Abstract
INTRODUCTION
Previous publications have shown conflicting results regarding body mass index (BMI) and prostate cancer (CaP) outcomes after definitive radiotherapy prior to the dose escalation era. Our goal is to determine whether increasing BMI is associated with CaP outcomes in men with localized CaP treated with dose escalated radiotherapy.
METHODS
We identified patients with localized (T1b-T4N0M0) CaP treated with definitive intensity modulated radiation therapy (IMRT) and image guidance (IGRT) from 2001–2010. BMI was analyzed as a continuous variable. Adjusting for confounders, multivariable competing risk and Cox proportional hazards regression models were used to assess the association between BMI category and the risk of biochemical failure (BF), distant metastasis (DM), cause-specific mortality (CSM) and overall mortality (OM).
RESULTS
Of the 1,442 patients identified, there were 20% with BMI<25 kg/m2, 48% with BMI 25–29.9 kg/m2, 23% with BMI 30–34.9 kg/m2, 6% with 35–39.9 kg/m2, and 4% with BMI≥40 kg/m2. Median follow-up was 47.6 months (range 1–145) with median age of 68 years (range 36–89). Median dose was 78Gy (range 76–80) and 30% of patients received androgen deprivation therapy. Increasing BMI was inversely associated with age (p<0.001) and pre-treatment PSA (p=0.018). On multivariable analysis, increasing BMI was associated with increased risk of BF (HR=1.03[95% CI 1.00–1.07], p=0.042), DM (HR=1.07[1.02–1.11], p=0.004), CSM (HR=1.15[1.07–1.23], p<0.001), and OM (HR=1.05[1.02–1.08], p=0.004).
CONCLUSION
For CaP patients receiving dose-escalated IMRT with IGRT, increasing BMI appears to be associated with an increased risk of biochemical failure, distant metastases development, cause-specific and overall survival.
Keywords: obesity, body mass index, prostate cancer, radiation therapy, IMRT
INTRODUCTION
Obesity is an epidemic affecting more than one-third of adults in the United States and the incidence has more than doubled in the last 40 years.1 Increasing body mass index (BMI) is associated with increasing prevalence for multiple conditions including hypertension, dyslipidemia, type 2 diabetes, and coronary heart disease, among others.2 Obesity has also been shown to be associated with an increased risk of cancers such as endometrial, breast, renal, and esophageal cancer.2, 3 However, the association with prostate cancer is unclear. Evidence linking obesity to prostate cancer incidence has been inconsistent.3–5 A recent meta-analysis showed decreased localized prostate cancer incidence and increased advanced prostate cancer incidence in obese men.6 Increasing BMI has also been associated with pathologic progression in men with low-risk prostate cancer undergoing active surveillance.7 Data supporting the association of BMI and prostate cancer mortality is compelling.4, 5 The Physicians’ Health Study shows that obese men had a significantly higher risk of prostate cancer mortality compared with men with a healthy BMI.8 A prospective study of more than 250,000 men showed that despite a lower incidence of prostate cancer, men with higher BMI have a significant elevation of prostate cancer mortality compared with those with normal BMI.4
Literature available for obese patients treated with external beam radiation therapy (EBRT) has yielded conflicting results; multiple studies have shown an increasing risk of biochemical failure (BF) in obese men9, 10 whereas others have not.11 The majority of patients in these studies were treated to 70Gy or less with the traditional four-field technique or 3D-conformal radiation therapy. Multiple studies have shown the cancer control benefit of dose escalation12, 13 and studies using brachytherapy for primary treatment of localized prostate cancer have not shown BMI to be associated with PSA failure.14, 15 In addition, Geinitz et al suggests that increase in daily shifts and difficulty of daily set-up in obese patients may attribute to inferior prostate cancer specific outcomes.11 It is important to provide further data on men with localized prostate cancer treated in the dose-escalation era with intensity modulated radiation therapy (IMRT) and daily image guidance (IGRT). Therefore, we sought to evaluate the effect of obesity on prostate cancer specific outcomes in a large cohort of patients treated with dose-escalated IMRT with daily IGRT.
METHODS
From 2001–2010, 1,442 patients with clinically localized (T1b-T4N0M0) prostate cancer were treated with definitive IMRT at our institution and had complete baseline analysis data available in our prospectively collected prostate cancer database. Informed consent was obtained for inclusion in the IRB approved database. Weight and height were documented prior to the initiation of EBRT. Patients were stratified using the National Comprehensive Cancer Network risk grouping criteria.16 Computed tomography (CT)-based planning was performed for all patients. Magnetic resonance imaging (MRI) simulation was routinely performed for all patients unless patients had a contraindication for MRI (e.g. pacemaker) and image fusion was performed. Both CT and MRI were used for delineation of the target volume.
All patients were treated with IMRT. The techniques for IMRT treatment have been previously described.17 Daily imaging was utilized for all patients. Methods include implantation of gold fiducial markers with electronic portal imaging device, cone beam CT scans, or continuous real-time tracking of implanted radiofrequency beacons. B-type acquisition ultrasound was also used prior to March 2010.
