Abstract
Rationale
Despite epidemiological and clinical data indicating marked gender differences in alcohol use disorders (AUDs), few preclinical studies have examined sex differences in animal models of AUDs.
Objective
The purpose of this study was to first characterize sex differences in ethanol consumption and reinforcement in an alcohol preferring (P) rat model of alcoholism, then use this model to screen pharmacological treatments for sex-specific effects.
Methods
Ethanol consumption was first assessed in male and female P rats under a three-bottle free-choice procedure. Next, ethanol’s reinforcing effects were assessed under a fixed-ratio 1 (FR1) schedule followed by a progressive-ratio (PR) schedule. Finally, the effects of two pharmacological treatments for AUDs, naltrexone (1 mg/kg) and topiramate (10 or 20 mg/kg), alone and in combination, were tested for sex-specific differences in their efficacy at reducing ethanol’s reinforcing effects.
Results
Although females initially had higher consumption of and preference for ethanol, male rats increased their consumption and preference over time and rapidly became equal to females. Following prolonged 24-hr/day access, males and females self-administered similar levels of ethanol under FR1 and PR schedules. In response to pharmacological treatment, we observed some sex differences and similarities, most notably, a more robust effect of the combination of naltrexone and topiramate in males as compared to females.
Conclusions
This model of selectively bred P rats may be useful for understanding sex differences in AUDs and related behavior and their underlying neurobiological mechanisms and treatment.
Keywords: Alcohol preferring (P) rats, Ethanol, Sex Differences, Topiramate, Naltrexone, Combination treatment
1. Introduction
Although rates of alcohol use disorders (AUDs) are greater among men than women, research indicates that women may be more vulnerable to certain aspects of AUDs (Brady and Randall, 1999; Substance Abuse and Mental Health Services Administration, 2004). Women often show an accelerated path to dependence, termed “telescoping”, and incur health consequences at a faster rate than men (i.e. liver cirrhosis, Hepatitis C, alcohol-induced brain damage; Mann et al. 2005; Brady and Randall, 1999; Lynch et al. 2002). Women also become intoxicated and have higher blood alcohol concentrations after lower quantities of alcohol consumed than men (Marshall et al. 1983), which may contribute to an increased vulnerability in women. There are also gender differences in ethanol-induced dopamine release, reuptake, and density of receptors in the reward pathway (Carroll, 2004; Blanchard and Glick, 1999) suggesting that the neurobiology of AUDs and its treatment may differ between males and females.
Despite these sex differences, females are very much underrepresented in research on AUDs, particularly in preclinical studies examining its neurobiological basis and potential treatments. This may be attributable in part to the belief that rodents may not be an appropriate model for studying sex differences in AUDs given that sex differences observed in rodents typically do not correspond to humans. Specifically, in contrast to the human data, which show similar or higher levels of consumption in men (Keyes et al. 2008), preclinical studies using outbred rat strains consistently report higher consumption and preference for ethanol in females (Lancaster and Spiegel, 1992; Almeida et al. 1998; Juárez and De Tomasi, 1999; Cailhol and Mormede, 2001; Vetter O’Hagan et al. 2009). Female rats and mice also acquire ethanol self-administration more quickly (Carroll and Anker, 2010; Melón et al., 2013) and escalate their intake over time to a greater extent than males (Melón et al. 2013). Notably, sex differences in ethanol intake are less robust or absent in some strains selectively bred for high ethanol drinking (i.e. Alcohol Preferring (P) rats, High Alcohol Drinking (HAD) rats, High-Ethanol Preferring (HEP); McKinzie et al. 1998; Dhaher et al. 2012; Terenina-Rigaldie et al. 2004; Mormede et al. 2004), depending on the parameters and methods used to measure ethanol drinking. The P line of rats has a phenotype with construct and face validity for an animal model of AUDs (Bell et al. 2006a; Bell et al. 2014), as well as pharmacological treatments for AUDs (Bell et al. 2012). Thus, we are now interested in investigating P rats as a model of sex differences specifically, as it is important to validate for future preclinical research. To date, only one other study has examined sex differences in ethanol consumption and preference using a long duration of continuous access in P rats (Bell et al. 2011). In this study, we wanted to further investigate these sex differences under prolonged ethanol intake, as well as across multiple self-administration paradigms and in response to pharmacological treatment.
To evaluate the potential utility of the P line of rats as a model for examining sex differences in AUDs and its treatment, we assessed ethanol drinking and self-administration behaviors through multiple paradigms and over an extended length of time (7–8 months). We first examined ethanol consumption in males and females given prolonged access to ethanol under a 24-hr/day three-bottle free-choice procedure, a paradigm that has been shown to result in physiologically relevant levels of ethanol consumption in P rats (Bell et al. 2006). We then assessed ethanol’s reinforcing effects in these same animals with a fixed-ratio 1 (FR1) schedule followed by a progressive-ratio (PR) schedule. Finally, we were interested in the effects of two pharmacological treatments for AUDs, naltrexone and topiramate, on the reinforcing effects of ethanol as these have shown promise in treating AUDs in humans, but whether their efficacy varies based on sex/gender is still unclear. We have shown previously that the combination of topiramate and naltrexone, as well as a 20 mg/kg dose of topiramate alone decreases ethanol consumption in males (Moore et al 2014), potentially through decreasing ethanol’s reinforcing effects. Following baseline responding for ethanol, these medications, alone and in combination, were tested for sex-specific differences in reducing ethanol’s reinforcing effects as assessed under a PR schedule. We used a within-subjects design, enabling us to compare the same cohort of males and females in multiple paradigms. Additionally, we began treatments after ~6 months of ethanol experience in order to more closely model the chronic exposure that is characteristic of human AUDs. As the targets of these treatments (i.e. GABA, glutamate, and opioid receptor) are altered over the course of chronic alcohol use, this model of prolonged access may also be more neurobiologically relevant (Eravci et al. 2000; Darstein et al. 1998; Hölter et al. 2000).
2. Materials and Methods
2. 1 Animals and Housing
Male (n=24) and female (n=24) P rats from the 73–74th generations were selectively bred at Indiana Alcohol Research Center’s Animal Production Core. Rats were single-housed in clear, polycarbonate cages within the same colony room maintained on a 12:12 light/dark cycle. Food and water were provided ad libitum. Rats were 11–12 weeks old upon arrival and were given a 1-week habituation period before ethanol consumption began. Animal health and weight was monitored daily by trained laboratory staff. All animal protocols were approved by the Animal Care and Use Committee at the University of Virginia. Rats were between 12–15 months at the end of the treatment phase; final weights were ~500g for males and ~ 320g for females. The same cohorts of males and females were used for each procedure, and these cohorts were run simultaneously.
