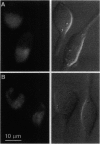
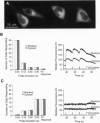
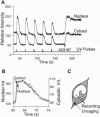
Abstract
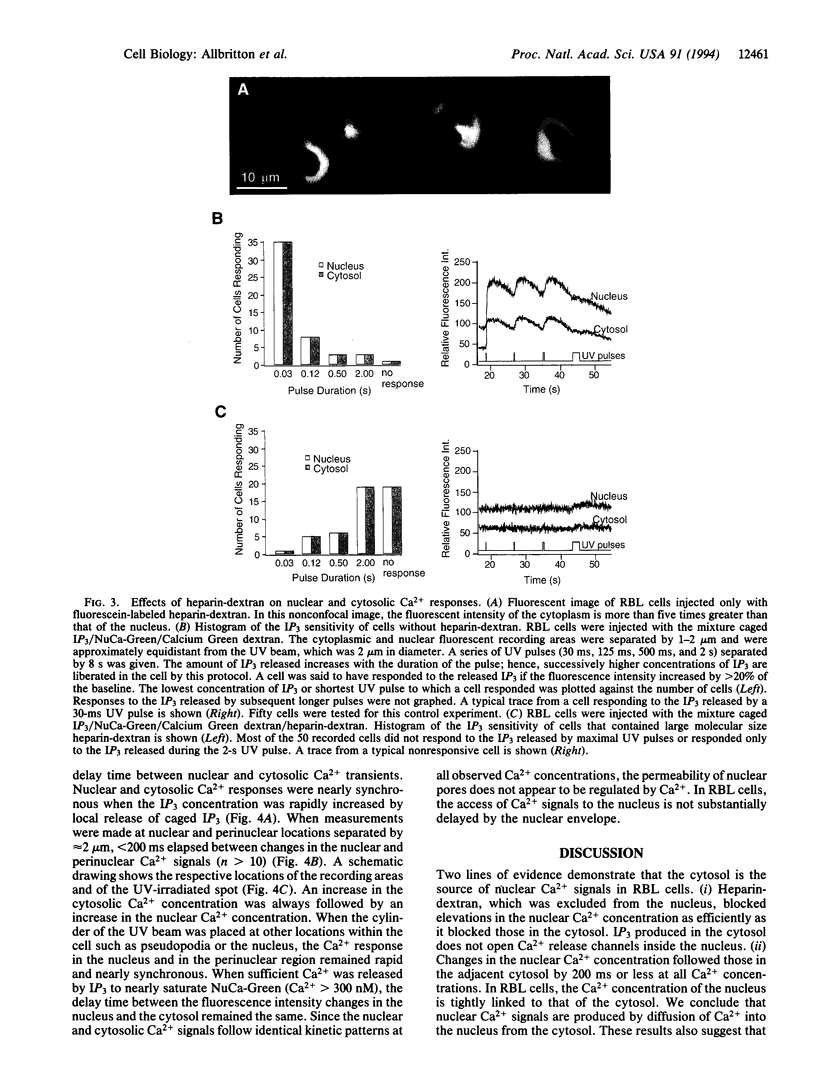
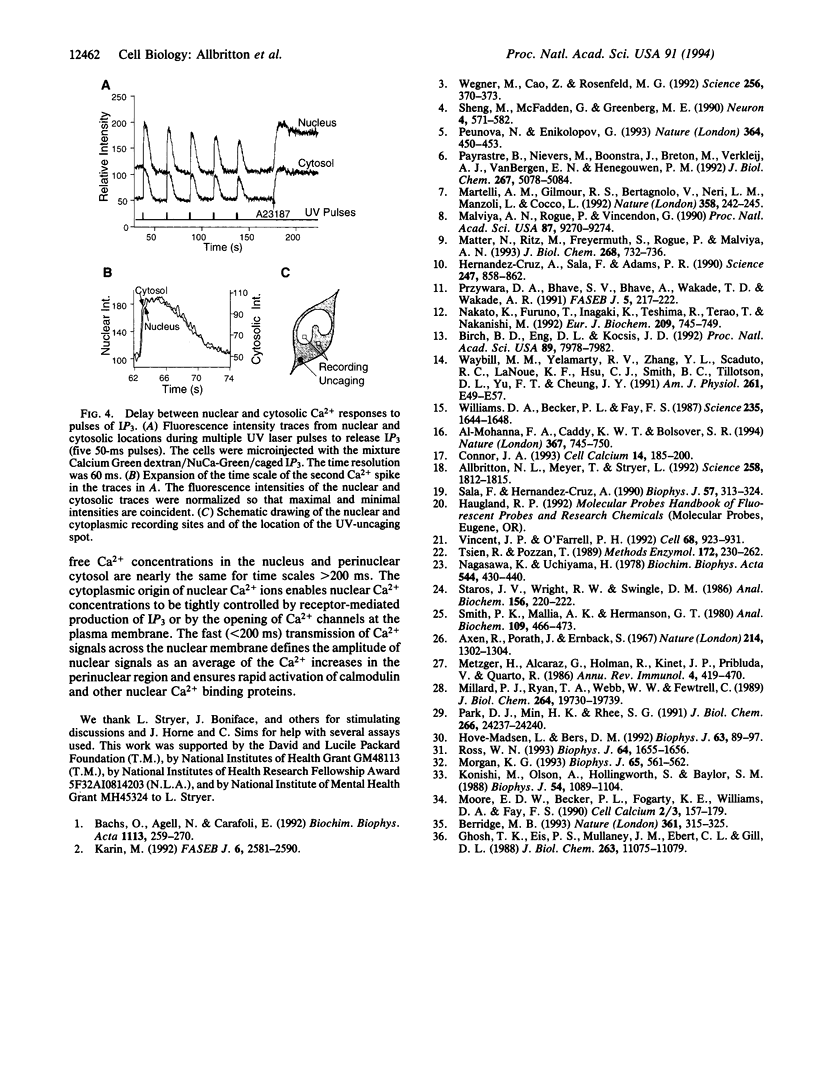
Transient increases of Ca2+ concentration in the nucleus regulate gene expression and other nuclear processes. We investigated whether nuclear Ca2+ signals could be regulated independently of the cytoplasm or were controlled by cytoplasmic Ca2+ signals. A fluorescent Ca2+ indicator that is targeted to the nucleus was synthesized by coupling a nuclear localization peptide to Calcium Green dextran, a 70-kDa Ca2+ indicator. Stimulation of rat basophilic leukemia cells by antigen or by photolytic uncaging of inositol 1,4,5-trisphosphate induced transient increases in nuclear and cytosolic Ca2+ concentrations. Elevations in the nuclear Ca2+ concentration followed those in the nearby perinuclear cytosol within 200 ms. Heparin-dextran, an inhibitor of the inositol 1,4,5-trisphosphate receptor that is excluded from the nucleus, was synthesized to specifically block the release of Ca2+ from cytosolic stores. Addition of this inhibitor suppressed Ca2+ transients in the nucleus and the cytosol. We conclude that the Ca2+ level in the nucleus is not independently controlled. Rather, nuclear Ca2+ increases follow cytosolic Ca2+ increases with a short delay most likely due to Ca2+ diffusion from the cytosol through the nuclear pores.
Full text
PDF

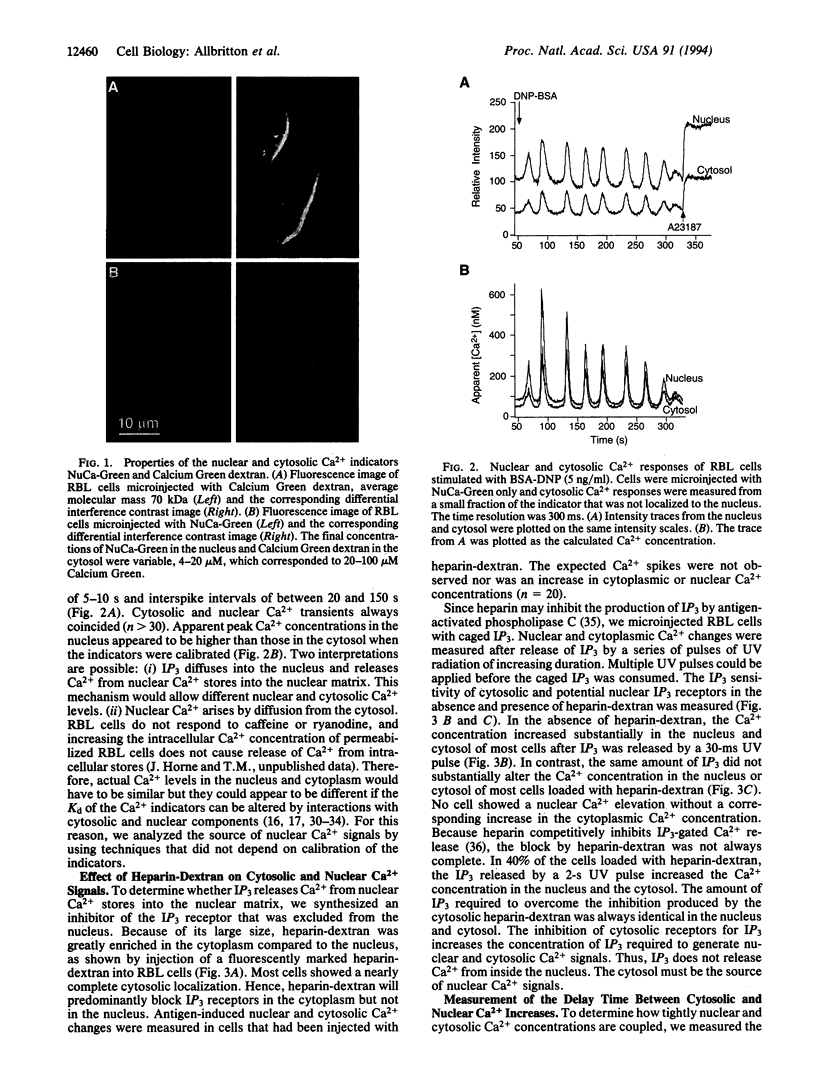
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bachs O., Agell N., Carafoli E. Calcium and calmodulin function in the cell nucleus. Biochim Biophys Acta. 1992 Aug 14;1113(2):259–270. doi: 10.1016/0304-4157(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Birch B. D., Eng D. L., Kocsis J. D. Intranuclear Ca2+ transients during neurite regeneration of an adult mammalian neuron. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7978–7982. doi: 10.1073/pnas.89.17.