Abstract
BACKGROUND
Cardiac manifestations of neonatal lupus (cardiac NL) include congenital heart block and cardiomyopathy. Several candidate biomarkers were evaluated in cases at risk for cardiac NL on the basis of potential roles in inflammation, fibrosis, and cardiac dysfunction: C-reactive protein (CRP); NT-pro-B-type natriuretic peptide (NT-proBNP); troponin I; matrix metalloproteinase (MMP)-2; urokinase plasminogen activator (uPA); urokinase plasminogen activator receptor (uPAR); plasminogen; and vitamin D.
OBJECTIVES
Identification of maternal and fetal biomarkers associated with development and morbidity of cardiac NL should provide clues to pathogenesis with translational implications for management.
METHODS
Cord and maternal blood samples (139 each) collected during pregnancies at risk for cardiac NL were available for study. Levels of cord and maternal CRP, cord NT-proBNP, and cord troponin I were evaluated using multiplex assays. Cord and maternal vitamin D were assessed by liquid chromatography-mass spectrometry. MMP-2, uPA, uPAR, and plasminogen were evaluated using ELISA.
RESULTS
Cord CRP, NT-proBNP, MMP-2, uPA, uPAR, and plasminogen levels were higher in cardiac NL-affected fetuses than in unaffected cases, independent of maternal rheumatic disease, season at highest risk of cardiac NL development, and medications taken during pregnancy. These biomarkers were positively associated with a disease severity score derived from known risk factors for mortality in cardiac NL. Maternal CRP and cord troponin I levels did not differ between the groups. Cord and maternal vitamin D levels were not significantly associated with cardiac NL, but average maternal vitamin D level during pregnancy was positively associated with longer time to postnatal pacemaker placement.
CONCLUSIONS
These data support the association of fetal reactive inflammatory and fibrotic components with development and morbidity of cardiac NL. Following CRP and NT-proBNP levels after birth can potentially monitor severity and progression of cardiac NL. MMP-2 and the uPA/uPAR/plasminogen cascade provide therapeutic targets to decrease fibrosis. Although decreased vitamin D did not confer increased risk, given the positive influence on postnatal outcomes, maternal levels should be optimized.
Keywords: antibody, congenital heart disease, heart block
Neonatal lupus (NL) is a form of passively acquired autoimmunity, typically presenting as cardiac and/or cutaneous disease. The former disease is clinically identified as congenital heart block and/or cardiomyopathy (1,2). Injury to the developing heart is postulated to occur secondary to a proinflammatory and profibrotic cascade initiated following transplacental passage of maternal autoantibodies to SSA/Ro and/or SSB/La ribonucleoproteins (2–4). Detection occurs most often between the gestational ages (GA) of 16 and 24 weeks of pregnancy (2). Cardiac NL is associated with a significant risk of mortality (17%; most occurring in the fetal or neonatal period) and morbidity (70% of survivors require pacemaker placement) (5,6). The case fatality rate is greatest in those with lower ventricular rates in utero and disease that extends beyond the atrioventricular (AV) node, such as endocardial fibroelastosis (EFE), dilated cardiomyopathy (DCM), and hydrops fetalis (5,6). The estimated risk of cardiac NL in anti-SSA/Ro–positive mothers with no prior affected pregnancies is approximately 2% and is 6- to 10-fold higher following a previously affected child (7–10). The low penetrance rate suggests that anti-SSA/Ro antibodies are necessary but insufficient for the development of disease and that fetal reactivity and in utero environment are likely contributory.
Several of the candidate biomarkers that have been associated with inflammation, fibrosis, and heart dysfunction may play a role in the development, progression, and severity of cardiac NL. C-reactive protein (CRP), a sensitive marker of inflammation, has been associated with coronary heart disease in adults and is elevated in fetal hypoxia and neonatal sepsis (11,12). N-terminal pro-B-type natriuretic peptide (NT-proBNP) is used in screening and diagnosis of congestive heart failure in adults and has been associated with fetal heart dysfunction in umbilical cord blood and amniotic fluid (13,14). Troponin I is a sensitive and specific marker for myocardial damage and necrosis (15). Elevated concentrations of matrix metalloproteinase (MMP)-2, a proinflammatory and profibrotic factor that activates transforming growth factor (TGF)-β, have been found in adult heart failure patients (16). MMP-2 is stimulated by urokinase plasminogen activator/urokinase plasminogen activator receptor (uPA/uPAR)-dependent plasminogen activation and plasmin generation, which have been associated with cardiac NL in a previous univariate analysis (17). Vitamin D has been shown to act as a negative regulator in TGF-β signaling and fibrosis and has been associated with cardiac inflammation, fibrosis, and dysfunction in rats (18,19). Recently, on the basis of indirect evidence and seasonal time of the vulnerable period during pregnancy, it was speculated that vitamin D levels might be decreased in mothers during pregnancies of cardiac NL children (10).
Identification of fetal and maternal biomarkers that associate with the development and morbidity of cardiac NL should provide clues to pathogenesis, with translational implications for management. Accordingly, this study used a U.S.-based cohort of anti-SSA/Ro-exposed cardiac NL-affected and -unaffected pregnancies to evaluate candidate biomarkers in umbilical cord blood of fetuses and in maternal blood from the time of pregnancy. It was previously reported that CRP, NT-proBNP, and troponin do not cross the placenta; thus, increases in cord blood biomarkers can be attributed to fetal production (20–22). The higher masses of MMP-2 (74 kDa), uPA (49 kDa), uPAR (37 kDa), and plasminogen (91 kDa) likely preclude placental transfer, and active transport of markers of collagen metabolism has not been reported in humans (23). Furthermore, MMP-2 and the plasminogen activators are expressed in placental tissue, and fetal and placental concentrations of uPA and uPAR are significantly higher than in maternal blood (24,25). Fetal vitamin D is entirely dependent on maternal stores and, because 25(OH) vitamin D diffuses across the placenta, cord and maternal blood levels correlate closely (26).