2.2 Drugs
Ethanol solutions (8, 10, 16% v/v) were prepared from 190 proof absolute ethyl alcohol (Pharmco-Aaper brand, Brookfield, CT, USA) and diluted using tap water. Naltrexone HCl and Topiramate HCl were purchased from Sigma-Aldrich (St. Louis, MO). All compounds were dissolved in 0.9% sodium chloride and sterile water. Doses of naltrexone (1 mg/kg) and topiramate (10, 20 g/kg) were chosen based on previous studies demonstrating the ability of these doses to reduce ethanol consumption/reinforcement in male P rats (Lynch et al. 2011; Moore et al. 2014).
2.3 Ethanol consumption: Three-bottle free choice access
Sex differences in ethanol consumption were examined under prolonged 24-hr/day access as previously described (Moore et al. 2014). Briefly, all animals were given access to two concentrations of ethanol solution (8 and 16% v/v) along with the bottle containing water. For three months, intake of ethanol, water, and ad libitum food were measured daily.
2.4 Ethanol self-administration under a FR1 and a PR schedule
Ethanol’s reinforcing effects were examined in males and females (n’s=23; 1 male and 1 female were lost to health complications) following the ethanol consumption period under a FR1 schedule as described previously (Moore et al. 2014). Briefly, ethanol was removed from the home cages and rats were trained to self-administer 10% v/v ethanol in operant testing chambers 6 days/week. During the session, a single response on the left (water-associated lever) or right (ethanol-associated lever) lever led to a delivery of 0.1 ml of either liquid. Animals were moved back into their polycarbonate cages following the end of each 60-minute session, where food and water were provided ad libitum.
Once responding stabilized under FR1 conditions (defined as variation less than 20% over three consecutive days), animals were placed on a PR schedule to measure motivation to obtain 0.1 ml deliveries of 10% v/v ethanol using methods previously described (Moore et al. 2014). With this schedule, animals must respond for deliveries of ethanol and water at higher levels for each subsequent delivery in the following steps: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, etc. After 30 minutes without a delivery on either lever, the session ended. Motivation for ethanol was determined by the breakpoint, or the last ratio completed (defined by the number of deliveries obtained within each session).
2.5 Effect of an ethanol deprivation period on PR responding
To examine “relapse-like” behavior, we used an alcohol deprivation effect (ADE) paradigm measuring changes in ethanol’s reinforcing effects following a deprivation. Increased consumption following a deprivation is believed to reflect propensity to relapse and previous work has demonstrated that an ADE occurs in both male and female P rats (Sinclair and Li, 1989; Bell et al. 2008). However, little is known regarding the deprivation’s effect on ethanol’s reinforcing effects or sex differences. After a minimum of 2 weeks and a 3-day stable baseline under a PR schedule, animals began a 7-day deprivation phase. This length was chosen based on previous studies showing 7 days as sufficient to induce an increase in ethanol intake (Sinclair and Li, 1989; Heyser et al. 1997). Animals remained in their home cages during the deprivation phase with PR testing resuming following the 7th day.
2.6 Effect of naltrexone and topiramate on ethanol reinforcement
The effects of topiramate, naltrexone, and the combination of topiramate and naltrexone on ethanol’s reinforcing effects were also investigated in these same rats (n=22 males, n=23 females; 1 additional male was lost to health complications). Methods used were previously described (Moore et al. 2014). Briefly, on test days, topiramate (10 or 20 mg/kg), naltrexone (1 mg/kg), their combination (10mg/kg topiramate/1 mg/kg naltrexone), or an equal volume of saline was administered intraperitoneally 30 minutes prior to the start of the session. Treatments were given in random order at least 7 days apart after re-establishing baseline responding. Under this PR schedule, which constrains the amount of ethanol received daily, some animals did not continue to reliably self-administer ethanol (i.e., deliveries were variable and low), and the PR data from these animals were excluded from analysis (4 males and 1 female). The majority of the animals, however, did maintain high levels of responding ethanol (12 deliveries; roughly 150 responses), despite only consuming low levels of ethanol for a prolonged period of time. Thus, our analysis of treatment effects on PR responding for ethanol focused on only the animals that were reliably responding for ethanol at high levels (n=18 males and n=22 females). Animals qualified for treatments after self-administering a minimum of 12 deliveries for a 3-day stable baseline. Each animal that qualified received between 2–3 treatments. The effects of these treatments on PR responding for ethanol have been previously published in a larger group of males (n=22; Moore et al. 2014); however, in this study, we focused on only a subset of this larger group that maintained high-levels of responding for ethanol. All other data from this male cohort (i.e. ethanol consumption, FR1 responding, PR responding at baseline, and ADE) have not previously been published. Importantly, the all the males presented in this study were run simultaneously with the females to allow for an examination of sex differences.
2.7 Control for sex difference in ethanol dose/delivery
Following the completion of the treatment phase, we adjusted the volume of ethanol/delivery to equalize for g/kg concentration. This was done to control for body weight differences between males and females. Each delivery of ethanol was 0.15 mg/kg with volumes ranging from 0.06–0.14 ml. After 20 days of habituation to the new dose, we measured baseline responding over 10-days.
2.8 Statistical Analysis
Ethanol consumption data were calculated using ethanol’s specific density ((ethanol% * ml consumed* 0.793)/kg body weight) and displayed as g/kg to reflect consumption relative to body weight. Preference scores were calculated as (ml of total ethanol solution consumed/total liquid consumption × 100). Sex differences in baseline ethanol consumption and preference under three-bottle free-choice, as well as deliveries obtained under FR1 and PR conditions were analyzed across all days using a repeated-measures analysis of variance (ANOVA). Additional analyses were conducted with values collapsed into 10-day averages. Sex differences in average intake or preference collapsed across all days were determined using a Student’s t-test. For pharmacological manipulations, the main dependent measure was the number of ethanol deliveries obtained during the PR sessions at baseline (the average number of deliveries on the three days preceding treatment) and on the day of treatment using a repeated measures ANOVA to detect changes from baseline. Following a significant interaction indicating differential treatment effects between sexes, separate analyses were conducted within each treatment comparing males and females also using repeated measures ANOVAs. Post-hoc comparisons with vehicle were conducted using Dunnett’s t-test. Similar statistics were used to analyze potential treatment side effects on food and water consumption in the home cage and water deliveries during the session. Statistical analyses were performed using SPSS (version 20) with 0.05 as the alpha level for statistical significance.
