Abstract
Transition metal-catalyzed cross-coupling reactions have become one of the most utilized carbon–carbon and carbon–heteroatom bond-forming reactions in chemical synthesis. More recently, nickel catalysis has been shown to participate in a wide variety of C–C bond forming reactions, most notably Negishi, Suzuki–Miyaura, Stille, Kumada, and Hiyama couplings1,2. Despite the tremendous advances in C–C fragment couplings, the ability to forge C–O bonds in a general fashion via nickel catalysis has been largely unsuccessful. The challenge for nickel-mediated alcohol couplings has been the mechanistic requirement for the critical C–O bond forming step (formally known as the reductive elimination step) to occur via a Ni(III) alkoxide intermediate. In this manuscript, we demonstrate that visible light-excited photoredox catalysts can modulate the preferred oxidation states of nickel alkoxides in an operative catalytic cycle, thereby providing transient access to Ni(III) species that readily participate in reductive elimination. Using this synergistic merger of photoredox and nickel catalysis, we have developed a highly efficient and general carbon–oxygen coupling reaction using abundant alcohols and aryl bromides. More significantly, we have developed a general strategy to “switch on” important yet elusive organometallic mechanisms via oxidation state modulations using only weak light and single-electron transfer (SET) catalysts.
Visible light-mediated photoredox catalysis has gained momentum over the last decade as a platform for the development of novel synthetic transformations via the implementation of non-traditional open-shell mechanisms. This catalysis field employs transition metal polypyridyl complexes or organic dyes that, upon excitation by visible light, readily engage in an array of single-electron transfer (SET) processes3–5 that often include oxidation, reduction, or redox pathways that have previously been elusive6–9. Indeed, several research groups have demonstrated that photoredox catalysis can be combined with transition metal catalysis to achieve a series of unique bond forming reactions that take advantage of the known reactivity of each individual mode of catalysis10–16. We recently questioned whether the combination of photoredox catalysis and transition metal catalysis might be capable of delivering fundamentally new organometallic reactivity by providing access to currently unknown or inaccessible mechanistic pathways.
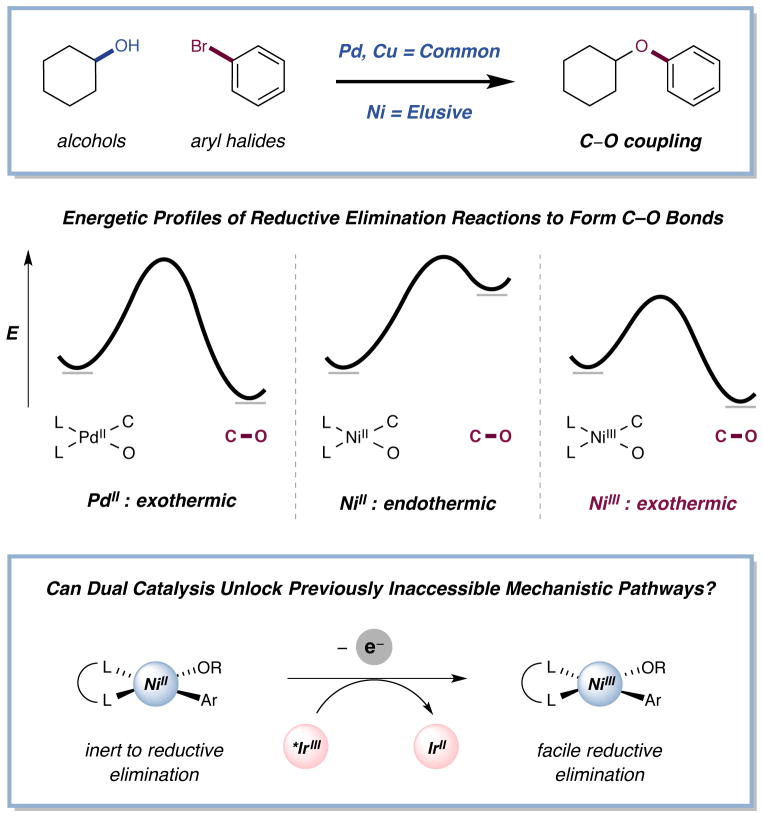
In a series of seminal studies, Hillhouse and coworkers demonstrated that Ni(II) alkoxide complexes do not readily undergo reductive elimination at ambient or elevated temperature and that stoichiometric conversion to a less stable Ni(III) system is required for productive C–O bond formation17–19. Similarly, Sanford and Mirica have recently demonstrated C–heteroatom reductive elimination when stoichiometrically accessing Ni(IV) intermediates20,21. In fact, computational studies have revealed that Ni(II) alkoxide reductive elimination is endothermic, in contrast to Pd, Pt, or alkyl