The target volumes for IMRT were previously described.18 Dose per fraction was 2Gy unless otherwise specified. Briefly, low-risk prostate cancer patients were treated to 76-78Gy to the prostate and proximal seminal vesicles (PTV1). Intermediate- risk patients were treated to 76-80Gy to PTV1 and ≥56Gy to the distal seminal vesicles (PTV2) in 38–40 fractions. High-risk patients were treated to 76-80Gy to PTV1 and ≥56Gy to PTV2 and regional lymph nodes in 38–40 fractions. More than 95% of the PTVs received the prescription dose.
BMI is defined as weight in kilograms divided by the square of height in meters and was evaluated as a continuous variable. Biochemical failure (BF) was defined according to the RTOG-ASTRO Phoenix consensus19 and rerun with the previous ASTRO consensus using three consecutive rises.20 The time to BF, distant metastasis (DM), cause specific mortality (CSM), and overall mortality (OM) was calculated from date of start of radiation. Patients without an event were censored at the time of their last PSA measurement. Patient deaths were coded as prostate cancer-related only if it was stated in the death certificate, medical record, or physician’s note.
Statistics
Baseline differences by BMI categories were assessed using Chi-square tests. Continuous BMI was used to assess an increasing linear relationship with each outcome. Cox proportional hazards regression21 was used for all endpoints to assess the association with BMI when considered alone and with other covariates. Results are presented as hazard ratios for Cox models. The proportional hazards assumption for each covariate was evaluated and the assumption held for all covariates except for T-stage and Gleason score for the BF outcome. For BF, we estimated hazard ratios for BMI and other covariates using a stratified Cox proportional hazards model22. Although adjustments were performed for all covariates, hazard ratios cannot be generated for T-stage and Gleason score because the proportional hazards assumption does not hold. In multivariable analysis (MVA), covariates included androgen deprivation therapy (ADT) use, pre-treatment prostate-specific antigen as a log-transformed variable (iPSA), and age. Gleason score and T-stage were included as covariates for DM, CSM, and OM, and as stratification variables for BF. For BF and OM, history of diabetes, heart disease, and hypertension were included as covariates. Comorbidities were not included in MVA models for DM or CSM because of the low number of events.
To illustrate the association of BMI and the outcomes, survival curves were generated from the Cox model results for increasing BMI values while holding other covariates at their mean values.23 For the stratified Cox model used for BF, as there is a separate baseline survival function for each strata, we estimated freedom from BF at an intermediate risk strata (T-stage=T2, Gleason score=7) while holding other covariates at their mean values. All statistical tests are two-sided and analyses were performed using SAS statistical software (version 9.3) and Stata (version 12).
RESULTS
Of the 1,442 patient identified, there were 284 men (20%) with BMI<25 kg/m2, 688 (48%) with BMI 25–29.9 kg/m2, 326 (23%) with BMI 30–34.9 kg/m2, 92 (6%) with 35–39.9 kg/m2, and 52 (4%) with BMI≥40 kg/m2. Median follow-up was 47.6 months (range 1–149) with median age of 68 years (range 36–89). Median dose was 78Gy (range 76–80) and 30% of patients received ADT. Increasing BMI was inversely associated with age (p<0.001), iPSA (p=0.018), and history of diabetes (p<0.001) and hypertension (p<0.001). All other characteristics were balanced. (Table 1) There were 146 BF, 56 DM, 17 CSM, and 133 deaths overall. BF predated DM and CSM in all men.
Table 1.
