Abstract
Altered resting-state brain activity, as a measure of functional connectivity, is commonly observed in chronic pain. Identifying a reliable signature pattern of altered resting-state activity for chronic pain could provide strong mechanistic insights and serve as a highly beneficial neuroimaging-based diagnostic tool. We collected and analyzed resting-state fMRI data from female patients with urologic chronic pelvic pain syndrome (UCPPS, N = 45) and matched healthy participants (N = 45) as part of a NIDDK funded multicenter project (www.mappnetwork.org). Using dual regression and seed-based analyses, we observed significantly decreased functional connectivity of the default mode network (DMN) to two regions in the posterior medial cortex (PMC): the posterior cingulate cortex (PCC) and left precuneus (TFCE, FWE corrected p<0.05). Further investigation revealed that patients demonstrated increased functional connectivity between the PCC and several brain regions implicated in pain, sensory, motor, and emotion regulation processes (e.g., insular cortex, dorsolateral prefrontal cortex, thalamus, globus pallidus, putamen, amygdala, hippocampus). The left precuneus demonstrated decreased functional connectivity to several regions of pain processing, reward, and higher executive functioning within the prefrontal (orbitofrontal, anterior cingulate, ventromedial prefrontal) and parietal cortices (angular gyrus, superior and inferior parietal lobules). The altered PMC connectivity was associated with several phenotype measures, including pain and urologic symptom intensity, depression, anxiety, quality of relationships and self-esteem levels in patients. Collectively, these findings indicate that in UCPPS patients, regions of the PMC are detached from the DMN, while neurological processes of self-referential thought and introspection may be joined to pain and emotion regulatory processes.
Keywords: UCPPS, interstitial cystitis, bladder pain syndrome, posterior cingulate cortex, default mode network, DMN, precuneus, dual regression, resting state, fMRI
1. Introduction
Accumulating evidence indicates that alterations in central nervous system (CNS) structure and function are associated with the manifestation and maintenance of chronic pain [1; 6; 33; 54]. Resting-state functional magnetic resonance imaging (RS-fMRI) is commonly used to examine CNS function by measuring the synchronicity of low-frequency oscillations in the blood oxygen level dependent (BOLD) signal across distinct brain regions [22]. Functional brain networks are collections of brain regions whose BOLD signal oscillations are significantly synchronous [9]. The default mode network (DMN) and salience network are among the most thoroughly researched [27; 50], and their topographies are altered by injury [12; 48; 56], disease [28], and cognitive processes [49]. In addition, functional network topography (including that of the DMN) and brain activity are altered across many chronic pain syndromes, including, fibromyalgia, chronic pelvic pain, chronic low back pain, and others [5; 11; 13; 41]. The overall picture of how these alterations contribute to chronic pain, however, remains unclear.
Pain is a complex sensory and cognitive experience, and pain processing involves multiple distributed brain regions and networks [16]. Therefore, we hypothesized that in chronic pain, functional connectivity (FC), which is a measure of the synchrony of BOLD signal oscillations, would be altered across multiple major resting-state (RS) networks (e.g., sensory-motor, default mode, salience and executive control networks). We also expected these networks to demonstrate altered FC to the individual brain regions involved in pain processing previously shown to be altered in chronic pain (e.g., somatosensory, motor, insular, prefrontal regions). We tested this hypothesis using RS-fMRI data acquired through the MAPP Research Network, a multicenter collaborative initiative of the National Institutes of Health for the study of urologic chronic pelvic pain syndrome (UCPPS). UCPPS is a debilitating disorder of pain in the pelvic region and urological symptoms (e.g. bladder pressure, urgency to void, increased frequency of urination) [10]. Previous studies of brain activity in chronic pelvic pain have revealed altered FC within and between specific brain regions [2; 19; 35]; however, a whole brain data-driven approach applied to a large patient data set has not yet been published, and may have implications for alterations in RS FC and chronic pain states in general. Using a data-driven approach, we observed significantly decreased FC of between regions of the posterior medial cortex (PMC), specifically the posterior cingulate cortex (PCC) and left precuneus, to the DMN in patients with UCPPS as compared with HCs. To further understand these alterations in DMN FC, we conducted seed-based FC analyses of the PCC and left precuneus which revealed additional alterations in FC, both within and outside the DMN, and implicated associations to several clinical and behavioral measures.
2. Methods
2.1. Data Collection
All data were obtained through a multicenter collaborative study, the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network* (www.mappnetwork.org) [15]. Patients with UCPPS and matched healthy controls (HC) participated in study visits that included extensive phenotyping, collection of biospecimens (blood and urine), and neuroimaging [37]. Extensive phenotype data included information on the duration of symptoms, intensity and locations of pain, medication use history, presence of urologic symptoms (e.g., urgency, frequency), and psychological symptoms (e.g., depression and anxiety).