METHODS
STUDY POPULATION
Subjects were identified from the Research Registry for Neonatal Lupus (RRNL), described elsewhere (27). The Institutional Review Board of the New York University School of Medicine approved the evaluation of deidentified information. Briefly, enrolled mothers have anti-SSA/Ro and/or anti-SSB/La antibodies and at least 1 child with NL. Maternal and cord blood collected during the time of a cardiac NL or unaffected pregnancy were analyzed. Cardiac NL was defined as previously described (6). Pregnancies complicated by first-degree block or transient sinus bradycardia were excluded from the dichotomous analyses comparing cardiac NL with unaffected fetuses because their benign features could dilute the effects of the more characteristic severe disease. A severity score of fetal outcomes was generated to associate potential biomarkers with disease morbidity (Table 1). For this score, sinus bradycardia and first-degree block cases were included as a potential outcome along the spectrum of cardiac NL. The extreme end of the spectrum included features associated with an increased risk of morbidity and mortality, such as lower fetal ventricular nadir (which requires earlier pacing) and extranodal disease (5,6,28). Cardiac NL-affected individuals were contacted between September 1, 2011, and June 1, 2014, to evaluate the need for cardiac medications (beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or digoxin).
TABLE 1.
Severity Scores for Potential Outcomes in Anti-SSA/Ro-Exposed Fetuses
| Outcome | Severity Score |
|---|---|
| Unaffected | 0 |
| Sinus bradycardia (transient) | 1 |
| First-degree AV block | 2 |
| Advanced AV block, no PPM at 6 months | 5 |
| Advanced AV block, PPM placed by 6 months | 6 |
| Advanced AV block, PPM placed by 1 week | 8 |
| 1 extranodal manifestation | 14 |
| >1 extranodal manifestation | 16 |
| Death | 20 |
Advanced AV block = second- or third-degree heart block; AV = atrioventricular; PPM = permanent pacemaker; extranodal manifestation = endocardial fibroelastosis, dilated cardiomyopathy, or hydrops fetalis.
SERUM ANALYSIS
CRP, NT-proBNP, and troponin I were evaluated using commercial Luminex assays (human cardiovascular disease panel 3, HCVD3MAG-67K, and panel 1, HCVD1MAG-67K; Millipore, Billerica, Massachusetts). ELISA was used to assess levels of MMP-2 according to the manufacturer’s instructions (R&D Biosystems, Minneapolis, Minnesota). Total 25(OH) vitamin D was assessed by quantitative high-performance liquid chromatography-tandem mass spectrometry.
STATISTICAL ANALYSIS
Univariate and multivariate analyses were applied to identify associations among serum biomarkers and outcome responses including cardiac NL, fetal echocardiographic EFE, DCM, and hydrops; requirement of and age at pacemaker placement; requirement of cardiac medications on long-term follow-up; death; and severity score. Cord blood uPA, uPAR, and plasminogen levels, which were previously reported in univariate analysis with cardiac NL by Briassouli et al. (17), were also included in the additional analyses. Logistic regression was applied to binary outcomes, and ordinary linear regression was applied to continuous outcomes. In univariate analysis, each biomarker, as a predictor, was regressed on each outcome. In multivariate analysis, both predictors and confounders were regressed on the outcomes in a variable selection procedure. Models with a p value of <0.05 and confounders with a p value of <0.2 were selected for the final multivariate analysis. Confounders included maternal use of fluorinated steroids (which cross the placenta, potentially affecting both the fetal and maternal inflammatory response); nonfluorinated steroids (which are inactivated by the placenta and affect only the maternal inflammatory response); hydroxychloroquine; and/or intravenous immunoglobulin (IVIg) during pregnancy; Caucasian race; maternal diagnosis of systemic lupus erythematosus (SLE) and/or Sjögren’s syndrome (SS); child’s sex; GA 20 weeks occurring in winter; GA at delivery; and age of cardiac NL individual at long-term follow-up. Results for CRP and NT-proBNP showed right skewness of their empirical distribution, and natural logarithms were used for normalization.
RESULTS
PATIENT DEMOGRAPHICS AND OUTCOMES
In total, cord blood samples were obtained from 139 anti-SSA/Ro-exposed fetuses, 59 (42%) with cardiac NL, 75 (54%) who were unaffected (including 7 with cutaneous NL), 2 (1%) with first-degree heart block, and 3 (2%) with sinus bradycardia during pregnancy. Maternal blood samples during pregnancy were obtained in 135 cases; 54 (40%) resulting in cardiac NL-affected fetuses, 76 (56%) in unaffected fetuses (10 with cutaneous NL), 2 (1%) with first-degree heart block, and 3 (2%) with sinus bradycardia. Demographics, medications used, and fetal outcomes are shown in Table 2.
TABLE 2.
