pain using a mouse model of CHS induced by a hapten, squaric acid dibutylester (SADBE). Preliminary results of this study are available in abstract form [35].
2. Methods
2.1. Animals
C57BL/6 male mice used in the study were, 2 to 3 months of age and weighed 20–30 g. Some of these were either of two types of transgenic mice, one having a green fluorescent protein (GFP) in neurons that expressed the MrgprA3 receptor for chloroquine (“MrgprA3+ neurons) [17] whereas the other type of mice exhibiting the GFP in neurons that expressed MrgprD receptor (MrgprD+ neurons) [53]. The breeders used to develop our colonies of these transgenic mice were provided by Dr. Xinzhong Dong’s laboratory at Johns Hopkins University. All the experimental procedures were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine and were consistent with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain.
2.2. Model of allergic contact dermatitis
Allergic contact dermatitis (ACD) or contact hypersensitivity (CHS) was elicited by using contact sensitizer squaric acid dibutylester (SADBE; Sigma, St. Louis, MO), as described previously [11; 34]. The mice were sensitized with 1% SADBE (25 μl) topically applied to the shaved abdomen once daily for three consecutive days. Five days later, mice were challenged with 1% SADBE (25 μl) topically applied, in different behavioral tests, either to the calf of one hind leg or to the right cheek or, for the in vitro studies, to the hairy skin of foot and the calf of hind leg once daily for two consecutive days. Separate groups of mice were challenged with the same amount of acetone alone and serviced as controls.
2.3. Behavioral testing
The behavioral tests of CHS were performed before the 1st challenge with SADBE (or vehicle) and 24 h after each challenge. For the “calf model” (lower hindlimb), behavioral responses were video recorded with a camcorder for 2 h. The video recording was played back offline in slow-motion to assess the time spent biting and licking the treated calf. The duration of each biting or licking action was measured and summed to provide the cumulative biting or licking time [23]. Licking behavior was manifested by a series of long-stroke bobbing of the head (about 4 Hz) during which the tongue was occasionally seen as moving across the skin. Biting behavior consisted of gnawing-like movements of the mandible (which apparently scraped across the skin at about 15 Hz) [23].
In some experiments, a selective antagonist of the chemokine receptor CXCR3, or its vehicle, was administered 1h before testing at 24 h after the 2nd SADBE challenge. The CXCR3 antagonist AMG487, a generous gift from Amgen (Thousand Oaks, CA) was injected subcutaneously at a location on the caudal back with a dose of 5 mg/kg, in a vehicle of 20% of hydroxypropyl-β-cyclodextrin (HPCD in saline; Sigma). The dose of AMG487 was chosen based on pilot tests and published dose-response findings [3; 24].
For the “cheek model”, the total number of spontaneous scratching bouts with the hind paw and wiping with the forepaw were measured for 30 min [41]. In some tests, either CXCL10 (2 μg/10 μl in PBS; R&D Systems or BioLegend) or its vehicle alone (10 μl PBS) was injected i.d. into the right cheek 24 h after the 1st SADBE challenge (when the skin was inflamed but in less fragile condition than after the 2nd challenge). Either AMG487 (5 mg/kg) in vehicle (20% of HPCD) or its vehicle alone was injected s.c. into the caudal back 1 h before the cheek injection of CXCL10.
2.4. Retrograde labeling of cutaneous neurons
For in vitro studies, DRG cell bodies were identified as cutaneous and as having innervated the area of CHS (or vehicle treatment) by the presence of a retrogradely transported red fluorescent dye, DiI (Invitrogen). Dil (1.7 mg/ml in 1% DMSO) was injected i.d. to the calf of one hind leg and hairy skin of foot of mice at three sites (10 μl per site) at least 1 week before the 1st challenge with SADBE or acetone vehicle.
2.5. Cultures of dissociated DRG neurons
At 24 h after the 2nd challenge, L3–L5 lumbar DRGs, ipsilateral to either the acetone- or SADBE-treated skin, were harvested and placed in oxygenated complete saline solution (CSS) for cleaning and then mincing. The CSS consisted of (in mM): 137 NaCl, 5.3 KCl, 1 MgCl2, 3 CaCl2, 25 Sorbitol, and 10 HEPES, adjusted to pH 7.2 with NaOH. For 20 min the DRGs were digested with 0.35 U/ml of Liberase TM (Roche Diagnostics Corp., Indianapolis, IN) and then for 15 min with 25 U/ml of Liberase TL (0.25 U/ml; Roche Diagnostics Corp.) and papain (30 U/ml, Worthington Biochemical, Lakewood, NJ) in CSS containing 0.5 mM EDTA at 37°C. The tissue was triturated with a fire-polished Pasteur pipette. The DRG neurons were suspended in DMEM medium containing 1 mg/ml trypsin inhibitor and 1 mg/ml bovine serum albumin (Sigma) and then plated onto poly-D-lysine/laminin coated glass coverslips (BioCoat, BD Biosciences, MA). The DMEM medium had equivalent amounts of DMEM and F12 (Gibco, Grand Island, MD) with 10% FCS (Sigma) and 1% penicillin and streptomycin (Invitrogen). The cells were maintained in 5% CO2 at 37°C in a humidified incubator and used within 24 h after plating.
2.6. Quantitative RT-PCR
The L3, L4, L5 DRG, ipsilateral to either the acetone- or SADBE-treated skin, were harvested 24 h after the first or the second challenge with SADBE (or acetone vehicle alone) and flash-frozen in liquid nitrogen. Total RNAs were extracted using the RNeasy Mini Kit (Qiagen). Quantitative RT-PCR was performed on a Bio-Rad machine using SYBER Green reagents (Life Technologies). The primers used were as follows: CXCR3 forward, 5′-AGA ATC ATC CTG GTC TGA GAC AA-3′ and reverse, 5′-AAG ATA GGG CAT GGC AGC-3′; CXCL10 forward, 5′-CCC ACG TGT TGA GAT CAT TG-3′ and reverse, 5′-CAC TGG GTA AAG GGG AGT GA-3′; GAPDH forward, 5′-CCA TGA CAA CTT TGG CAT TG-3′ and reverse, 5′-CCT GCT TCA CCA CCT TCT TG-3′ [44; 50]. Each sample was performed in triplicates. The expression level of the target genes was quantified relative to the level of GAPDH gene expression using the 2−ΔΔCT method.