2.1.1. Participants
Data from 45 females with UCPPS (henceforth referred to as “patients”) and 45 age-matched HC females were used in the present study. The included scans were collected at multiple sites as follows: Northwestern University (NU) 5 HC, 2 UCPPS; University of California at Los Angeles (UCLA) 10 HC, 9 UCPPS; University of Michigan (UM) 13 HC, 15 UCPPS; University of Alabama at Birmingham (UAB) 7 HC, 9 UCPPS; Stanford University (SU) 10 HC, 10 UCPPS. Patient and HC data were selected from a larger MRI data set of 279 total participants who underwent neuroimaging scans from March 2010 through November 2012 as part of the MAPP Research Network. The data used in the present study were selected from the total MAPP neuroimaging data set to include only female patients without comorbid syndromes of fibromyalgia (FM), chronic fatigue syndrome (CFS), and irritable bowel syndrome (IBS). UCPPS in females consists of interstitial cystitis / bladder pain syndrome (IC/BPS) defined as chronic unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms, in the absence of infection or other identifiable causes. General patient inclusion criteria were therefore as follows: the presence of pain, pressure, or discomfort (present intensity rating > 1 on a 0–10 scale) perceived to be related to the bladder and/or pelvic region; associated with lower urinary tract symptoms; present for the majority of the time during any 3 months in the previous 6 months; and present for the majority of the time during the most recent 3 months. Patients were allowed to continue their standard medications during the study. All HC participants were required to have no pain in the pelvic or bladder region, and no chronic pain in more than one non-urologic region. Additional inclusion / exclusion criteria for all participants undergoing neuroimaging study procedures were as follows: no current major psychiatric disorder or other psychiatric, vision or hearing disorders that would interfere with study participation; no history of neurological disease including stroke or seizure disorders; no claustrophobia; and no metallic implants or devices that would be contraindicative to scanning procedures. (For additional details on general inclusion / exclusion criteria and study protocols of the MAPP Research Network study see: [37].) All procedures were approved by the Institutional Review Boards at each site, and written informed consent was obtained from all participants prior to study procedures.
2.1.2. Neuroimaging Data Collection
MRI scanning was performed at multiple sites using different scanner technology (3T Siemens Trio (NU and UCLA), 3T Phillips Ingenia (UM), 3T Philips Achieva (UAB), and 3T GE Discovery (SU)). Trans-MAPP neuroimaging data was collected, quality controlled and archived according to multi-site imaging procedures developed collaboratively between the MAPP Research Network, the UCLA PAIN repository and the UCLA Laboratory of Neuroimaging. Detailed procedures and description of the repository are available at PAINrepository.org. Scanner compatible acquisition parameters were developed based on recommendations from fBIRN (https://xwiki.nbirn.org:8443/bin/view/Function-BIRN/FBIRN_Best_Practices) and all sites were required to complete and pass a site qualification including a set of pilot scans of a human volunteer; the initial scans were reviewed for quality control by the UCLA site, and recommendations and adjustments were made as necessary prior to commencement of study scans. A high resolution structural image was acquired from each subject with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2200 ms, echo time (TE) = 3.26 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 13 mm voxel size. RS scans were acquired while subjects rested with eyes closed for 10 minutes in 30 – 40 slice* whole brain volumes, slice thickness = 4 mm, slice gap = 0.5 mm, TR = 2000 ms, TE = 28 ms, flip angle = 77°, field of view (FOV) = 220 × 200, 3.43 × 3.43 mm in-slice voxel size*, 64 × 64 voxel matrices*. (*Number of slices varied among sites: NU 36, UCLA 40, UM 30, SU 32 and UAB 32. UM's sequence used 2.75 × 2.75 mm in-plane voxel size and 80 x 80 voxel matrices to acquire the same FOV as other sites.) Before entering the scanner, subjects were asked to empty their bladder. Physiological data (heart rate and respiration) were monitored and recorded at some of the scanning sites; however, because these data were not consistently collected across all sites and all participants, alternative methods for physiological noise regression were used in the analyses presented here.
2.2. Neuroimaging Analysis
2.2.1. MRI Image Preprocessing
Structural and functional images were reconstructed and preprocessed using FSL [Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Center for FMRIB, University of Oxford, Oxford, UK)]. Structural brain (T1) images were separated from skull and surrounding tissue using FSL's brain extraction tool. Functional images were preprocessed using FSL's FMRI Expert Analysis Tool (FEAT) software as follows: deletion of the first 5 volumes, motion correction (MCFLIRT), registration to structural (brain-extracted) images [7 degrees of freedom (DOF) to subject structural image and 12 DOF to standard space image], spatial smoothing (6-mm FWHM), temporal filtering (high-pass temporal filter of 0.01 Hz to eliminate linear drift). Voxel-wise noise-regression of cerebrospinal fluid (CSF), white matter (WM) signal, movement, and the mean global brain signal was performed using a custom, in-house MATLAB script (MATLAB 2012, MathWorks, Natick, MA, USA).
2.2.2. Independent Components Analysis (ICA)
An independent components analysis (ICA) was conducted using FSL's MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components). Rather than use a standard template that best characterizes the network organization of HC participants, we identified RS networks specific to our population. We concatenated the preprocessed RS-fMRI data from all 90 subjects, and included these data in an ICA analysis. The ICA was restricted to 30 components. RS networks were identified and distinguished from artifactual components (i.e., those due to physiologic noise or movement) by visual inspection, which was based on templates of standard RS networks from Shirer et al. (2012) [49]. Components identified as valid networks, based on a standardized template matching, were used as spatial regressors in the dual regression analysis. Noise components were similarly regressed from the dual regression analysis.
2.2.3. Dual Regression: Creation of Back-reconstructed Spatiotemporal Maps
As done previously we used FSL software to perform dual regression analysis to measure differences in functional brain networks in patients as compared with HCs [7; 20; 21; 61]. While several functional brain networks have been established for healthy populations [50], these networks might not accurately depict brain function in pathological states. Thus, the use of standard network templates may be biased and not ideal for assessing clinical states. Dual regression avoids this issue by basing its analysis on networks derived from the sample population (i.e., a combination of healthy participants and those with chronic pain) [20; 61]. Dual regression also automatically regresses out signals related to noise components.
Briefly, dual regression uses a selected network map (identified from the ICA) as a spatial regressor to extract a time series of activity from each subject's functional data. Then, the time series for each subject is used as a temporal regressor to back-reconstruct an individual's spatial map for the given network (i.e., “back-reconstructed spatiotemporal map”). This was conducted serially and automatically for each of the 30 components identified by the group ICA (for both valid components and noise components).