Clinical and Demographic Characteristics of Anti-SSA/Ro-Exposed Cardiac NL and Unaffected Cases
| Characteristic | Available Cord Blood
|
Available Maternal Blood During Pregnancy
|
||||
|---|---|---|---|---|---|---|
| Cardiac NL (n = 59) | Unaffected (n = 75) | p Value | Cardiac NL (n = 54) | Unaffected (n = 76) | p Value | |
|
| ||||||
| Maternal caucasian race | 44 (75%) | 48/73 (66%) | 0.34 | 40 (70%) | 55 (72%) | 1.00 |
|
| ||||||
| Maternal diagnosis | ||||||
| Asymptomatic/UAS | 30 (51%) | 29 (39%) | 24 (44%) | 34 (45%) | 1.00* | |
| SS | 19 (32%) | 24 (32%) | 0.17* | 19 (35%) | 21 (28%) | |
| SLE | 3 (5%) | 7 (9%) | 4 (7%) | 6 (8%) | ||
| SLE and SS | 6 (10%) | 12 (16%) | 6 (11%) | 15 (20%) | ||
| Unknown | 1 (2%) | 3 (4%) | 1 (2%) | 0 (0%) | ||
|
| ||||||
| Maternal anti-SSB/La+ | 32/56 (57%) | 29/49 (59%) | 0.85 | 30/51 (59%) | 33/53 (62%) | 0.84 |
|
| ||||||
| Maternal medications during pregnancy | ||||||
| Fluorinated steroids | 39/58 (67%) | 6/67 (9%) | <0.001 | 38/53 (72%) | 5/72 (7%) | <0.001 |
| Nonfluorinated steroids | 5/58 (9%) | 11/67 (16%) | 0.28 | 4/53 (8%) | 10/71 (14%) | 0.39 |
| IVIg | 6/58 (10%) | 17 (23%) | 0.07 | 3/53 (6%) | 21/76 (28%) | 0.02 |
| Hydroxychloroquine | 5/57 (9%) | 26/67 (39%) | <0.001 | 8/53 (15%) | 37/71 (52%) | <0.001 |
|
| ||||||
| Child female sex | 34/58 (59%) | 35/73 (48%) | 0.29 | 30/52 (58%) | 29/68 (43%) | 0.14 |
| GA 20 weeks during winter | 13 (22%) | 21/70 (30%) | 0.32 | 12 (22%) | 18/70 (26%) | 0.68 |
| Mean ± SD GA (weeks) at time of birth | 36.7 ± 2.1 | 38.3 ± 1.7 | <0.001 | 36.5 ± 3.0 | 38.4 ± 1.78 | <0.001 |
| Fetal echo EFE | 11/53 (21%) | 13/48 (27%) | ||||
| Fetal echo DCM | 4/50 (8%) | 3/47 (6%) | ||||
| Fetal echo hydrops | 6/51 (12%) | 6/47 (13%) | ||||
|
| ||||||
| Pacemaker requirement | 38/54 (70%) | 32/48 (67%) | ||||
| Number of weeks after birth of pacemaker | 0.43 (0.14–1.29) | 0.57 (0–1.36) | ||||
|
| ||||||
| Receiving cardiac meds at follow-up | 7/44 (16%) | 4/40 (10%) | ||||
| Average age (yrs) at follow-up (mean ± SD) | 6.0 ± 5.3 | 4.1 ± 3.8 | ||||
|
| ||||||
| Child death | 1 (2%) | 4 (7%) | ||||
Values are n (%), n/N (%), median (interquartile range), or mean ± SD.
Comparison of asymptomatic/UAS and all other maternal diagnoses combined in known cases.
Cardiac meds = use of beta-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or digoxin; DCM = dilated cardiomyopathy; echo = echocardiography; EFE = endocardial fibroelastosis; GA = gestational age; IVIg = intravenous immunoglobulin; IQR = interquartile range; NL = neonatal lupus; SLE = systemic lupus erythematosus; SS = Sjögren’s syndrome; UAS = undifferentiated autoimmune syndrome.
C-REACTIVE PROTEIN
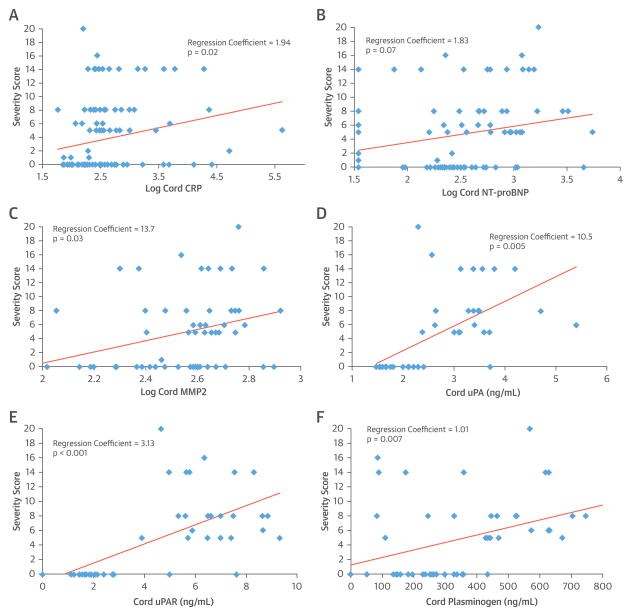
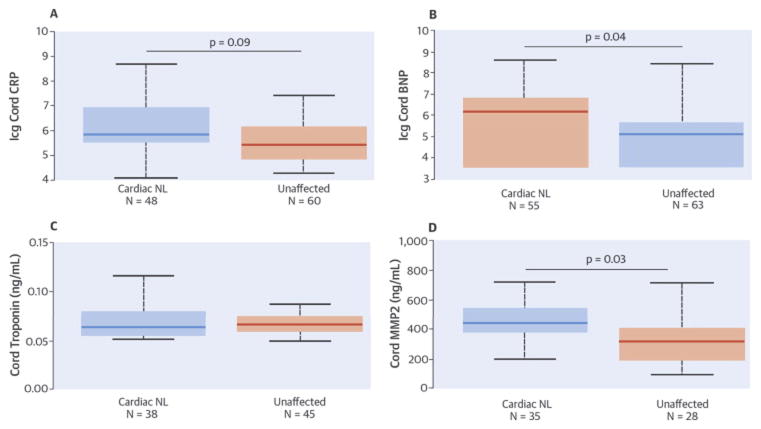
Median cord CRP levels were higher in cardiac NL fetuses than in unaffected cases (Table 3). Log-transformed levels of cord CRP were positively associated with cardiac NL in univariate (odds ratio [OR]: 1.57, OR per SD: 1.90, p = 0.009) and multivariate (OR: 1.50, OR per SD: 1.78, p = 0.09) analyses (Central Illustration). Log cord CRP was significantly associated with severity score in univariate and multivariate analyses (regression coefficient [RC]: 2.18, p = 0.02 and 1.94, p = 0.02, respectively) (Central Illustration, panel A).