Ni(II) variants which are exothermic (Fig. 1)22. Indeed, the use of palladium and copper catalysis to generate aryl ethers from alcohols, as demonstrated by Buchwald, Hartwig, and others, is well precedented and broadly employed23–25. The utilization of nickel catalysis in C–O bond construction would be an important complementary method, considering the diversity of electrophiles amenable to nickel cross couplings, such as alkyl halides and aryl pseudohalides1,2. Hartwig and coworkers have reported a Ni(COD)2 catalyzed aryl etherification at elevated temperature using three preformed oxides, namely sodium methoxide, ethoxide, and tert-butyldimethylsiloxide with electron-deficient aryl bromides26. However, a general Ni-catalyzed C–O coupling strategy under mild conditions has thus far been elusive and no examples with alcohols have been documented. We recently questioned whether photoredox catalysis might be used to modulate the range of nickel alkoxide oxidation states that can be accessed during a conventional catalytic cycle. More specifically, we recognized that it should be possible to utilize the photonic energy of weak visible light to thermodynamically expand the number of Ni-catalyst oxidation states via two discrete photocatalyst SET events: (i) oxidation of Ni(II) to the elusive Ni(III)-alkoxide complex (which we assumed would facilitate the critical C–O bond forming step) and thereafter (ii) reduction of Ni(I) to Ni(0), to enable the subsequent aryl bromide oxidative addition steps. If successful, we recognized that this new synergistic catalysis pathway might provide (a) the first general example of a nickel-catalyzed C–O coupling reaction employing simple alcohols and aryl halides, and (b) a demonstration of a new strategy by which elusive organometallic couplings can be “switched on” via oxidation state modulation.
Figure 1. Modulating oxidation states of nickel enables challenging carbon–heteroatom coupling.
Palladium(II) catalyzed C–O reductive elimination is an exothermic process and well precedented. Nickel(II) C–O reductive elimination is thermodynamically disfavored – we postulated that accessing Ni(III) by photoredox-mediated oxidation state manipulation could switch on C–O coupling in a general fashion, circumventing this thermodynamic restriction.
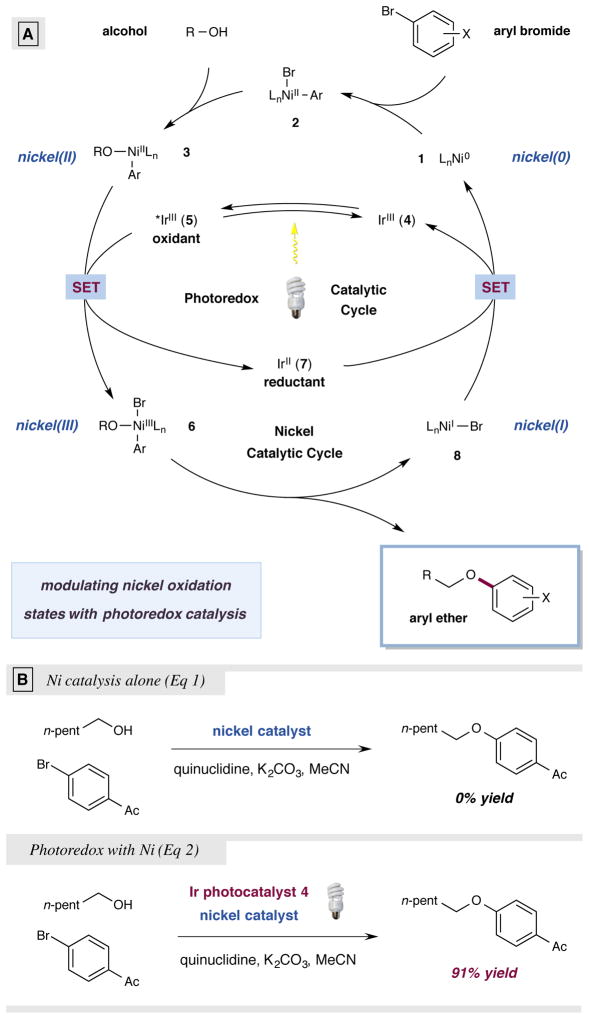
A detailed description of our proposed mechanistic cycle for the photoredox/nickel-catalyzed C–O coupling is outlined in Fig. 2A. Oxidative addition of Ni(0) 1 into an aryl bromide would deliver a Ni(II) aryl complex 227. At this stage, ligand exchange (displacement of the bromide ion with the substrate alcohol) would produce the Ni(II) aryl alkoxide 3 –an organometallic species which traditionally represents a catalytic “dead end.” At the same time, visible light irradiation of heteroleptic iridium(III) photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 [dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine, dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine] (4) would produce the long-lived photoexcited *IrIII state 5 (τ = 2.3 μs)28. At this juncture, we