2.2.4. Statistical Group Comparison using Randomise
We used FSL's randomise permutation-testing tool (two-sample t-test for patient group versus HC group) to conduct statistical comparisons to identify differences in functional brain networks, based on the initial dual regression outputs (back-reconstructed spatiotemporal maps for each individual). Spatial network ICA maps for each of the non-artifactual components were generated by running a one-sample t-test including all patient and HC scans (randomise). The ICA maps were then thresholded [voxel-based thresholding, family wise error (FWE) corrected, p < 0.05] and binarized to make a mask for each component. Second, the masks were used in a two-sample t-test comparison (randomise), with statistical analysis for each component restricted to within the regions of the component's network. Between-group significant differences were determined using threshold-free cluster enhancement (TFCE), (FWE corrected p < 0.05)[51].
2.2.5. Seed-Based Functional Connectivity Analyses
To further investigate the results of our dual regression analysis, we conducted seed-based FC analyses. This allowed us to determine how altered FC within the DMN, identified through our dual regression analysis, was related to brain regions outside of the DMN. Significant clusters from the dual regression analysis (TFCE, FWE corrected p < 0.05) were used as regions of interest in our whole-brain seed-based FC analyses. The seed-based analysis was performed twice, separately for each PMC seed region identified earlier in the ICA / dual regression analysis: the posterior cingulate cortex (PCC) and left precuneus. For each seed region, we performed the following steps. First, we created a seed mask and then used the seed mask to extract the time course of activity within the seed region for each subject's preprocessed RS image (preprocessing and voxel-wise noise-regression was performed as described above). For each individual subject, the seed time courses were then used as a regressor of interest in a whole-brain seed-based FC analysis using FSL's FEAT [100s high pass filter cut-off, custom regressors for subject's motion (from preprocessing step) and the subject's seed time course]. The resulting individual subject whole-brain FC maps were contrasted between patients and controls using a two-sample t-test using FSL's FLAME for mixed effects (Gaussianized T/F statistic images determined for significance using Z > 2.3 and cluster correction p < 0.05)[59]. Significant results indicated brain regions to which the patients’ PCC and left precuneus demonstrated FC that was significantly different than HC.
We used the Automated Anatomical Labeling (AAL) atlas to identify the anatomical boundaries of regions that showed significantly altered FC to the PCC or the left precuneus in patients as compared with HCs (WFU PickAtlas) [53]. This allowed us to separate each significant cluster into a distinct anatomically defined region of interest (ROI). To identify each cluster's anatomical boundaries, we first binarized the FC map results, and then masked the binarized FC maps with brain regions as defined by the AAL atlas. This produced 10 anatomical ROIs (aROIs) for the PCC seed, and 11 aROIs for the left precuneus seed. For each subject, we measured the pairwise Pearson's linear correlation coefficients between the mean time series for each seed region (PCC and left precuneus) and the mean time series extracted from each of their respective aROIs (MATLAB). Across all patients (N=45), we then ran a correlation analysis between the seed-aROI correlation coefficients and behavioral measures described below (MATLAB).
2.2.6. Clinical and Behavioral Covariates
For each of 21 seed ROI - aROI connections (10 for the PCC seed and 11 for the L. Precuneus seed), we assessed associations of FC strength to 17 phenotype measures (clinical and behavioral measures collected within 48 hours of the scan visit). Eight clinical measures included: duration of symptoms, symptom severity (Symptom Measures Questionnaire, SYMQ), sensory pain score (McGill Pain Questionnaire, MPQ), affective pain score (MPQ), pain from comorbid symptoms (Comorbid Symptom Index, CMSI), pelvic pain (Genito-Urinary Pain Index, GUPI), urinary symptoms (GUPI), and quality of life (QOL) related to urinary symptoms and pain (GUPI). Nine behavioral measures included: anxiety (Hospital Anxiety and Depression Scale, HADS), depression (HADS), positive affect (Positive and Negative Affect Scale (PANAS), negative affect (PANAS), experience of traumatic life events before age 17 (Childhood Traumatic Event Scale, CTES), experience of traumatic life events within the last 3 years (CTES), level of self-esteem (Self Esteem and Relationships, SEAR), quality of overall relationships (SEAR), and quality of sex relationships (SEAR). Two demographic measures, age and BMI, were correlated with several seed-aROI pairs in a preliminary analysis and these correlations were therefore regressed prior to the final correlation analysis (MATLAB). Since the behavioral correlation analysis was primarily an exploratory analysis, we did not correct for multiple comparisons (p < 0.05).
3. Results
3.1. Participant Demographics
Patients ranged in age from 21.2–65.1 years (mean, std 39.4 years +/− 11.4); HC participants ranged in age from 20.9–57.3 years (mean, std 37.9 years +/− 10.5), not statistically different from the patient group. Racial distribution for patients (41 white, 1 white–Hispanic, 2 African-American, and 1 combination of African and Native American) was similar to that of HC participants (37 white, 2 white–Hispanic, 2 Asian, 2 African-American, 1 other/not reported, 1 combination of 3 races (white, African-American, and Native American) (Table 1).
Table 1.
Phenotype data: Demographic, clinical and behavioral measures for patients and healthy controls.