Patient Baseline and Treatment Characteristics
| Characteristic | ALL | BMI | p-value | ||||
|---|---|---|---|---|---|---|---|
|
<25 kg/m2 |
25–29.9 kg/m2 |
30–34.9 kg/m2 |
35–39.9 kg/m) |
≥40 kg/m2 |
|||
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Total | 284 (20%) | 688 (48%) | 326 (23%) | 92 (6%) | 52 (4%) | ||
|
Age at Start of RT |
<0.001 | ||||||
| 36–59 yrs | 241 (17%) | 30 (11%) | 112 (16%) | 64 (20%) | 19 (21%) | 16 (31%) | |
| 60–69 yrs | 590 (41%) | 102 (36%) | 266 (39%) | 159 (49%) | 42 (46%) | 21 (40%) | |
| 70–79 yrs | 539 (37%) | 128 (45%) | 278(40%) | 90 (28%) | 28 (30%) | 15 (29%) | |
| ≥80 yrs | 72 (5%) | 24 (8%) | 32 (5%) | 13 (4%) | 3 (3%) | 0 (0%) | |
| Gleason Score | 0.339 | ||||||
| 6 | 640 (44%) | 129 (45%) | 311 (45%) | 146 (45%) | 36 (39%) | 18 (35%) | |
| 7 | 571 (40%) | 107 (38%) | 269 (39%) | 128 (39%) | 46 (50%) | 21 (40%) | |
| 8–10 | 231 (16%) | 48 (17%) | 108 (16%) | 52 (16%) | 10 (11%) | 13 (25%) | |
| T-stage | 0.083 | ||||||
| T1 | 946 (66%) | 178 (63%) | 434 (63%) | 228 (70%) | 68 (74%) | 38 (73%) | |
| T2 | 412 (29%) | 92 (32%) | 210 (31%) | 82 (25%) | 19 (21%) | 9 (17%) | |
| T3/4 | 84 (6%) | 14 (5%) | 44 (6%) | 16 (5%) | 5 (5%) | 5 (10%) | |
| iPSA, ng/mL | 0.018 | ||||||
| 0-<10 | 1055 (73%) | 198 (70%) | 502 (73%) | 240 (74%) | 77 (84%) | 38 (73%) | |
| 10-<20 | 273 (19%) | 53 (19%) | 143 (21%) | 61 (19%) | 6 (6%) | 10 (19%) | |
| 20+ | 114 (8%) | 33 (12%) | 43 (6%) | 25 (8%) | 9 (10%) | 4 (8%) | |
| Risk Group | 0.697 | ||||||
| Low | 458 (32%) | 93 (33%) | 218 (32%) | 101 (31%) | 32 (35%) | 14 (27%) | |
| Intermediate | 646 (45%) | 117 (41%) | 317 (46%) | 151 (46%) | 40 (43%) | 21 (40%) | |
| High | 338 (23%) | 74 (26%) | 153 (22%) | 74 (23%) | 20 (22%) | 17 (33%) | |
| ADT Use | 0.142 | ||||||
| No | 1003 (70%) | 202 (71%) | 483 (70%) | 218 (67%) | 70 (76%) | 30 (58%) | |
| Yes | 439 (30%) | 82 (29%) | 205 (30%) | 108 (33%) | 22 (24%) | 22 (42%) | |
| Dose, cGy | 0.199 | ||||||
| 7600 | 688 (48%) | 122 (43%) | 345 (50%) | 158 (49%) | 38 (41%) | 25 (48%) | |
| 7800 | 323 (22%) | 71 (25%) | 142 (21%) | 67 (21%) | 30 (33%) | 13 (25%) | |
| 8000 | 431 (30%) | 91 (32%) | 201 (29%) | 101 (31%) | 24 (26%) | 14 (27%) | |
| Comorbidities | |||||||
| Diabetes | 275 (19%) | 39 (14%) | 101 (15%) | 80 (25%) | 28 (30%) | 27 (52%) | <0.001 |
| Hypertension | 834 (58%) | 139 (49%) | 370 (54%) | 215 (66%) | 69 (75%) | 41 (79%) | <0.001 |
| Heart Disease | 260 (18%) | 51 (18%) | 126 (18%) | 60 (18%) | 16 (17%) | 7 (13%) | 0.934 |
BMI, body mass index; ADT, androgen deprivation therapy; iPSA, pre-treatment prostate specific antigen
PSA failure
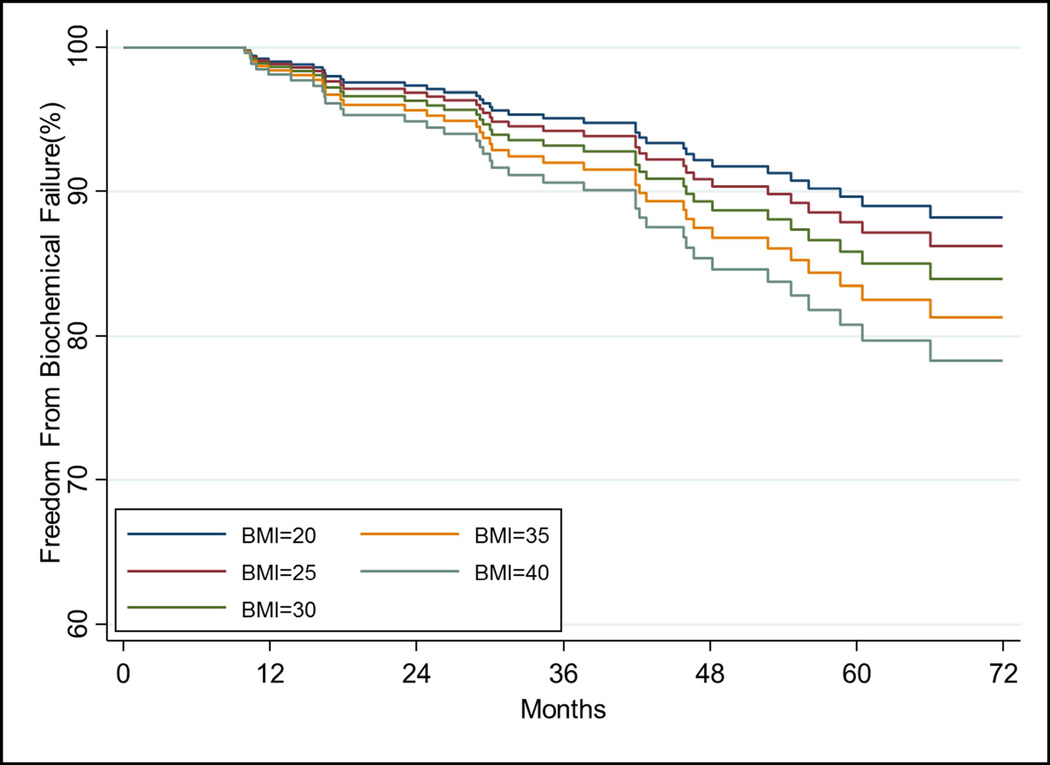
On univariate analysis (UVA) BMI as a continuous variable was not associated with increased risk of BF (p=0.063). However, after accounting for covariates, increasing BMI and iPSA were significant for increased risk of BF (all p<0.05, Table 2). Cumulative incidence of BF at 5 years was 11.4% overall (95% CI:9.4–13.7%). The relationship of increasing BMI on estimated freedom from BF over time, adjusted for other covariates, is shown in Figure 1A.