2.7. Fluorescence-activated cell sorting (FACS) analysis
The surface expression of CXCR3 on DRG neurons was measured using FACs analysis. DRG neurons were acutely dissociated as described and stained with Alexa Fluor 488 mouse monoclonal anti-GFAP (1:50; BD Biosciences) and APC hamster monoclonal anti-mouse CXCR3-173 (1:500; BD Pharmingen; Cat. # 562266) for 40 min at 4°C. APC Hamster IgG1 (BD Pharmingen; Cat. # 553974) served as controls. The specificity of APC hamster monoclonal anti-mouse CXCR3-173 for CXCR3 was tested in CXCR3 knockout mice previously [47]. The portion of glial cells was first gated out using an excitation wavelength for fluorescence of 448 nm. The remaining GFAP negative cells were subsequently gated using an excitation fluorescence wavelength of 647 nm. Only the Dil-labeled neurons were counted.
2.8. Single-cell reverse-transcription PCR
After cell sorting, the individual CXCR3 immunopositive neurons were harvested in a PCR tube. For the negative control, a sample of the FACs solution without any cell contents was used. The total RNA was extracted from individual DRG neuron using an RNeasy Plus Micro kit (Qiagen) as described [36]. The RNA was reverse transcribed using Superscript Reverse Transcriptase II (Invitrogen) according to the manufacturer’s instructions. PCR amplification was then performed using a Titanium TaqPCR Kit (Clontech). The CXCR3 primers were the same as those used for the above quantitative RT-PCR. Beta III-tubulin was used as an internal control. The PCR products were displayed on an ethidium bromide-stained 2% agarose gel.
2.9. Calcium imaging
Calcium imaging was performed on cultured mouse DRG neurons, as described [37]. Only small-diameter neurons (≤ 25 μm) were used that were labeled as cutaneous by the presence of DiI and innervated the chemically treated areas. In the experiments using transgenic mice, some neurons were additionally labeled by a GFP indicating that it expressed either MrgprA3 or MrgprD and innervated the epidermis[17; 53]. DRG neurons were first loaded with 2 μM Fura 2-acetoxymethyl ester (Invitrogen) in the dark for 45 min at 37°C and subsequently washed twice in a HEPES buffer containing (in mM): 145 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES (adjusted to pH 7.4 with NaOH). Neurons were then incubated with 10 μg/ml isolectin B4 conjugated to fluorescein isothiocyanate (IB4-FITC; Sigma) for 10 min, washed with HEPES buffer, and visualized with FITC filters. Only neurons with a complete ring of FITC stain around the perimeter of the soma were considered as IB4 positive neurons (IB4+). After the detection of the fluorescence of IB4-FITC, these cells were alternatively excited at 340 nm and 380 nm using a Polychrome V Monochromator (TILL Photonics). Images were recorded at 2-s intervals at a room temperature of 20–22°C using a cooled CCD camera (Sensicam, PCO, Germany) that was controlled by a computer with Image Workbench 5.2 software (Indec Biosystems, CA). The ratio of 340 nm to 380 nm fluorescence intensity [R(340/380)] within a certain region of interest was used as a relative measure of the intracellular concentration of calcium ([Ca2+]i). At the end of the experiment, the viability of the neurons was confirmed by an increase in [Ca2+]i evoked by a 5-s application of 50 mM K+. Cells were considered to be responsive to a chemical if an increase in R340/380 was equal or greater than 15% above baseline [50]. All agents were dissolved in HEPES buffer and applied locally to the neuronal cell bodies through a micropipette with a tip diameter of 100-μm-diameter and connected to an 8-channel pressure-controlled drug application system (AutoMate Scientific, CA). The Ca2+-free external solution was equivalent to the normal HEPES buffer but with CaCl2 removed and 1 mM EGTA added [37]. The dose of each chemical was chosen based on previous findings [24; 26; 32]. Mouse recombinant CXCL10 (50 nM, R&D Systems) or AMG487 (10 μM in 0.1% DMSO) was added to HEPES buffer. AMG487 was shown to suppress CXCR3-mediated Ca2+ responses in a dose dependent manner and the dose of 10 μM AMG487 was able to inhibit about 60% of CXCR3-mediated Ca2+ response [24]. Histamine (His; 100 μM; 30 s) or the TRPV1 agonist, capsaicin (CAP; 1 μM; 10 s), was applied at the end of recordings to identify His- and CAP- sensitive nociceptors, respectively.
2.10. Electrophysiological recordings
Whole-cell recordings were made from small-diameter (≤25 μm), DiI-labeled DRG neurons – typically those that had been identified as responsive to CXCL10 using calcium imaging. Whole-cell current clamp experiments were performed at room temperature of 20–22°C by means of a Multiclamp 700A amplifier and pClamp 9 software (Molecular Device, Sunnyvale, CA), as described [36; 37]. Signals were sampled at either 10 kHz or 20 kHz and were filtered at 2 kHz. The patch pipettes were pulled from borosilicate glass capillaries with a P97 horizontal puller (Sutter Instruments, Novarto, CA). The patch pipettes, after filled with internal solution, had a resistance of 3–4 MΩ and their series resistance was routinely compensated at 60–80%. The DRG neurons were continuously perfused with HEPES buffer. The internal solution contained (in mM): K+-gluconate 120, KCl 30, MgCl2·6H2O 2, HEPES 10, MgATP 2, CaCl2·2H2O 1, EGTA 11, with pH adjusted to 7.2 using Tris-base. Only neurons with a resting membrane potential more negative than −40 mV were included in the study.
2.11. Statistical analysis
Data were presented as means ± SEM. Student’s t-test was used to test the significance of differences between means between two groups. Comparisons for more than three groups were carried out using a one-way analysis of variance (ANOVA) followed by Bonferroni post test comparisons. Comparisons of proportions were made using Fisher’s exact test. The probability criterion for significant differences was p < 0.05. The type of statistical tests used for each comparison was indicated in the figure legends.