| Demographics | Patients (N) | Mean | Healthy Controls (N) | Mean |
|---|---|---|---|---|
| Age (years) | 45 | 40.3 | 45 | 38.1 |
| Duration of UCPPS (years) | 45 | 9.42 | ||
|
Race / Ethnicity | ||||
| White | 41 | 37 | ||
| White-Hispanic | 1 | 2 | ||
| Black | 2 | 2 | ||
| Asian | 0 | 2 | ||
| Other | 1 | 2 | ||
| Endometriosis | 9 | 0 | ||
| Diagnosis of IC / BPS | 37 | 0 | ||
| Diagnosis of CPPS | 5 | 0 | ||
| RICE case (binary, yes) | 24 | 0 | ||
| TMJD | 8 | 0 | ||
| Vulvodynia | 13 | 0 | ||
|
Medication Type | ||||
| none | 22 | 42 | ||
| peripheral | 6 | 2 | ||
| central | 12 | 1 | ||
| opioid | 5 | 0 | ||
|
No. painful body sites (BPI)* | ||||
| within pelvic region | 42 | 2.1 | 6 | 0.26 |
| beyond pelvic region | 26 | 2.15 | 13 | 0.71 |
| Behavioral Measures at time of MRI Scan Visit: | Patients (N) | Mean | Healthy Controls (N) | Mean |
|---|---|---|---|---|
| Pain Sensory Score (MPQ) | 40 | 9.34 | 41 | 0.46 |
| Pain Affect Score (MPQ) | 40 | 2.275 | 41 | 0.73 |
| Present Pain Severity (SYMQ) | 44 | 3.95 | 42 | 0.07 |
| Anxiety Score (HADS) | 44 | 5.77 | 45 | 3.977 |
| Depression Score (HADS) | 44 | 4.45 | 45 | 1.933 |
| Pain Score (GUPI) | 44 | 12.56 | 45 | 0.577 |
| Urinary Symptom Score (GUPI) | 44 | 5.63 | 45 | 0.77 |
| Qualtiy of Life (GUPI) | 44 | 7.318 | 44 | 0.409 |
| Total Score (GUPI) | 45 | 25.51 | 45 | 1.75 |
| CTES (prior to age 17) | 44 | 1.73 | 45 | 1.02 |
| CTES (within last 3 years) | 44 | 1.49 | 45 | 1.15 |
| SEAR sexual relationships | 43 | 37.37 | 40 | 68.34 |
| SEAR overall relationships | 43 | 72.96 | 39 | 87.82 |
| SEAR self esteem | 45 | 47.77 | 41 | 55.69 |
| SEAR total | 42 | 46.37 | 40 | 68.29 |
Abbreviations: IC / BPS, interstitial cystitis / bladder pain syndrome; CPPS, chronic pelvic pain syndrome; TMJD, temporomandibular joint disorder; RICE, RAND Interstitial Cystitis Epidemiology; BPI, Brief Pain Inventory; MPQ, McGill Pain Questionnaire; SYMQ, Symptom Measures Questionnaire; HADS, Hospital Anxiety and Depression Scale; GUPI, Genito-Urinary Pain Index; CTES, Childhood Traumatic Events Scale [sum (items 1-6) effect of traumatic life events prior to age 17; and sum (items 7-13) effect of traumatic life events within the last 3 years]; SEAR, Self-Esteem and Relationships Scale.
BPI was collected at the baseline visit, prior to the scan visit. BPI regions indicated by several of the healthy controls were primarily located in the head, back and abdomen regions and likely due to general (non-chronic) aches and pains.
3.2. ICA Networks
We identified 17 valid (i.e., non-artifactual) RS networks from the group ICA results including the cerebellum, right executive control network (ECN), primary visual, medial sensory motor, precuneus /DMN, ventral DMN, anterior DMN, visuospatial/left ECN, lateral visuospatial, higher visual, language/dorsal DMN, lateral sensory motor, salience, basal ganglia, medial visuospatial, auditory, and medial temporal lobe (MTL) networks (Supplementary Fig. 1.). Each network was identified based on how its brain regions overlapped with regions included in previously established RS networks. Notably, a number of functional networks are reliably produced in resting-state fMRI research, which are not included in the atlas from Shirer et al. 2012. We elected to include these additional networks, and did not restrict ourselves to the Shirer et al. 2014 atlas, for network identification. For example, some of the networks that we identified represent subdivisions of networks (e.g., medial and lateral sensory motor) [60], combinations of networks (e.g., visuospatial / left ECN), and regional networks (e.g., cerebellum) [50].
3.3. Dual Regression Results
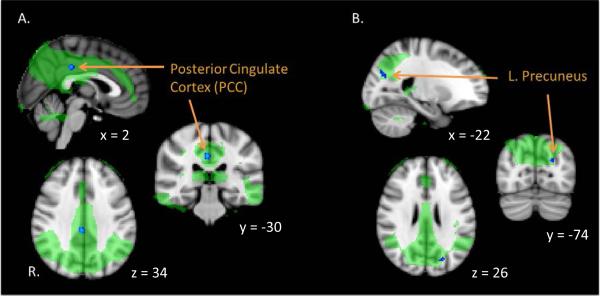
The dual regression analysis revealed regions of significant alterations in FC between patients and HCs. The main finding of statistical significance was observed within the DMN (precuneus / DMN shown in Supplementary Figure 1). Regions within the PCC (peak voxel coordinates: 4, −30, 36) and left precuneus (peak voxel coordinates: −22, −74, 26) demonstrated decreased FC to the DMN in patients as compared with HCs (TFCE, FWE corrected p < 0.05) (Fig. 1). No other networks showed significant between-group differences.
Figure 1. Decreased regional functional connectivity (FC) of the posterior medial cortex (PMC) to the default mode network (DMN).
Dual regression analysis (masked for the DMN) demonstrated decreased FC within a region of the PCC (peak voxel, x = 4, y = −30, z = 36) (A) and left precuneus (peak voxel, x = −22, y = −74, z = 26) (B) to the DMN network in patients. DMN shown in green. Regions of decreased connectivity to the network in blue (TFCE, FWE corrected p<0.05).
