Abstract
Dopamine and acetylcholine are two principal transmitters in the striatum and are usually balanced to modulate local neural activity and maintain striatal homeostasis. In this study, we investigated the role of dopamine and muscarinic acetylcholine receptors in the regulation of a central signaling protein, i.e., the mitogen-activated protein kinase (MAPK). We focused on the synaptic pool of MAPKs based on the fact that these kinases reside in peripheral synaptic structures in addition to their somatic locations. We found that a systemic injection of a dopamine D1 receptor (D1R) agonist SKF81297 enhanced phosphorylation of extracellular signal-regulated kinases (ERKs), a prototypic subclass of MAPKs, in the adult rat striatum. Similar results were observed in another dopamine responsive region, the medial prefrontal cortex (mPFC). The dopamine D2 receptor agonist quinpirole had no such effects. Pretreatment with a positive allosteric modulator (PAM) of muscarinic acetylcholine M4 receptors (M4Rs), VU0152100, attenuated the D1R agonist-stimulated ERK phosphorylation in the two regions, while the PAM itself did not alter basal ERK phosphorylation. All drug treatments had no effect on phosphorylation of c-Jun N-terminal kinases (JNKs), another MAPK subclass, in the striatum and mPFC. These results demonstrate that dopamine and acetylcholine are integrated to control synaptic ERK, although not JNK, activation in striatal and mPFC neurons in vivo. Activation of M4Rs exerts an inhibitory effect on the D1R-mediated upregulation of synaptic ERK phosphorylation.
Keywords: Basal ganglia; M4 receptor; D1 dopamine receptor; extracellular signal-regulated kinase; c-Jun N-terminal kinase; stress-activated protein kinase; RRID, AB_476693; RRID, AB_331646; RRID, AB_10695746; RRID, AB_2140557; RRID, AB_10693936; RRID, nif-0000-30467
Graphical Abstract
The mitogen-activated protein kinase (MAPK) is expressed in postmitotic neurons of adult mammalian brains (Nozaki et al., 2001). As key serine/threonine protein kinases, MAPKs play a central role in cell signaling and synaptic plasticity and are linked to various neurological and psychiatric disorders (Sweatt, 2004; Thomas and Huganir, 2004; Wang et al., 2007). At least three subfamilies of MAPKs have been identified, which are extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) also known as stress-activated protein kinases, and p38 MAPKs (Pearson et al., 2001; Volmat and Pouyssegur, 2001). All of these MAPKs are activated through a module cascade involving initial activation of small GTPases (Ras or Rac) and subsequent three-tiered protein kinase systems.
ERKs represent prototypic MAPKs and have been most extensively investigated in neural activity. Traditionally, ERKs translocate into the nucleus following activation and there they activate transcription factors to regulate gene expression (Treisman, 1996). Additionally, ERKs reside in neuronal peripheries, such as postsynaptic dendritic spines (Ortiz et al., 1995; Boggio et al., 2007; Casar et al., 2009), as an important sub-pool of the kinases at synaptic sites sensitive to changing synaptic input and implicated in the regulation of synaptic transmission and plasticity. Clearly, the ERK's territory covers both nuclear and synaptic locations.
ERKs are sensitive substrates of psychostimulants and are important for drug action (Wang et al., 2007). The two common stimulants (cocaine and amphetamine) consistently increased ERK phosphorylation in the striatum in a number of early studies (Choe et al., 2002; Choe and Wang, 2002; Zhang et al., 2004; Jenab et al., 2005; Valjent et al., 2000; 2004; 2005; 2006). Selective activation of dopamine D1 receptors (D1Rs) with the D1R agonist also elevated ERK phosphorylation in the striatum and dentate gyrus (Gangarossa and Valjent, 2012; Gangarossa et al., 2011; 2013). Recently, amphetamine was demonstrated to activate a specific pool of ERKs, i.e., the synaptic ERKs, in the striatum (Mao et al., 2013). Thus, ERKs are readily activated by dopamine stimulants, although exact roles of D1Rs and dopamine D2 receptors (D2Rs), two subtypes enriched in the striatum, in linking dopamine to synaptic ERKs are less clear.
Acetylcholine is a principal transmitter in the striatum in addition to dopamine (Bolam et al., 1984). This transmitter provides the region with a strategic drive to balance dopamine and maintain local homeostasis. As such, activation of muscarinic cholinergic receptors, a predominant subfamily of cholinergic receptors in the striatum, suppressed motor responses to dopamine stimulation, while blockade of muscarinic receptors augmented them (Chou et al., 1992; Morelli et al., 1993; Wang and McGinty, 1997). Non-selective muscarinic receptor antagonists (atropine or scopolamine) also enhanced the efficiency of dopamine in stimulating gene expression in the striatum (Chou et al., 1992; Bernard et al., 1993; Morelli et al., 1993; Wang and McGinty, 1996; 1997). However, at present, whether acetylcholine interacts with dopamine to regulate ERKs in the synaptic location remains elusive. Moreover, the muscarinic receptor subtype responsible for these events needs further investigation. In this regard, the muscarinic M4 receptor (M4R) subtype is particularly intriguing. These Gi-coupled receptors are co-expressed with Gs-coupled D1Rs in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001) and are thought to antagonize D1R activity, although whether M4Rs interact with D1Rs to regulate synaptic ERK phosphorylation is unclear.