hypothesized that the nickel and photoredox cycles would merge via single-electron transfer (SET) between the Ni(II) complex 3 and the highly oxidizing photo-excited *Ir(III) 5 to generate the critical Ni(III) aryl alkoxide 6 and the reduced Ir(II) photocatalyst 7. Based on the established redox potentials of these catalytic intermediates, we envisioned this key electron transfer step to be kinetically and thermodynamically favorable under standard reaction conditions (E1/2red [*IrIII/IrII] = +1.21 V vs. SCE in CH3CN, E1/2red [NiIII/NiII] = +0.71 V vs. Ag/AgCl in CH2Cl2 for (bpy)NiII(Mes)OMe)28,29. Once formed, we assumed the transient Ni(III) complex 6 would rapidly undergo reductive elimination to forge the desired C–O bond, while delivering the aryl ether product and Ni(I) complex 8. At this stage, we envisioned both catalytic cycles to merge for a second time, enabling the single-electron reduction of Ni(I) to Ni(0) by Ir(II) species 7 (E1/2red [IrIII/IrII] = −1.37 V vs. SCE in CH3CN), thereby completing the nickel and photoredox catalytic cycles at the same moment28.
Figure 2. Photoredox catalysis switches on challenging nickel-catalyzed C–O coupling: proposed mechanism.
(a) The catalytic cycle begins with oxidative addition of Ni(0) 1 into an aryl bromide to give Ni(II) aryl 2. Ligand exchange with an alcohol under basic conditions produces Ni(II) aryl alkoxide 3. Excitation of photocatalyst 4 gives the excited state 5, which can then oxidize 3 to the key Ni(III) intermediate 6 and generate Ir(II) 7. Ni(III) 6 readily reductively eliminates to form the aryl ether product and Ni(I) 8. A second SET event closes the two catalytic cycles, regenerating Ni(0) 1 and Ir(III) 4. (b) Nickel-catalyzed C–O reductive elimination can be turned on by addition of a photoredox catalyst and visible light.
Based on this synergistic catalysis design plan, we began our investigations into the proposed nickel-catalyzed aryl–alcohol etherification using 1-hexanol and 4-bromoacetophenone. As expected, the use of traditional nickel cross-coupling conditions (i.e. without photoredox), led to no observable product despite the implementation of a range of Ni(II) and Ni(0) complexes (Fig. 2B, Eq 1). In contrast, we were delighted to find that the desired C–O bond could be forged in excellent yield (86%) at room temperature via the introduction of the photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 in the presence of Ni(COD)2, dtbbpy, quinuclidine, K2CO3, and blue LEDs as the visible light source. Given the inherent advantages of employing a bench-stable nickel(II) catalyst in lieu of Ni(COD)2, we hypothesized that a catalytically active Ni(0) species 1 might be accessible in situ via two SET reductions of (dtbbpy)Ni(II)Cl2 using the iridium photocatalyst (E1/2red [IrIII/IrII] = −1.37 V vs. SCE in CH3CN, E1/2red [NiII/Ni0] = −1.2 V vs. SCE in DMF)28,30. In this vein, we hypothesized that quinuclidine may also serve as a sacrificial reductant and were pleased to observe that with NiCl2(dtbbpy) the desired fragment etherification was accomplished in 91% yield (Fig. 2B, Eq 2).