Table 2.
Association between BF and BMI and covariates
| Variable | HR* | 95% CI | p-value | |
|---|---|---|---|---|
| BMI, continuous(kg/m2) | 1.03 | 1.00–1.07 | 0.042 | |
| Log iPSA(ng/mL) | 2.02 | 1.60–2.52 | <0.001 | |
| Age(years) | 1.00 | 0.98–1.02 | 0.992 | |
| ADT Use(yes vs no) | 0.74 | 0.47–1.17 | 0.192 | |
| Comorbidities(yes vs no) | ||||
| Diabetes | 1.09 | 0.71–1.69 | 0.691 | |
| Hypertension | 1.17 | 0.83–1.66 | 0.373 | |
| Heart Disease | 0.80 | 0.51–1.25 | 0.322 | |
BF, biochemical failure; BMI, body mass index; HR, hazard ratio; ADT, androgen deprivation therapy; iPSA, pre-treatment prostate specific antigen.
Hazard ratios are from a single stratified Cox proportional hazards model, adjusted for T-stage and Gleason score as stratification variables.
Figure 1.
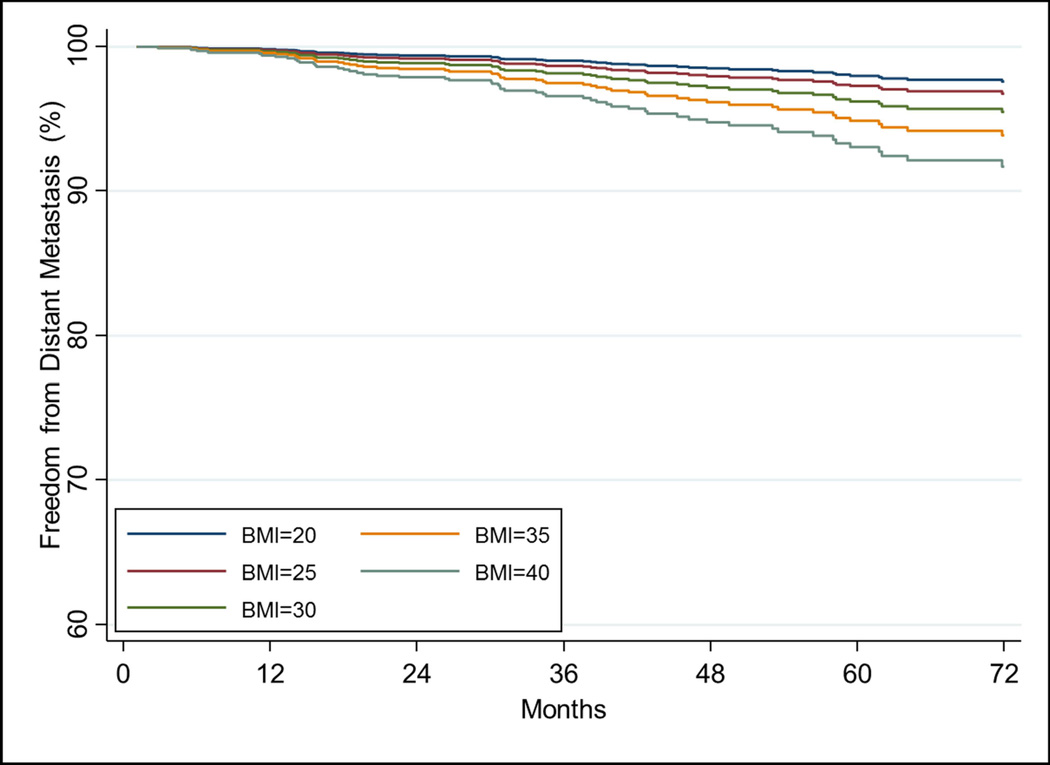
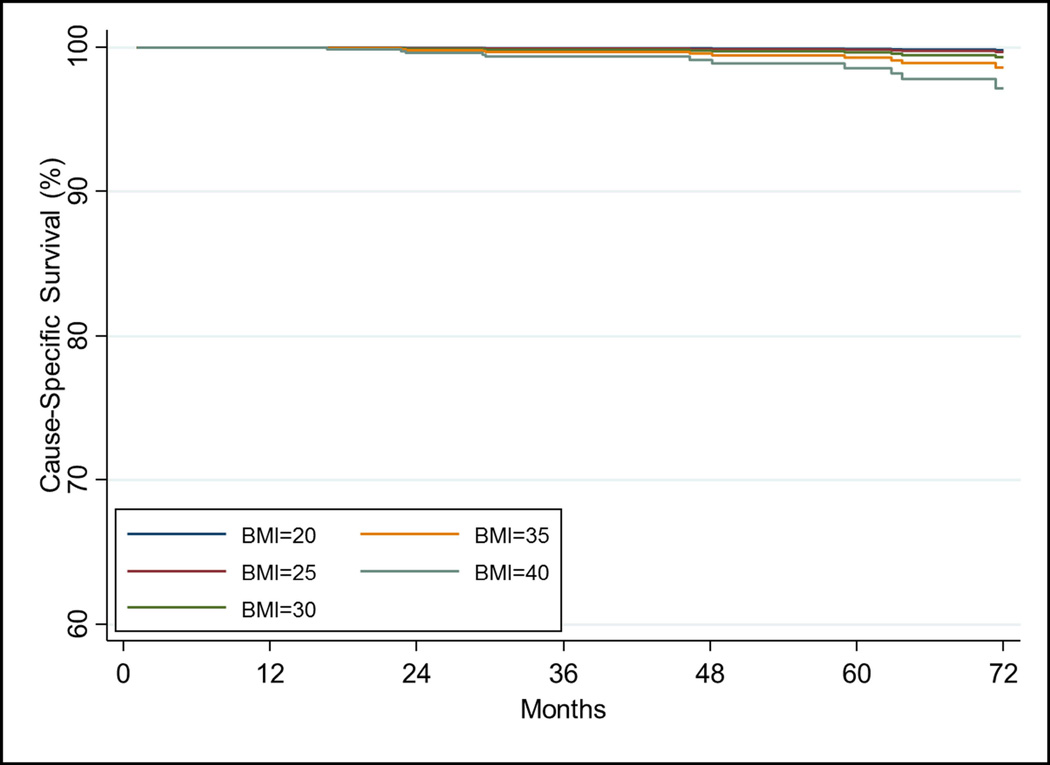
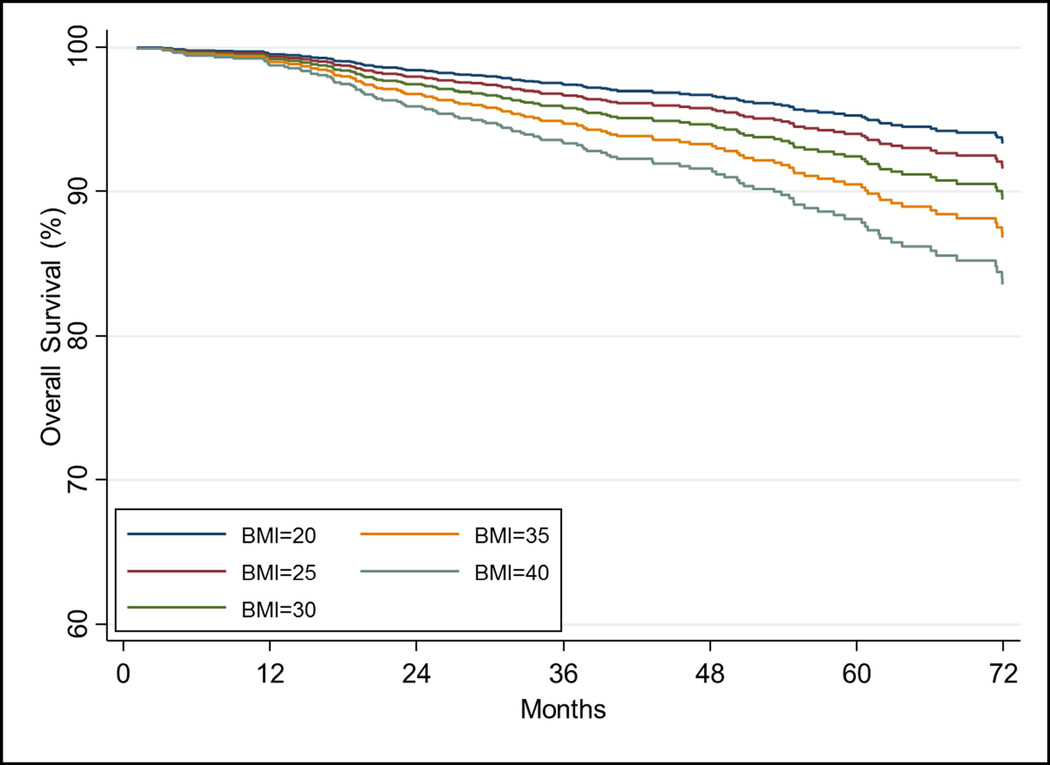
The relationship of increasing BMI on a) freedom from biochemical failure using the stratified Cox model using T-stage=2 and Gleason score=7 and other covariates at their mean value. The relationship of increasing BMI on b) freedom from distant metastasis, c) cause-specific survival, and d) overall survival estimated from the Cox model while holding other covariates at the mean value.