3. Results
3.1. CHS upregulated neuronal expression of CXCL10 and CXCR3
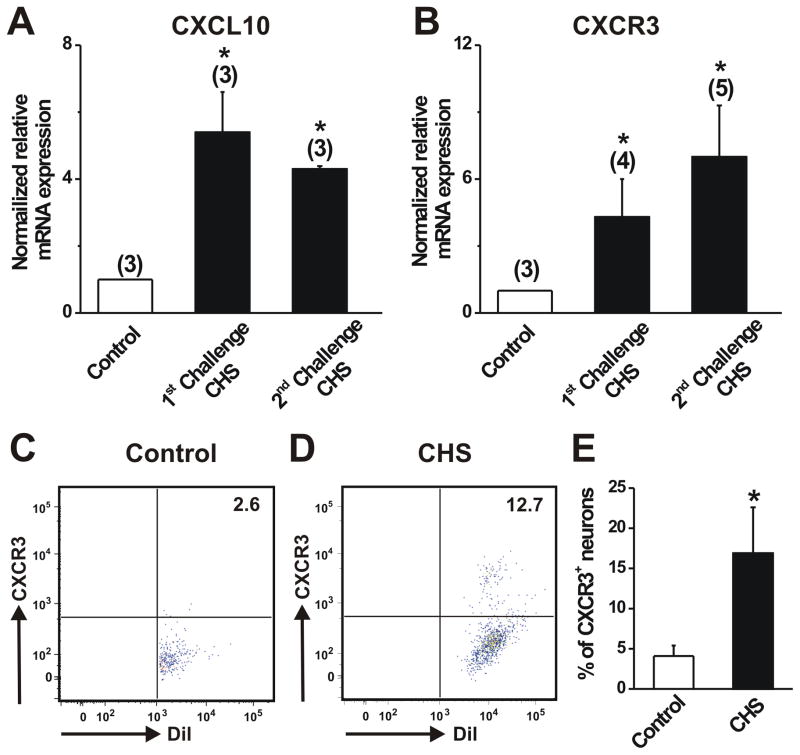
The mean levels of CXCL10 and CXCR3 mRNA expression in the DRG were significantly greater for SADBE- vs. vehicle treated skin both after the first as well as the second challenge (Fig. 1A, B). At the protein level, FACs analysis revealed that a significantly larger percentage of Dil-labeled cutaneous DRG neurons of CHS mice stained CXCR3 compared with controls (Fig. 1C–E), indicating an increased number of cutaneous sensory neurons expressing CXCR3 after the development of CHS. In contrast, when Dil-labeled cutaneous DRG neurons from either vehicle or SADBE-treated mice were treated with the control IgG1 antibody, no CXCR3 staining was observed in these neurons (data not shown), indicating that the anit-CXCR3 used for FACs was specific for CXCR3. Single-cell RT-PCR confirmed that all the tested CXCR3 immunopositive neurons obtained after FACs possessed CXCR3 mRNA (data not shown).
Figure 1.
Effects of CHS on the expression of CXCR3 and its ligand CXCL10 in DRG. A–B, Quantitative RT-PCR showed a significant increase in the mRNA expression levels of CXCL10 and CXCR3 in L3–L5 DRG from CHS mice compared to those from acetone-treated control animals. The number of animals in each group is given in parentheses. * p < 0.05, CHS vs. control, unpaired t test. C–D, Representative scatter plot depicting the expression of CXCR3 on the surface of the Dil-labeled cutaneous DRG neurons obtained from control (acetone-treated) and CHS (SADBE-treated) mice 24 h after the 2nd challenge. DRG neurons were stained with monoclonal antibody against CXCR3. The number in the upper right corner of each box indicates the percentage of DiI-labeled neurons stained with CXCR3. E, FACS analysis revealed that the mean percentage of DRG neurons stained with CXCR3 from CHS mice was significantly greater as compared to control animals. Twelve mice were used per condition. *p < 0.05 versus control, Fisher’s exact test.
3.2. CHS increased the incidence and magnitude of CXCR3-mediated Ca2+ responses to CXCL10 in cutaneous DRG neurons with pruriceptive, nociceptive properties
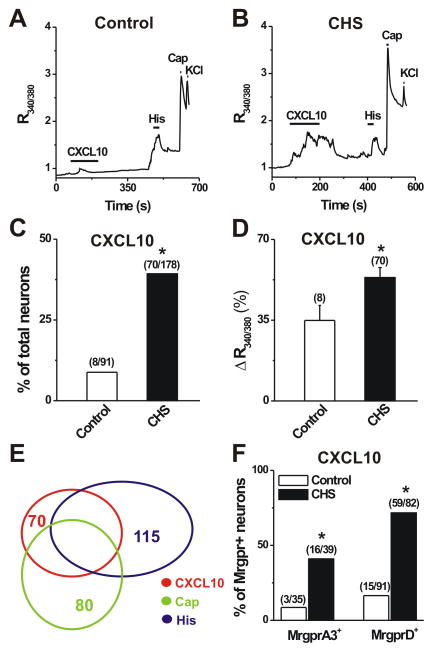
To determine whether the function of CXCL10/CXCR3 signaling in primary sensory neurons was enhanced under skin inflammatory conditions, we compared Ca2+ responses evoked by CXCL10 between the Dil-labeled cutaneous small-diameter DRG neurons from control and CHS mice using ratiometric Ca2+ imaging (Fig. 2). Few cutaneous DRG neurons from control mice responded to CXCL10 (50 nM; 2 min; Fig. 2A). In contrast, there were significantly more neurons responsive to CXCL10 in CHS mice (Fig. 2B, C). Moreover, the magnitude of CXCL10-induced Ca2+ response in neurons from CHS mice was significantly greater than that in neurons from control animals (Fig. 2D). Of CXCL10-responsive neurons (CXCL10+) in CHS mice, 52.9% (37 of 70) and 64.3% (45 of 70) showed a [Ca2+]i rise in response to capsaicin (CAP, 1 μM) and histamine (His, 1 mM), respectively (Fig. 2A, E). Thus, transactivation of TRPV1 by CXCL10 may in part contribute to an increase in the peak of CXCL10-induced Ca2+ response observed in DRG neurons from CHS mice. In addition, of the CXCL10+/CAP+ responsive neurons, 67.6 % (25 of 37) responded to histamine (Fig. 2E). When the dissociated neurons were stained with IB4-FITC, 51 of 61 (83.6%) CXCL10-responsive neurons were IB4+. Competence of viable cells was confirmed by a robust Ca2+ response observed in all cells exposed to high K+ levels (Fig. 2A, B).