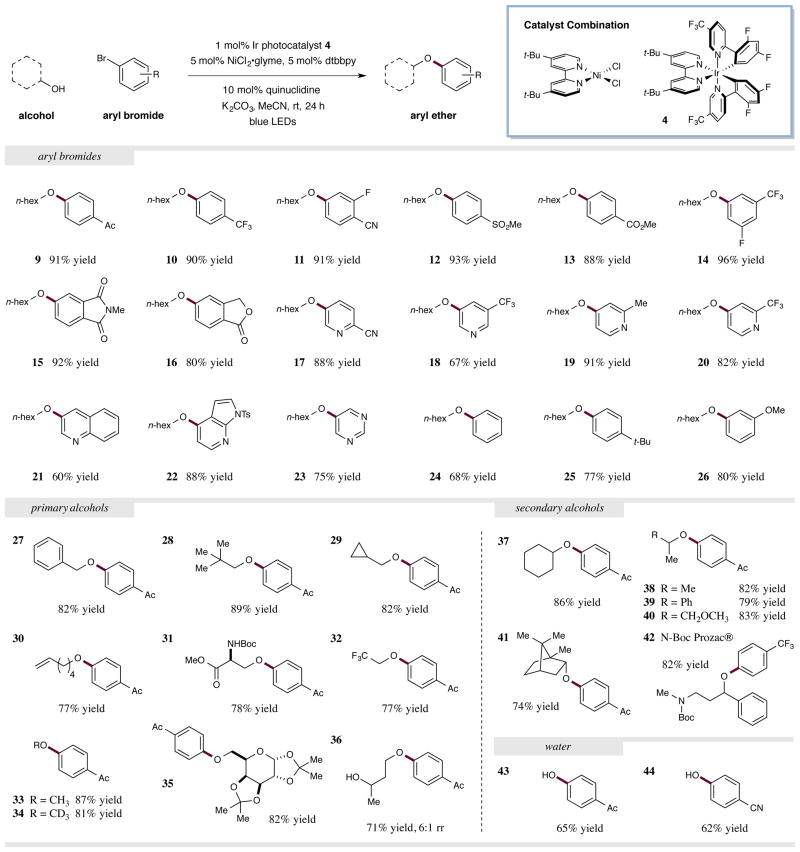
With the optimal conditions in hand, we next sought to explore the scope of the aryl bromide component in this new nickel-catalyzed C–O coupling reaction. As shown in Fig. 3, a diverse array of electron-deficient bromoarenes with a variety of functional groups (ketones, trifluoromethyls, nitriles, sulfones, esters) perform well using this synergistic protocol (9–14, 88–96% yield). Moreover, 3-bromoanisole was found to be a competent substrate in this transformation, demonstrating the diversity of substituents that can be tolerated (26, 80% yield). Notably, bicyclic aromatics such as phthalimides and phthalides couple with high levels of efficiency (15 and 16, 92% and 80% yield). With respect to heteroaromatic coupling partners, we have found that a range of pyridines with substitution at the 2 and 3 positions (nitrile, trifluoromethyl, methyl) are effective electrophiles in this protocol (17–20, 67–91% yield). Furthermore, quinolines, azaindoles, and pyrimidines can be employed without loss in efficiency (21–23, 60–88% yield). Perhaps most important, this transform is not limited to electron-deficient aryl bromides. For example, phenyl and tert-butylphenyl can be readily incorporated in this nickel etherification (24 and 25, 68% and 77% yield). It should be noted that comparable yields of aryl ether product 13 were achieved when either coupling partner was employed in excess (1.5 equiv.) (see Supplementary Information).
Figure 3. Alcohol and aryl halide scope in the photoredox-nickel catalyzed C–O coupling reaction.