Here we conducted a series of experiments to clarify the role of dopamine-muscarinic interplay in regulating ERKs at defined synaptic sites in two primary dopamine responsive regions, the striatum and medial prefrontal cortex (mPFC). We first assessed effects of D1R and D2R agonists on ERK phosphorylation. We then examined effects of a recently available positive allosteric modulator (PAM) for M4Rs on constitutive ERK phosphorylation. Finally, the effect of the M4R PAM on the D1R agonist-stimulated ERK phosphorylation was investigated. In parallel, synaptic JNK phosphorylation was tested in all experiments as comparisons.
MATERIALS AND METHODS
Animals
We used adult male Wistar rats weighing 275-335 g (Charles River, New York, NY). Animals were housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% on a 12-h/12-h light/dark cycle with food and water available ad libitum. Rats were allowed 6-7 days of habituation to the animal colony before pharmacological experiments. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Drug administration and experimental arrangement
All pharmacological agents used in this study were administered via an intraperitoneal (i.p.) injection. A set of three pharmacological studies were carried out. In the first study, we investigated effects of D1R and D2R stimulation on synaptic ERK1/2 and JNK phosphorylation in three groups of rats. These rats received a single dose of saline, the D1R agonist SKF81297 (3 mg/kg), or the D2R agonist quinpirole (3 mg/kg). They were then sacrificed 20 min after drug injection for immunoblot analysis of changes in ERK1/2 and JNK phosphorylation in the striatum and mPFC. In the second study, we examined effects of M4R stimulation on ERK1/2 and JNK phosphorylation. Three groups of rats were treated with vehicle or VU0152100, a systemically active PAM of M4Rs (Brady et al., 2008), at 6 or 60 mg/kg (20 min prior to brain tissue collection). Finally, we tested effects of VU0152100 on the D1R agonist-induced ERK1/2 phosphorylation. Three groups of rats were treated with vehicle + saline, vehicle + SKF81297 (3 mg/kg), and VU0152100 (60 mg/kg) + SKF81297 (3 mg/kg). The first injection was made 20 min prior to the second injection and rats were sacrificed 20 min after the second drug injection. Doses of drugs were calculated as the salt. The effectiveness of chosen doses following a systemic injection has been well demonstrated in previous behavioral and neurochemical studies conducted in this and other laboratories (Wang and McGinty, 1996; Zhang et al., 2007; Brady et al., 2008).
Synaptosomal protein preparation
Rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and decapitated. After rat brains were removed, coronal slices were cut. From slices, the entire striatum containing the caudate putamen and nucleus accumbens and the mPFC which included the anterior cingulate, prelimbic cortex and infralimbic cortex were dissected for following synaptosomal membrane preparations at 4°C. Striatal and mPFC tissues were homogenized in isotonic sucrose homogenization buffer (SHB) containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease/phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). These homogenates were centrifuged at 800 g (10 min). We collected the supernatant and had it centrifuged at 10,000 g (30 min) to obtain crude synaptosomal plasma membranes in the pellet. The pellet was washed once with 1 volume of SHB and centrifuged at 10,000 g (15 min). We then resuspended and solubilized the final synaptosomal pellet which contained both presynaptic and postsynaptic membranes in SHB containing 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS), 1% deoxycholic acid, 1 mM dithiothreitol and the protease/phosphatase inhibitor cocktail with gentle rotation (1 h, 4°C). Protein concentrations were determined. Samples were stored at −80°C until use.
Western blot analysis
Immunoblots were performed as described previously (Yang et al., 2006; Zhang et al., 2007). Briefly, the equal amount of protein was loaded and separated on SDS NuPAGE Novex 4-12% gels (Invitrogen, Carlsbad, CA). Proteins were transferred to the polyvinylidene fluoride membrane (Millipore, Bedford, MA). The membrane was blocked in a blocking buffer (3% nonfat dry milk in phosphate-buffered saline (PBS) and 0.1% Tween-20) for 1 h, followed by incubation in the blocking buffer containing a primary antibody overnight at 4°C. The membrane was then incubated in a horseradish peroxidase-linked secondary antibody (Jackson Immunoresearch Laboratory, West Grove, PA) for 1 h at 1:5,000. Immunoblots were developed with the enhanced chemiluminescence reagents (GE Healthcare Life Sciences, Piscataway, NJ). MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. X-ray film images of all immunoblots were measured using NIH ImageJ analysis software (RRID: nif-0000-30467). β-Actin was used as a loading control in Western blot analysis.