3.4. Seed-based Functional Connectivity
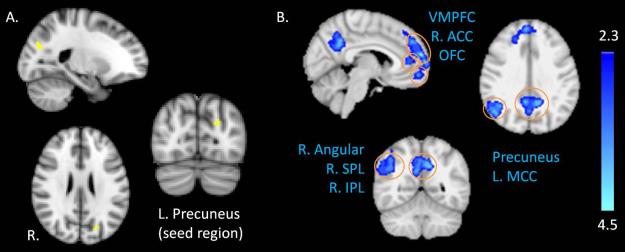
The seed-based FC analysis of the PCC seed demonstrated increased FC to a large cluster which encompassed 10 brain regions: the left anterior insular cortex, right mid-insular cortex, left hippocampus, left amygdala, bilateral putamen, bilateral globus pallidus, right thalamus and left dorsolateral prefrontal cortex (DLPFC) (Z > 2.3, cluster correction p < 0.05) (Fig. 2). In contrast, the seed-based FC analysis with the left precuneus seed demonstrated decreased FC to large clusters which encompassed 11 brain regions: the bilateral ventromedial prefrontal cortex (VMPFC), bilateral orbitofrontal cortex (OFC), right anterior cingulate cortex (ACC), left mid-cingulate cortex (MCC), right superior parietal lobule (SPL), right inferior parietal lobule (IPL), right angular gyrus, and bilateral precuneus (Z > 2.3, cluster correction p < 0.05) (Fig. 3). (Anatomically-masked regions (aROIs) are shown in Supplementary Fig. 2 and 3.) Specifically, mean z-score values indicated greater positive PCC seed connectivity (correlated) in patients as compared with negative (anticorrelated) PCC seed connectivity in HCs. Mean z-score values also indicated that left precuneus seed connectivity was negative (anticorrelated) in HCs, and this negative connectivity was nearly eliminated in patients (values approaching zero). The negative z-score values were still present in a subsequent analysis without global signal regression, indicating that these values represent true negative correlations or anticorrelations [23]. (Supplementary Table 1.)
Figure 2. Whole-brain, seed-based functional connectivity (FC) of the PCC seed region.
Seed-based analysis revealed increased FC of the PCC seed region (peak voxel, x = 4, y = −30, z = 36) (A) in relation to several regions (B), including the bilateral insular cortex, bilateral globus pallidus, bilateral putamen, right thalamus, left amygdala, left hippocampus, and left dorsolateral prefrontal cortex (DLPFC) (Z > 2.3, cluster corrected p < 0.05).
Figure 3. Whole-brain, seed-based functional connectivity (FC) of the left precuneus seed region.
Seed-based analysis revealed decreased FC of the left precuneus seed region (peak voxel, x = −22, y = −74, z = 26) (A) in relation to several regions (B), including the bilateral ventromedial prefrontal cortex (VMPFC), right anterior cingulate cortex (ACC), bilateral orbitofrontal cortex (OFC), bilateral precuneus cortex, left mid-cingulate cortex (MCC), right angular gyrus, and right superior and inferior parietal lobule (SPL, IPL respectively) (Z > 2.3, cluster corrected p < 0.05).
3.5. Seed ROI - aROI FC Associations with Clinical and Behavioral Phenotype Measures
We observed several positive and negative associations among the 21 seed ROI - aROI connections and 17 phenotype measures. Post-hoc analyses revealed significant correlation between several measures; therefore, our inclusion of 17 phenotype measures in actuality only represented approximately 7 distinct measurements (see Table 2). For example, measures of pain and symptom intensity were moderately to strongly correlated (SYMQ, GUPI, MPQ), measures of behavior and affect were all moderately correlated (HADS and PANAS), CTES subscales were moderately correlated, and SEAR subscales were moderately to strongly correlated. From a preliminary analysis, we observed that FC of the PCC to right insula, right putamen, right globus pallidus, and left DLPFC all were positively associated with age, and FC of the left precuneus to left mid-cingulate (MCC) showed a strong positive association with BMI, along with several other associations (see Table 3). Therefore, the demographic measures of age and BMI were regressed from the final correlation analysis.
Table 2.
Associations between clinical and behavioral phenotype measures.
| r value | p value | |
|---|---|---|
| SYMQ × GUPI pain | 0.698 | < 0.001 |
| SYMQ × GUPI urinary | 0.429 | 0.003 |
| SYMQ × MPQ sensory | 0.526 | < 0.001 |
| MPQ sensory × GUPI pain | 0.587 | < 0.001 |
| MPQ sensory × GUPI urinary | 0.556 | < 0.001 |
| MPQ affective × GUPI pain | 0.307 | 0.04 |
| MPQ affective × GUPI urinary | 0.309 | 0.039 |
| HADS Anx × HADS Dep | 0.458 | 0.002 |
| HADS Anx × PANAS Pos | −0.386 | 0.009 |
| HADS Anx × PANAS Neg | 0.613 | < 0.001 |
| HADS Dep × PANAS Pos | −0.502 | < 0.001 |
| HADS Dep × PANAS Neg | 0.588 | < 0.001 |
| CTES < age 17 × CTES last 3 years | 0.412 | 0.005 |
| SEAR s.e. × SEAR s.c. | 0.68 | < 0.001 |
| SEAR s.e. × SEAR s.r | 0.305 | 0.041 |
| SEAR s.e. × SEAR o.r. | 0.482 | 0.001 |
| SEAR s.c. × SEAR s.r. | 0.377 | 0.011 |
| SEAR s.c. × SEAR o.r. | 0.936 | < 0.001 |
Several significant correlations were observed between clinical and behavioral phenotype measures. Significant correlations (p < 0.05) with Pearson's correlation coefficient (r) greater than or equal to 0.6 were considered “strong” associations, while significant correlations with r values greater than 0.3 but less than 0.6 were considered to be “moderate” associations. Non-significant associations not shown. SYMQ, Symptom Measures Questionnaire; GUPI, Genito-Urinary Pain Index; MPQ, McGill Pain Questionnaire; HADS, Hospital Anxiety and Depression Scale; PANAS, Positive and Negative Affect Score; CTES, Childhood Traumatic Events Scale; SEAR, Self-Esteem and Relationships Scale (s.e., self-esteem; s.c., self-confidence; s.r. quality of sex relationships; o.r., overall quality of relationships).
Table 3.