A broad range of aryl bromides and alcohols are efficiently coupled to produce aryl ethers under the standard reaction conditions (top, generalized reaction). The aryl bromide scope includes electron deficient and electron neutral arenes and heteroarenes with diverse functionalities. Both primary and secondary alcohols are proficient coupling partners under the standard conditions. Water can be employed as the nucleophile to generate phenol derivatives in a single step. Isolated yields are indicated below each entry. See Supplementary Information for experimental details.
We next turned our attention to the alcohol reaction component. As continued in Fig. 3, we were delighted to discover that a host of primary alcohols were effective coupling partners, including substrates that incorporate benzyl, tert-butyl, cyclopropyl, and alkene functionalities (27–30, 77–89% yield). Moreover, carbamates and ester groups are tolerated, as exemplified by the etherification of a serine derivative in 83% yield (31). Interestingly, trifluoroethanol couples proficiently in this reaction despite its diminished nucleophilicity (32, 77% yield). Both methanol and d4-methanol are effective substrates, providing a valuable strategy for installing H3CO– and D3CO– groups on arenes (33 and 34, 87% and 81% yield).
Relatively complex substrates are also tolerated in this transformation as demonstrated by the etherification of a protected pyranose in excellent yield (35, 82% yield). Notably, secondary alcohols are equally effective in this etherification protocol – alkyl, benzyl, and protected ether functionalities all being amenable to this technology (37–40, 79–86% yield). Moreover, secondary alcohols possessing β-quaternary carbons could be employed with good levels of efficiency (41, 74% yield). In the case of a substrate possessing both a primary and secondary alcohol, the less hindered site is arylated predominantly, as demonstrated by product 36 (71% yield, 6:1 rr). This nickel-catalyzed process can be applied to the expedient synthesis of medicinally relevant molecules, such as the antidepressant Prozac® in three chemical steps (42, 82% yield for coupling step). It is important to note that using H2O as the nucleophile delivers the corresponding phenol product in a single step from commercially available materials (43 and 44, 65% and 62% yield).
Based on our proposed design plan (Fig. 2A), we initiated mechanistic studies to determine if the critical Ni(III) oxidation state was indeed operative in this new photoredox protocol. As shown in Fig. 4A, we preformed the stable Ni(II)(aryl)(alkoxide) complex 45 to examine whether C–O reductive elimination might be achieved under various photoredox or non-photoredox conditions. Importantly, in the absence of either light or photocatalyst, none of the desired aryl ether product 46 was observed. However, when complex 45 was exposed to Ir[dF(CF3)ppy]2(dtbbpy)PF6 (4) and a visible light source, the desired ether product 46 was formed in 59% yield. We attribute this result to the formation of a transient Ni(III) complex 47 via the single-electron reduction of *IrIII, thereby enabling the favorable Ni(III) C–O reductive elimination, a result which is consistent with the seminal studies of Hillhouse. Furthermore, cyclic voltammetry of complex 45 showed an irreversible oxidation at +0.83 V vs. SCE in CH3CN (Fig. 4B), which we attribute to the oxidation of Ni(II) to Ni(III) (45 to 47). As such, we expect the oxidation of Ni(II) aryl alkoxides such as 3 to be readily accomplished by *IrIII photocatalyst 5 (E1/2red [*IrIII/IrII] = +1.21 V vs. SCE)28. Finally, Stern–Volmer fluorescence quenching experiments have demonstrated that the emission intensity of *IrIII 5 is diminished in the presence of Ni(II) complex 45, presumably signifying an oxidation event to form Ni(III) 47 (see Supplementary Information).
Figure 4. Mechanistic studies support intermediacy of transient Ni(III) complex to enable C–O reductive elimination.
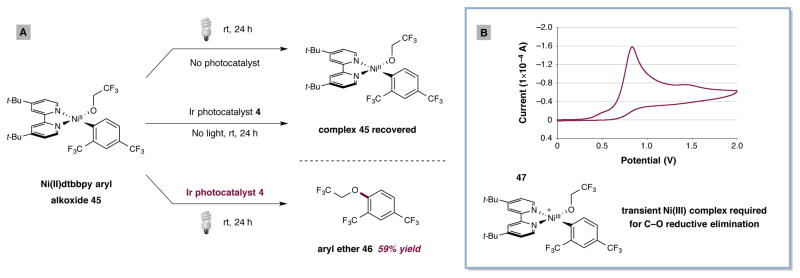
(a) Reductive elimination to form C–O bond only occurs in the presence of photocatalyst and light. Reactions performed on 5.55 μmol scale with 41 mol% photocatalyst 1 and blue LEDs. See supplementary information for experimental details. (b) Cyclic voltammogram of 45 shows NiIII/NiII couple at +0.83 V vs. SCE in CH3CN with 0.1M tetrabutylammonium hexafluorophosphate as the supporting electrolyte at 100 mVs−1.