3. Results
3.1 Ethanol consumption and preference during three-bottle, free-choice access
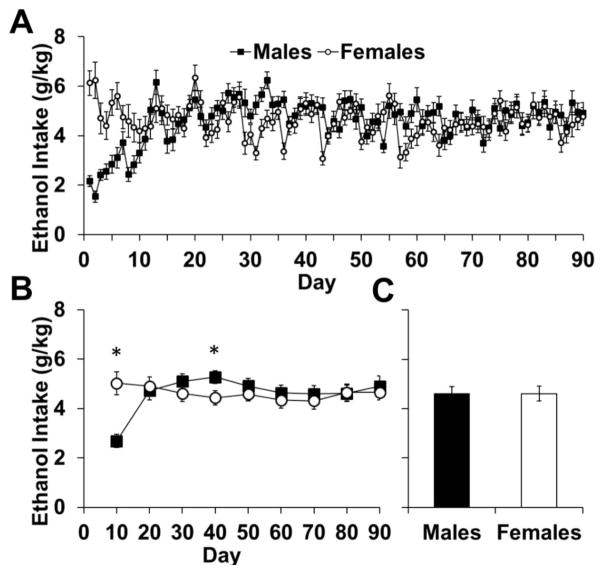
Females readily consumed higher levels of ethanol; however males rapidly increased their intake to levels comparable to females (Fig 1a–c). An analysis of ethanol consumption over 90-days showed an effect of day (F89,4094=6.54, p<0.001) and an interaction of sex-by-day (F89,4094=8.72, p<0.001; Fig 1a). To determine the loci of sex differences, we averaged daily consumption into 10-day blocks. Analyses within each of these blocks showed a sex difference in the first 10-day block (p<0.05; Fig 1b), where females consumed more ethanol than males, and in the block from 30–40 days (p< 0.05), where males consumed more ethanol than females. However, an analysis of average daily intake overall showed no difference between males and females (p>0.05; Fig 1c).
Fig. 1.
Ethanol intake of male (n=24) and female (n=24) rats during three-bottle free choice consumption. (A) Data are plotted as mean (± SEM) daily ethanol consumption (g/kg) for each day. (B) Data are plotted as mean (± SEM) daily ethanol consumption (g/kg) for blocks of 10 days each. (C) Data are plotted as the mean (± SEM) ethanol consumption across all 90 days of ethanol intake. An asterisk (*) represents a significant difference between males and females (p<0.05)
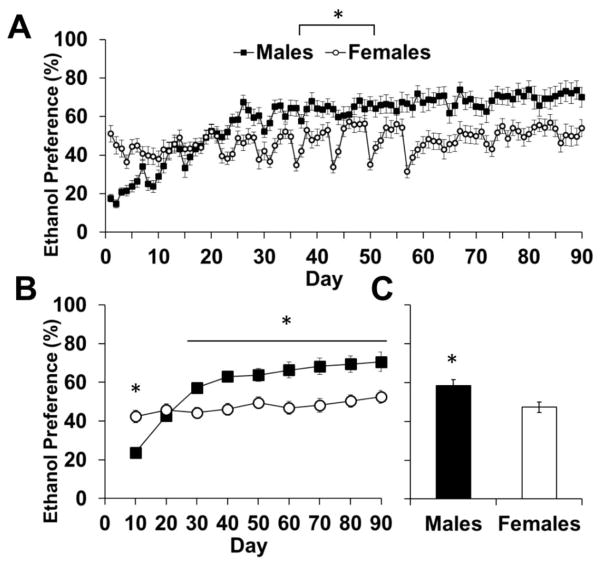
Mirroring the intake data, males initially had lower ethanol preference scores than females, but quickly increased their preference over time, surpassing the females. Females’ ethanol preference score stayed relatively stable and was lower than males overall (Fig 2a–c). An analysis of ethanol preference over 90-days showed an effect of day (F89,4094=18.73, p<0.001) and an interaction of sex-by-day (F89,4094=10.75, p<0.001; Fig 2a). Analyses of each 10-day block showed a sex difference in ethanol preference for all blocks (p<0.05), except for the 2nd block (days 11–20; p>0.05; Fig 2b). Overall preference was higher in males than females (p>0.05; Fig 2c). Thus, while females initially had higher consumption of and preference for ethanol, males quickly increased their levels of consumption to equal females, and increased their preference to a higher level than females.
Fig. 2.
Total ethanol preference of male (n=24) and female (n=24) rats during three-bottle free choice consumption. (A) Data are plotted as mean (± SEM) daily ethanol preference (%) for each day. (B) Data are plotted as mean (± SEM) daily ethanol preference (%) for blocks of 10 days each. (C) Data are plotted as the mean (± SEM) ethanol preference of all 90 days of ethanol consumption
3.2 Comparison of 8% v/v and 16% v/v consumption and preference
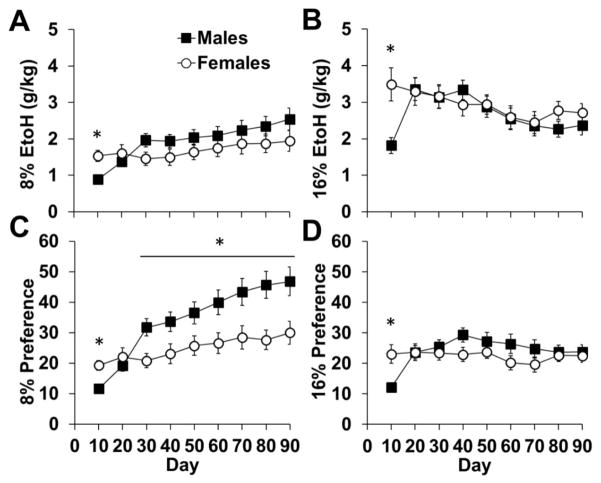
To identify sex differences within concentrations of ethanol, we analyzed consumption of 8% and 16% solutions separately. An analysis of 8% ethanol across 90-days revealed an effect of day (F89,4094=5.12, p<0.001) and an interaction of day-by-sex (F89,4094=3.25, p<0.001); however average intake overall did not differ between males and females (p>0.05; Table 1). Within the 10-day blocks, females consumed more 8% than males in the first block (p<0.05; Fig 3a), and males tended to drink more than females in the 3rd block (p=0.05). An analysis of 16% ethanol across 90-days also revealed an effect of day (F89,4094=4.42, p<0.001) and an interaction of day-by-sex (F89,4094=4.28, p<0.001), but no difference in average intake overall (p>0.05). Within each block, there was an effect of sex for days 1–10 only (p<0.05; Fig 3b), with females consuming more 16% ethanol than males.