Distant metastasis
On UVA, increasing BMI was associated with increased risk of DM (HR=1.05[1.00–1.10], p=0.032). On MVA, this associated remained statistically significant (HR=1.07[1.02–1.11], p=0.004, Table 3).Other significant variables on MVA were Gleason score, T-stage, and iPSA (all p<0.05). Figure 1B shows the impact of increasing BMI on freedom from distant metastasis estimated from the Cox model while holding other covariates at the mean values. DM rate at 5 years is 4.7%(95% CI:3.5–6.1%) for the entire cohort.
Table 3.
Association between DM and BMI and covariates
| Variable | HR | 95% CI | p-value | |
|---|---|---|---|---|
| BMI, continuous(kg/m2) | 1.07 | 1.02–1.11 | 0.004 | |
| Gleason Score | ||||
| 7 vs 6 | 2.48 | 1.14–5.36 | 0.220 | |
| 8–10 vs 6 | 3.78 | 1.43–9.96 | 0.007 | |
| T-stage | ||||
| T2 vs T1c | 2.55 | 1.40–4.65 | 0.002 | |
| T3/4 vs T1c | 3.52 | 1.47–8.42 | 0.005 | |
| Log iPSA(ng/mL) | 1.94 | 1.40–2.67 | <0.001 | |
| Age(years) | 1.02 | 0.98–1.05 | 0.322 | |
| ADT Use(yes vs no) | 0.71 | 0.34–1.45 | 0.347 | |
DM, distant metastasis; CSM, cause-specific mortality; OM, overall mortality; BMI, body mass index; HR, hazard ratio; ADT, androgen deprivation therapy; iPSA, pre-treatment prostate specific antigen.
Cause specific mortality
CSM at 5 years was 0.8%(0.4–1.6%) overall. On UVA, BMI was significantly associated with CSM when analyzed as a continuous variable (HR=1.11[1.04–1.18], p=0.001). On MVA, increasing BMI was significantly associated with increased CSM (p<0.001, Table 4). T-stage was also statistically significant for CSM (p=0.002). The relationship of increasing BMI on cause specific survival is shown in Figure 1C after adjusting for other covariates using the Cox model.
Table 4.
Assocation between CSM and BMI and covariates
| Variable | HR | 95% CI | p-value | |
|---|---|---|---|---|
| BMI, continuous(kg/m2) | 1.15 | 1.07–1.23 | <0.001 | |
| Gleason Score | ||||
| 7 vs 6 | 3.15 | 0.48–14.65 | 0.143 | |
| 8–10 vs 6 | 2.59 | 0.42–15.98 | 0.304 | |
| T-stage | ||||
| T2 vs T1c | 6.29 | 1.90–20.80 | 0.003 | |
| T3/4 vs T1c | 6.47 | 1.16–36.08 | 0.033 | |
| Log iPSA(ng/mL) | 2.41 | 1.29–4.50 | 0.006 | |
| Age(years) | 1.06 | 0.99–1.14 | 0.089 | |
| ADT Use(yes vs no) | 1.25 | 0.37–4.27 | 0.722 | |
DM, distant metastasis; CSM, cause-specific mortality; OM, overall mortality; BMI, body mass index; HR, hazard ratio; ADT, androgen deprivation therapy; iPSA, pre-treatment prostate specific antigen.
Overall mortality
OM at 5 years for the entire cohort is 8.5%(6.8–10.3%). BMI was significantly associated with OM on UVA as a continuous variable (HR1.03[1.00–1.07], p=0.025). After adjusting for covariates, increasing BMI was significant when analyzed as a continuous variable (p=0.004, Table 5). Other significant factors include age, iPSA, and history of diabetes and hypertension (all p<0.05). Figure 1D shows the impact of increasing BMI on overall survival over time after adjusting for covariates.
Table 5.
