Abstract
Background
Cervical cancer screening and follow-up guidelines have changed considerably in recent years, but to the authors' knowledge few published reports exist to estimate the impact of these changes in community-based settings. The authors examined the patterns and results of cervical cancer testing and follow-up over a decade in 4 geographically diverse US health care systems to inform future evaluation of changes resulting from increased uptake of the human papillomavirus (HPV) vaccination.
Methods
The authors studied women aged 21 to 65 years who were members of one of these health systems at any time between1998 and 2007. Data were collected and standardized across sites, based on receipt of Papanicolaou (Pap) and HPV tests, HPV vaccination, cervical biopsies, and treatment of cervical dysplasia. Annual rates (per 1000 person-years) of Pap testing, HPV testing, and cervical biopsy and treatment procedures were calculated. Screening intervals and trends in the results of screening Pap tests and cervical biopsies also were examined.
Results
Pap testing rates decreased (from 483 per 1000 person-years in 2000 to 412 per 1000 person-years in 2007) and HPV testing rates increased over the study period. Screening frequency varied across health care systems, and many women continued to receive annual testing. All 4 sites moved to less frequent screening over the study period without marked changes in the overall use of cervical biopsy or treatment.
Conclusions
Despite differences over time and across health plans in rates of cervical cancer testing and follow-up cervical procedures, the authors found no notable differences in Pap test results, diagnostic or treatment procedure rates, or pathological outcomes. This finding suggests that the longer screening intervals did not lead to more procedures or more cancer diagnoses.
Keywords: Keywords: cervical intraepithelial neoplasia, cervical cancer screening, Papanicolaou test, human papillomavirus testing, screening guideline, implementation science, health care delivery systems
Introduction
Cervical cancer screening plays a central role in women's preventive health care. Its effectiveness derives from the detection and treatment of precancerous and asymptomatic invasive lesions. The effectiveness of screening with the Papanicolaou (Pap) test using conventional cytological techniques is well established1-5 and has been historically recommended and performed annually in the United States. Between 2002 and 2003, and later in 2012, updated guidelines by the American College of Obstetricians and Gynecologists, the American Cancer Society, and the US Preventive Services Task Force recommended longer screening intervals, an older age at initiation, and a younger age for discontinuation of screening.6-9 These changes occurred in tandem with the introduction of new screening technologies, including replacing traditional slide-based tests with liquid-based cytology,10-11 the use of automated laboratory Pap test review protocols, routine testing for the high-risk oncogenic strains of the human papillomavirus (HPV),12-13 and revised follow-up protocols for abnormal Pap tests.14-15
To the best of our knowledge, there are few published reports regarding recent secular trends in the use and outcomes of cervical cancer testing in US community-based settings;16-22 even fewer studies have been able to provide longitudinal, population-based data encompassing the time period of change in testing technology and guidelines to help gauge the impact on screening practices and outcomes in community settings. This information is needed to help assess the effectiveness of cervical cancer screening delivery and provide a baseline to evaluate future changes in testing practices and results related to HPV vaccination.
The aim of the current study was to examine trends in cervical cancer screening and follow-up in the United States using data from 4 geographically diverse HMO Research Network (HMORN) health systems over a 10-year period (1998–2007). The objectives were to determine the rates of Pap and HPV testing, cervical biopsy, and treatment, overall and by health system, time period, and age group. We also examined whether screening intervals changed after the introduction of new screening guidelines and testing approaches.
Materials and Methods
Setting and Population
The current retrospective cohort study was conducted as part of the multicenter Screening Effectiveness And Research in Community-Based Healthcare (SEARCH) project. The data were derived from 4 health systems that participate in the HMORN/Cancer Research Network: Group Health Cooperative (Washington), Kaiser Permanente Northwest (Oregon/Washington), Kaiser Permanente Hawaii, and Reliant Medical Group (Massachusetts). Women aged 21 to 65 years who were members of one of these health systems at any time between 1998 and 2007 were included in this analysis. We excluded women with Common Procedural Terminology (CPT) codes documenting prior hysterectomy or International Classification of Diseases for Oncology codes for cervical, vaginal, or endometrial cancer diagnosis. The Institutional Review Boards of the participating sites approved the study protocol.