Associations between demographic measures and seed-ROI functional connectivity.
| Correlations with Age | r value | p value |
|---|---|---|
| PCC - R. Insula × age | 0.33 | < 0.02 |
| PCC - R. Putamen × age | 0.32 | < 0.03 |
| PCC - R. Globus Pallidus × age | 0.37 | < 0.02 |
| PCC - L. DLPFC × age | 0.33 | <0.03 |
| L. Precuneus - R. Precuneus × age | 0.29 | < 0.05 |
| Correlations with BMI | ||
| L. Precuneus - R. Precuneus × BMI | 0.43 | < 0.004 |
| L. Precuneus - L. Precuneus × BMI | 0.44 | < 0.003 |
| L. Precuneus - R. Angular × BMI | 0.38 | < 0.02 |
| L. Precuneus - L. MCC × BMI | 0.6 | < 0.00001 |
Age was correlated with posterior cingulate cortex (PCC) FC to 4 aROIs, and with left precuneus FC to the right precuneus. BMI was correlated with left precuneus FC to 4 aROIs. L. DLPFC, left dorsolateral prefrontal cortex; MCC, left medial cingulate cortex.
3.5.1. PCC - aROI FC Associations with Phenotype Measures
Several positive and negative associations were observed between PCC - aROI FC strengths and phenotype measures (see Table 4A). PCC - left amygdala FC was positively associated with several measures of urologic symptom and pain severity (GUPI, SYMQ, MPQ). PCC - left hippocampus FC was negatively associated with levels of anxiety and depression (HADS). PCC - left putamen FC was positively associated with the experience of childhood (prior to age 17) traumatic events (CTES) and negatively associated with levels of self-confidence and positive overall relationships (SEAR). PCC - right thalamus FC was negatively associated with levels of self-esteem (SEAR).
Table 4A. Associations between ROI-aROI connectivity and phenotype measures (clinical and behavioral).
Clinical and Behavioral Measure Associations with PCC - aROI Connectivity
| Measure / aROI | L. Amygdala | L. Hippocampus | L. Putamen | R. Thalamus |
|---|---|---|---|---|
| GUPI pain | r=0.30, p=0.043 | |||
| GUPI urinary | r=0.37, p=0.012 | |||
| MPQ sensory | r=0.41, p=0.0085 | |||
| SYMQ severity | r=0.32, p=0.035 | |||
| HADS anxiety | r= −0.38, p=0.010 | |||
| HADS depression | r= −0.30, p=0.048 | |||
| CTES prior to age 17 | r=0.33, p=0.029 | |||
| SEAR self confidence | r=−0.31, p=0.044 | |||
| SEAR self esteem | r=−0.29, p=0.049 | |||
| SEAR relationships | r=−0.34, p=0.024 |
3.5.2. Left precuneus - aROI FC Associations with Phenotype Measures
Several positive and negative associations were observed between left precuneus - aROIs FC strengths and phenotype measures (see Table 4B). Left precuneus - left OFC FC was positively associated with urologic symptom severity, while left precuneus - right OFC FC was negatively associated with urologic symptom severity (GUPI). Left precuneus - right IPL FC and left precuneus – right SPL FC were both positively associated with patient-reported quality of sexual relationships (SEAR). Left precuneus - right precuneus FC was negatively associated with patient-reported levels of self-esteem (SEAR).
Table 4B. Associations between ROI-aROI connectivity and phenotype measures (clinical and behavioral).
Clinical and Behavioral Measure Associations with Left Precuneus - aROI Connectivity
| Measure / aROI | L. OFC | R. OFC | R. SPL | R. IPL | R. Precuneus |
|---|---|---|---|---|---|
| GUPI urinary | r=0.31, p=0.042 | r= −0.30, p=0.047 | |||
| SEAR sex relation. | r=0.32, p=0.038 | r=0.35, p=0.020 | |||
| SEAR self esteem | r=−0.30, p=0.042 |
Associations between phenotype measures and aROI to A) PCC seed ROI and B) left precuneus seed ROI connections are shown. All correlations shown are p < 0.05, not corrected for multiple comparisons. See text for complete list of tested phenotype measures. SYMQ, Symptom Measures Questionnaire; MPQ, McGill Pain Questionnaire; GUPI, Genito-Urinary Pain Index; HADS, Hospital Anxiety and Depression Scale; CTES, Childhood Traumatic Event Scale; SEAR, Self Esteem and Relationships. R. and L. OFC, right and left orbitofrontal cortex; R. SPL, right superior parietal lobe, R. IPL, right inferior parietal lobe.
4. Discussion
4.1. Altered Regional PMC-DMN Functional Connectivity in UCPPS
Through the combined efforts of a multi-site, NIDDK-sponsored initiative for the study of urologic chronic pelvic pain syndrome, we have identified significantly decreased FC between regions of the PMC and the DMN in a large sample of female patients with UCPPS. Further, the specific regions within the PMC, the PCC and left precuneus, exhibit significantly altered FC to multiple regions outside of the DMN as well. Specifically, we observed that the PCC is decoupled from the DMN and instead appears to join with other brain regions implicated in pain, sensory, motor, and emotion regulatory processes (e.g., insula, putamen, amygdala, and hippocampus). Additionally, we observed that a region within the left precuneus is similarly decoupled from the DMN, as well as from regions within the prefrontal and parietal lobes. Through our use of a whole brain, data-driven analysis, we offer a pattern of altered RS activity that ties together previous findings of altered RS activity within the insular and parietal cortices [5; 19; 35] and regions of altered gray matter structure within the thalamus, hippocampus, amygdala, insula, cingulate cortex, and putamen [2; 3; 34].