TABLE 3.
Cord Blood Biomarkers Associated With Cardiac NL
| Biomarker | Cardiac NL (n) | Unaffected (95% CI) (n) | OR, OR per SD |
|---|---|---|---|
| CRP (mg/l) | 0.35 (0.25–1.00) (48) | 0.23 (0.12–0.48) (60) | 1.50, 1.78* (LT) (0.93–2.41) |
| NT-proBNP (pg/ml) | 467.8 (34.3–930.5) (55) | 165.2 (34.3–295.2) (63) | 1.49, 1.76† (LT) (1.02–2.18) |
| MMP-2 (ng/ml) | 447.2 ± 144.4 (35) | 325.1 ± 170.4 (28) | 1.01, 2.36† (1.30–23.24) |
| uPA (ng/ml) | 3.38 ± 0.66 (27) | 1.93 ± 0.48 (22) | 62.2, 46.61‡ (6.71–577.47) |
| uPAR (ng/ml) | 6.57 ± 1.37 (27) | 2.07 ± 1.58 (22) | 4.01, 41.98‡ (1.94–8.28) |
| Plasminogen (ng/ml) | 448.9 ± 215.5 (27) | 231.4 ± 102.8 (22) | 1.01, 15.68‡ (2.12–83.31) |
Values are mean ± SD.
p < 0.10.
p < 0.05.
p < 0.01.
Covariates were CRP (fluorinated steroid exposure, fluorinated steroid exposure at time of birth, maternal IVIg, maternal hydroxychloroquine, maternal non-fluorinated steroid, sex, week of delivery); NT-proBNP (fluorinated steroid exposure at birth, maternal IVIg, maternal hydroxychloroquine, gestational age 20 weeks during winter, week of delivery); MMP-2 (maternal fluorinated steroid, maternal diagnosis of SLE or SS, sex); uPA (no confounders selected on the basis of p < 0.2); uPAR (no confounders selected on the basis of p < 0.2); and plasminogen (maternal hydroxychloroquine, maternal nonfluorinated steroid, Caucasian race, week of delivery).
CRP = C-reactive protein; LT = log-transformed; MMP-2 = matrix metalloproteinase-2; MV = multivariate; NT-proBNP = N-terminal pro-brain natriuretic peptide; OR = odds ratio per the unit increase of log-transformed value (or original value for uPA/uPAR); OR per SD = odds ratio per the increase of SD of log-transformed value (or original value for uPA/uPAR); uPA = urokinase plasminogen activator; uPAR = urokinase plasminogen activator receptor. Other abbreviations as in Table 1.
There were no statistical differences between average maternal CRP levels during 48 cardiac NL and 60 unaffected pregnancies (26.4 mg/l [12.3 to 45.2 mg/l] and 19.5 mg/l [9.2 to 42.0 mg/l], respectively). Moreover, there were no statistical differences between maternal CRP levels when analyses were limited to GA 16 to 24 weeks in 16 cardiac NL pregnancies and in 38 unaffected pregnancies (14.1 mg/l [11.4 to 34.7 mg/l] and 17.3 mg/l [10.6 to 28.7 mg/l], respectively).
No associations with cord or maternal blood CRP were identified with extranodal disease, pacemaker requirement, age at pacemaker placement, need for cardiac medications on follow-up, or child death.
NT-proBNP AND TROPONIN I
Median cord NT-proBNP levels were higher in cardiac NL cases than in unaffected fetuses (Table 3). Log cord NT-proBNP level was positively associated with cardiac NL in univariate (OR: 1.59, OR per SD: 1.93, p = 0.001) and multivariate (OR: 1.49, OR per SD: 1.76, p = 0.04) analysis (Central Illustration). NT-proBNP was particularly high in 6 cases with hydrops. However, the association with cardiac NL remained significant, excluding those cases (OR: 1.54, OR per SD: 1.83, p = 0.003 univariate and OR: 1.48, OR per SD: 1.73, p = 0.04 multivariate). Log cord NT-proBNP was associated with severity score in univariate and multivariate analyses (RC: 2.79, p = 0.002 and 1.83, p = 0.07 respectively) (Central Illustration, panel B). No associations were identified between NT-proBNP and extranodal disease or postnatal outcomes.
Mean troponin I concentration was 0.070 ± 0.02 ng/ml in cardiac NL and 0.072 ± 0.02 ng/ml in unaffected cord bloods (not significant) (Central Illustration). No significant associations were identified between troponin I and extranodal disease, postnatal outcomes, or severity score.