Antibody characterization
Table I listed all primary antibodies used in the present study. These antibodies include rabbit polyclonal antibodies against phosphorylated ERK1/2 (pERK1/2), ERK1/2, phosphorylated JNK (pJNK), JNK, or β-actin. The antibody against β-actin was purchased from Sigma-Aldrich (St. Louis, MO) and other antibodies were purchased from Cell Signaling Technology (Beverly, MA). The antibody against pERK1/2 recognizes ERK1/2 proteins phosphorylated at Thr202 and Tyr204 and therefore show two corresponding bands in Western blot from rat striatal and mPFC samples: 42 (pERK2) and 44 (pERK1) kDa bands. The anti-ERK1/2 antibody recognizes ERK1 (44 kDa) and ERK2 (42 kDa) in striatal and mPFC extracts. The antibody against pJNK recognizes JNK proteins phosphorylated at Thr183 and Tyr185. In immunoblots using striatal and mPFC samples, this antibody detected two bands at 46 (pJNK1) and 54 (pJNK2/3) kDa. The anti-JNK antibody recognizes JNK1 at 46 kDa and JNK2/3 at 54 kDa. The β-actin antibody detects a 42 kDa band in our samples as well as in a widely variety of tissues and species in immunoblot analysis. All antibodies have been widely used in research with Western blots. In the absence of primary antibody, incubation of secondary antibody alone at the dilutions employed in the procedure produced no detectable immunoreactivity.
Table I.
Primary Antibodies Used
| Antigen | Description of Immunogen | Source, host species, catalog No., clone or lot No., RRID | Concentration used (μg/ml) | Primary antibody blocking solution |
|---|---|---|---|---|
| β-Actin | C-terminal actin fragment: Ser-Gly-Pro-Ser-Ile-Val-His-Arg-Lys-Cys-Phe | Sigma-Aldrich (St. Louis, MO), rabbit polyclonal, A2066, AB_476693 | 0.2 | 3% Nonfat rrilk-PBS 0.1% Tween-20 |
| pERK1/2 | A synthetic phosphopeptide corresponding to residues surrounding Thr202/Tyr204 of human p44 MAP kinase | Cell Signaling Technology (Beverly, MA), rabbit polyclonal, 9101S, lot No. 28, AB_331646 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| ERK1/2 | A synthetic peptide corresponding to a sequence in the C-terminus of rat p44 MAP Kinase | Cell Signaling Technology, rabbit polyclonal, 9102S, lot No. 23, AB_10695746 | 0.5 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| pJNK | A synthetic phosphopeptide corresponding to residues surrounding Thr183/Tyr185 of human SAPK/JNK. | Cell Signaling Technology, rabbit polyclonal, 9251L, lot No. 21, AB_2140557 | 1 | 3% Nonfat milk-PBS 0.1% Tween-20 |
| JNK | A recombinant human JNK2 protein | Cell Signaling Technology, rabbit polyclonal, 9252S, lot No. 12, AB_10693936 | 0.5 | 3% Nonfat milk-PBS 0.1% Tween-20 |
Pharmacological agents
Pharmacological agents, including (±)-6-chloro-PB hydrobromide (SKF81297) and (-)-quinpirole hydrochloride, were purchased from Sigma-Aldrich. VU0152100 [3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide] was purchased from Axon Medchem (Reston, VA). All agents were freshly prepared at the day of experiments. VU0152100 was dissolved in 10% Tween 80 and dH2O with the pH adjusted to approximately 7.0 using 1 N NaOH. Other agents were dissolved in physiological saline.
Statistics
The results are presented as means ± SEM. The data were evaluated using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. Probability levels of < 0.05 were considered statistically significant.
RESULTS