Complimentary to previous findings in chronic pelvic pain populations, we have identified a multi-region pattern of altered FC in UCPPS, centered around the PCC and left precuneus, and including regions involved in pain processing and emotion regulation. Urological chronic pelvic pain syndrome is characterized by pain in the pelvic region accompanied by urological symptoms (e.g., bladder pressure, urgency to void, increased frequency of urination) [10]. It often occurs with unknown etiology [8]. Altered CNS structure and function occurs in patients with chronic pelvic pain, indicating that these changes may play a role in the pathophysiology of pain and urologic symptoms [2; 19]. For example, altered FC within the insular cortex is correlated with pain intensity in males with chronic prostatitis [19]. In females with chronic pelvic pain, alterations in the lowest RS frequency band (0.01-0.027 Hz) occur within the sensory, motor, and insular cortices, and these regions demonstrate altered connectivity to the midbrain, cerebellum, and parietal lobe [35]. Anatomically, decreased gray matter density within the thalamus, cingulate cortex, putamen, and insula is observed in females with painful endometriosis [2], while increased gray matter density within the somatosensory cortex, amygdala, and hippocampus is observed in females with chronic pelvic pain [3; 34].
Our correlation analyses between FC strength and phenotype measures support the behavioral significance of altered FC between the PCC and precuneus regions. Particularly, our observation of increased PCC - left amygdala FC in patients was associated with increased pain and urinary symptom severity. This association may reflect enhanced levels of prevention [31] [52] and fear-avoidance behaviors [45; 57] in UCPPS as part of the chronic pain experience [30]. The hippocampus is implicated in default mode network processes and is functionally connected to the PCC [28]. Therefore it is not surprising that we observed increased FC of the PCC to the hippocampus in our patient group. This connection is decreased in Alzheimer's disease and is related to dysfunctional episodic memory and consolidation [28]. Conversely, this connection is increased in UCPPS, perhaps reflecting increased processes of memory consolidation related to the pain experience. The negative correlation of hippocampus - PCC FC with behavioral measures of anxiety and depression suggests a within group (among patients) effect.
Both the PCC and precuneus regions have been described as functional “hubs” or core regions of the DMN [24; 44; 55]. Thus, our primary observation of decreased FC of the PCC and left precuneus to the DMN, suggests some general DMN dysfunction in UCPPS. DMN connectivity is altered in several psychopathological states (for review: [58]) and in chronic pain, including chronic low back pain [4; 38; 47], complex regional pain syndrome [11], and fibromyalgia. For example, increased FC between the DMN and the right insular cortex occurs in fibromyalgia and can be partially reversed by effective therapeutics [29; 40; 41]. Interestingly, the level of connectivity between the precuneus and DMN has been described previously as reflecting the level of engagement with one's surroundings [55]. Following this notion, our findings may suggest a similar state of disengagement and increased introspection in UCPPS. In contrast to previous neuroimaging studies of chronic pelvic pain, we did not find significant changes in the sensory-motor network. We also did not observe altered FC to regions of the somatosensory or motor cortices in our follow-up PCC and left precuneus seed-based analyses. However, this may have been due to differences in our analysis approach since, rather than being directly influenced by DMN connections, somatosensory and motor regions may alternatively be affected via top-down influences of the insular and anterior cingulate cortices [17; 39]. Regions of the PMC, including the PCC and precuneus, are generally implicated in self-referential thought processes [14; 18]. Our observations of increased PCC FC to regions related to aspects of pain processing and emotion suggest the integration of these self-referential thought processes with the chronic pain experience. For example, our observation of increased PCC - anterior insular cortex FC, may reflect integration of self-referential thought processes with emotional valence of pain [46].
Chronic pain, and pelvic pain syndromes especially, are linked to sociopsychological issues and history of childhood traumatic events, and are exacerbated by stressful relationships and situations [25; 26; 42]. We observed that increased PCC FC to several regions was related to decreased levels of self-confidence, self-esteem and quality of relationships and increased experience of childhood traumatic events. These observations may therefore represent an increased integration of stress and self-devaluation with one's neural processes of self-representation [43]. We also observed interesting positive correlation between the FC of the left precuneus to right SPL and right IPL regions and quality of sex relationships. Thus, while in the HC group the left precuneus is normally anticorrelated with these regions, in patients this anticorrelation is extinguished and may be related to lower quality of sex relationships. Ultimately, the suggested implications of these behavioral correlations with altered PCC and left precuneus FC should be interpreted cautiously because they were observed through secondary exploratory analyses.
4.2. Limitations
As a multi-site study, we used standardized scan protocols and rigorous quality control standards across sites, but additional sources of between-site variability may still exist. However, results from a subsequent analysis with site effects regressed were the same as those presented here (without site regressed). (Supplementary Fig. 4.) Our patient population excluded major comorbid conditions (FM, IBS, CFS). Future studies will need to determine whether our findings are generalizable to chronic pain patients with mixed symptomology. Previous neuroimaging studies of chronic visceral pain indicate differences in RS brain activity based on sex [32], therefore our findings in a female population might not generalize to males. We assessed 17 validated RS networks for differences in FC, and this may be argued as a non-specific approach. However, strikingly, the PCC and left precuneus were the only regions of significant difference found. Thus, these regions may represent the most robust alterations across RS networks in UCPPS. In contrast to our observation of decreased left precuneus to vmPFC FC in our patient population, increased FC of DMN regions the PCC / precuneus to the medial prefrontal cortex has been linked to enhanced levels of rumination in patients with temporomandibular disorder [36]. However, the precuneus region we identified was at a location superior within the posterior medial cortex, as compared with the PCC / precuneus region identified previously, which may have contributed to these different findings in two distinct chronic pain populations. Lastly, our exploratory correlation analyses of phenotype measures and seed ROI - aROI FC were intentionally broad and not corrected for multiple comparisons, therefore, our observed associations warrant further investigation as a priori hypotheses.
4.3. Conclusions
Our findings contribute to the growing body of evidence indicating that altered neurological function plays a role in UCPPS. Decreased FC among DMN hubs of the PCC and precuneus indicates an overall emotional disconnectedness from a patient's surroundings.