Association between OM and BMI and covariates
| Variable | HR | 95% CI | p-value | |
|---|---|---|---|---|
| BMI, continuous(kg/m2) | 1.05 | 1.02–1.08 | 0.004 | |
| Gleason Score | ||||
| 7 vs 6 | 1.19 | 0.78–1.80 | 0.417 | |
| 8–10 vs 6 | 1.18 | 0.65–2.14 | 0.595 | |
| T-stage | ||||
| T2 vs T1c | 1.21 | 0.82–1.78 | 0.327 | |
| T3/4 vs T1c | 1.44 | 0.75–2.76 | 0.269 | |
| Log iPSA(ng/mL) | 1.35 | 1.06–1.73 | 0.016 | |
| Age(years) | 1.08 | 1.05–1.11 | <0.001 | |
| ADT Use(yes vs no) | 1.04 | 0.66–1.65 | 0.858 | |
| Comorbidities(yes vs no) | ||||
| Diabetes | 1.54 | 1.03–2.28 | 0.034 | |
| Hypertension | 1.48 | 1.01–2.17 | 0.045 | |
| Heart Disease | 1.19 | 0.81–1.76 | 0.373 | |
DM, distant metastasis; CSM, cause-specific mortality; OM, overall mortality; BMI, body mass index; HR, hazard ratio; ADT, androgen deprivation therapy; iPSA, pre-treatment prostate specific antigen.
DISCUSSION
To our knowledge, no previous study has investigated the association between BMI and prostate cancer specific outcomes among patients treated with definitive EBRT in the era of dose-escalated IMRT with daily IGRT. This study supports the association of obesity with increased risk of prostate-cancer specific outcomes and overall mortality in patients treated with definitive dose-escalated IMRT with daily IGRT.
Multiple technical and biologic mechanisms have been proposed for the association between increasing BMI and increase in prostate-cancer specific outcomes. Previous studies have postulated that worse outcomes could partly be attributed to technical issues such as difficulty of daily setup due to abdominal adipose tissue obscuring skin tattoos and the lack of image guidance in the cohorts of patients examined.9, 10 This is often based on reports of the strong correlation of increased shifts in obese men compared to their normal weight counterparts and patient adjustments on up to 80% of treatment days when they are treated with daily IGRT.24, 25 All patients in our study underwent daily localization with either radiofrequency transponders which track the prostate in real-time or with imaging prior to their treatment to verify prostate position. This would prevent any geographical miss in our patient population and the potential for under-dosing the CTV which may have occurred in previous studies. Therefore the association of increased BMI and worse prostate-cancer specific outcomes seen in this cohort of patients is unlikely to be explained by technical misses.
Another hypothesized technical mechanism for worse prostate-specific outcomes with increasing BMI is the use of lower radiation doses in the treatment of prostate cancer in previous studies with the median radiation dose often at 70Gy or below10, 11. Multiple prospective, randomized trials have demonstrated superior cancer control utilizing dose escalation,12, 13 with studies showing that the most significant therapeutic factor affecting BF after definitive RT is radiation dose.26 Despite the fact that all patients in our study were treated with dose escalation (≥76Gy), an association with worse BF, DM development, CSM, and OM was seen with increasing BMI. Studies examining the effect of BMI on BF after brachytherapy (which delivers an ablative dose to the prostate) for localized prostate cancer failed to show a relationship.14, 15 It is possible that the significant dose escalation achieved with prostate brachytherapy can mitigate the detrimental effects of increasing BMI.
Another theory for increased risk of DM, CSM, and OM with high BMI is hemodilution of PSA from increased blood volume because the total volume of PSA does not differ by BMI.27 Patients with higher BMI have been shown to have lower serum PSA compared to men with normal weight28 which is also supported by our analysis. Hemodilution may cause PSA levels to meet threshold for biopsy later than their normal weight counterparts and may promote the diagnosis of higher risk disease at presentation29, as well as a decreased incidence of prostate cancer diagnosis.4 In our cohort, however, there was no statistical difference in clinical T-stage or Gleason score between the men in various BMI groups, suggesting that hemodilution may not significantly impact results for this cohort.
One biological mechanism for worse survival outcomes in obese men is due to more rapid progression to DM after BF. In a study describing patients who underwent radical prostatectomy and received ADT prior to the onset of metastases in the SEARCH database, patients with higher BMI were more likely to develop metastases and had a trend for greater risk of progression to castration-resistant prostate cancer.30 In addition, case reports have shown prostate cancers to have a more aggressive biology at the time of relapse with decreased PSA production31.
Studies have linked increased BMI with more aggressive prostate cancer biology.32, 33 However, no significant difference in Gleason scores within the BMI groups in our study was seen. Other obesity-related biological mechanisms include higher levels of insulin32 and IGF-134 leading to elevated circulating growth factors, decreased levels of testosterone, and higher levels of estrogen35, as well as high inflammatory factors such as leptin36 and IL-637. Changes in these factors all lead to increased tumor proliferation, reduced tumor apoptosis, and transformation into androgen independence27. This can in turn transition to prostate cancer progression leading to death.