D1R activation enhanced synaptic ERK phosphorylation
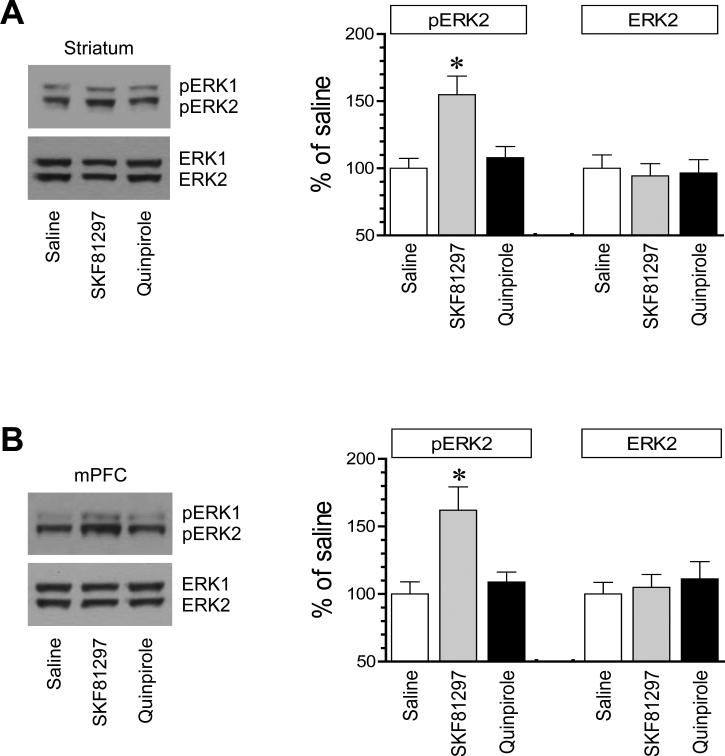
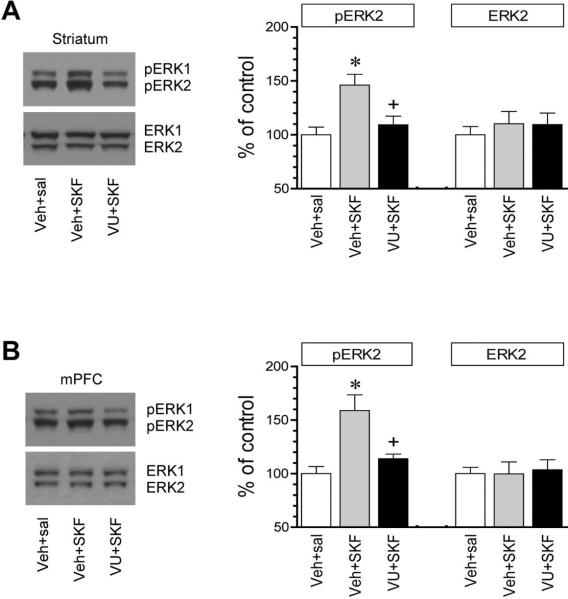
We first investigated the role of two dopamine receptors in regulating ERK phosphorylation in the striatum. We focused on the distinct pool of ERK in the synaptic fraction, i.e., the synaptosomal fraction containing synaptic and immediate extrasynaptic contents. Rats were treated with a single dose of the D1R agonist SKF81297 (3 mg/kg, i.p.) or the D2R agonist quinpirole (3 mg/kg, i.p.). We then sacrificed rats 20 min after drug injection to collect brain tissue for immunoblot analysis. SKF81297 induced a moderate increase in ERK1/2 phosphorylation. In quantification analysis, pERK2 protein levels were significantly higher in SKF81297-treated rats compared to saline-treated rats (Fig. 1A). In contrast, quinpirole did not alter ERK1/2 phosphorylation. The two agonists had no effect on a total amount of ERK1/2 proteins. These data demonstrate that selective D1R activation upregulates ERK1/2 phosphorylation in the synaptic fraction of striatal neurons.
Figure 1. Effects of dopamine receptor agonists on synaptic ERK1/2 phosphorylation in the rat forebrain.
(A) Effects of SKF81297 and quinpirole on ERK1/2 phosphorylation in the striatum. (B) Effects of SKF81297 and quinpirole on ERK1/2 phosphorylation in the mPFC. Two bands were typically observed for pERK1/2 and ERK1/2 signals (upper bands = pERK1 and ERK1 with 44 kDa; lower bands = pERK2 and ERK2 with 42 kDa). Note a significant increase in pERK2 levels in both forebrain regions in SKF81297- but not quinpirole-treated rats. Representative immunoblots are shown left of the quantified data. Rats received a single dose of SKF81297 (3 mg/kg, i.p.) or quinpirole (3 mg/kg, i.p.) and were sacrificed 20 min after drug injection for immunoblot analysis with synaptosomal proteins. The pERK2 data were analyzed with one-way analysis of variance: F(2,15) = 8.3, n = 18, P < 0.05 and F(2,12) = 7.7, n = 15, P < 0.05, respectively in the striatum and mPFC. The ERK2 data were also analyzed with one-way analysis of variance: F(2,15) = 0.92, n = 18, P > 0.05 and F(2,12) = 0.76, n = 15, P > 0.05, respectively in the striatum and mPFC. Data are presented as means ± SEM (n = 5-6 per group). *P < 0.05 versus saline.
The mPFC is another dopamine responsive region in the forebrain and is actively involved in stimulant action (Steketee, 2003; Van den Oever et al., 2010). We thus monitored changes in ERK1/2 phosphorylation in this region. Similar to the striatum, SKF81297 induced a significant increase in pERK1/2 levels, while the agonist did not affect cellular levels of ERK1/2 (Fig. 1B). Quinpirole was still ineffective. Thus, like the striatum, the mPFC is another area where ERK1/2 phosphorylation is sensitive to D1R but not D2R signals.
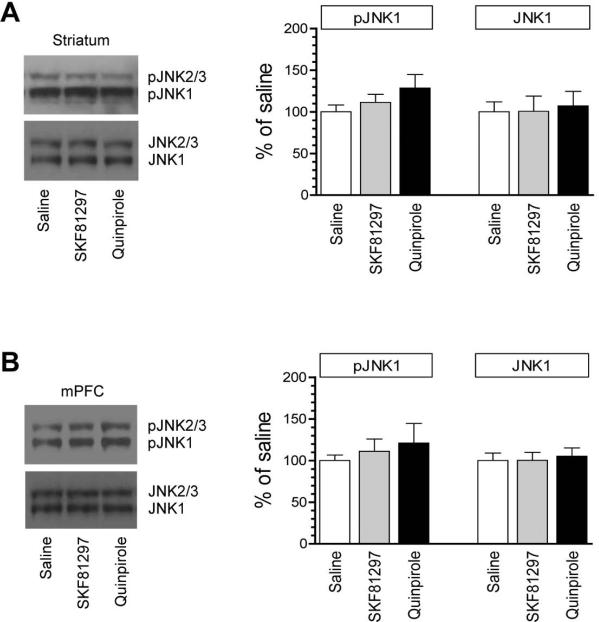
We next analyzed JNK phosphorylation in the same synaptosomal samples. As shown in Fig. 2A, SKF81297 did not alter JNK phosphorylation in the striatum. Neither did quinpirole. In the mPFC, pJNK levels showed a minimal change in response to all drug treatments (Fig. 2B). The total amount of JNK proteins remained stable in the two regions following administration of SKF81297 or quinpirole. Thus, unlike ERK, JNK in the synaptosomal fraction of striatal and mPFC neurons is less sensitive to dopamine stimulation.