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992 Jul;63(1):89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Signal transduction from cell surface to nucleus in development and disease. FASEB J. 1992 May;6(8):2581–2590. doi: 10.1096/fasebj.6.8.1317309. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A. M., Gilmour R. S., Bertagnolo V., Neri L. M., Manzoli L., Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature. 1992 Jul 16;358(6383):242–245. doi: 10.1038/358242a0. [DOI] [PubMed] [Google Scholar]
- Matter N., Ritz M. F., Freyermuth S., Rogue P., Malviya A. N. Stimulation of nuclear protein kinase C leads to phosphorylation of nuclear inositol 1,4,5-trisphosphate receptor and accelerated calcium release by inositol 1,4,5-trisphosphate from isolated rat liver nuclei. J Biol Chem. 1993 Jan 5;268(1):732–736. [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Ryan T. A., Webb W. W., Fewtrell C. Immunoglobulin E receptor cross-linking induces oscillations in intracellular free ionized calcium in individual tumor mast cells. J Biol Chem. 1989 Nov 25;264(33):19730–19739. [PubMed] [Google Scholar]
- Moore E. D., Becker P. L., Fogarty K. E., Williams D. A., Fay F. S. Ca2+ imaging in single living cells: theoretical and practical issues. Cell Calcium. 1990 Feb-Mar;11(2-3):157–179. doi: 10.1016/0143-4160(90)90068-6. [DOI] [PubMed] [Google Scholar]
- Morgan K. G. Ca2+i versus [Ca2+]i. Biophys J. 1993 Aug;65(2):561–562. doi: 10.1016/S0006-3495(93)81087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Uchiyama H. Preparation and properties of biologically active fluorescent heparins. Biochim Biophys Acta. 1978 Dec 1;544(2):430–440. doi: 10.1016/0304-4165(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Nakato K., Furuno T., Inagaki K., Teshima R., Terao T., Nakanishi M. Cytosolic and intranuclear calcium signals in rat basophilic leukemia cells as revealed by a confocal fluorescence microscope. Eur J Biochem. 1992 Oct 15;209(2):745–749. doi: 10.1111/j.1432-1033.1992.tb17343.x. [DOI] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. IgE-induced tyrosine phosphorylation of phospholipase C-gamma 1 in rat basophilic leukemia cells. J Biol Chem. 1991 Dec 25;266(36):24237–24240. [PubMed] [Google Scholar]