Data Collection
We derived data for the study from electronic health plan databases and medical records. We used health plan enrollment files to enable women to enter and exit the cohort throughout the study period. To calculate rates of testing and outcomes, we collected monthly membership data from health plan enrollment files; enrollment gaps of <3 months were treated as continuous enrollment.23 Analytic data were extracted from the standardized HMORN Virtual Data Warehouse files at each site.24 Data that were unavailable in the Virtual Data Warehouse were extracted from local clinical laboratory information systems or other on-site data resources and mapped to a common data standard for analysis. Pap test dates and results were collected from semistructured and unstructured cytology reports at 3 sites; at the fourth site, this information was extracted from a coded cytology data set. One site provided Pap test data beginning in 2000 and could not provide complete cervical pathology data for all study years. Data regarding the receipt of HPV tests were obtained from laboratory databases. We obtained data regarding excisional and ablative treatments and hysterectomy from electronic databases using CPT codes (see Supporting Information Table S1). HPV vaccination status was obtained from immunization registries, and data concerning cervical biopsies were obtained from pathology databases. Pathology reports on cervical biopsies at 2 study sites were reviewed and coded by trained abstractors according to a standard protocol. At the third site, pathologists coded results. For each test, the most severe conclusive diagnostic category was used in the analysis. Information regarding cancer diagnoses came from tumor registries.
During the study period, liquid-based cytology replaced conventional cytology at all health plans (in 2006 at sites A and D and in 2004 at sites B and C). Sites A, B, and C switched to SurePath (Becton, Dickinson and Company, Franklin Lakes, NJ), whereas site D switched to ThinPrep (Hologic Inc, Marlborough, Mass).
Statistical Analysis
We calculated annual Pap testing and HPV testing rates, restricted to 1 test type per woman for each calendar year, and annual Pap screening rates. We defined a screening Pap test as one with no abnormal Pap test result in the previous 9 months.17-19,25,26 Thus, women had to be enrolled during the prior 9 months for a test to qualify as screening. We calculated rates of cervical biopsy and treatment by health plan and age group; for the cervical biopsy rates we excluded pathology records in which CPT codes documented a cervical treatment procedure within 10 days before or after the biopsy date, assuming that these records were treatments, not diagnostic biopsies. Data were analyzed for each site separately and combined. Women who underwent total hysterectomy or had a diagnosis of cervical, vaginal, or endometrial cancer during the study period were removed from the analysis after the procedure or diagnosis date. We tested for time trends in rates using log-linear binomial regression. We did not age-standardize rates; age distributions of women in our health plans were reasonably stable over time, both within and across health plans (see Supporting Information Table S2). We compared mean numbers of tests and rate differences across sites using generalized linear models. Differences across age groups for Pap text and biopsy results were compared using generalized estimating equation models.
Among women aged ≥30 years in 1998 with 10 years of prospective continuous health plan membership, we calculated the mean and median number of Pap screening tests for 1998 through 2007. We restricted this analysis to women who were aged ≥30 years at the outset because screening guidelines for this age group were more stable over the study period than those for younger women.
We compared the time intervals between routine cytological screening before and after the changes in screening guidelines by determining the time from the last cervical screen with a negative result in women who had undergone Pap tests in 2002 and 2007. These analyses were restricted to women with ≥4.5 years of continuous prior health plan membership to detect intervals of up to 4 calendar years. To calculate calendar year screening intervals, we included tests ≤6 months on either side of each interval year (eg, a Pap reported as “2 years prior” was received 1.50–2.49 years before the index test).17
Among routine screening Pap tests, we examined the distribution of Pap results (each as a percentage of all results) overall and by age group, health plan, and time period. Among the cervical biopsies after screening Pap tests, we examined the distribution of pathology results similarly. If a woman underwent multiple Pap tests or biopsies on the same date, we selected the most severe result. To create a baseline for later examination of population changes after HPV vaccination uptake, we excluded 5956 women with any HPV vaccination noted in our vaccine databases (0.5% of the cohort) from these Pap and cervical pathology distributions.
Results
Guidelines for cervical cancer screening and follow-up varied by health plan and changed over the study period (see Supporting Information Table S3). Three sites developed their own screening guidelines; 1 site adopted the guidelines of the state in which it was located.27 During this period, the recommended age for screening initiation rose slightly and the screening cessation age declined at some sites. In addition, all site guidelines recommended less frequent screening (every 2–3 years) after a specified number of negative annual screens. Between 2005 and 2007, guidelines for sites A, B, and C added HPV reflex testing after PAP test results indicating atypical squamous cells of undetermined significance (ASCUS).
Rates of Pap Testing, Pap Screening, and HPV Testing
This analysis included 956,028 women with 4,279,283 person-years. Pap testing rates decreased at all 4 health plans (Fig. 1) from an average overall rate of 483 per 1000 person-years in 2000 (the earliest year with data from all 4 plans) to 412 per 1000 person-years in 2007. The rate decreases were small at sites A and B (P for trend .12 and .23, respectively) and were larger at sites C and D (P< .01 for trend). Rates were consistently highest at site C (P< .01).
Figure 1.