Figure 2. Effects of dopamine receptor agonists on synaptic JNK phosphorylation in the rat forebrain.
(A) Effects of SKF81297 and quinpirole on JNK phosphorylation in the striatum. (B) Effects of SKF81297 and quinpirole on JNK phosphorylation in the mPFC. Two bands were usually seen for pJNK and JNK signals (upper bands = pJNK2/3 and JNK2/3 with 54 kDa; lower bands = pJNK1 and JNK1 with 46 kDa). Representative immunoblots are shown left of the quantified data. Rats received a single dose of SKF81297 (3 mg/kg, i.p.) or quinpirole (3 mg/kg, i.p.) and were sacrificed 20 min after drug injection for immunoblot analysis with synaptosomal proteins. The pJNK1 data were analyzed with one-way analysis of variance: F(2,15) = 0.27, n = 18, P > 0.05 and F(2,12) = 0.67, n = 15, P > 0.05, respectively in the striatum and mPFC. The JNK1 data were also analyzed with one-way analysis of variance: F(2,15) = 0.91, n = 18, P > 0.05 and F(2,12) = 0.90, n = 15, P > 0.05, respectively in the striatum and mPFC. Data are presented as means ± SEM (n = 5-6 per group).
M4R activation unaffected ERK and JNK phosphorylation
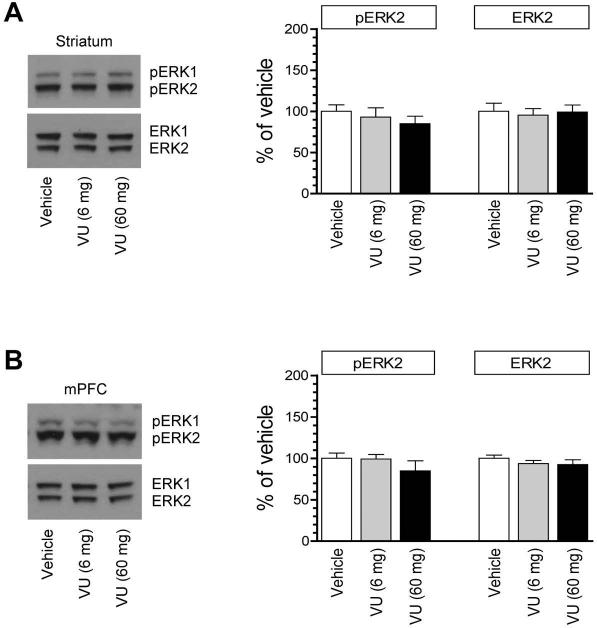
Acetylcholine is a primary transmitter in the striatum in addition to dopamine. Through activating the M4R, a major muscarinic cholinergic receptor subtype in the striatum that is predominantly co-expressed with D1Rs in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001), acetylcholine balances dopamine input and contributes to maintaining the homeostasis of striatal neurons. To determine the participation of M4Rs in synaptic ERK activity, we examined effects of VU0152100, a centrally active PAM of M4Rs (Brady et al., 2008), on ERK1/2 phosphorylation in synaptic samples. A VU0152100 injection at 6 mg/kg (i.p.) did not alter basal levels of pERK1/2 in the striatum (Fig. 3A). VU0152100 at a higher dose (60 mg/kg) still showed no significant effect. Similar responses of ERK phosphorylation were observed in the mPFC (Fig. 3B). Total ERK1/2 abundance in the two areas was not altered by VU0152100. These findings indicate that pharmacological activation of M4Rs by an exogenous PAM has a limited influence over ERK1/2 phosphorylation in both striatal and mPFC neurons under normal conditions. The high subtype selectivity of VU0152100 was demonstrated in early studies in which VU0152100 (56.6 mg/kg, i.p.) markedly blocked motor responses to dopamine stimulation in wild type rats and mice but not in M4R knockout mice (Brady et al., 2008; Byun et al., 2014).
Figure 3. Effects of the M4R PAM on synaptic ERK1/2 phosphorylation in the rat forebrain.
(A) Effects of VU0152100 (VU) on ERK1/2 phosphorylation in the striatum. (B) Effects of VU0152100 on ERK1/2 phosphorylation in the mPFC. Representative immunoblots are shown left of the quantified data. Rats received a single dose of VU0152100 (6 or 60 mg/kg, i.p.) and were sacrificed 20 min after drug injection for immunoblot analysis with synaptosomal proteins. The pERK2 data were analyzed with one-way analysis of variance: F(2,9) = 0.56, n = 12, P > 0.05 and F(2,9) = 0.41, n = 12, P > 0.05, respectively in the striatum and mPFC. The ERK2 data were also analyzed with one-way analysis of variance: F(2,9) = 0.93, n = 12, P > 0.05 and F(2,9) = 0.48, n = 12, P > 0.05, respectively in the striatum and mPFC. Data are presented as means ± SEM (n = 4 per group).