Figure 2.
Effects of CHS on the incidence and magnitude of CXCL10- evoked Ca2+ responses in DRG neurons with pruriceptive or nociceptive properties. A–B, Representative traces of changes in [Ca2+]i (R340/380) evoked by CXCL10 (50 nM, 2 min) in cutaneous (DiI labeled) DRG neurons from control (A) and CHS (B) mice. Application of histamine (His; 100 μM, 30 s), capsaicin (CAP; 10 s) and KCl (50 mM, 5 s) evoked Ca2+ transients, respectively. Black bars above the traces indicate the timing of chemical application. C, The percentage of cutaneous DRG neurons that responded to CXCL10 was significant greater for CHS mice than for acetone treated control mice. p < 0.05 versus control, Fisher’s exact test. Numbers of responsive neurons divided by total number tested are given in parentheses. D, The mean magnitude of Ca2+ response evoked by CXCL10 was significantly great for neurons from CHS- than control mice. Magnitude is expressed as peak percentage increase over base – see methods). *p < 0.05 versus control, unpaired t test. E, Venn diagram showed the overlap of CXCL10-, capsaicin (CAP)-, and histamine (His)-responsive neurons from CHS mice. F, The percentages of CXCL10-responsive MrgprA3+ and MrgprD+ neurons were significantly higher in CHS- than in control mice. The numbers of responsive cells vs. total tested are in parentheses. *p < 0.05 versus control, Fisher’s exact test.
Since MrgprA3+ and MrgprD+ neurons were shown to play a role in mediating allergic itch [17; 34], we next tested whether CXCL10 directly activated MrgprA3+ and MrgprD+ pruriceptive nociceptors under the condition of skin inflammation. Local application of CXCL10 evoked a robust Ca2+ response in a greater percentage of MrgprA3+ or MrgprD+ neurons from CHS- vs. control mice (Fig. 2F). These data suggest that an increased number of cutaneous DRG neurons express functional CXCR3 receptors following CHS.
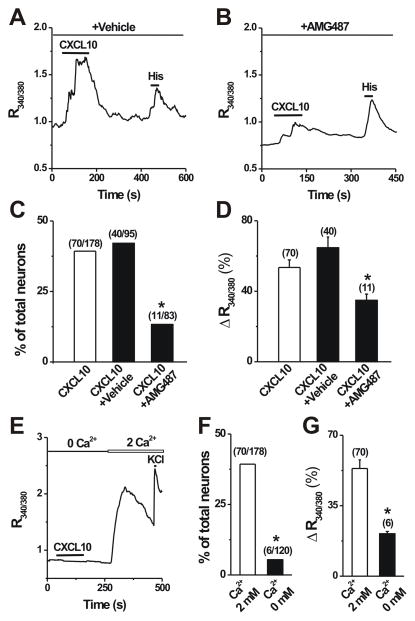
To test whether neuronal CXCR3 mediated the CXCL10-induced [Ca2+]i response, DRG neurons were pretreated with a specific CXCR3 antagonist, AMG487 (10 μM in a vehicle of 0.1% DMSO), for 5 min. AMG487 at this dose was shown to block approximately 60% of the CXCR3-mediated Ca2+ response [24]. In accordance with previous findings [24], the proportion of CXCL10-responsive neurons was significantly decreased by 67.5% in the presence of AMG487 as compared with the effects of vehicle alone (Fig. 3A–C). In addition, AMG487 significantly attenuated the magnitude of CXCL10-induced Ca2+ response (Fig. 3D). By contrast, vehicle alone had no such significant effects (Fig. 3C, D). These results suggest that CXCL10 induces a [Ca2+]i increase via the activation of CXCR3 expressed on DRG neurons. To determine the Ca2+ source of CXCL10-evoked [Ca2+]i increase, Ca2+ response was measured in Ca2+-free extracelluar solution. Removal of extracellular Ca2+ significantly reduced the percentage of CXCL10-responsive neurons (Fig. 3E, F) and the mean magnitude of CXCL10-evoked [Ca2+]i rise (Fig. 3G), indicating that CXCL10 induces Ca2+ increase mainly through calcium influx from extracellular space.
Figure 3.
The dependence of CXCL10-induced Ca2+ responses on functional CXCR3 receptors and the presence of extracellular Ca2+. A–B, Representative Ca2+ response to CXCL10 (50 nM, 2 min) in the presence of a specific CXCR3 antagonist AMG487 (10 μM in 0.1% DMSO vehicle) vs. in the presence of the vehicle alone. C–D, Pretreatment with AMG487 for 3 min but not vehicle significantly reduced the percentage of CXCL10-responsive neurons (Fisher’s exact test) and the mean magnitude of CXCL10-evoked Ca2+ response (unpaired t test). *p < 0.05 versus vehicle. E, Representative Ca2+ response in a neuron to CXCL10 in the absence of extracellular Ca2+ (0 mM) and KCl (50 mM) in presence of 2 mM extracellular Ca2+, respectively. F–G, Removal of extracelluar Ca2+ almost abolished CXCL10-evoked Ca2+ rise. Numbers of neurons tested are in parentheses.*p < 0.05 versus 2 mM Ca2+; Fisher’s exact test (F) or unpaired t test (G).