- Payrastre B., Nievers M., Boonstra J., Breton M., Verkleij A. J., Van Bergen en Henegouwen P. M. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J Biol Chem. 1992 Mar 15;267(8):5078–5084. [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993 Jul 29;364(6436):450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Przywara D. A., Bhave S. V., Bhave A., Wakade T. D., Wakade A. R. Stimulated rise in neuronal calcium is faster and greater in the nucleus than the cytosol. FASEB J. 1991 Feb;5(2):217–222. doi: 10.1096/fasebj.5.2.2004666. [DOI] [PubMed] [Google Scholar]
- Ross W. N. Calcium on the level. Biophys J. 1993 Jun;64(6):1655–1656. doi: 10.1016/S0006-3495(93)81537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Mallia A. K., Hermanson G. T. Colorimetric method for the assay of heparin content in immobilized heparin preparations. Anal Biochem. 1980 Dec;109(2):466–473. doi: 10.1016/0003-2697(80)90679-x. [DOI] [PubMed] [Google Scholar]
- Staros J. V., Wright R. W., Swingle D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986 Jul;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Waybill M. M., Yelamarty R. V., Zhang Y. L., Scaduto R. C., Jr, LaNoue K. F., Hsu C. J., Smith B. C., Tillotson D. L., Yu F. T., Cheung J. Y. Nuclear calcium gradients in cultured rat hepatocytes. Am J Physiol. 1991 Jul;261(1 Pt 1):E49–E57. doi: 10.1152/ajpendo.1991.261.1.E49. [DOI] [PubMed] [Google Scholar]
- Wegner M., Cao Z., Rosenfeld M. G. Calcium-regulated phosphorylation within the leucine zipper of C/EBP beta. Science. 1992 Apr 17;256(5055):370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Becker P. L., Fay F. S. Regional changes in calcium underlying contraction of single smooth muscle cells. Science. 1987 Mar 27;235(4796):1644–1648. doi: 10.1126/science.3103219. [DOI] [PubMed] [Google Scholar]
- al-Mohanna F. A., Caddy K. W., Bolsover S. R. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994 Feb 24;367(6465):745–750. doi: 10.1038/367745a0. [DOI] [PubMed] [Google Scholar]