Table 1.
Ethanol consumption and preference during 24-hour three bottle free access (mean ± SEM; n’s = 24 for males and females). An asterisk (*) represents a significant difference between males and females (p<0.05)
| All Days (1–90) | First 10 days | Days 11–90 | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Total g/kg | 4.61(0.3) | 4.61 (0.3) | 2.7 (0.3) | 5.0 (0.5)* | 4.9 (0.3) | 4.6 (0.3) |
| 8% g/kg | 1.9 (0.2) | 1.7 (0.2) | 0.9 (0.1) | 1.5 (0.2)* | 2.1 (0.2) | 1.7 (0.2) |
| 16% g/kg | 2.7 (0.2) | 2.9 (0.3) | 1.8 (0.2) | 3.5 (0.5)* | 2.8 (0.2) | 2.9 (0.3) |
| EtOH Preference (%) | 58.3(3.1) | 47.3 (2.6)* | 23.7 (2.4) | 42.4 (3.2)* | 62.6 (3.3) | 47.9 (2.6)* |
| 8% Preference (%) | 34.3 (2.6) | 24.9 (2.4) | 11.6 (0.9) | 19.3 (1.6)* | 37.1 (2.9) | 25.6 (2.6)* |
| 16% Preference (%) | 24.0 (2.0) | 22.4 (2.0) | 12.1 (1.6) | 23.0 (3.0)* | 25.5 (2.1) | 22.3 (2.0) |
Fig. 3.
Ethanol consumption (A and B) and preference (C and D) within different concentrations of ethanol (8 and 16%). Data are plotted as mean (± SEM) daily ethanol consumption (g/kg) for blocks of 10 days each. An asterisk (*) represents a significant difference between males (n=24) and females (n=24; p<0.05).
Similar effects were observed for preference for the two concentrations of ethanol. An analysis of 8% ethanol preference across 90-days revealed an effect of day (F89,4094=9.79, p<0.001) and an interaction of day-by-sex (F89,4094=4.60, p<0.001). Preference for 8% was higher in males than females overall (p<0.05; Table 1). Within the 10-day blocks, there was an effect of sex for days 1–10, with females having a higher preference for 8% than males, and males having a higher preference for 8% than females for each block from day 31–90 (p<0.05; Fig 3c). An analysis of 16% ethanol across the 90-day testing period revealed an effect of day (F89,4094=3.33, p<0.001) and an interaction of day-by-sex (F89,4094=4.02, p<0.001); however average preference overall did not differ between males and females (p>0.05). Within the 10-day blocks, there was a significant effect of sex for days 1–10, with females having a higher preference for 16% than males (p<0.05; Fig 3d). Following the initial 10 days, males steadily increased their preference for 16% ethanol a similar level as females, even being slightly higher than females within the block of days 31–40, however, this effect did not reach statistical significance (p=0.06). Thus, males low preference for ethanol quickly increased, mirroring the increase in consumption. Within the different concentrations of ethanol, males preferred the lower concentration of ethanol (8%), but females showed an equal preference for the low and high (16%).
3.3 Ethanol self-administration under a FR1 and a PR schedule
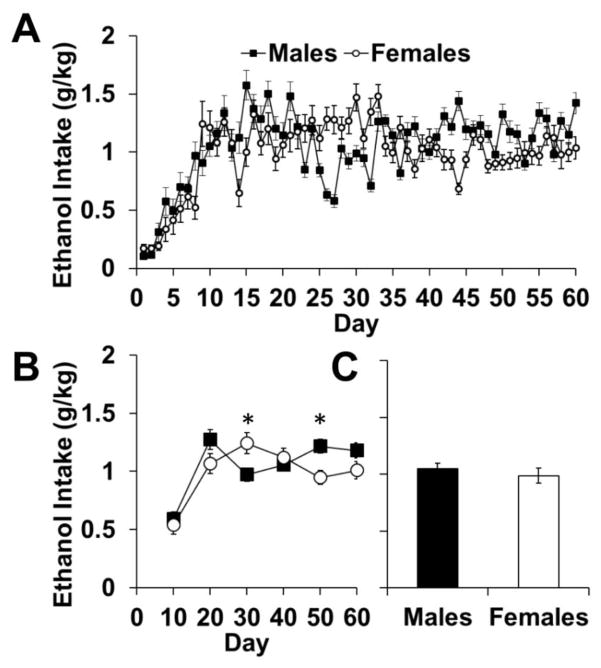
Under an FR1 schedule, males and females self-administered similar levels of ethanol (Fig 4a–c). An analysis of all 60 days showed an effect of day (F59,2419=23.267, p<0.001) and an interaction of day-by-sex (F59,2419=7.555, p<0.001). Analysis of the 10-day blocks showed differences in intake on the third and fifth blocks (p’s<0.05; Fig 4b), reflecting different and non-systematic fluctuation patterns between males and females. However, average daily intake overall showed no difference between males and females (p>0.05; Fig 4c).
Fig. 4.
Ethanol self-administration under a fixed ratio 1 schedule of reinforcement. (A) Data are plotted as mean (± SEM) daily ethanol consumption (g/kg) for each daily testing session. (B) Data are plotted as mean (± SEM) daily ethanol consumption (g/kg) for blocks of 10 days each. (C) Data are plotted as the mean (± SEM) ethanol preference of all 60 days of FR1 ethanol consumption. An asterisk (*) represents a significant difference between males (n=23) and females (n=23; p<0.05).