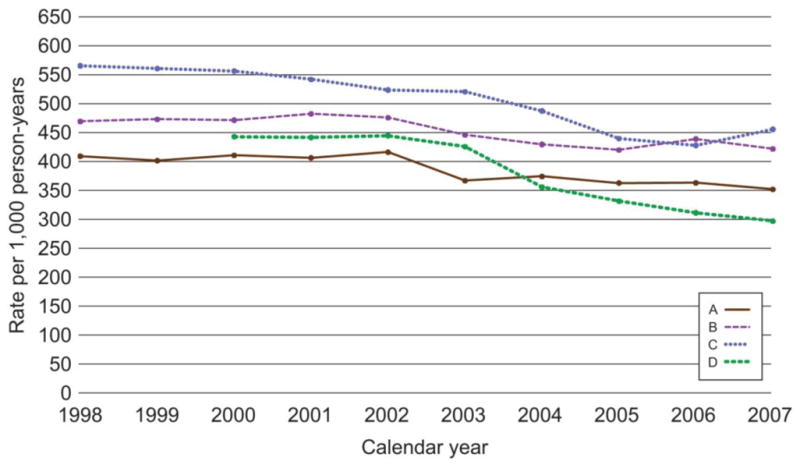
Annual rates per 1000 person-years of Papanicolaou testing in 4 managed care plans for the period 1998 through 2007. P value tests for trend were as follows: site A: P= .12; site B: P= .23; site C: P<0.01; and site D: P<0.01.
Pap screening tests (ie, no abnormal Pap test results within the previous 9 months) accounted for approximately 79% of all tests from 1998 through 2007. The overall rate in 2001 was 378 per 1000 person-years (earliest year with data from all 4 plans) and 321 per 1000 person-years in 2007 (data not shown). Trends over time by site were similar to those for overall Pap testing (site A: P= .17; site B: P= .71; site C: P< .01; and site D: P= .05).
HPV reflex testing (an HPV test was performed if Pap test result indicated an ASCUS finding) began between 2002 and 2004 at sites A, B, and C; the rates increased over time (Fig. 2). HPV co-testing (an HPV test was ordered with every Pap test) began at all 3 health plans after 2007. At site D, HPV co-testing began in 2005 and rapidly increased to 130 per 1000 person-years in 2007.
Figure 2.
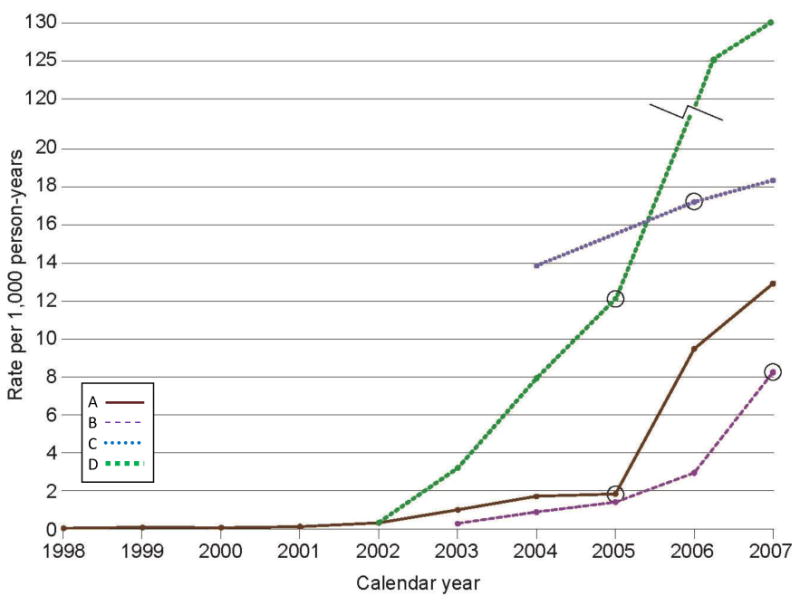
Annual rates per 1000 person-years of human papillomavirus testing in 4 managed care plans for the period 1998 through 2007. The circles indicate the calendar year in which human papillomavirus testing became the guideline in each health plan: 2005 for sites A and D, 2006 for site C, and 2007 for site B. The rates for Site D in 2006 and 2007 are 125 and 130 per 1000 person-years, respectively.
Frequency of Pap Screening
The Pap screening interval varied across health plans in both 2002 and 2007 (Fig. 3). Among women receiving Pap tests in 2002, annual screening at sites A, B, and C was performed on 32%, 60%, and 71% of women, respectively. We had insufficient data for analysis at site D for 2002. In each of the 3 health plans, Pap screening intervals increased between 2002 and 2007. A higher percentage of women at sites A, B, and C had 3-year testing intervals in 2007 compared with 2002. Site A demonstrated the longest average screening intervals for both years. In 2007, >50% of women at sites C and D were still receiving annual Pap screening compared to 23% at site A and 40% of women at site B. At all sites, >90% of women with screening tests in 2007 and a prior negative screen had received that previous test within the prior 4 years.