MATRIX METALLOPROTEINASE-2
Mean cord MMP-2 levels were significantly associated with cardiac NL in univariate (OR: 1.01, OR per SD: 2.39, p = 0.006) and multivariate (OR: 1.01, OR per SD: 2.36, p = 0.03) analyses (Table 3, Central Illustration), and with severity score in univariate and multivariate analyses (RC: 1.01, p = 0.01, and RC: 1.01, p = 0.03, respectively) (Central Illustration, panel C). Cord MMP-2 was not associated with extranodal disease or postnatal outcomes.
uPA, uPAR, AND PLASMINOGEN
Elevated cord blood levels of uPA, uPAR, and plasminogen have previously been associated with cardiac NL in univariate analysis (17). In multivariate analysis of the same samples, cord uPA (OR: 62.2, OR per SD: 46.61, p < 0.001), uPAR (OR: 4.01, OR per SD: 41.98, p < 0.001), and plasminogen (OR: 1.01, OR per SD: 15.68, p = 0.004) remained associated with cardiac NL versus unaffected cases. uPA, uPAR, and plasminogen levels were also positively associated with severity score. RC values in univariate and multivariate analyses for uPA were 33.7 (p < 0.01) and 10.5 (p = 0.005), respectively; 3.72 (p < 0.001) and 3.13 (p < 0.001) for uPAR, respectively; and 1.01 (p = 0.01) and 1.01 (p = 0.07) for plasminogen, respectively (Central Illustration, panels D to F). No additional associations with extranodal disease or postnatal outcomes were observed.
VITAMIN D
There were no significant differences in cord blood levels of 25(OH) vitamin D between cardiac NL and unaffected fetuses (23.8 ± 8.2 ng/ml vs. 22.7 ± 8.3 ng/ml, respectively). Likewise, there were no associations between concentrations of cord vitamin D and extranodal disease, postnatal outcome, or severity score.
The maternal level of vitamin D was analyzed in the average of all samples available for each pregnancy, as well as in blood drawn at GA 16 to 24 weeks. In both cases, levels did not distinguish between cardiac NL and unaffected pregnancies. The average vitamin D concentration was 33.5 ± 11.0 ng/ml in cardiac NL and 33.5 ± 13.6 ng/ml in unaffected cases. Vitamin D at GA 16 to 24 weeks was 37.7 ± 10.9 ng/ml in cardiac NL and 36.6 ± 17.1 ng/ml in unaffected cases. Maternal vitamin D at GA 16 to 24 weeks was lower in 5 cases with EFE than in 10 cardiac NL cases without EFE (28.8 ± 8.6 vs. 42.4 ± 9.8, respectively, OR: 0.81, OR per SD: 0.04, p = 0.08) in univariate analysis only. Although levels of maternal vitamin D were not associated with pacemaker requirement, higher average levels of maternal vitamin D during pregnancy were associated with later age of pacemaker placement in univariate and multivariate analyses (RC: 3.53, p = 0.008 in both analyses) (Figure 1). There were no further associations between maternal vitamin D and other postnatal outcomes or severity score.
FIGURE 1.
Association of Cord Blood Biomarkers with Cardiac-NL Morbidity
Multivariate associations of serum biomarkers in anti-SSA/Ro-exposed cord blood with cardiac-NL morbidity derived from a severity score of potential clinical outcomes. (A) Log CRP; (B) log NT-proBNP; (C) MMP2 (ng/ml); (D) uPA (ng/ml); (E) uPAR (ng/ml); (F) plasminogen (ng/ml). Covariates: CRP: fluorinated steroid exposure, fluorinated steroid exposure at time of birth, maternal IVIg; NT-proBNP: fluorinated steroid exposure at birth, maternal hydroxychloroquine, gestational age 20 weeks during winter, week of delivery; MMP2: maternal fluorinated steroid, maternal hydroxychloroquine, gestational age 20 weeks during winter; uPA: fluorinated steroid, maternal IVIg, maternal nonfluorinated steroid, sex, gestational age 20 weeks during winter, week of delivery; uPAR: fluorinated steroid, maternal IVIg, maternal nonfluorinated steroid, maternal diagnosis of SLE or SS, sex, gestational age 20 weeks during winter, week of delivery. Plasminogen: maternal IVIg, maternal nonfluorinated steroid, sex, gestational age 20 weeks during winter. SS = Sjögren’s syndrome; uPA = urokinase plasminogen activator; uPAR = urokinase plasminogen activator receptor.
DISCUSSION
Data from the largest biorepository to date of neonates exposed to maternal anti-SSA/Ro antibodies support tissue inflammation and fibrosis in the pathogenesis and morbidity of cardiac NL. Specifically, cord blood CRP, NT-proBNP, MMP-2, uPA, uPAR, and plasminogen levels were higher in neonates with cardiac NL than in unaffected neonates on multivariate analyses (Table 3) and correlated with a disease severity score derived from previously identified risk factors for mortality. Associations were independent of maternal rheumatic disease, season at highest risk of cardiac NL development, and medications during pregnancy. Cord troponin I levels did not differ between groups. Although vitamin D levels in cord blood and maternal blood during pregnancy were equivalent in cardiac NL and unaffected pregnancies, higher maternal vitamin D during pregnancy was associated with a later age of pacemaker implantation in multivariate analysis.
Cord blood CRP elevations have been previously associated with neonatal sepsis and hypoxia (11,12,29). This study is the first to identify a fetal acute phase response to passively acquired autoimmunity. The absence of a significant association between maternal CRP and cardiac NL, paired with the fact that CRP does not cross the placenta, demonstrates that the fetus specifically generates the acute phase response.