3.3. CXCL10 increased the membrane excitability of DRG neurons innervating CHS skin through neuronal CXCR3
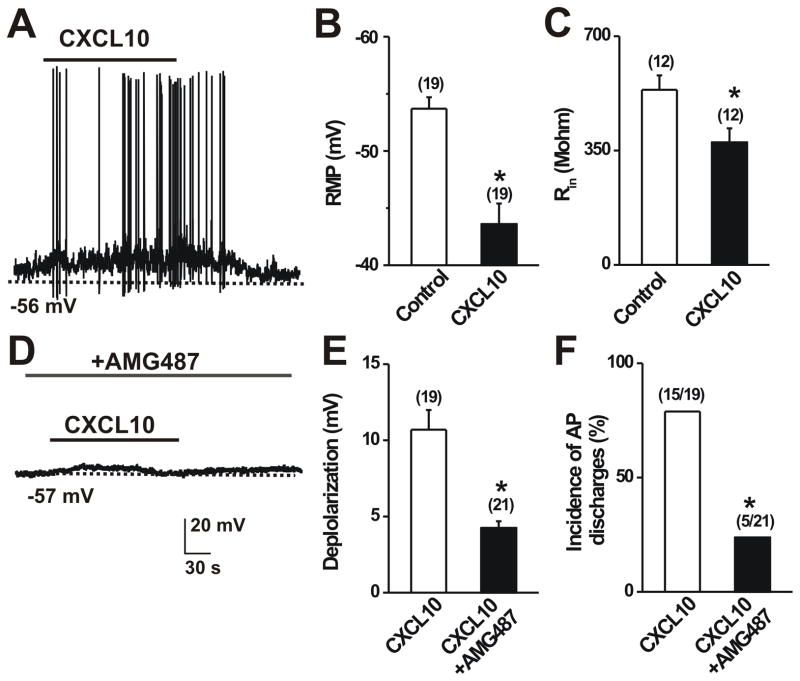
To determine whether CXCL10 directly altered the membrane excitability of neurons innervating SADBE-treated skin of mice, whole-cell current clamp recordings were made on DiI-labeled DRG neurons with CXCL10 responsiveness initially identified by calcium imaging. Local application of CXCL10 caused a significant depolarization of the resting membrane potential (RMP) of DRG neurons (Fig. 4A, B). Furthermore, membrane depolarization was sufficient to induce action potential discharges upon CXCL10 activation in the majority of these activated neurons (Fig. 4A, F). The mean input resistance was markedly reduced (Fig. 4C) during exposure to CXCL10, suggesting an increase in the opening of resting ion channels. Pretreatment with AMG487 (10 μM) for 3 min significantly attenuated CXCL10-induced membrane depolarization (Fig. 4E). Moreover, AMG487 markedly reduced the percentage of neurons that fired with APs in response to CXCL10 (Fig. 4F). These findings suggest that neuronal CXCR3 mediates the direct excitatory effect of CXCL10 on primary sensory neurons after CHS.
Figure 4.
CXCL10 increased neuronal membrane excitability after CHS. In each panel, neurons were tested that exhibited DiI labeling transported from SADBE challenged skin. A, Typical current clamp recordings of CXCL10-induced membrane potential depolarization and action potential discharges. Black bars above the traces indicates the duration of CXCL10 (50 nM; 2 min) application. B–C, Mean resting membrane potential (RMP) and mean input resistance (Rin) before (control) and during application of CXCL10. CXCL10 significantly depolarized the RMP and reduced the Rin. *p < 0.05 versus control, paired t tests. D, Representative current clamp recordings of CXCL10 application in presence of AMG487 (10 μM). E–F, Pretreatment with AMG487 significantly reduced the mean magnitude of CXCL10-induced membrane depolarization (paired t test) and decreased the incidence (percentage) of neurons exhibiting action potentials (Fisher’s Exact test). The number of cells responding and/or tested are in parentheses. *p < 0.05 versus control.
3.4. CXCL10/CXCR3 signaling mediated itch- but not pain-like behaviors in CHS mice
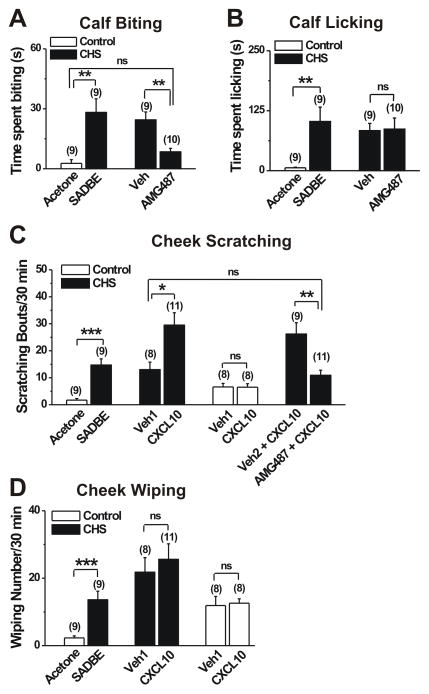
To determine whether CXCR3 was involved in itch and pain that accompany CHS, we examined the effects of AMG487, a specific CXCR3 antagonist, on spontaneous itch- and pain-like behaviors on the calf of mice 24 h after the 2nd SADBE challenge. Consistent with our previous study [34], mice challenged with SADBE exhibited a significant increase in the cumulative durations of site-directed, spontaneous biting and licking (itch- and pain-like behaviors, respectively directed) in comparison with mice treated with only the acetone vehicle (Fig. 5A, B). The mean cumulative duration of spontaneous biting observed on the calf of CHS mice was significantly reduced 1 h after s.c. injection of AMG487 as compared to vehicle (20% HPCD) (Fig. 5A). There was no significant difference in the mean cumulative duration of spontaneous biting between acetone-treated animals and AMG487-treated CHS mice. By contrast, s.c. injection of AMG487 did not significantly affect the mean cumulative duration of spontaneous licking on the calf of CHS mice (Fig. 5B) compared with vehicle (20% HPCD).
Figure 5.
