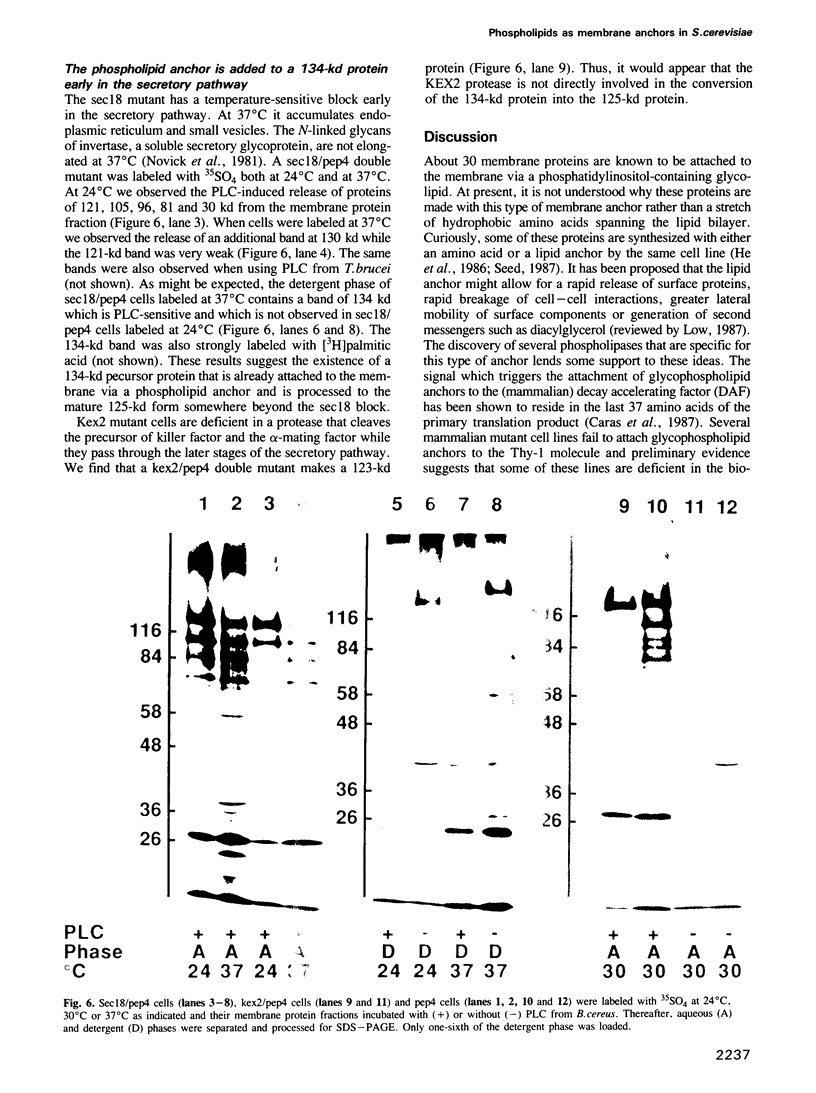
Abstract
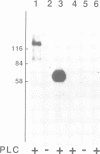
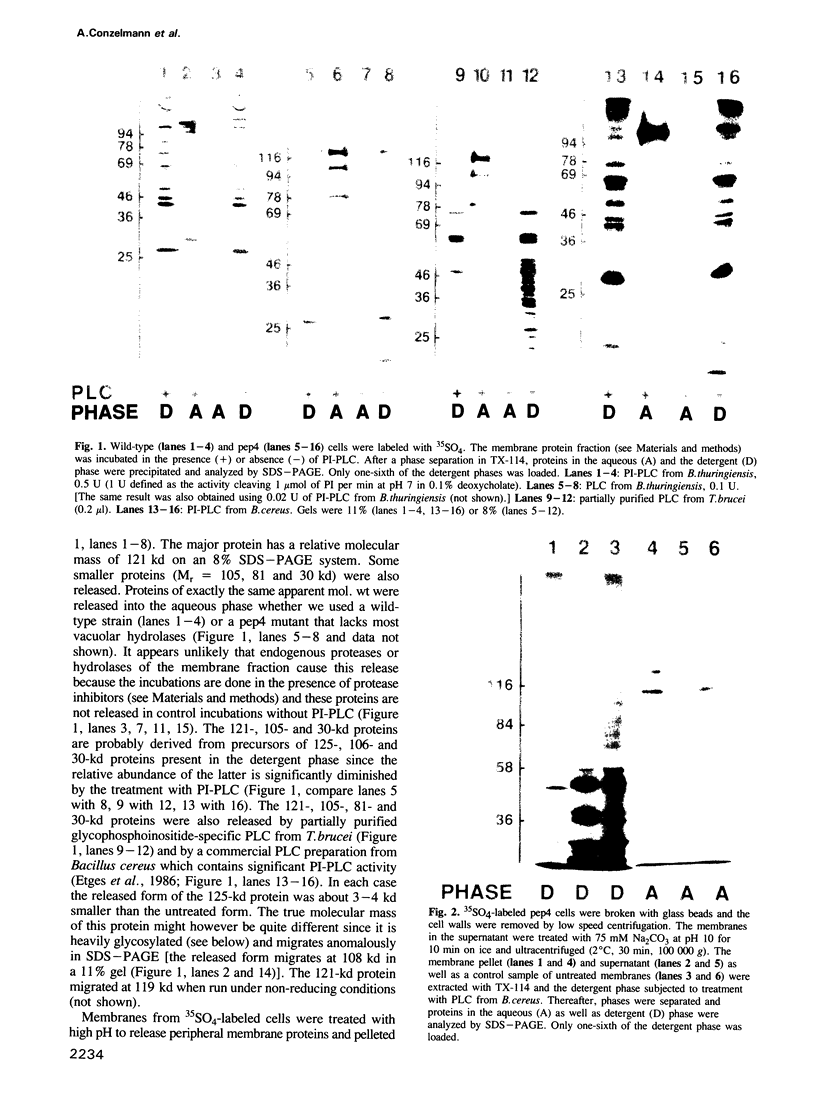
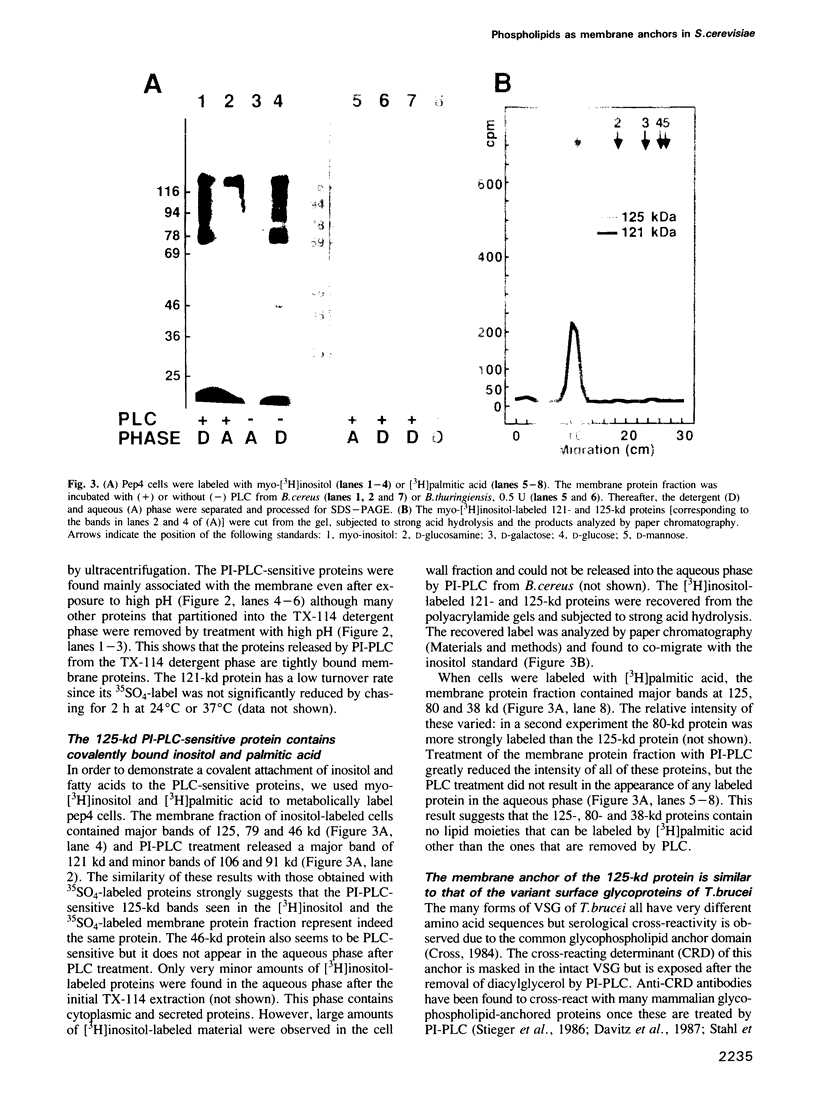
A number of plasma membrane glycoproteins of mammalian and protozoan origin are released from cells by phosphatidylinositol-specific phospholipase C. Some of these proteins have been shown to be attached to the lipid bilayer via a covalently linked, structurally complex glycophospholipid. Here we establish the existence of similarly linked glycoproteins in the yeast Saccharomyces cerevisiae. The most abundant of these is a tightly membrane-bound glycoprotein of 125 kd. The detergent-binding moiety of this protein can be removed by phosphatidylinositol-specific phospholipase C of bacterial origin or from Trypanosoma brucei. Metabolic labeling indicates that the protein contains covalently attached fatty acid and inositol. It also contains the cross-reacting determinant (CRD), an antigen found previously on the glycophospholipid anchor of protozoan and mammalian origin. Treatment of the protein with endoglycosidases F and H results in a 95-kd species. In the secretion mutant sec18, grown at 37 degrees C, the vesicular transport of glycoproteins is reversibly blocked between the rough endoplasmic reticulum and the Golgi apparatus. We find that sec18 cells, when grown at 37 degrees C, do add phospholipid anchors to newly synthesized glycoproteins. This indicates that these anchors are added in the rough endoplasmic reticulum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bülow R., Overath P. Purification and characterization of the membrane-form variant surface glycoprotein hydrolase of Trypanosoma brucei. J Biol Chem. 1986 Sep 5;261(25):11918–11923. [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Carter H. E., Strobach D. R., Hawthorne J. N. Biochemistry of the sphingolipids. 