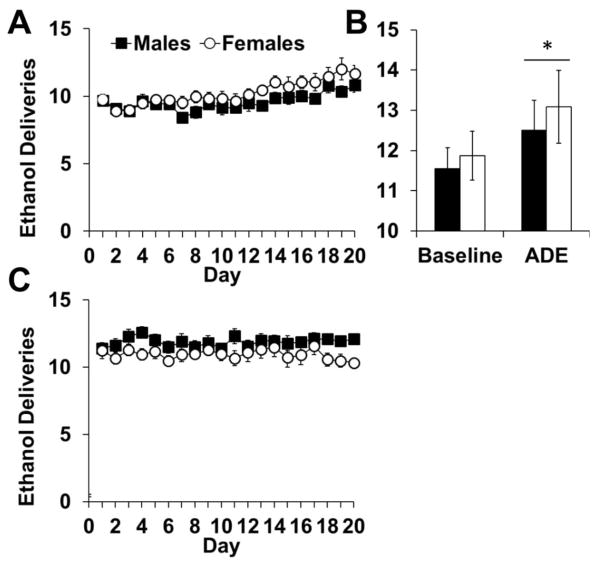
Males and females also responded for similar levels of ethanol under a PR schedule (Fig 5a), and showed a similar increase in ethanol breakpoint over time and in response to a deprivation period (Fig 5b). Under this PR schedule animals consumed low, non-pharmacological levels of ethanol in each session (average baseline: males mean=1.3 ± 0.16 ml/session; female mean=1.36 ± 0.2 ml/session). An analysis of ethanol deliveries obtained for the first 20 days of PR responding showed an effect of day (F19,760=6.930, p<0.0001) but no day-by-sex interaction (p>0.05; Fig 5a), demonstrating slight increases in deliveries over time by both males and females. While an analysis of baseline ethanol deliveries (3 days prior to deprivation) showed no effects of day or sex (p>0.05), an analysis of deliveries at baseline and after the 7-day deprivation period showed an effect of day (F1,43=6.290, p<0.05; Fig 5b), but not sex (p>0.05). Thus, males and females respond at similar levels for ethanol under both a FR1 and PR schedule, and display a similar increase in motivation for ethanol following a 7-day deprivation period.
Fig. 5.
Responding for ethanol under a PR schedule of reinforcement (n=22, males; n=23, females). (A) Data for 20 days of stable PR are plotted as mean (± SEM) daily ethanol deliveries obtained. (B) Responding after a brief 7-day withdrawal period. Data are plotted as mean (± SEM) daily ethanol deliveries during 3 baseline testing sessions and the testing session following deprivation. ADE=Alcohol Deprivation Effect (C) Responding for ethanol when the dose was adjusted for body weight. Data are plotted as mean (± SEM) daily ethanol deliveries obtained. An asterisk (*) represents an increased number of deliveries from baseline.
As a control for body weight differences (females weighed 100–200 g less than males), we adjusted the volume/delivery to equalize dose between the sexes. An analysis of ethanol deliveries obtained over 20 days of PR responding showed no effects of day and no interaction of day-by-sex (p’s >0.05; Fig 5c). Thus, small differences in dose did not affect PR responding.
3.4 Effects of naltrexone and topiramate on ethanol reinforcement
In males and females, topiramate (20 mg/kg dose), naltrexone, and the combination of topiramate (10 mg/kg) and naltrexone reduced motivation for ethanol; with an enhanced effect of the combination on reducing motivation for ethanol in males compared to females (Fig 6a–b). Ethanol deliveries obtained at baseline did not differ between males and females (p’s > 0.05). Comparison of ethanol deliveries at baseline and on the day of treatment revealed a main effect of day (F1,97=172.818, p<0.0001) and interactions of day-by-sex (F1,97=910.217, p<0.01), day-by-treatment (F4,97=14.626, p<0.0001), and day-by-sex-by-treatment (F4,97=2.472, p<0.05). Post-hoc analysis showed that all treatments, except for topiramate alone (10mg/kg), decreased breakpoint from vehicle (p’s<0.05; Fig 6a–b;10 mg/kg topiramate data not shown in 6a for simplicity of figure). Topiramate alone (20 mg/kg) reduced ethanol deliveries from vehicle in males (p<0.05); however in females, this decrease did not reach statistical significance (p=0.07). All other treatments produced significant decreases from vehicle for both males and females (p’s<0.05). Additionally, following treatment with the combination of naltrexone and topiramate, males showed a significantly lower breakpoint from females (day-by-sex-by-drug interaction, F1,40=10.43, p<0.01), indicating an enhanced effect of this combination treatment in males (Fig 6a–b).
Fig. 6.
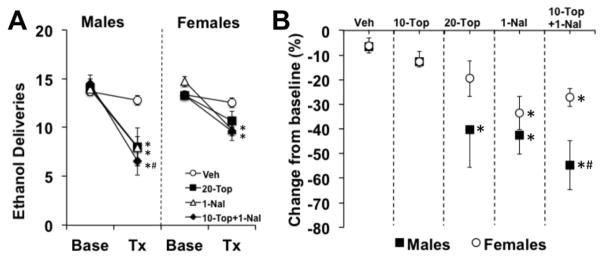
Treatment effects on PR responding for ethanol. (A) Data are plotted as average ethanol deliveries obtained under a PR schedule (± SEM) at baseline (Base) and after acute treatment (Tx). (B) Data are plotted as average percent change from baseline deliveries obtained (± SEM). For both panels, each data point represents an n between 6 and 15. An asterisk (*) represents a significant difference from vehicle (p<0.05), and a pound sign (#) represents a significant difference from females (p<0.05).
To determine any non-specific effects of these treatments, we analyzed food and water intake, and water deliveries obtained in the testing chambers at baseline and on the day of treatment. There was a mild decrease in food intake on the day of treatment indicated by a day-by-treatment interaction(F4,97=25.71, p<0.01), however, post-hoc tests showed no significant effects of treatment compared to vehicle, suggesting that each treatment reduced food intake very mildly (p’s > 0.05; Table 2). Water intake in the home cage was mildly, but differentially affected by treatments in males and females. While there was a significant interaction of day-by-sex-by-treatment on water intake in the home cage (F4,97=2.84, p<0.05), there was no main effect of day, or interactions of day-by-sex or day-by-treatment (p’s > 0.05). Further analyses showed that differences in water intake following treatment was not significant within each treatment compared to vehicle, nor between sexes (p’s > 0.05). Water deliveries obtained during the session were decreased following treatment with topiramate alone (20 mg/kg) and naltrexone alone (day–by-treatment interaction, F4,97=4.54, p<0.01; p’s < 0.05 compared to vehicle). There were no effects of sex on treatment effects on water deliveries (no interactions of day-by-sex or day-by-sex-by-treatment; p’s > 0.05; Table 2).