The contribution of CRP to the pathogenesis of cardiac NL is supported by in vitro and in vivo observations. In adult heart disease, CRP polarizes macrophages to an inflammatory M1 phenotype (30). Aside from the liver, CRP is also produced by macrophages located within atherosclerotic plaques (31). In autopsies of cardiac NL fetuses, macrophages have been persistently identified in the region of cardiac conduction tissue (32). Coculture of macrophages with anti-SSA/Ro opsonized apoptotic cardiocytes resulted in release of proinflammatory and profibrosing cytokines that differentiated cardiac fibroblasts into scarring phenotypes (33). In addition to being part of a general inflammatory response, elevated CRP in cardiac NL may play a pathogenic role in disease development and severity, both as a marker of infiltrating macrophages in cardiac NL tissue and by its action on the macrophages to promote an inflammatory phenotype.
NT-proBNP elevation in cord blood has previously been correlated with volume overload in neonates with congenital heart defects (13). In adults, higher BNP after myocardial infarction is associated with adverse left ventricular remodeling (34). The finding of elevated NT-proBNP in cardiac NL is consistent with the heart dysfunction that occurs and may be associated with the further development of heart abnormalities due to abnormal remodeling. Amniotic fluid NT-proBNP has been correlated with echocardiography in twin-twin transfusion syndrome and has been proposed as a marker for fetal cardiac health (14,35). Because fetal echocardiograms may underestimate pathologic findings in cardiac NL (36), a sensitive method to predict clinical manifestations in utero, such as amniotic fluid NT-proBNP, could provide utility in guiding and monitoring treatment of cardiac NL.
Cord troponin I was not associated with cardiac NL or morbidity. It is possible that necrosis of tissue that precedes cardiomyocyte calcification occurs only during the target GA of disease onset, and troponin I levels may normalize by the time of childbirth. Alternatively, the troponin I assay used may not have been sufficiently sensitive to detect smaller differences due to heart failure or myocarditis that novel high-sensitivity tests would potentially discern.
MMP-2 is a proinflammatory and profibrotic member of a family of zinc- and calcium-dependent endopeptidases. It is expressed by macrophages and fibroblasts undergoing transition to myofibroblasts. Increased MMP-2 has been associated with cardiac fibrosis in animals and heart failure in adults (16,37). MMP-2 activates TGF-β, which has been identified in the extracellular fibrous matrix of AV nodal regions in cardiac NL (3). MMP inhibitors decrease fibrosis and attenuate ventricular dysfunction and remodeling in dogs with chronic heart failure (38). Thus, MMP inhibitors could be a novel therapeutic option to regulate antibody-induced inflammation and fibrosis in cardiac NL, as well as to potentially influence the remodeling of the fetal heart after the initial insult.
MMP-2 is activated by plasmin, the end product of the uPA/uPAR/plasminogen pathway, induced by macrophage accumulation in cardiac tissues (37). Studies by Briassouli et al. (4,17) revealed that uPA/uPAR inhibition attenuated TGF-β activation in supernatants of apoptotic cardiocytes exposed to anti-SSA/Ro and that cord blood uPA, uPAR, and plasminogen were elevated in cardiac NL cases in univariate analysis (4,17). In applying multivariate analysis to the previously reported results, uPA, uPAR, and plasminogen remained significantly associated with development of cardiac NL and associated with disease severity. These findings reinforce the role of this profibrotic pathway in cardiac NL, which could also be a new target for treatment.
Vitamin D has become a topic of interest in autoimmunity and cardiac disease, as its numerous immunomodulatory effects influence both innate and adaptive mechanisms (39). Low levels of vitamin D have been reported in autoimmune diseases, including SLE and scleroderma (40,41). Vitamin D receptor signaling is impaired in scleroderma, and reduced receptor expression with decreased vitamin D levels enhances the sensitivity of scleroderma fibroblasts to the profibrotic effects of TGF-β (18). Animal studies indicate that vitamin D deficiency can result in cardiac inflammation, fibrosis, apoptosis, and systolic dysfunction (19). In a Swedish NL cohort, a season-of-birth pattern was reported in which GA of 18 to 24 weeks occurring in winter correlated with a higher proportion of children with cardiac NL (10). This led the authors to hypothesize that low maternal vitamin D in winter months may contribute to disease development. In the present study, there were no differences in season of gestational vulnerability or vitamin D level in cord or maternal bloods between cardiac NL and unaffected cases. In a small subgroup of cardiac NL cases with maternal blood obtained during GA 16 to 24 weeks, a negative trend in vitamin D with development of EFE was noted on univariate analysis. Among patients requiring a pacemaker, higher maternal vitamin D was associated with later age of placement on multivariate analysis. Given the known role of TGF-β in cardiac NL, there remains reason to consider vitamin D as a potential modifiable risk factor in disease morbidity, and further study is warranted.
The association of our studied analytes with severity of cardiac NL underscores their potential clinical utility as biomarkers to identify and treat sicker cases. Furthermore, a recent report from the Swedish cardiac NL cohort identified 13 anti-SSA/Ro-exposed cases diagnosed after birth (age 4 months to 43 years of age), 11 with normal heart rates in utero (42). These findings support the hypothesis that immune-mediated inflammation and fibrosis in cardiac tissues can continue postnatally, resulting in late progression to cardiac NL. Accordingly, biomarkers may be important monitoring tools for anti-SSA/Ro-exposed infants to identify those at risk for late disease progression.
This study represents the largest reported cohort of anti-SSA/Ro-exposed pregnancies with available cord and maternal blood, but several limitations are acknowledged. The sample size remains a shortcoming in the investigation of prospective biomarkers in cardiac NL. Due to the rarity of the disease and finite quantities of available blood, not all analytes could be measured in each sample, and the GA at sampling also varied among cases. Rates of highly negative outcomes, such as extranodal disease, use of postnatal cardiac medications, and death are often <10%, limiting the power to significantly identify differences in biomarkers between groups. Blood from the most severe cases resulting in death in utero were also not available for study. Furthermore, fetal echocardiographic data was unavailable in 15% of all pregnancies. The RRNL is a U.S.-based registry and despite nearly universal prenatal vitamin therapy, enrollees can have a wide range of sun exposure within various climates throughout the country. Furthermore, differing clinical practices amongst the various sites may limit the interpretation of timing of pacemaker placement. These factors may have had undocumented effects on associations with maternal vitamin D levels, compared to the more homogeneous Swedish cohort.