CXCL10 and CXCR3 mediated itch- but not pain-like behaviors after CHS. A–B, The effects of CHS on the calf on the cumulative durations of site-directed biting and licking (itch- and pain-like behaviors, respectively). The mean durations (time spent) of each behavior were significantly increased 24 h after the 2nd challenge with SADBE (CHS, closed bars) vs. c acetone vehicle treatment (control, open bars)). Prior subcutaneous injection of the CXCR3 antagonist AMG487 (5 mg/kg in 20% HPCD) significantly attenuated the spontaneous biting but not the licking behaviors to the level of acetone-treated mice. In contrast, prior injection of just the antagonist vehicle (Veh; 20% HPCD) had no significant effects on either type of behavior. The cumulative durations of behaviors were quantified for 2 h starting 1h of after AMG487 or vehicle injection. C–D, The effects of CHS on the cheek on spontaneous and CXCL10-evoked, site-directed scratching with the hind limb and wiping with the forepaw (itch- and pain-like behaviors, respectively) 24 h after the 1st challenge with SADBE (CHS, closed bars) or acetone vehicle (control, open bars). The mean numbers of each type of spontaneous behavior were significantly greater after challenge with SADBE (vs. acetone alone), Intradermal (i.d.) injection of CXCL10 (2 μg/10 μl in PBS vehicle) into the SADBE challenged cheek significantly increased the site-directed scratching (C) but not the wiping (D) in comparison with the non-significant effects of injecting the PBS vehicle alone (Veh1; PBS). When injected into the acetone control cheek, CXCL10 evoked no significant differences beyond PBS vehicle alone. The exogenous CXCL10-evoked scratching in the SADBE challenged cheek was almost abolished by preinjection, 1 h before, with AMG487 (5 mg/kg; s.c.) in comparison with prior injection of the antagonist’s vehicle 20% HPCD (Veh2). There was no significant difference (ns) in scratching bouts between PBS (Veh1)-treated and AMG487-treated CHS mice. The number of bouts of scratching or wipes was quantified for 30 min immediately following CXCL10 injection. The number of animals tested is in the parentheses. *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Bonferroni post test.
To explore the behavioral effects of CXCL10 after CHS, we used the cheek model which, like the calf model, allows the differentiation of side-directed itch- and pain-like behaviors in response to pruritic and algesic chemical stimuli [41]. At 24 h after the 1st challenge, mice with SADBE-induced CHS displayed more site-directed spontaneous scratching and wiping behaviors than acetone-treated control mice (Fig. 5C, D). Intradermal (i.d.) injection of CXCL10 (2 μg/10 μl) into the cheek of CHS mice significantly increased the number of scratching bouts but not the number of wipes as compared to the injection of vehicle (PBS) (Fig. 5C, D). In contrast, injection of CXCL10 to control mice did not significantly change the number of scratching and wipes as compared to vehicle (PBS) (Fig, 5C, D). These results suggest that CXCL10 elicits more itch than pain within an area of CHS. We next asked whether CXCR3 was required for CXCL10-evoked scratching in CHS mice. S.c injection of the CXCR3 antagonist, AMG487 (5 mg/kg) but not its vehicle (20% HPCD) completely inhibited the exogenous CXCL10-evoked scratching (Fig. 5C). No significant differences in scratching bouts were observed between PBS (Veh1)-treated and AMG487-treated CHS mice. These findings indicate that CXCL10 acts on CXCR3 to trigger itch behaviors in vivo under the inflammatory skin conditions.
4. Discussion
Our findings support the hypothesis that CXCL10/CXCR3 signaling is enhanced in cutaneous sensory neurons and that CXCL10 evokes itch, but not pain sensation, by directly activating CXCR3 expressed on cutaneous, small diameter, primary sensory neurons.
Although CXCR3 is typically expressed in specific subsets of immune cells, CXCR3 and its agonist CXCL10 have also been detected in DRG neurons [1]. Moreover, CXCL10 can directly excite nociceptive primary sensory neurons though neuronal CXCR3 [1; 32]. However, few studies addressed the role of neuronal CXCL10/CXCR3 signaling in the context of skin inflammation. This study provides translational and functional evidence that support the increased CXCL10/CXCR3 signaling within DRG after the development of ACD. First, RT-PCR revealed an upregulation of CXCL10 and CXCR3 mRNA expression in DRG innervating SADBE-treated skin. Second, at the protein level, FACS showed that the increased number of cutaneous DRG neurons became CXCR3+ following CHS. Since there is no reliable commercial primary anti-CXCR3 available for immunohistochemical staining in mouse tissues, we were unable to immunostain CXCR3 expressing neurons to obtain histological information about the expression pattern of CXCR3 in DRG neurons. Instead, we employed FACS to evaluate the CXCR3 expression on the surface of DRG neurons. Finally, consistent with the anatomical observations of increased CXCR3 expression, calcium imaging experiments clearly demonstrated a considerable degree of functional CXCR3 upregulation in cutaneous DRG neurons that innervated a skin area of CHS. A larger proportion of dissociated cutaneous DRG neurons became responsive to CXCL10 after CHS that they seldom exhibited under normal conditions. Moreover, this Ca2+ response was remarkably attenuated by a CXCR3 antagonist. The upregulated CXCL10/CXCR3 signaling within DRG was also observed in the context of inflammatory/neuropathic pain [1; 10; 43]. Two variants of CXCR3 (CXCR3-A and CXCR3-B) have been identified [40]. Further investigations are needed to determine which variant of CXCR3 is upregulated following CHS.
The mechanisms by which CHS induces the upregulation of CXCL10/CXCR3 signaling in the cell bodies of sensory neurons require further study. It is probably due to the activation of a signaling cascade of cytokines and other mediators upstream of CXCL10/CXCR3 expression, such as IFN-γ or nuclear transcription factors [30; 49; 51]. FACS analysis revealed that many more DRG neurons began to express CXCR3 after CHS. And, many more sensory neurons from CHS mice became responsive to CXCL10 in calcium imaging experiments. Thus, it is likely that these additional neurons develop responsive to CXCL10 that they previously did not have as a result of cutaneous inflammation. Moreover, these findings implied the possibility that neurons that were originally non-pruriceptive (not responsive to pruritic chemicals) might become pruriceptive after they become responsive to CXCL10.
Recently we functionally characterized a subset of cutaneous sensory neurons that became hyperexcitable after CHS [34]. Here, we provide a cellular mechanism that might contribute to neuronal hyperexcitability and thus the itch behavior that accompanies this inflammatory disorder. Our findings support a direct action of CXCL10 on small-diameter sensory neurons innervating the area of inflamed skin. First, calcium imaging experiments revealed that CXCL10 directly evoked a calcium response though neuronal CXCR3 in a subset of small-diameter DRG neurons.. In addition, our electrophysiological analyses provide novel evidence that CXCL10 directly depolarized the membrane potential and triggered action potential discharges in these cutaneous neurons after CHS. However, the ionic mechanisms underlying CXCL10-induced neuronal activation remain to be determined. In murine microglia, CXCL10 was shown to trigger a Cl− current via CXCR3 [38]. Our preliminary data showed that CXCL10 induced an inward current at resting membrane potential of DRG neurons [35]. Moreover, this current was modulated by intracellular Cl− and inhibited by the general Cl− channel antagonists (unpublished data) [35]. Therefore, it is possible that the neuronal excitatory effects of CXCL10 are mediated by the triggering of a Cl− current downstream to the activation of CXCR3. Indeed, previous studies have demonstrated that Cl− channels can be activated by several pruritogen and algogen, such as endothelin, bradykinin, and histamine [5; 26]. Additionally, DRG neurons express multiple voltage-gated and TRP channels that may also be involved in CXCL10-induced neuronal activation. These mechanisms await further investigation.