Table 2.
Nonspecific treatment effects on water deliveries, food, and water consumption in the home cage at baseline and after acute treatment (Mean ± SEM). An asterisk (*) represents a significant difference from vehicle (p<0.05).
| Operant Behavior | Home Cage Consumption | |||||||
|---|---|---|---|---|---|---|---|---|
| Water Deliveries | Food Consumption (g) | Water Consumption (g) | ||||||
| Treatment | Sex | n | Baseline | Acute | Baseline | Acute | Baseline | Acute |
| Vehicle | Males | 13 | 4.1 (1.0) | 3.7 (1.3) | 17.8 (0.4) | 18.4 (1.1) | 19.0 (0.6) | 19.2 (1.4) |
| Females | 15 | 6.4 (0.4) | 6.1 (1.5) | 14.4 (0.5) | 14.0 (0.6) | 21.7 (1.3) | 21.6 (1.6) | |
| 10-Top | Males | 13 | 4.4 (0.4) | 4.7 (0.4) | 18.6 (0.7) | 15.5 (1.4) | 20.1 (0.7) | 21.0 (1.2) |
| Females | 16 | 6.3 (0.4) | 5.9 (0.4) | 15.2 (0.5) | 13.3 (0.8) | 22.6 (1.6) | 21.8 (1.2) | |
| 20-Top | Males | 6 | 2.8 (0.5) | 2.2 (0.7)* | 16.4 (0.7) | 12.8 (1.0) | 19.7 (0.7) | 19.8 (1.2) |
| Females | 9 | 4.6 (0.4) | 6.0 (0.7)* | 15.8 (1.0) | 12.9 (1.3) | 27.0 (2.9) | 29.8(3.5) | |
| Nal | Males | 10 | 3.9 (0.5) | 2.3 0.6)* | 18.2 (0.7) | 15.5 (1.0) | 20.8 (0.8) | 17.6 (2.0) |
| Females | 9 | 6.1 (0.4) | 3.6 (0.4)* | 15.1 (0.8) | 13.9 (0.8) | 20.9 (1.5) | 23.3 (1.8) | |
| 10-Top +Nal | Males | 7 | 3.6 (0.5) | 3.0 (1.0) | 17.9 (0.9) | 14.1 (1.9) | 18.3 (0.4) | 19.3 (1.4) |
| Females | 9 | 5.8 (0.3) | 5.0 (0.5) | 15.4 (0.6) | 11.4 (1.2) | 22.0 (1.6) | 18.6 (1.7) | |
4 Discussion
We used a battery of tests to characterize sex differences in P rats in order to determine the utility of this strain as a model of sex differences in AUDs. While we did observe a sex difference in ethanol consumption initially, with females consuming higher levels than males, this difference disappeared within 10 days of access. Preference for ethanol was also initially higher in females, but this switched to a higher preference in males. Self-administration of ethanol under FR1 and PR schedules showed no systematic sex differences, and both sexes showed a similar increase in motivation for ethanol following a deprivation period. Following treatment with naltrexone and topiramate, two medications used to treat AUDS in humans, we observed sex differences and similarities, most notably, a more robust effect of the combination of naltrexone and topiramate in males. These findings begin to characterize sex differences within multiple paradigms that model AUDs, and suggest that this P rat model may be useful for gaining a greater understanding of the sex differences in AUDs and its treatment.
The most striking sex difference observed in this study was the initial higher consumption of ethanol by females. Notably, this difference disappeared within 10 days as males reached the female level of consumption. Studies in P rats or other selectively bred lines have also observed higher initial ethanol consumption in females, followed eventually by no difference between the sexes (P rats, Bell et al. 2006; HAD rats, Terenina-Rigaldie et al. 2004; Mormede et al. 2004). However, these data are in contrast to another study using adult P rats, which found persistently higher intake in females for 6 weeks (P rats: Bell et al. 2011). This may be due to methodological differences, in that the Bell et al. (2011) study used 15% and 30% concentrations of ethanol, while the current study used 8% and 16% concentrations of ethanol. Other studies have shown that as the ethanol concentration is increased, females increase their preference and consumption, where males’ preference stays the same or decreases (Vetter O’Hagen et al 2009; Almeida et al. 1998; Sherrill et al. 2011). This results in greater sex differences in ethanol consumed in studies that use higher ethanol concentrations. Through showing similar levels of g/kg intake in males as other studies (e.g. 4.7 ± 0.3 in the current study compared to ~4–6 g/kg in Bell et al. 2011), as well as equivalent intake in females, our methods may reduce the confound of higher intake in females and thus serve as a good model for preclinical studies of sex differences in AUDs.
The initial sex difference in ethanol consumption observed here could be reflective of the “telescoping” effect observed in women (Greenfield, 2002; Hernandez-Avila et al. 2004). The telescoping hypothesis has recently come under scrutiny as multiple surveys of the general population found no escalated use in women overall (Keyes et al. 2010). However, as the initial effect was seen in treatment populations, this could reflect an additional vulnerability of women predisposed to AUDs (Wilsnack et al. 2014). As the P rats are an animal model of alcoholism, this effect may manifest in high initial use and preference that results in chronic consumption and likely dependence. However, further research is necessary to examine this possibility since we did not measure dependence in this current study.
In parallel to the consumption data, females immediately showed a higher preference score for ethanol, but were quickly surpassed by ethanol preference in males. Overall, males and females drank similar amounts of fluid (29.8 ml/day ± 0.7 and 27.4 ml/day ±0.5 in males and females, respectively), which is interesting considering females had body weights well below males. While females had a slightly lower preference score for ethanol overall, this is likely due to slightly higher water intake in females (with the exception of the first 10 days of 24-hr/day access, when males were drinking much less ethanol and more water; see Table 1). The low preference scores in females are likely not due to an aversion to ethanol as females’ preference for the two concentrations were equivalent (24.9% ± 2.4 and 22.4% ± 2.0 for 8% and 16%, respectively) as was g/kg intake between males and females. Overall, these data showing equal ethanol consumption and slightly higher preference in males support the validity of this line of rats as a model for sex differences in AUDs.