Figure 3.
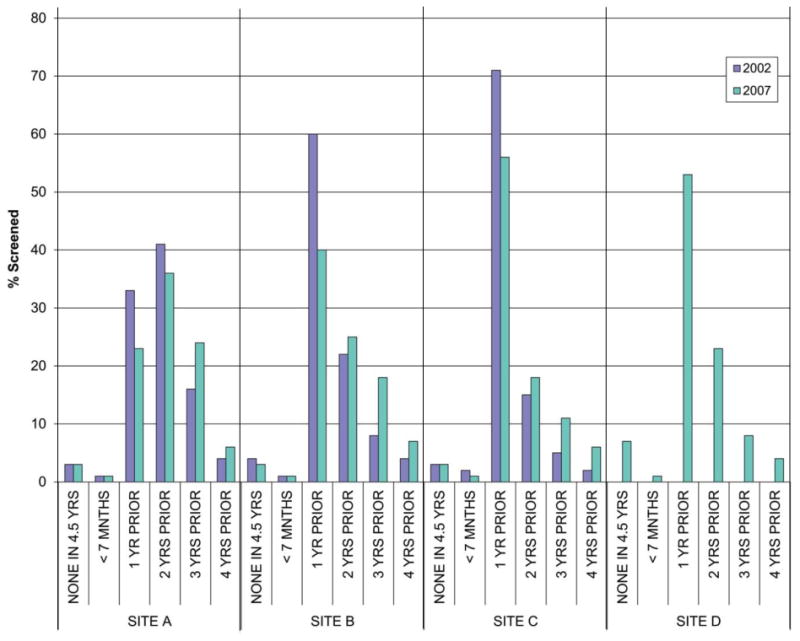
Estimated time from last routine cervical screen with a negative result among women undergoing Papanicolaou test screening in 2002 and 2007 by health plan. Site D did not have 4.5 years' worth of data for the 2002 calculation.
Among women aged ≥30 years who were members for the entire period between 1998 and 2007, the average number of screening Pap tests ± and standard deviation were 3.8 ± 2.3, 4.3 ± 2.9, and 5.3 ± 3.4, respectively, in sites A, B, and C (P< .01 for all comparisons). Site D contributed 8 years of data during this period (2000–2007); the average number of Pap tests was 3.5 ± 2.6 among members aged ≥30 years enrolled continuously for that period.
Pap Screening Results
Pap screening laboratory results were found to be broadly similar across the 4 health plans and over the time period of the study. Among the 1,227,627 routine Pap screening tests across all sites, 94% were normal (range, 91%–96%); 3.0% were ASCUS or atypical squamous cells, cannot rule out a high-grade lesion (ASC-H) (range, 1.8%–3.6%); and 0.20% of Pap tests had a result suggesting cancer or severe dysplasia (including high-grade squamous intraepithelial lesion, adenocarcinoma in situ, or invasive cancer) (range, .13%–.23%) (Table 1). The percentages with ASCUS, ASC-H, low-grade squamous intraepithelial lesion all decreased with increasing age (P< .01 for all).
Table 1.
Outcomesa of Routine Cervical Pap Test Screeningb in 4 Managed Care Plans, Overall and by Age Group, As a Percentage of Screened Women (1998-2007)c
| Age group (yrs) | Number of Pap tests | Results, % | ||||||
|---|---|---|---|---|---|---|---|---|
| Unsatisfactory/null null result | Normal cytology | ASCUS/ASC-Hd | AGC | LSIL | HSIL | Invasive cancer/AIS | ||
| 21-30 | 242,728 | 1.9 | 92.3 | 3.6 | 0.2 | 1.7 | 0.33 | 0.004 |
| 31-40 | 313,686 | 1.8 | 93.8 | 3.0 | 0.4 | 0.8 | 0.25 | 0.007 |
| 41-50 | 333,263 | 1.7 | 93.1 | 3.2 | 1.3 | 0.5 | 0.14 | 0.010 |
| 51-60 | 267,458 | 1.7 | 94.9 | 2.4 | 0.6 | 0.3 | 0.07 | 0.020 |
| 61-65 | 70,492 | 1.8 | 95.7 | 1.8 | 0.4 | 0.2 | 0.07 | 0.028 |
| Overall | 1,227,627 | 1.8 | 93.7 | 3.0 | 0.6 | 0.7 | 0.19 | 0.011 |
Abbreviations: AGC, atypical glandular cells; AIS, adenocarcinoma in situ; ASC-H, atypical squamous cells-cannot exclude high-grade lesion; ASCUS, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIS, los-grade squamous intraepithelial lesion; Pap, Papanicolaou.