This study showed that CXCL10-evoked Ca2+ response in DRG neurons depended on the presence of extracellular Ca2+, suggesting that Ca2+ influx from extracellular space is the main source of the increase in [Ca2+]i. Moreover, CXCL10 directly depolarized the membrane potential of DRG neurons, which might result in the activation of voltage-gated Ca2+ channels (VGCCs) and a subsequent influx of extracellular calcium through VGCCs. In addition, other Ca2+ permeable mechanisms such as certain TRP channels [14] may be also involved in CXCL10-induced Ca2+ influx in DRG neurons.
Although the role of CXCL10/CXCR3 signaling in the cause of ACD inflammation is well established [7; 44], little is known of its contribution to itch and pain that accompany this disorder. The upregulated excitatory CXCL10/CXCR3 signaling has been implicated in the chronic inflammatory pain state [1]. In present study of CHS, the murine model of ACD, we observed that systemically administration of a specific CXCR3 antagonist attenuated spontaneous itch but had no effects on pain-like behavior. In addition, cutaneous injection of CXCL10 evoked site-directed itch- but not pain-like behaviors in the animals with CHS. In contrast, CXCL10 failed to elicit both types of behaviors in control animals, which is likely attributable to the paucity of functional CXCR3 expressed on cutaneous DRG neurons under normal conditions. The findings indicate that CXCL10/CXCR3 signaling mediates itch but not pain in the context of CHS. Since these behaviors were assessed right after administration of a CXCR3 antagonist, the antipruritic effects of CXCR3 antagonist may be due to the immediate, inhibitory effects on hyperexcitable CXCR3-expressing pruriceptive cutaneous sensory neurons (as observed in our in vitro experiments) rather than secondary to the reduction in the cutaneous inflammation. These data suggest that CXCL10/CXCR3 signaling may contribute to allergic itch through the direct excitation of pruriceptive DRG neurons. In this study, spontaneous scratching directed to site of CHS on the cheek may appear less than that elicited by injection of the cheek of untreated, healthy mice with commonly used pruritogens, such as choloroquine, BAM 8-22 and histamine [28; 41; 50]. The pruritogen-evoked stronger scratching behavior may be related to the concentration of injected vs. endogenous pruritogens. When the lower doses of pruritogen are of histamine (5 μg/10 μl) or BAM8-22 (1 μg/10 μl) was injected into the cheek of control mice[12], the numbers of scratching bouts are comparable to those occurring spontaneously in our CHS mice.
The present study did not identify which subtypes of sensory neurons were the major elements responsible for CXCR3-mediated itch in the setting of skin inflammation. We found that a significant number of MrgprA3+ and MrgprD+ neurons became responsive to CXCL10 following CHS. These two distinct types of cutaneous sensory have been implicated in acute and chronic itch [17; 27]. Therefore, it is possible that CXCL10 activates a larger percentage of these itch-mediating sensory neurons during CHS, leading to neuronal hyperexcitability and thus partially contributes to pruritus accompanying CHS. Since mouse MrgprA3 and MrgprC11 (or MrgprX1) are mostly co-expressed in the same DRG neurons [52], CXCR3 is likely expressed in the MrgprC11 subset of MrgApr3 neurons. However, it is unclear whether the CXCL10+ neurons responded to other common algogens and pruritogens besides capsaicin and histamine, such as BAM8-22, chloroquine, and AITC. Further study is required to characterize fully the CXCL10+ population.
Epidermal cells, especially keratinocytes, appear to be a major source of CXCL10 in vivo during CHS [9; 15; 46]. In addition to keratinocytes, CXCL10 was detected in the cell bodies of DRG neurons [1]. Previous studies have proposed that chemokines can be packaged into secretory vesicles and released by DRG neurons upon depolarization [1]. We here showed that CXCL10 expression was upregulated in the DRG following CHS. Moreover, CHS increased the incidence of activation of cutaneous DRG neurons by CXCL10. Therefore, we postulate that the increased CXCR3 activation in DRG neurons of CHS mice in vivo might, in part, result from enhanced tonic release of CXCL10 from sensory neurons, in addition to keratinocytes, in a paracrine or autocrine manner.
Although our results support a neuronal mechanism of CXCR3 for allergic itch, we cannot exclude the involvements of non-neuronal cells. CXCR3 is expressed on activated T cells and is thought to recruit T cells to inflammation sites where a variety of proinflammatory mediators are released [29; 33], such as substance P, nerve growth factor and interleukin-6 [25]. It seems likely that at least some of these mediators might assist in the development of allergic itch. Future studies, including those using neuronal-specific CXCR3 knockout mice, are required to determine the relative contributions of sensory neurons and immune cells to allergic itch accompanying CHS.
In conclusion, our findings indicate that the increased CXCL10/CXCR3 signaling in sensory neurons may contribute to the pathogenesis of the chronic itch accompanying CHS. We suggest that neuronal CXCR3 emerges as a molecular target for pharmacological agents that can be developed to treat the sensory symptoms of inflammatory disease, such as ACD, in humans.