Under FR1 testing conditions, male and females obtained an equal level of ethanol intake, with non-systematic differences observed in patterns of intake over time. Additionally, under a PR schedule, males and females obtained similar ethanol deliveries both when receiving a standard 0.1 ml delivery and when the dose/delivery was adjusted for body weight differences. Males and females also showed similar significant, although very modest, increases in motivation for ethanol following an ethanol deprivation period. This is in concordance with previous research in P rats that found no sex differences in the ADE on ethanol consumption (McKinzie et al. 1998). However these conclusions are limited by the fact that only one method was used to test ADE in these animals. Future studies with different relapse paradigms, such as longer or repeated deprivations, should be done to further characterize any potential sex differences.
This study was the first of our knowledge to preclinically screen for sex differences in the efficacies of pharmacological treatments that are being investigated for use in humans. In response to the different treatments, we found a sex difference in the efficacy of the combination of naltrexone and topiramate where the combination was more effective and selective in males. Topiramate alone at the higher dose (20 mg/kg) also reduced responding for ethanol in males, while only tending to decrease responding in females. Topiramate’s mechanism of action is on GABA/glutamate release in mesolimbic areas, and is thought to normalize these pathways following prolonged alcohol use (Johnson, 2004). In preclinical models, males present with more severe withdrawal symptoms, such as seizure susceptibility, which manifest from dysfunction in the GABA/glutamate neurotransmission (Devaud and Chadda, 2001; Tsai and Coyle, 1998). As topiramate effectively reduced ethanol’s reinforcing effects in males, but not females, an alternate neurotransmitter pathway may more strongly mediate ethanol’s reinforcing effects in females.
While treatment with naltrexone decreased ethanol’s reinforcing effects in both sexes, we saw no influence of sex on treatment efficacy of naltrexone alone. This is in contrast to some human data suggesting that it is more beneficial at reducing alcohol use in males (Garbutt et al. 2005; Pettinati et al. 2008); however other studies report no gender differences in response to naltrexone treatment (Sable et al. 2006; Anton et al. 2006). Naltrexone is proposed to reduce ethanol’s reinforcing effects through blockade of μ-opioid receptors, preventing ethanol-induced dopamine release (Unterwald, 2008). With known gender differences in striatal dopamine release in humans (Munro et al. 2006), it is important to investigate sex-specific effects in response to naltrexone and other medications targeting dopamine; however, our data suggest that this treatment has similar efficacy in males and females.
One goal of a combination treatment using low doses of both naltrexone and topiramate was to minimize side effects while maximizing its effect on the reinforcing properties of ethanol. All treatments seemed to have a mild effect on food intake, which is not surprising, as topiramate and naltrexone have both been studied as weight loss drugs (Bray et al. 2003; Greenway et al. 2010). Interestingly, topiramate alone (20 mg/kg) and naltrexone alone reduced water deliveries obtained within the session. However, the combination of treatments prevented this non-selective side effect seen when either treatment was used alone.
Some limitations of this study were that limited doses of each medication were tested, these effects were tested acutely, and not all rats received each medication. These doses were chosen based on their ability to decrease motivation for ethanol in males (Moore et al. 2014), and to potentially see a synergistic effect of the combination treatment. Due to time constraints and strict baseline criteria, not all animals received each treatment. As the animals were randomized to treatments and compared to their own baseline, this should not affect the interpretation of the results; however the large variation in the number of animals per treatment group must be taken into account. There is some evidence for sex-specific dose-responses to certain medications (Gear et al. 1999); however there is currently a large gap in the knowledge of sex differences within clinical pharmacology (Anderson, 2008). The likelihood of sex-specific, dose-dependent effects highlights the need to use preclinical models to screen treatment efficacy. After screening with acute administration, chronic administration would be the next step on modeling human AUD treatment. Further research is needed to delineate any potential differences in efficacies based on sex-specific targets or a dose-effect curve shift between sexes.
An additional limitation of the present study is that gonadal hormones were not measured as a factor of ethanol intake. While some studies implicate differences in ethanol’s reinforcing properties in different stages of the estrous cycle, estrous cycle effects aren’t seen in freely cycling females (Roberts et al. 1998). The main interest in these effects would be on whether fluctuations in hormones due to stage of estrous would change the efficacy in pharmacological treatments. However, our experimental design (i.e. each treatment was received only once by a subset of animals) was such that these effects could not be investigated fully. Future research investigating the mechanisms underlying these sex differences in treatment effects should consider estrous cycle phases.
While we can’t say with certainty that our females stayed asynchronous in their cycles, we measured ethanol self-administration each day under both FR and PR schedules and saw no fluctuating patterns in females that would indicate shifts in ethanol’s reinforcing properties. Under the 24-hr/day consumption, we did notice a regularity with which the female’s intake and preference dip during the first 2 months of access. While looking through the schedule, this corresponded to when the 16% and 8% ethanol bottles were switched. It is possible that females initially have a stronger place preference than males, a research question that would have to be addressed with a different experimental design.
5 Conclusions
This research across multiple self-administration paradigms supports the use of P rats to investigate sex differences in AUDs and its treatment. Gender differences in AUDs in the US population are closing (Keyes et al. 2008), and research suggests that within treatment-seeking populations, these differences are even fewer (Ross, 1989; Shuckit et al. 1994). For example, within an inpatient treatment population, women and men alcoholics were similar in alcohol/drug history, treatment completion, and relapse rates (Wallen, 1992; Rubin et al. 1996; Foster et al. 2000; Mann et al. 2005; Walitzer and Dearing, 2006). Additionally, despite historically higher rates among men, many studies show that women are at equal risk for the heritability of alcohol dependence (Heath et al. 1997; Prescott et al. 1999; Knopik et al. 2012). As clear gender differences exist in the use of alcohol and prevalence and severity of alcohol use disorders, it is imperative to use preclinical models to characterize neurobiological differences in disease parameters and screen for efficacious pharmacological interventions for both genders. By establishing an animal model of sex differences in AUDs, research can focus on neurobiologically based sex-specific treatments.
Highlights.