If a woman underwent multiple Pap tests on the same date, the most severe result was selected using the following order: invasive cancer/AIS, HSIL or higher, LSIL, AGC, ASCUS/ASC-H, normal, and unsatisfactory.
Defined as no abnormal Pap test result within the 9 months leading up to the screening Pap test.
Excluded 5956 women with prior human papillomavirus vaccination; excluded 106,829 women with non-screening Pap tests.
ASC-H was made a separate category in 2004.
Rates of Biopsy and Treatment
The 2007 cervical biopsy rate (including endocervical curettage) for sites A, B, and C combined was 19.6 per 1000 person-years. Rates were fairly constant in 1998 through 2007 at sites A and B (Fig. 4). The cervical biopsy rate at site C in 1998 and 1999 was relatively high but declined by approximately 50% by the end of the study period to a rate similar to those observed at the other 2 sites.
Figure 4.
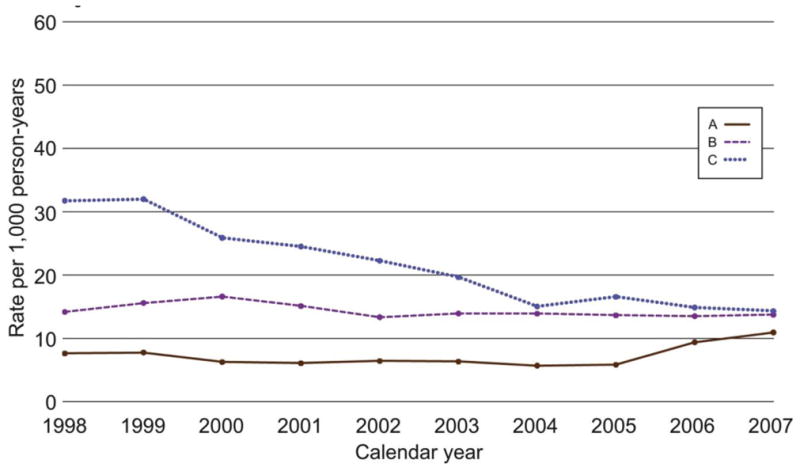
Annual rates per 1000 person-years of diagnostic cervical biopsy in 3 managed care plans for the period 1998 through 2007.
The overall cervical treatment rate including excisional and ablative procedures for all years was 2.26 per 1000 person-years and did not change over the 10-year study period. The treatment rate declined at site B (P< .01 for trend) but was fairly stable at site A. The rates at sites C and D had an upward trajectory (Fig. 5).
Figure 5.
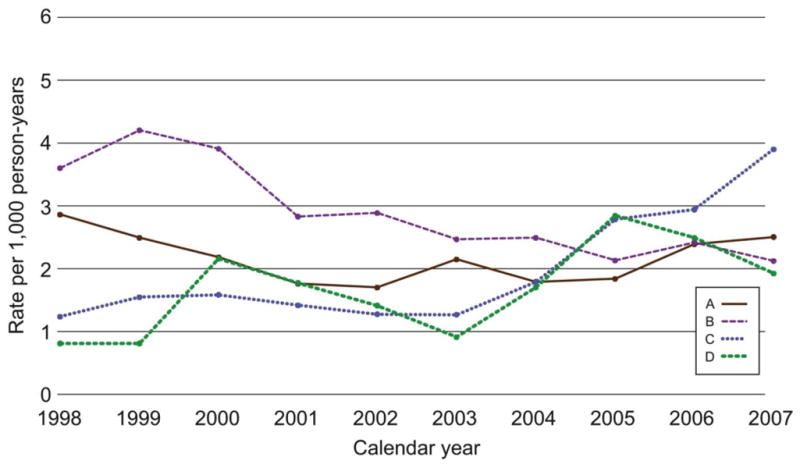
Annual rates per 1000 person-years of cervical surgical treatment in 4 managed care plans for the period 1998 through 2007.
Biopsy Results
Laboratory results of the 46,657 cervical biopsies were similar during this period across the 3 health plans for which there were complete pathology data (sites A-C). Approximately 79% of biopsies (range, 57% in women aged 21-30 years to ≥90% for women aged >50 years) were coded as normal (Table 2). Cervical intraepithelial neoplasia (CIN) type 1 (CIN1), CIN-2, and CIN-3 diagnoses were much more common in younger women than in older women. The percentage of biopsies with a result suggesting cancer or severe dysplasia (CIN3+) was 1.9%, 2.1%, and 3.0%, respectively, at sites A, B, and C, and was 2.3% overall. The percentage of biopsies containing cancer increased with age (P< .01).
Table 2.