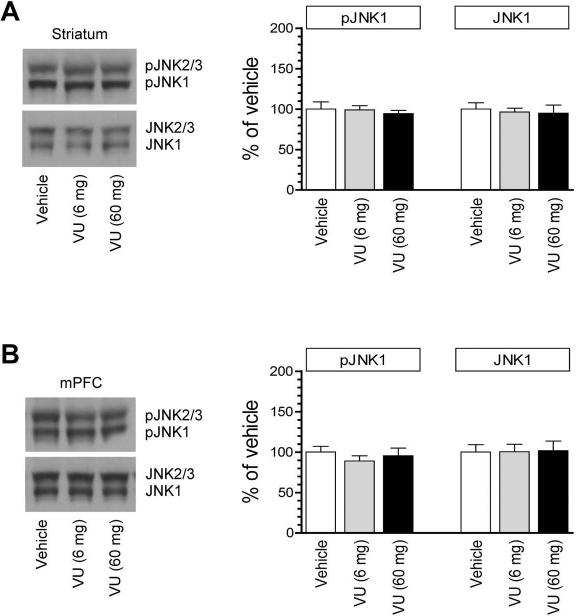
We next examined the effect of M4R activation on synaptic JNK phosphorylation. VU0152100 at the two doses did not affect pJNK levels in the striatum (Fig. 4A). Similarly, the PAM at either dose had little effects on JNK phosphorylation in the mPFC (Fig. 4B). Cellular levels of JNK remained unchanged in response to VU0152100. Thus, activation of M4Rs by VU0152100 is unable to alter the synaptic pool of JNK in its constitutive phosphorylation.
Figure 4. Effects of the M4R PAM on synaptic JNK phosphorylation in the rat forebrain.
(A) Effects of VU0152100 (VU) on JNK phosphorylation in the striatum. (B) Effects of VU0152100 on JNK phosphorylation in the mPFC. Representative immunoblots are shown left of the quantified data. Rats received a single dose of VU0152100 (6 or 60 mg/kg, i.p.) and were sacrificed 20 min after drug injection for immunoblot analysis with synaptosomal proteins. The pJNK1 data were analyzed with one-way analysis of variance: F(2,9) = 0.81, n = 12, P > 0.05 and F(2,9) = 0.62, n = 12, P > 0.05, respectively in the striatum and mPFC. The JNK1 data were also analyzed with one-way analysis of variance: F(2,9) = 0.89, n = 12, P > 0.05 and F(2,9) = 0.99, n = 12, P > 0.05, respectively in the striatum and mPFC. Data are presented as means ± SEM (n = 4 per group).
M4R activation suppressed SKF81297-stimulated ERK phosphorylation
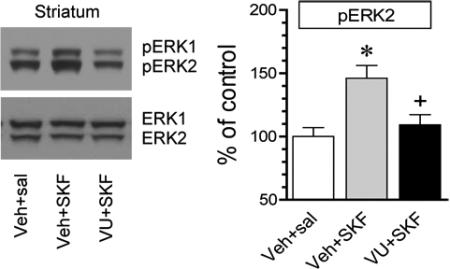
To determine the effect of VU0152100 on the D1R-stimulated ERK phosphorylation, we subjected rats to an injection of VU0152100 (60 mg/kg, i.p.) 20 min prior to SKF81297 (3 mg/kg, i.p.) and sacrificed rats 20 min after SKF81297 administration. Interestingly, VU0152100 showed a significant impact on the SKF81297 action in stimulating ERK phosphorylation. A prior injection of VU0152100 blocked the increase in ERK1/2 phosphorylation induced by SKF81297 in the synaptosomal fraction of striatal neurons (Fig. 5A). Similarly in the mPFC, SKF81297 no longer elevated ERK1/2 phosphorylation in rats pretreated with VU0152100 relative to rats pretreated with vehicle (Fig. 5B). Clearly, activation of VU0152100-sensitive M4Rs counteracts D1Rs in stimulating ERK1/2 phosphorylation in the synaptic location in both brain regions.
Figure 5. Effects of VU0152100 on D1R agonist-stimulated ERK1/2 phosphorylation in the rat forebrain.
(A) Effects of VU0152100 (VU) on SKF81297 (SKF)-stimulated ERK1/2 phosphorylation in the striatum. (B) Effects of VU0152100 on SKF81297-stimulated ERK1/2 phosphorylation in the mPFC. Note that VU0152100 reduced the SKF81297-stimulated ERK1/2 phosphorylation in both regions. Representative immunoblots are shown left to the quantified data. Rats were given a single injection of vehicle (Veh) or VU0152100 (60 mg/kg, i.p.) 20 min prior to saline (sal) or SKF81297 (3 mg/kg, i.p.) and sacrificed 20 min after drug injection for immunoblot analysis with synaptosomal proteins. The pERK2 data were analyzed with one-way analysis of variance: F(2,9) = 8.3, n = 12, P < 0.05 and F(2,9) = 10.1, n = 12, P < 0.05, respectively in the striatum and mPFC. The ERK2 data were also analyzed with one-way analysis of variance: F(2,9) = 0.74, n = 12, P > 0.05 and F(2,9) = 0.95, n = 12, P > 0.05, respectively in the striatum and mPFC. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus vehicle + saline. +P < 0.05 versus vehicle + SKF81297.