Meanwhile, increased FC between the PCC and several regions outside of the DMN suggests a shift of introspective and self-referential thought towards affective and sensory dimensions of pain and emotion regulation. Although our UCPPS patient population excluded major comorbid conditions, our findings complement previous neuroimaging findings in chronic pain, including fibromyalgia, chronic low back pain and chronic visceral pain. Thus, the intertwined neurological processes of self-referential thought, sensory and affective dimensions of pain, and emotion regulation may be relevant to chronic pain in general.
Supplementary Material
5. Acknowledgements
Thanks to Cody Ashe-McNally for his technical expertise in coordinating and running the cross-site quality control of all MAPP Research Network neuroimaging data. Special thanks to Jeff Alger for his expertise as a physicist at UCLA in oversight and coordination of the multi-site collection of neuroimaging data.
Support:
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316). This study was supported by additional NIH grants (K24 DA29262, T32 GM89626) and the Redlich Pain Research Endowment.
Footnotes
Conflict of Interest Statement:
Dr. Daniel Clauw declares receipt of grants from Pfizer, Eli Lilly, UCB, Astra Zeneca, Merck, J &J, Nuvo, Jazz, Abbott, Cerephex, Iroko, Tonix, Theravance, IMC, Zynerba, Sammumed, and receipt of consulting fees or honorarium from Pfizer, Cypress, Biosciences, Forest, Merck, Nuvo, and Cerephex. Dr. Harris declares consultancy for Pfizer Inc. Dr. Greicius declares SBGneuro stock/stock options. All other authors have no conflicts of interest to declare.
References
- 1.Apkarian AV. Cortical pathophysiology of chronic pain. Novartis Found Symp. 2004;261:239–245. discussion 245-261. [PubMed] [Google Scholar]
- 2.As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Vania Apkarian A, Mayer EA, Clauw DJ, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP Network Study. Pain. 2014 doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9(9):e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain. 2014;15(2):197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckmann CF, Mackay CE, Filippini N, S.M. S. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. OHBM. 2009 [Google Scholar]
- 8.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of urology. 2011;186(2):540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 10.Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. The Journal of urology. 2007;177(2):450–456. doi: 10.1016/j.juro.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Bolwerk A, Seifert F, Maihofner C. Altered resting-state functional connectivity in complex regional pain syndrome. J Pain. 2013;14(10):1107–1115. e1108. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. Journal of neurology, neurosurgery, and psychiatry. 2010;81(7):806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 14.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 15.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC urology. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 17.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. Journal of cognitive neuroscience. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 18.Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, Chang C, Greicius MD, Menon V, Kennedy DP, Parvizi J. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci U S A. 2011;108(7):3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. The Journal of urology. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippini N, Rao A, Wetten S, Gibson RA, Borrie M, Guzman D, Kertesz A, Loy-English I, Williams J, Nichols T, Whitcher B, Matthews PM. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer's disease. Neuroimage. 2009;44(3):724–728. doi: 10.1016/j.neuroimage.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 23.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Fry RP, Beard RW, Crisp AH, McGuigan S. Sociopsychological factors in women with chronic pelvic pain with and without pelvic venous congestion. Journal of psychosomatic research. 1997;42(1):71–85. doi: 10.1016/s0022-3999(96)00231-0. [DOI] [PubMed] [Google Scholar]
- 26.Ginting JV, Tripp DA, Nickel JC, Fitzgerald MP, Mayer R. Spousal support decreases the negative impact of pain on mental quality of life in women with interstitial cystitis/painful bladder syndrome. BJU international. 2011;108(5):713–717. doi: 10.1111/j.1464-410X.2010.09846.x. [DOI] [PubMed] [Google Scholar]
- 27.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 30.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins ET. Beyond pleasure and pain. The American psychologist. 1997;52(12):1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- 32.Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33(29):11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis and rheumatism. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Interstitial Cystitis/Painful Bladder Syndrome Patients. The Journal of urology. 2014 doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff B, Labus J, Jiang Z, Farmer M, Apkarian AV, Mackey S, Martucci KT, Clauw D, Harris RE, Deutsch G, Ness T, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity within sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2014 doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34(11):3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ. The MAPP research network: design, patient characterization and operations. BMC urology. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Zhang JH, Yi T, Tang WJ, Wang SW, Dong JC. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupuncture in medicine : journal of the British Medical Acupuncture Society. 2014;32(2):102–108. doi: 10.1136/acupmed-2013-010423. [DOI] [PubMed] [Google Scholar]
- 39.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and rheumatism. 2012;64(7):2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. The Journal of urology. 2010;184(4):1358–1363. doi: 10.1016/j.juro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose MJ, Klenerman L, Atchison L, Slade PD. An application of the fear avoidance model to three chronic pain problems. Behaviour research and therapy. 1992;30(4):359–365. doi: 10.1016/0005-7967(92)90047-k. [DOI] [PubMed] [Google Scholar]
- 46.Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64(7):1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- 47.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134(Pt 8):2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 49.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 52.Strauman TJ, Detloff AM, Sestokas R, Smith DV, Goetz EL, Rivera C, Kwapil L. What shall I be, what must I be: neural correlates of personal goal activation. Frontiers in integrative neuroscience. 2012;6:123. doi: 10.3389/fnint.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 54.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate Classification of Structural MRI Data Detects Chronic Low Back Pain. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, Soddu A, Perlbarg V, Ledoux D, Brichant JF, Moonen G, Maquet P, Greicius MD, Laureys S, Boly M. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 58.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 59.Worsley KJ. Jezzard P, Matthews PM, Smith SM, editors. Statistical Analysis of Activation Images. Functional MRI: An Introduction to Methods. 2001 [Google Scholar]
- 60.Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage. 2011;55(1):24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.