Outcomesa of Cervical Biopsies in 3 Managed Care Plans, Overall and by Age Group, as a Percentage of All Cervical Biopsies (1998-2007)b
| Age group | Number of biopsies | Result, % | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | CIN-1 | CIN-2 | CIN-3/carcinoma in situ | Squamous cell carcinoma | Adeno-carcinoma/AIS | Unknown | ||
| 21-30 | 10,598 | 56.9 | 31.8 | 6.3 | 3.1 | 0.1 | 0.1 | 1.8 |
| 31-40 | 10,038 | 75.4 | 15.7 | 3.7 | 2.8 | 0.3 | 0.3 | 1.8 |
| 41-50 | 14,292 | 88.1 | 7.4 | 1.2 | 1.0 | 0.3 | 0.3 | 1.8 |
| 51-60 | 9,883 | 90.8 | 4.4 | 0.8 | 0.6 | 0.3 | 0.3 | 2.7 |
| 61-65 | 1,846 | 89.9 | 3.8 | 0.5 | 0.7 | 0.5 | 0.9 | 3.7 |
| Overall | 46,657 | 78.9 | 13.9 | 2.8 | 1.8 | 0.2 | 0.3 | 2.0 |
Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; SCC, squamous cell carcinoma.
For women who underwent multiple biopsies on the same date, the most severe result was selected using the following order: adenocarcinoma/AIS, SCC, CIN-3/SSC in situ, CIN-2, CIN-1, normal, and unknown.
Excluded 824 women with prior human papillomavirus vaccination; excluded 17,065 records with treatment received within 10 days before or after the pathology collection date.
Invasive Cervical Cancer
Diagnoses of invasive cervical cancer were rare in the current study cohort, with 366 total cancers diagnosed over the course of 10 years at sites A, B, and C and 8 years in site D (2000–2007). The overall rate of cervical cancer diagnosis during the study period was 10 per 100,000 person-years for this population of women aged 21 years to 65 years and did not vary significantly over time (8 per 100,000 person-years in 1998 vs 12 per 100,000 person-years in 2007).
Discussion
From 1998 through 2007, all 4 health plans in the current study extended the intervals of Pap screening, transitioned from conventional cytology using manual slide interpretation to liquid-based cytology and automated slide interpretation, and incorporated HPV testing. There was considerable variability across plans with regard to rates of cervical cancer screening and follow-up procedures. Despite considerable changes to the health plans' screening programs and guidelines, we did not identify any notable differences in Pap test results, biopsy rates, or pathological outcomes over the study period, suggesting that less intense screening had no adverse effects on outcomes.
The varying health plan guidelines regarding recommended screening intervals were generally reflected in the actual observed screening intervals. As expected, the site with the longest recommended screening interval at the beginning of the study (2–3 years) had the lowest percentage of women screened annually in both 2002 and 2007. At another site, the percentage of women receiving annual screening declined considerably after the recommended screening interval was lengthened to 3 years. However, at 2 sites, a majority of women screened in 2007 had also been screened the previous year despite health plan guidelines to screen every 1 to 3 years, suggesting that many providers and/or patients continued to prefer annual pelvic examination and highlighting the time it can take to implement new screening guidelines.
In the data from the current study, the rates of Pap testing decreased over time in tandem with extended screening intervals, but the decline in testing was only statistically significant at the 2 health plans with guidelines that had continued to include more frequent testing intervals (every 1-3 years). Not surprisingly, one of these 2 health plans also had the highest testing rate at the beginning and at the end of the study period. Thus, the decline in testing was most apparent in the health systems with the highest levels before the change in guidelines. Although the exact reasons for these differences are not known, they may be related to differences in patient and provider preferences or patient-level, provider-level, and/or system-level adherence to the national guidelines.
The health plan with the highest rates and frequency of Pap testing also had the highest percentages of abnormal biopsies, although the differences among sites narrowed over time. This may be due to differences in the aggressiveness of treatment of patients with an abnormal Pap test result, or in the pathological interpretation of biopsies but requires further study.
To the best of our knowledge, few studies are available for direct comparison. Schabert et al, analyzing medical claims for women with commercial health insurance through a large US fee-for-service health plan, reported that among women of all ages who had routine Pap screening in 2000, 43% were screened again the next year, 17% underwent biennial screening, and 8% had triennial screening.28 Among women aged 21 to 65 years in New Mexico, the median time to previous Pap screening test was 1.5 years in 2008.16 In the 2005 National Health Interview Survey (NHIS), 53% of women reported having had ≥1 Pap test every year, 17% reported biennial screening, and 11% reported triennial screening;29 NHIS findings for the year 2000 were similar. Similar to the results of the current study, these results reflect a heavy reliance on annual screening during 2000 through 2008.
Both the current study and that by Cuzick et al16 report Pap screening results that can be used as baseline data for the evaluation of the impact of HPV vaccination. The differences in the percentage of women with abnormal results (approximately 4.5% in the current study compared with approximately 7.5% in women in New Mexican) illustrate the importance of obtaining population-specific baseline estimates for this evaluation.