DISCUSSION
We investigated the role of dopamine and muscarinic acetylcholine receptors in the modulation of MAPK phosphorylation in the dopamine responsive regions of adult rat brains in vivo. The synaptic fraction of MAPKs was the focus of entire study. We found that activation of D1Rs by a systemic injection of a D1R agonist SKF81297 increased synaptic ERK1/2 phosphorylation in the striatum and mPFC, while the D2R agonist quinpirole did not. Activation of M4Rs by an M4R activator VU0152100 attenuated the ERK1/2 phosphorylation induced by SKF81297. Both dopamine and muscarinic receptor agents had no effect on JNK phosphorylation in the two regions. These data indicate that the synaptic pool of ERK1/2 but not JNK is regulated by dopamine and acetylcholine. The two transmitters seem to oppositely control ERK1/2 via a mechanism involving the D1R-M4R interplay.
A large number of early studies have documented the stimulating effect of dopamine indirect agonists such as cocaine and amphetamine on ERK1/2 phosphorylation in the striatum (Choe et al., 2002; Choe and Wang, 2002; Zhang et al., 2004; Jenab et al., 2005; Valjent et al., 2000; 2004; 2005; 2006; Sun et al., 2007). Most of these studies have targeted nuclear ERK1/2 with immunohistochemistry or have used whole cell homogenates in immunoblots. Recently, ERK1/2 phosphorylation in crude synaptosomal membranes of the rat prefrontal cortex or synaptic membranes of the rat striatum and mPFC was observed in response to cocaine (Fumagalli et al., 2009) or amphetamine (Mao et al., 2013). These findings identify the synaptic structure as a sensitive site where ERK positively responds to dopamine signals. Since the D1R agonist like stimulants readily activated synaptic ERK (this study), the stimulant-induced ERK1/2 phosphorylation is believed to be mediated through a signaling pathway initiated by D1Rs (Valjent et al., 2000; Bertran-Gonzalez et al., 2008; Shi and McGinty, 2011).
An important finding in this study is that the M4R acts as an essential regulator of ERK1/2 phosphorylation at synaptic sites. In the striatum, the M4R is a principal muscarinic receptor subtype and is known to co-express with D1Rs in striatonigral projection neurons (Ince et al., 1997; Santiago and Potter, 2001). Given that D1Rs upregulate ERK1/2 phosphorylation, M4Rs are likely to downregulate it based on numerous neurochemical and behavioral studies which show a delicate balance between dopamine and acetylcholine, i.e., usually the antagonistic relationship, in controlling striatal neuronal activity and behavior (Di Chiara et al., 1994). Indeed, our data support this likelihood. Activation of M4Rs with a subtype-selective M4R PAM suppressed ERK1/2 responses to the D1R agonist. Thus, the dopamine-acetylcholine balance works well in our model in which local M4Rs inhibit D1Rs in activation of ERK1/2. Of note, the dopamine-acetylcholine balance in the regulation of ERK1/2 was largely activity-dependent since the M4R PAM had no significant effect on constitutive ERK1/2 phosphorylation under normal conditions. In addition to M4Rs, muscarinic M1 receptors are expressed in striatal neurons (Di Chiara et al., 1994). Their roles in regulating synaptic ERK activity can be investigated in future studies.
The mPFC is another dopamine responsive area where acetylcholine regulates ERK1/2 phosphorylation. This six-layered structure (I-VI) is innervated by dopaminergic fibers primarily from the ventral tegmental area and by diffuse cholinergic afferents primarily from basal forebrain cholinergic neurons, whereas massive mPFC glutamatergic efferents project to the striatum in a topographical manner (Heidbreder and Groenewegen, 2003; de Almeida et al., 2008; Henny and Jones, 2008; Bloem et al., 2014). D1Rs were present in pyramidal neurons in all layers (Bergson et al., 1995; de Almeida et al., 2008), while M4Rs also localize to mPFC neurons (Levey et al., 1991; Volpicelli and Levey, 2004). Thus, acetylcholine through activating M4Rs is believed to affect dopamine input to control mPFC neurons. In fact, we found that activation of M4Rs inhibited the efficacy of D1Rs in activating ERK1/2. This was seen at synaptic sites. Thus, D1R and M4R signals converge onto synaptic ERK1/2 and antagonistically control ERK1/2 activity in the synaptic microdomain.
Most of known ERK1/2 substrates exist in the nucleus (Treisman, 1996; Kosako et al., 2009). Through regulating nuclear gene expression, ERK1/2 transcriptionally modulate synaptic transmission and plasticity (Treisman, 1996; Sweatt, 2004; Thomas and Huganir, 2004). Regarding the synaptic sub-pool of ERK1/2, a number of local substrates have also been identified, which include protein kinase C alpha (Debata et al., 2010), Kv4.2 potassium channels (Adams et al., 2000; Schrader et al., 2006), presynaptic synapsin I (Jovanovic et al., 1996), postsynaptic density-93 (Guo et al., 2012), and postsynaptic density-95 (Sabio et al., 2004). Recent work indicates that a synaptic glutamate receptor, i.e., metabotropic glutamate receptor 5, is a biochemical substrate of MAPKs (Park et al., 2013). However, at present, whether and how ERK1/2 regulate function of these synaptic substrates have been incompletely investigated. Future studies will focus on the functional aspect of the ERK1/2 linkage to these synaptic substrates.