A further series of experiments were performed utilizing Ni(COD)2 as the nickel catalyst in lieu of NiCl2 (see supplementary text). In the presence of this Ni(0) catalyst, the reaction proceeds with yields that are comparable to the Ni(II) precatalyst; however, when the iridium photocatalyst is omitted, the desired C–O coupling is not observed. This result suggests again that reductive elimination does not occur from the Ni(II) oxidation state as a single-electron oxidation to Ni(III) is required to enable product formation. Interestingly, when quinuclidine is omitted, the reaction efficiency is greatly diminished (8% yield over 24 hours). This effect can possibly be attributed to the amine functioning as an electron shuttle, facilitating reduction of Ni(I) and oxidation of Ni(II); however, mechanistic studies are currently ongoing to elucidate the role of quinuclidine in more detail.
In summary, we have developed a catalytic strategy for accessing transient Ni(III) complexes through the use of visible light-mediated photoredox catalysis for applications in various challenging C–O cross couplings. This method of modulating transition metal oxidation states has enabled a previously elusive transformation within the realm of nickel catalysis. We anticipate that this new mechanistic paradigm will find application in utilizing nickel and other transition metals in a series of challenging bond constructions.
Supplementary Material
Acknowledgments
Financial support was provided by NIHGMS (R01 GM093213-01) and kind gifts from Merck, AbbVie, and Bristol-Myers Squibb. J.A.T. thanks Bristol-Myers Squibb for a Graduate Fellowship. J.D.C. thanks Marie Curie Actions for an International Outgoing Fellowship. The authors thank Eric R. Welin for assistance in preparing Ni(II) complexes.
Footnotes
Author Contributions J.A.T., J.D.C., and V.W.S. performed and analyzed experiments. J.A.T., J.D.C., V.W.S., and D.W.C.M. designed experiments to develop this reaction and probe its utility, and also prepared this manuscript.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Tasker SZ, Standley EA, Jamison TF. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netherton MR, Fu GC. Nickel-catalyzed cross-couplings of unactivated alkyl halides and pseudohalides with organometallic compounds. Adv Synth Catal. 2004;346:1525–1532. [Google Scholar]
- 3.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem Soc Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 4.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz DM, Yoon TP. Solar synthesis: Prospects in visible light photocatalysis. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicewicz DA, MacMillan DWC. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ischay MA, Anzovino ME, Du J, Yoon TP. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J Am Chem Soc. 2008;130:12886–12887. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- 8.Narayanam JMR, Tucker JW, Stephenson CRJ. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J Am Chem Soc. 2009;131:8756–8757. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- 9.Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science. 2013;339:1593–1596. doi: 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkinson MN, Sahoo B, Li JL, Glorius F. Dual catalysis sees the light: combining photoredox with organo-, acid, and transition-metal catalysis. Chem Eur J. 2014;20:3874–3886. doi: 10.1002/chem.201304823. [DOI] [PubMed] [Google Scholar]
- 11.Osawa M, Nagai H, Akita M. Photo-activation of Pd-catalyzed Sonogashira coupling using a Ru/bipyridine complex as energy transfer agent. Dalton Trans. 2007:827–829. doi: 10.1039/b618007h. [DOI] [PubMed] [Google Scholar]