The finding of lengthened screening intervals in 2007 compared with 2002 noted in the current study, without an increasing percentage of women with intervals of ≥4 years, is encouraging, suggesting that, in regularly screened women, the lengthened screening intervals did not negatively affect the receipt of screening as recommended by guidelines. Compared with the current study finding that <10% of routinely screened women had not been previously screened for ≥4 years, Schabert et al found that among women screened in 2000, 4% subsequently had a 4-year screening interval and 27% were not rescreened for ≥5 years (although 4.6% of the latter group had evidence of hysterectomy after the initial screen).28 This percentage was 11% in the NHIS sample.
Rates of Pap screening and follow-up diagnostic testing in the fee-for-service setting in the study by Schabert et al demonstrated temporal patterns somewhat similar to the results of the current study. Schabert et al reported age-adjusted rates for women aged 20 to 64 years of 539 per 1000 women in 2000 and 515 per 1000 women in 2004.28 These rates are higher than the current study's reported 2000 and 2004 Pap screening rates: 378 and 344 per 1000 person-years, respectively. However, the rates reported by Schabert et al include multiple tests per year for some enrollees and only included those women with at least 1 medical claim for a routine cervical cancer screening. Kruzikas et al20 reported that rates of cervical diagnostic procedures remained relatively stable from 2001 through 2006, which is similar to 2 of the 4 health plans in the current study.
The current study has certain limitations. The cervical cancer screening and follow-up reported herein may not be representative of patterns in the entire US population, which includes women who are uninsured and those in fee-for-service arrangements. However, the current study populations had socioeconomic and racial/ethnic profiles similar to those women in their local communities. Cervical treatments were identified from CPT codes and may have been underascertained in some health plans. However, we were able to provide a more complete picture than is available elsewhere because the health plans in the current study regularly update cancer screening guidelines in tandem with national consensus guidelines, have high-quality electronic medical record data, and have relatively stable member populations.
Although many previous studies have modeled event rates, treatment protocols, and outcomes for cervical HPV-related disease using algorithms based on published literature,30 to the best of our knowledge only a few reports to date have provided detailed information regarding screening, diagnosis, and follow-up in populations with documented health service information. The results of the current study add to the literature, providing important data with which to inform modeling studies and local and national policy decisions on cervical cancer screening as well as providing a baseline to permit the future evaluation of changes resulting from the uptake of HPV vaccination.
Supplementary Material
Acknowledgments
Funding Support: Supported by National Institutes of Health grant CA148576 to Drs. Buist and Doubeni. Some data infrastructure elements were developed for the HMO Research Network Virtual Data Warehouse (grant U19CA79689). Cancer and vital status data were supported in part by contract N01-CN-67009 and by contract N01-PC-35142 from the Surveillance, Epidemiology, and End Results (SEER) program.
Footnotes
Conflict of Interest Disclosures: Dr. Weinmann was the Principal Investigator on a grant from Glaxo-SmithKline to study outcomes after surgery for cervical dysplasia, which was awarded to her institution. Dr. Doubeni has acted as a paid consultant for Exact Sciences.
References
- 1.Day NE. Effect of cervical cancer screening in Scandinavia. Obstet Gynecol. 1984 May;63(5):714–718. [PubMed] [Google Scholar]
- 2.Parkin DM, Nguyen-Dinh X, Day NE. The impact of screening on the incidence of cervical cancer in England and Wales. Br J Obstet Gynaecol. 1985 Feb;92(2):150–157. doi: 10.1111/j.1471-0528.1985.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson L, Adami HO. Cytologic screening for cancer of the uterine cervix in Sweden evaluated by identification and simulation. Br J Cancer. 1990 Jun;61(6):903–908. doi: 10.1038/bjc.1990.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurdsson K. Effect of organized screening on the risk of cervical cancer. Evaluation of screening activity in Iceland, 1964-1991. Int J Cancer. 1993 Jun 9;54(4):563–570. doi: 10.1002/ijc.2910540408. [DOI] [PubMed] [Google Scholar]
- 5.Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987 May 30;1(8544):1247–1249. doi: 10.1016/s0140-6736(87)92695-x. [DOI] [PubMed] [Google Scholar]
- 6.Screening for cervical cancer: recommendations and rationale. Am Fam Physician. 2003;67:1759–1768. [PubMed] [Google Scholar]
- 7.American College of Obstetrics and Gynecology Practice Bulletin 45: Cervical cancer screening, August, 2003. Ostet Gynecol. 2003;102:417–427. [Google Scholar]