SIGNIFICANCE.
In this study, we found that stimulation of a dopamine receptor (D1 subtype) can activate a specific synaptic pool of the extracellular signal-regulated kinase (ERK), an enzyme critical for intracellular signaling process, in two rat brain regions called the striatum and medial prefrontal cortex. An agent that enhances activity of a muscarinic cholinergic receptor (M4 subtype) was able to suppress the response of ERK to dopamine stimulation. These results support a D1-M4 interaction model that regulates ERK particularly at synaptic sites. Malfunction of the D1-M4 interaction may be linked to various psychiatric disorders such as drug addiction and depression.
Acknowledgement
This work was supported by NIH grants R01DA10355 and R01MH61469.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: BX, LMM, DZJ, and JQW. Acquisition of data: BX and LMM. Analysis and interpretation of data: BX, LMM, DZJ, and JQW. Drafting and critical revision of the manuscript: BX, LMM, DZJ, and JQW.
Footnotes
Conflict of interest
The authors declare that there are no potential conflicts of interest.
REFERENCES
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur J Neurosci. 1993;5:1218–1225. doi: 10.1111/j.1460-9568.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem B, Schoppink L, Rotaru DC, Faiz A, Hendriks P, Mansvelder HD, van de Berg WD, Wouterlood FG. Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J Neurosci. 2014;34:16234–16246. doi: 10.1523/JNEUROSCI.3011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Glustetto M. Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE. 2007;2(7):e604. doi: 10.1371/journal.pone.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarnic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casar B, Arozarena I, Sanz-Moreno V, Pinto A, Agudo-Ibanez L, Marais R, Lewis RE, Berciano MT, Crespo P. MTRas subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol Cell Biol. 2009;29:1338–1353. doi: 10.1128/MCB.01359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. Neuroreport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- Chou H, Ogawa N, Asanuma M, Hirata H, Mori A. Muscarinic cholinergic receptor-mediated modulation on striatal c-fos mRNA expression induced by levodopa in rat brain. J Neural Transm. 1992;90:171–181. doi: 10.1007/BF01250959. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- Debata PR, Ranasinghe B, Berliner A, Curcio GM, Tantry SJ, Ponimaskin E, Banerjee P. Erk1/2-dependent phosphorylation of PKCalpha at threonine 638 in hippocampal 5-HT(1A) receptor-mediated signaling. Biochem Biophys Res Commun. 2010;397:401–406. doi: 10.1016/j.bbrc.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Racagni G, Riva MA. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19:402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Di Benedetto M, O'Sullivan GJ, Dunleavy M, Alcacer C, Bonito-Oliva A, Henshall DC, Waddington JL, Valjent E, Fisone G. Convulsant doses of a dopamine D1 receptor agonist result in Erk-dependent increases in Zif/268 and Arc/Arg3.1 expression in mouse dentate gyrus. PLoS One. 2011;6:e19415. doi: 10.1371/journal.pone.0019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Perroy J, Valjent E. Combinatorial topography and cell-type specific regulation of the ERK pathway by dopaminergic agonists in the mouse striatum. Brain Struct Funct. 2013;218:405–419. doi: 10.1007/s00429-012-0405-6. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Valjent E. Regulation of the ERK pathway in the dentate gyrus by in vivo dopamine D1 receptor stimulation requires glutamatergic transmission. Neuropharmacology. 2012;63:1107–1117. doi: 10.1016/j.neuropharm.2012.06.062. [DOI] [PubMed] [Google Scholar]
- Guo ML, Xue B, Jin DZ, Mao LM, Wang JQ. Interactions and phosphorylation of postsynaptic density 93 (PSD-93) by extracellular signal-regulated kinase (ERK). Brain Res. 2012;1465:18–25. doi: 10.1016/j.brainres.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones B. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16:1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Blockade of muscarinic receptors potentiates D1 dependent turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats but does not influence D2 mediated response. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154:637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation of physiological functions. Endo Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, Kuhlendahl F, Leal-Ortiz S, Goedert M, Garner C, Cuenda A. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem J. 2004;380:19–30. doi: 10.1042/BJ20031628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290:C852–861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology. 2011;214:653–663. doi: 10.1007/s00213-010-2068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of acute cocaine on ERK and DARPP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res. 2007;1178:12–19. doi: 10.1016/j.brainres.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JG, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell. 2001;93:71–79. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao LM. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D(1) dopamine receptor agonist SKF-81297 in the intact rat striatum. Neuroscience. 1996;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, Liu X, Wang JQ. A signaling mechanism from Gαq-protein-coupled glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J Neurosci. 2006;26:971–980. doi: 10.1523/JNEUROSCI.4423-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Parelkar NK, Arora A, Yang L, Haines M, Fibuch EE, Wang JQ. In vivo regulation of Homer1a expression in the striatum by cocaine. Mol Pharmacol. 2007;71:1148–1158. doi: 10.1124/mol.106.028399. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]