Limitations of the current study include the single institution retrospective design and small numbers of prostate cancer deaths with broad hazard ratio confidence intervals indicative of wide variability. In a NIH-AARP study linking questionnaire respondents to state cancer registries,4 there were 10 times as many prostate cancer deaths as seen in our cohort. However, there were nearly 7 times as many prostate cancer cases and the impact of obesity in men treated with RT could not be estimated because no treatment information was provided. Additionally, inclusions for prostate cancer deaths can be nuanced and be difficult to determine with absolute certainty outside of a prospective trial. Therefore despite stringent definitions of CSM in our cohort, care should be taken when applying to the general prostate population. Other limitations include height and weight data was generally taken at the time of initial consult which can be months before initiation of definitive EBRT and therefore may not represent the BMI of the patient at the time of radiation initiation. BMI changes over time were also not accounted for and weight gain has been shown to increase risk of prostate cancer mortality4. Other measures of obesity such as abdominal visceral adipose tissue and waist circumference, which may be a more accurate measurement of obesity38, were not assessed. Lastly, adjustments for multiple comparisons across outcomes were not performed because the outcomes are related.
CONCLUSION
To the authors’ knowledge, this is the first study to report the association between obesity and prostate-cancer outcomes after dose-escalated IMRT with daily localization for localized prostate cancer. Increasing BMI appears to be associated with an increased risk of biochemical failure, distant metastases development, and prostate-cancer specific and overall mortality. Further studies should be conducted to better elucidate this relationship.
Acknowledgments
Funding sources: This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH and in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI or NIH or Varian Medical Systems, Inc.
Footnotes
Financial disclosures: None
We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this paper.
REFERENCES
- 1.Fryar CD, Carrol MD, Ogden CL. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2009–2010. Available from URL: http://www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.htm.
- 2.National Heart L, and Blood Institute. NIH Publication [serial online] 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obese Adults: The Evidence Report; p. 228. [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 5.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 6.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 7.Bhindi B, Kulkarni GS, Finelli A, et al. Obesity Is Associated with Risk of Progression for Low-risk Prostate Cancers Managed Expectantly. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strom SS, Kamat AM, Gruschkus SK, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 10.Stroup SP, Cullen J, Auge BK, L'Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–1009. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 11.Geinitz H, Thamm R, Mueller T, et al. Impact of body mass index on outcomes after conformal radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:16–22. doi: 10.1016/j.ijrobp.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efstathiou JA, Skowronski RY, Coen JJ, Grocela JA, Hirsch AE, Zietman AL. Body mass index and prostate-specific antigen failure following brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71:1302–1308. doi: 10.1016/j.ijrobp.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 15.Merrick GS, Butler WM, Wallner KE, et al. Influence of body mass index on biochemical outcome after permanent prostate brachytherapy. Urology. 2005;65:95–100. doi: 10.1016/j.urology.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 16.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 17.Price RA, Murphy S, McNeeley SW, et al. A method for increased dose conformity and segment reduction for SMLC delivered IMRT treatment of the prostate. Int J Radiat Oncol Biol Phys. 2003;57:843–852. doi: 10.1016/s0360-3016(03)00711-9. [DOI] [PubMed] [Google Scholar]
- 18.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 21.Cox D. Regression models and life-tables (with discussion) JR Stat Soc B. 1972;34:187–202. [Google Scholar]
- 22.Collett D. Modelling Survival Data in Medical Research. Second Edition. New York, NY: CRC Press; 2003. [Google Scholar]
- 23.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 24.Wong JR, Gao Z, Merrick S, et al. Potential for higher treatment failure in obese patients: correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys. 2009;75:49–55. doi: 10.1016/j.ijrobp.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 25.Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M., 3rd Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100:1283–1292. doi: 10.1002/cncr.20093. [DOI] [PubMed] [Google Scholar]
- 27.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 29.Kheterpal E, Sammon JD, Diaz M, et al. Effect of metabolic syndrome on pathologic features of prostate cancer. Urol Oncol. 2013;31:1054–1059. doi: 10.1016/j.urolonc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Keto CJ, Aronson WJ, Terris MK, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110:492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schriefer P, Steurer S, Huland H, Graefen M. Is undetectable prostate-specific antigen always reliable to rule out prostate cancer recurrence after radical prostatectomy? J Clin Oncol. 2012;30:e341–e344. doi: 10.1200/JCO.2012.43.1767. [DOI] [PubMed] [Google Scholar]
- 32.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 33.Williams SB, Salami S, Regan MM, et al. Selective detection of histologically aggressive prostate cancer: an Early Detection Research Network Prediction model to reduce unnecessary prostate biopsies with validation in the Prostate Cancer Prevention Trial. Cancer. 2012;118:2651–2658. doi: 10.1002/cncr.26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 35.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol Cell Endocrinol. 2012;351:269–278. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Hoda MR, Popken G. Mitogenic and anti-apoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU Int. 2008;102:383–388. doi: 10.1111/j.1464-410X.2008.07534.x. [DOI] [PubMed] [Google Scholar]
- 37.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 38.Zilli T, Chagnon M, Van Nguyen T, et al. Influence of abdominal adiposity, waist circumference, and body mass index on clinical and pathologic findings in patients treated with radiotherapy for localized prostate cancer. Cancer. 2010;116:5650–5658. doi: 10.1002/cncr.25539. [DOI] [PubMed] [Google Scholar]