CONCLUSIONS
CRP and NT-proBNP in cord blood are significantly associated with development and severity of cardiac NL and can be considered markers to identify worsening heart function after birth. Elevations of MMP-2, uPA, uPAR, and plasminogen suggest therapies aimed at decreasing fibrosis. The role of vitamin D is unclear, but optimizing maternal levels could become routine in the management of all anti-SSA/Ro-positive pregnancies.
Figure 2.
CENTRAL ILLUSTRATION Biomarkers in Cardiac Neonatal Lupus: Association of Cord Blood Biomarkers With Cardiac-Neonatal Lupus Development
Multivariate associations of serum biomarkers in anti-SSA/Ro-exposed cord blood of cardiac NL and unaffected fetuses. (A) log CRP; (B) log NT-proBNP; (C) troponin I (ng/ml); (D) MMP-2 (ng/ml). Covariates were CRP (fluorinated steroid exposure, fluorinated steroid exposure at the time of birth, maternal IVIg, maternal hydroxychloroquine, maternal nonfluorinated steroid, sex, week of delivery), NT-proBNP (fluorinated steroid exposure at birth, maternal IVIg, maternal hydroxychloroquine, gestational age of 20 weeks during winter, and week of delivery), and MMP-2 (maternal fluorinated steroid, maternal diagnosis of SLE or SS, sex). CRP = C-reactive protein; IVIg = intravenous immunoglobulin; MMP-2 = matrix metalloproteinase-2; NL = neonatal lupus; NT-pro-BNP = N-terminal pro-B-type natriuretic peptide; SLE = systemic lupus erythematosus.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Inflammation and fibrosis are central to the pathogenesis of cardiac complications of neonatal lupus. Several related biomarkers in the cord blood of affected fetuses are associated with disease severity.
TRANSLATIONAL OUTLOOK
Future studies should examine the value of biomarkers of inflammation as surrogates for progressive cardiac dysfunction in the evaluation of antifibrotic therapeutic strategies for treating affected individuals.
Acknowledgments
The authors acknowledge the efforts of Androo Markham for handling of samples, and Amanda Zink for preparation of the manuscript.
Dr. Saxena is supported by the American Heart Association Founders affiliate Clinical Research Program award 11CRP795008, NYU CTSI National Institutes of Health/National Center for Research Resources core resources grant 5UL1RR029893, and an SLE Lupus Foundation MD Scientist fellowship grant. Dr. Buyon is supported by National Institute of Arthritis and Musculoskeletal and Skin Disease contract N01-AR-4-2220-11-0-1 and grant 5R37AR042455, National Institute of Child Health and Human Development grants R01 HD079951-01A1 and R03 HD069986, and the Lupus Foundation of America LFA Lifeline Program.
ABBREVIATIONS AND ACRONYMS
- DCM
dilated cardiomyopathy
- EFE
endocardial fibroelastosis
- GA
gestational age
- MMP
matrix metalloproteinase
- NL
neonatal lupus
- RRNL
Research Registry for Neonatal Lupus
- SLE
systemic lupus erythematosus
- SS
Sjögren’s syndrome
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Scott JS, Maddison PJ, Taylor PV, et al. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309:209–12. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- 2.Buyon JP, Friedman DM. Neonatal lupus. In: Lahita RG, Tsokos G, Buyon JP, et al., editors. Systemic Lupus Erythematosus. 5. New York, NY: Academic Press; 2010. pp. 541–71. [Google Scholar]
- 3.Clancy RM, Backer CB, Yin X, et al. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]
- 4.Briassouli P, Rifkin D, Clancy RM, et al. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-β and potentiates fibrosis. J Immunol. 2011;187:5392–401. doi: 10.4049/jimmunol.1101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliasson H, Sonesson SE, Sharland G, et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–26. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 6.Izmirly PM, Saxena A, Kim MY, et al. Maternal and fetal factors associated with mortality and morbidity in a multiracial/ethnic registry of anti-SSA/Ro associated cardiac neonatal lupus. Circulation. 2011;124:1927–35. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brucato A, Frassi M, Franceschini F, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Costedoat-Chalumeau N, Amoura Z, Lupoglazoff JM, et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum. 2004;50:3187–94. doi: 10.1002/art.20554. [DOI] [PubMed] [Google Scholar]
- 9.Llanos C, Izmirly PM, Katholi M, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosi A, Salomonsson S, Eliasson H, et al. Development of heart block in children of SSA/SSB-autoantibody-positive women is associated with maternal age and displays a season-of-birth pattern. Ann Rheum Dis. 2012;71:334–40. doi: 10.1136/annrheumdis-2011-200207. [DOI] [PubMed] [Google Scholar]
- 11.Prashant A, Vishwanath P, Kulkarni P, et al. Comparative assessment of cytokines and other inflammatory markers for the early diagnosis of neonatal sepsis—a case control study. PLoS One. 2013;8:e68426. doi: 10.1371/journal.pone.0068426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loukovaara M, Leinonen P, Teramo K, et al. Fetal hypoxia is associated with elevated cord serum C-reactive protein levels in diabetic pregnancies. Biol Neonate. 2004;85:237–42. doi: 10.1159/000076132. [DOI] [PubMed] [Google Scholar]
- 13.Kocylowski RD, Dubiel M, Gudmudsson S, et al. Biochemical tissue-specific injury kljmarkers of the heart and brain in postpartum cord blood. Am J Obstet Gynecol. 2009;200:273, e1–25. doi: 10.1016/j.ajog.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Merz WM, Gembruch U. Old tool—new application: NT-proBNP in fetal medicine. Ultrasound Obstet Gynecol. 2014;44:377–85. doi: 10.1002/uog.13443. [DOI] [PubMed] [Google Scholar]