- 8.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002 Nov;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 9.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jun;156(12):880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. W312. [DOI] [PubMed] [Google Scholar]
- 10.Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, Bulten J, Arbyn M. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009 Oct 28;302(16):1757–1764. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 11.Strander B, Andersson-Ellstrom A, Milsom I, Radberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program : a prospective randomized study. Cancer. 2007 Oct 25;111(5):285–291. doi: 10.1002/cncr.22953. [DOI] [PubMed] [Google Scholar]
- 12.Scott DR, Hagmar B, Maddox P, Hjerpe A, Dillner J, Cuzick J, Sherman ME, Stoler MH, Kurman RJ, Kiviat NB, Manos MM, Schiffman M. Use of human papillomavirus DNA testing to compare equivocal cervical cytologic interpretations in the United States, Scandinavia, and the United Kingdom. Cancer. 2002 Feb 25;96(1):14–20. doi: 10.1002/cncr.10317. [DOI] [PubMed] [Google Scholar]
- 13.Wright TC, Schiffman M, Solomon D, Cox JT, Farcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2007;197:346–355. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 14.Wright TC, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for women with cervical cytological abnormalities and cervical cancer precursors: Part 1. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 15.Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J. Myers O, Hunt WC, Robertson M, Joste NE, Castle PE, Benard VB, Wheeler CM. A population-based evaluation of cervical screening in the United States: 2008-2011. Cancer Epi Biomarkers Prev. 2014 May;23(5):765–773. doi: 10.1158/1055-9965.EPI-13-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insinga RP, Glass AG, Rush BB. Pap screening in a U.S. health plan. Cancer Epidemiol Biomarkers Prev. 2004 Mar;13(3):355–360. [PubMed] [Google Scholar]
- 18.Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus--related disease. Am J Obstet Gynecol. 2004 Jul;191(1):114–120. doi: 10.1016/j.ajog.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol. 2004 Jul;191(1):105–113. doi: 10.1016/j.ajog.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Kruzikas D, Smith JS, Harley C, Buzinec P. Costs associated with management of cervical human papillomavirus-related conditions. Cancer Epidemiol Biomarkers Prev. 2012 Sep;21(9):1469–1478. doi: 10.1158/1055-9965.EPI-11-1019. [DOI] [PubMed] [Google Scholar]
- 21.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011 Jul;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC, Raine-Bennett T, Gage JC, Kinney WK. Five-year risks of CIN 3+ and cervical cancer among women with HPV testing of ASC-US Pap results. J Low Genit Tract Dis. 2013 Apr;17(5 Suppl 1):S36–S42. doi: 10.1097/LGT.0b013e3182854253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field TS, Cernieux J, Buist D, Geiger A, Lamerato L, Hart G, Bachman D, Krajenta R, Greene S, Hornbrook MC, Ansell G, Herrinton L, Reed G. Retention of enrollees following a cancer diagnosis within health maintenance organizations in the Cancer Research Network. J Natl Cancer Inst. 2004 Jan 21;96(2):148–152. doi: 10.1093/jnci/djh010. [DOI] [PubMed] [Google Scholar]
- 24.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. eGEMs (Generating Evidence & Methods to improve patient outcomes) 2014;2 doi: 10.13063/2327-9214.1049. Available at: http://repository.academyhealth.org/egems/vol2/iss1/2. (1, Article 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol. 2000 Aug;96(2):219–223. doi: 10.1016/s0029-7844(00)00882-6. [DOI] [PubMed] [Google Scholar]
- 26.Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, Mielzynska-Lohnas I, Rush BB, Schiffman M. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003 Jan 1;95(1):46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 27.Buist DS, Tuzzio L, Greenlee RT, Field T, Williams A, Weinmann S, Stout NK, Kamineni A, Doubeni CA. 18th Annual HMO Research Network Conference. Seattle, WA: 2012. Apr 30, Clinical Guideline Development, Implementation, and Dissemination: Connection Points for Researchers and Health Care Delivery Systems for Comparative Effectiveness Research. [Google Scholar]
- 28.Schabert VF, Ye X, Insinga RP, Singhal PK, Riedel AA. Five-year routine cervical cancer screening rates and intervals in a US health plan. Curr Med Res Opin. 2008 Sep;24(9):2429–2435. doi: 10.1185/03007990802281671. [DOI] [PubMed] [Google Scholar]
- 29.Eltoum IA, Roberson J. Impact of HPV testing, HPV vaccine development, and changing screening frequency on national Pap test volume: projections from the National Health Interview Survey (NHIS) Cancer. 2007 Feb 25;111(1):34–40. doi: 10.1002/cncr.22487. [DOI] [PubMed] [Google Scholar]
- 30.Stout NK, Goldhaber-Fiebert JD, Ortendahl JD, Goldie SJ. Trade-offs in cervical cancer prevention: balancing benefits and risks. Arch Intern Med. 2008 Sep 22;168(17):1881–1889. doi: 10.1001/archinte.168.17.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