18. Complete structure of tetrasaccharide phytoglycolipid. Biochemistry. 1969 Jan;8(1):383–388. doi: 10.1021/bi00829a053. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Bron C., Hyman R. No glycolipid anchors are added to Thy-1 glycoprotein in Thy-1-negative mutant thymoma cells of four different complementation classes. Mol Cell Biol. 1988 Feb;8(2):674–678. doi: 10.1128/mcb.8.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Structure of the variant glycoproteins and surface coat of Trypanosoma brucei. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):3–12. doi: 10.1098/rstb.1984.0104. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. The phenotype of five classes of T lymphoma mutants. Defective glycophospholipid anchoring, rapid degradation, and secretion of Thy-1 glycoprotein. J Biol Chem. 1988 Jan 25;263(3):1288–1294. [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Fox J. A., Duszenko M., Ferguson M. A., Low M. G., Cross G. A. Purification and characterization of a novel glycan-phosphatidylinositol-specific phospholipase C from Trypanosoma brucei. J Biol Chem. 1986 Nov 25;261(33):15767–15771. [PubMed] [Google Scholar]
- Fox J. A., Soliz N. M., Saltiel A. R. Purification of a phosphatidylinositol-glycan-specific phospholipase C from liver plasma membranes: a possible target of insulin action. Proc Natl Acad Sci U S A. 1987 May;84(9):2663–2667. doi: 10.1073/pnas.84.