Acknowledgments
L.Q. designed the experiments, conducted calcium imaging and electrophysiological experiments, and wrote the manuscript; K.F. and S.S. carried out the behavioral experiments and analyzed the behavioral data; J. Y. performed quantitative RT-PCR; R.H.L. designed the research, supervised the project and edited the manuscript. This work was supported by NIH grants NS047399 and NS014624 (RHL). L. Qu is the recipient of a fellowship from the Canadian Institutes of Health Research (CIHR). We thank Dr. Xinzhong Dong (John Hopkins University, Baltimore) for providing the donor breeders we used to generate our colonies of MrgprA3 and MrgprD transgenic mice. We also thank Amgen Inc. (Thousand Oaks, CA) for the gift of AMG487.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. 2010;65(7):805–821. doi: 10.1111/j.1398-9995.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 3.Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer. 2009;100(11):1755–1764. doi: 10.1038/sj.bjc.6605078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(22):9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, Raouf R, Wood JN, Oh U. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15(7):1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 6.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120(1):1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 7.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 8.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89(4):1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flier J, Boorsma DM, Bruynzeel DP, Van Beek PJ, Stoof TJ, Scheper RJ, Willemze R, Tensen CP. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113(4):574–578. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 10.Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148(3):509–518. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu K, Qu L, Shimada SG, Nie H, LaMotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neuroscience letters. 2014;579:190–194. doi: 10.1016/j.neulet.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu K, Qu L, Shimada SG, Nie H, Lamotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett. 2014 doi: 10.1016/j.neulet.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk JO, Maibach HI. Horizons in pharmacologic intervention in allergic contact dermatitis. J Am Acad Dermatol. 1994;31(6):999–1014. doi: 10.1016/s0190-9622(94)70272-1. [DOI] [PubMed] [Google Scholar]
- 14.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harbor perspectives in biology. 2010;2(10):a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goebeler M, Trautmann A, Voss A, Brocker EV, Toksoy A, Gillitzer R. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol. 2001;158(2):431–440. doi: 10.1016/s0002-9440(10)63986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunology today. 1998;19(1):37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 17.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope JC, Campbell F, Hopkins SJ. Deficiency of IL-2 or IL-6 reduces lymphocyte proliferation, but only IL-6 deficiency decreases the contact hypersensitivity response. Eur J Immunol. 2000;30(1):197–203. doi: 10.1002/1521-4141(200001)30:1<197::AID-IMMU197>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, Hansen JB, Dissing S, Malling HJ, Skov PS, Poulsen LK. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol. 2000;165(3):1548–1556. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- 20.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79(17):11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo S, Pastore S, Fujisawa H, Shivji GM, McKenzie RC, Dinarello CA, Sauder DN. Interleukin-1 receptor antagonist suppresses contact hypersensitivity. J Invest Dermatol. 1995;105(3):334–338. doi: 10.1111/1523-1747.ep12320329. [DOI] [PubMed] [Google Scholar]
- 22.Kondo S, Pastore S, Shivji GM, McKenzie RC, Sauder DN. Characterization of epidermal cytokine profiles in sensitization and elicitation phases of allergic contact dermatitis as well as irritant contact dermatitis in mouse skin. Lymphokine Cytokine Res. 1994;13(6):367–375. [PubMed] [Google Scholar]
- 23.LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol. 2011;20(10):778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laragione T, Brenner M, Sherry B, Gulko PS. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion in rheumatoid arthritis. Arthritis Rheum. 2011;63(11):3274–3283. doi: 10.1002/art.30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27(9):3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. The Journal of clinical investigation. 2010;120(4):1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32(42):14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. The Journal of clinical investigation. 2012;122(6):2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105(10):3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 31.Nelson TE, Gruol DL. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J Neuroimmunol. 2004;156(1–2):74–87. doi: 10.1016/j.jneuroim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21(14):5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. The Journal of clinical investigation. 1998;101(4):746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, Lamotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137(Pt 4):1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu L, Fu K, Shimada SG, LaMotte RH. CXCL10 directly sensitizes cutaneous nociceptors through CXCR3 and contributes to allergic itch in a mouse model of allergic contact dermatitis. Washington DC: Society for Neuroscience; 2014. Abstract Program No. 534.19. [Google Scholar]
- 36.Qu L, Li Y, Pan X, Zhang P, LaMotte RH, Ma C. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J Neurosci. 2012;32(28):9554–9562. doi: 10.1523/JNEUROSCI.6355-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu L, Zhang P, LaMotte RH, Ma C. Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain Behav Immun. 2011;25(7):1399–1407. doi: 10.1016/j.bbi.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HW, Kettenmann H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J Immunol. 2002;168(7):3221–3226. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- 39.Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130(5):1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, Lazzeri E, Angeli R, Rotondi M, Fili L, Parronchi P, Serio M, Maggi E, Romagnani S, Annunziato F. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. The Journal of allergy and clinical immunology. 2005;116(6):1372–1379. doi: 10.1016/j.jaci.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139(3):681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183(4):1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strong JA, Xie W, Coyle DE, Zhang JM. Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PLoS One. 2012;7(7):e40779. doi: 10.1371/journal.pone.0040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suga H, Sugaya M, Miyagaki T, Ohmatsu H, Okochi H, Sato S. CXCR3 deficiency prolongs Th1-type contact hypersensitivity. J Immunol. 2013;190(12):6059–6070. doi: 10.4049/jimmunol.1201606. [DOI] [PubMed] [Google Scholar]
- 45.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177(6):1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokuriki A, Seo N, Ito T, Kumakiri M, Takigawa M, Tokura Y. Dominant expression of CXCR3 is associated with induced expression of IP-10 at hapten-challenged sites of murine contact hypersensitivity: a possible role for interferon-gamma-producing CD8(+) T cells in IP-10 expression. J Dermatol Sci. 2002;28(3):234–241. doi: 10.1016/s0923-1811(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 47.Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, Lu B, Gerard C, Hancock WW, Schreiber RD. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86(1):137–147. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphal GA, Schnuch A, Moessner R, Konig IR, Kranke B, Hallier E, Ziegler A, Reich K. Cytokine gene polymorphisms in allergic contact dermatitis. Contact Dermatitis. 2003;48(2):93–98. doi: 10.1034/j.1600-0536.2003.480208.x. [DOI] [PubMed] [Google Scholar]
- 49.White FA, Miller RJ. Insights into the regulation of chemokine receptors by molecular signaling pathways: functional roles in neuropathic pain. Brain Behav Immun. 2010;24(6):859–865. doi: 10.1016/j.bbi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14(5):595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang CH, Fang IM, Lin CP, Yang CM, Chen MS. Effects of the NF-kappaB inhibitor pyrrolidine dithiocarbamate on experimentally induced autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2005;46(4):1339–1347. doi: 10.1167/iovs.04-0640. [DOI] [PubMed] [Google Scholar]
- 52.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]