- 12.Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. Room-temperature C–H arylation: merger of Pd-catalyzed C–H functionalization and visible-light photocatalysis. J Am Chem Soc. 2011;133:18566–18569. doi: 10.1021/ja208068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y, Sanford MS. Merging visible-light photocatalysis and transition-metal catalysis in the copper-catalyzed trifluoromethylation of boronic acids with CF3I. J Am Chem Soc. 2012;134:9034–9037. doi: 10.1021/ja301553c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahoo B, Hopkinson MN, Glorius F. Combining gold and photoredox catalysis: visible light-mediated oxy- and aminoarylation of alkenes. J Am Chem Soc. 2013;135:5505–5508. doi: 10.1021/ja400311h. [DOI] [PubMed] [Google Scholar]
- 15.Tellis JC, Primer DN, Molander GA. Single-electron transmetalation in organoboron cross-coupling by photo-redox/nickel dual catalysis. Science. 2014;345:433–436. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsunaga PT, Hillhouse GL, Rheingold AL. Oxygen-atom transfer from nitrous oxide to a nickel metallacycle. Synthesis, structure, and reactions of [cyclic] (2,2′-bipyridine)Ni(OCH2CH2CH2CH2) J Am Chem Soc. 1993;115:2075–2077. [Google Scholar]
- 18.Matsunaga PT, Mavropoulos JC, Hillhouse GL. Oxygen-atom transfer from nitrous oxide (N=N=O) to nickel alkyls. Syntheses and reactions of nickel(II) alkoxides. Polyhedron. 1995;14:175–185. [Google Scholar]
- 19.Han R, Hillhouse GL. Carbon–oxygen reductive-elimination from nickel(II) oxametallacycles and factors that control formation of ether, aldehyde, alcohol, or ester products. J Am Chem Soc. 1997;119:8135–8136. [Google Scholar]
- 20.Camasso NM, Sanford MS. Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes. Science. 347:1218–1220. doi: 10.1126/science.aaa4526. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Schultz JW, Rath NP, Mirica LM. Aromatic methoxylation and hydroxylation by organometallic high-valent nickel complexes. J Am Chem Soc. doi: 10.1021/jacs.5b04082. [DOI] [PubMed] [Google Scholar]
- 22.Macgregor SA, Neave GW, Smith C. Theoretical studies on C–heteroatom bond formation via reductive elimination from group 10 M(PH3)2(CH3)(X) species (X = CH3, NH2, OH, SH) and the determination of metal–X bond strengths using density functional theory. Faraday Discuss. 2003;124:111–127. doi: 10.1039/b212309f. [DOI] [PubMed] [Google Scholar]
- 23.Torraca KE, Huang X, Parrish CA, Buchwald SL. An efficient intermolecular palladium-catalyzed synthesis of aryl ethers. J Am Chem Soc. 2001;123:10770–10771. doi: 10.1021/ja016863p. [DOI] [PubMed] [Google Scholar]
- 24.Wolter M, Nordmann G, Job GE, Buchwald SL. Copper-catalyzed coupling of aryl iodides with aliphatic alcohols. Org Lett. 2002;4:973–976. doi: 10.1021/ol025548k. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C–C, C–N, and C–O bond-forming cross-couplings. J Org Chem. 2002;67:5553–5566. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 26.Mann G, Hartwig JF. Nickel- vs. palladium-catalyzed synthesis of protected phenols from aryl halides. J Org Chem. 1997;62:5413–5418. [Google Scholar]
- 27.Amatore C, Jutand A. Rates and mechanism of biphenyl synthesis catalyzed by electrogenerated coordinatively unsaturated nickel complexes. Organometallics. 1988;7:2203–2214. [Google Scholar]
- 28.Lowry MS, Goldsmith JI, Slinker JD, Rohl R, Pascal RA, Malliaras GG, Bernhard S. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem Mater. 2005;17:5712–5719. [Google Scholar]
- 29.Klein A, Kaiser A, Wielandt W, Belaj F, Wendel E, Bertagnolli H, Záliš S. Halide ligands–more than just σ-donors? A structural and spectroscopic study of homologous organonickel complexes. Inorg Chem. 2008;47:11324–11333. doi: 10.1021/ic8007365. [DOI] [PubMed] [Google Scholar]
- 30.Durandetti M, Devaud M, Perichon J. Investigation of the reductive coupling of aryl halides and/or ethylchloroacetate electrocatalyzed by the precursor NiX2(bpy) with X− = Cl−, Br− or MeSO3− and bpy = 2,2′-dipyridyl. New J Chem. 1996;20:659–667. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.