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. T., Barbet J., Chaix J. C., Goridis C. Phosphatidylinositol is involved in the membrane attachment of NCAM-120, the smallest component of the neural cell adhesion molecule. EMBO J. 1986 Oct;5(10):2489–2494. doi: 10.1002/j.1460-2075.1986.tb04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. L., Hereld D., Bangs J. D., Hart G. W., Englund P. T. Identification of a glycolipid precursor of the Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1986 Sep 15;261(26):12147–12153. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Ballou C. E. Complete structures of the glycophospholipids of mycobacteria. Biochemistry. 1965 Jul;4(7):1395–1404. doi: 10.1021/bi00883a026. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5793–5797. doi: 10.1073/pnas.83.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Schmitz B., Klein R. A., Duncan I. A., Egge H., Gunawan J., Peter-Katalinic J., Dabrowski U., Dabrowski J. MS and NMR analysis of the cross-reacting determinant glycan from Trypanosoma brucei brucei MITat 1.6 variant specific glycoprotein. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1055–1063. doi: 10.1016/0006-291x(87)90754-6. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger A., Cardoso de Almeida M. L., Blatter M. C., Brodbeck U., Bordier C. The membrane-anchoring systems of vertebrate acetylcholinesterase and variant surface glycoproteins of African trypanosomes share a common antigenic determinant. FEBS Lett. 1986 Apr 21;199(2):182–186. doi: 10.1016/0014-5793(86)80476-8. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Wen D., Schlesinger M. J. Fatty acid-acylated proteins in secretory mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Apr;4(4):688–694. doi: 10.1128/mcb.4.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]