- 15.Amsterdam EA, Lewis WR. Identification of low risk patients with chest pain in the emergency department: another look at cardiac troponins. J Am Coll Cardiol. 1998;32:15–6. doi: 10.1016/s0735-1097(98)00179-x. [DOI] [PubMed] [Google Scholar]
- 16.Koitabashi N, Arai M, Niwano K, et al. Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail. 2008;10:373–9. doi: 10.1016/j.ejheart.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Briassouli P, Halushka MK, Reed JH, et al. A central role of plasmin in cardiac injury initiated by fetal exposure to maternal anti-Ro autoantibodies. Rheumatology (Oxford) 2013;52:1448–53. doi: 10.1093/rheumatology/ket156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerr P, Vollath S, Palumbo-Zerr K, et al. Vitamin D receptor regulates TGFβ signalling in systemic sclerosis. Ann Rheum Dis. 2015;74:e20. doi: 10.1136/annrheumdis-2013-204378. [DOI] [PubMed] [Google Scholar]
- 19.Assalin HB, Rafacho BP, dos Santos PP, et al. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ Heart Fail. 2013;6:809–16. doi: 10.1161/CIRCHEARTFAILURE.112.000298. [DOI] [PubMed] [Google Scholar]
- 20.O’Callaghan C, Franklin P, Elliot TS, et al. C reactive protein concentrations in neonates: determination by a latex enhanced immunoassay. J Clin Pathol. 1984;37:1027–8. doi: 10.1136/jcp.37.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bar-Oz B, Lev-Sagie A, Arad I, et al. N-terminal pro-B-type natriuretic peptide concentrations in mothers just before delivery, in cord blood, and in newborns. Clin Chem. 2005;51:926–7. doi: 10.1373/clinchem.2005.048892. [DOI] [PubMed] [Google Scholar]
- 22.Fleming SM, O’Gorman T, Finn J, et al. Cardiac troponin I in pre-eclampsia and gestational hypertension. BJOG. 2000;107:1417–20. doi: 10.1111/j.1471-0528.2000.tb11658.x. [DOI] [PubMed] [Google Scholar]
- 23.McDonald EA, Cheng L, Jarilla B, et al. Maternal infection with Schistosoma japonicum induces a profibrotic response in neonates. Infect Immun. 2014;82:350–5. doi: 10.1128/IAI.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaka K, Usuda S, Ito H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24:53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 25.Uszynski M, Perlik M, Uszyński W, et al. Urokinase plasminogen activator (uPA) and its receptor (uPAR) in gestational tissues: measurements and clinical implications. Eur J Obstet Gynecol Reprod Biol. 2004;114:54–8. doi: 10.1016/j.ejogrb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Salle BL, Delvin EE, Lapillonne A, et al. Peri-natal metabolism of vitamin D. Am J Clin Nutr. 2000;71:1317S–24. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 27.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 28.Jaeggi ET, Silverman ED, Laskin C, et al. Prolongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block: a prospective observational study on the effects of maternal antibodies on 165 fetuses. J Am Coll Cardiol. 2011;57:1487–92. doi: 10.1016/j.jacc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Romero R, Shim JY, et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85–90. doi: 10.1080/jmf.14.2.85.90. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol. 2011;31:1397–402. doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasojima K, Schwab C, McGeer EG, et al. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy MR, Kapur RP, Molad Y, et al. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 33.Clancy RM, Askanase AD, Kapur RP, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol. 2002;169:2156–63. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 34.Crilley J, Farrer M. Left ventricular remodelling and brain natriuretic peptide after first myocardial infarction. Heart. 2001;86:638–42. doi: 10.1136/heart.86.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habli M, Cnota J, Michelfelder E, et al. The relationship between amniotic fluid levels of brain-type natriuretic peptide and recipient cardiomyopathy in twin-twin transfusion syndrome. Am J Obstet Gynecol. 2010;203:404, e1–7. doi: 10.1016/j.ajog.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 36.Llanos C, Friedman DM, Saxena A, et al. Anatomical and pathological findings in hearts from fetuses and infants with cardiac manifestations of neonatal lupus. Rheumatology (Oxford) 2012;51:1086–92. doi: 10.1093/rheumatology/ker515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stempien-Otero A, Plawman A, Meznarich J, et al. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. J Biol Chem. 2006;281:15345–51. doi: 10.1074/jbc.M512818200. [DOI] [PubMed] [Google Scholar]
- 38.Morita H, Khanal S, Rastogi S, et al. Selective matrix metalloproteinase inhibition attenuates progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H2522–7. doi: 10.1152/ajpheart.00932.2005. [DOI] [PubMed] [Google Scholar]
- 39.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–6. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Arnson Y, Amital H, Agmon-Levin N, et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev. 2011;10:490–4. doi: 10.1016/j.autrev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Bergman G, Skog A, Tingström J, et al. Late development of complete atrioventricular block may be immune mediated and congenital in origin. Acta Paediatr. 2014;103:275–81. doi: 10.1111/apa.12483. [DOI] [PubMed] [Google Scholar]