Preclinical studies of sex differences in alcohol use disorders are imperative
We characterized ethanol behaviors in male and female alcohol preferring (P) rats
While females consumed more ethanol initially, after 10 days, differences ceased
A combination of naltrexone and topiramate was more efficacious in males
This model may aid in understanding sex differences across phases of alcohol use
Acknowledgments
This work was supported by NIAAA grant R01AA016554 (WJL). P rats for this study were provided by the Indiana Alcohol Research Center and funded by NIAAA grant 5P50AA007611-159005. NIAAA had no further role in study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to publish.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabough R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group . Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2006;25:502–507. [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. REVIEW: The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006a;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in periadolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006b;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, McBride WJ. Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 2008;42:407–416. doi: 10.1016/j.alcohol.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharm Bio Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharm Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: Modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Glick SD. Recent Developments in Alcoholism. Springer; US: 1995. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats; pp. 231–241. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiat Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. AlcoholClin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Darstein M, Albrecht C, Lopez-Francos L, Knörle R, Hölter SM, Spanagel R, Feuerstein TJ. Release and accumulation of neurotransmitters in the rat brain – acute effects of ethanol in vitro and effects of long-term voluntary ethanol intake. Alcohol Clin Exp Res. 1998;22:704–709. [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav. 2012;102:540–548. doi: 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eravci M, Schulz O, Grospietsch T, Pinna G, Brödel O, Meinhold H, Baumgartner A. Gene expression of receptors and enzymes involved in GABAergic and glutamatergic neurotransmission in the CNS of rats behaviourally dependent on ethanol. Br J Pharmacol. 2000;131:423–432. doi: 10.1038/sj.bjp.0703596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JH, Peters TJ, Marshall EJ. Quality of life measures and outcome in alcohol-dependent men and women. Alcohol. 2000;22:45–52. doi: 10.1016/s0741-8329(00)00102-6. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW Vivitrex Study Group . Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender-and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF. Women and alcohol use disorders. Harvard Rev Psychiat. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-, and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased Ethanol Self-Administration after a Period of Imposed Ethanol Deprivation in Rats Trained in a Limited Access Paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacol. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Hölter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology. 2000;153:93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Johnson BA. An overview of the development of medications including novel anticonvulsants for the treatment of alcohol dependence. Expert Opin Pharmacother. 2004;5:1943–1955. doi: 10.1517/14656566.5.9.1943. [DOI] [PubMed] [Google Scholar]
- Juárez J, De Tomasi EB. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiat. 2001;158:308–311. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry. 2010;167:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: reevaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiat. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Loi B, Colombo G, Maccioni P, Carai MA, Franconi F, Gessa GL. High alcohol intake in female Sardinian alcohol-preferring rats. Alcohol. 2014;48:345–351. doi: 10.1016/j.alcohol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology. 2011;217:3–12. doi: 10.1007/s00213-011-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Marshall AW, Kingstone D, Boss M, Morgan MY. Ethanol elimination in males and females: Relationship to menstrual cycle and body composition. Hepatology. 1983;3:701–703. doi: 10.1002/hep.1840030513. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Estrous synchrony and its mediation by Airborne chemical communication (Rattus norvegicus) Horm Behav. 1978;10:264–276. doi: 10.1016/0018-506x(78)90071-5. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK. The Alcohol Deprivation Effect in the Alcohol-Preferring P Rat under Free-Drinking and Operant Access Conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, Boehm SL., II Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharm Biochem Behav. 2013;104:177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede P, Colas A, Jones BC. High ethanol preferring rats fail to show dependence following short-or long-term ethanol exposure. Alcohol Alcohol. 2004;39:183–189. doi: 10.1093/alcalc/agh037. [DOI] [PubMed] [Google Scholar]
- Moore CF, Protzuk OA, Johnson BA, Lynch WJ. The efficacy of a low dose combination of topiramate and naltrexone on ethanol reinforcement and consumption in rat models. Pharmacol Biochem Behav. 2014;116:107–115. doi: 10.1016/j.pbb.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiat. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O’Brien CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34:378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex Differences in the Sources of Genetic Liability to Alcohol Abuse and Dependence in a Population-Based Sample of US Twins. Alcohol Clin Exp Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism: Clinical and Experimental Research. 1998;22(7):1564–1569. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of Concurrent Access to Multiple Ethanol Concentrations and Repeated Deprivations on Alcohol Intake of Alcohol-Preferring Rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Ross HE. Alcohol and drug abuse in treated alcoholics: A comparison of men and women. Alcohol Clin Exp Res. 1989;13:810–816. doi: 10.1111/j.1530-0277.1989.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Rubin A, Stout RL, Longabaugh R. Gender differences in relapse situations. Addiction. 1996;91:111–120. [PubMed] [Google Scholar]
- Sable HJ, Bell RL, Rodd ZA, McBride WJ. Effects of naltrexone on the acquisition of alcohol intake in male and female periadolescent and adult alcohol-preferring (P) rats. IJAMH. 2006;18:139–150. doi: 10.1515/ijamh.2006.18.1.139. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Daeppen JB, Tipp JE, Hesselbrock M, Bucholz KK. The clinical course of alcohol-related problems in alcohol dependent and nonalcohol dependent drinking women and men. J Stud Alcohol Drugs. 1998;59:581. doi: 10.15288/jsa.1998.59.581. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Overview of Findings from the 2003 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2004. [Google Scholar]
- Terenina-Rigaldie E, Jones BC, Mormède P. The High-Ethanol Preferring rat as a model to study the shift between alcohol abuse and dependence. Eur J Pharmacol. 2004;504:199–206. doi: 10.1016/j.ejphar.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Toalston JE, Oster SM, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA. Effects of alcohol and saccharin deprivations on concurrent ethanol and saccharin operant self-administration by alcohol-preferring (P) rats. Alcohol. 2008;42:277–284. doi: 10.1016/j.alcohol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Unterwald EM. Naltrexone in the treatment of alcohol dependence. J Addict Med. 2008;2:121–127. doi: 10.1097/ADM.0b013e318182b20f. [DOI] [PubMed] [Google Scholar]
- Vetter O’Hagen CS, Varlinskaya EL, Spear LP. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen J. A comparison of male and female clients in substance abuse treatment. J Subst Abuse Treat. 1992;9:243–248. doi: 10.1016/0740-5472(92)90067-x. [DOI] [PubMed] [Google Scholar]
- Wilsnack SC, Wilsnack RW, Kantor LW. Focus on: Women and the Costs of Alcohol use. Alcohol res. 2014;35:219. [PMC free article] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bieńkowski P. Effect of repeated treatment with topiramate on voluntary alcohol intake and beta-endorphin plasma level in Warsaw alcohol high-preferring rats. Psychopharmacology. 2013;225:265–281. doi: 10.1007/s00213-012-2812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]