Abstract
The objectives of this study were to assess the role of LFA-1 in enteric antigen (EAg)-induced activation of T-cells in vitro and in vivo and to define the importance of this integrin in promoting trafficking of T-cells to the MLNs and colon. We found that EAg-pulsed dendritic cells (DCs) induced proliferation of LFA-1-deficient (CD11a−/−) CD4+ T-cells that was very similar to that induced using WT T-cells suggesting that LFA-1 is not required for activation/proliferation of T-cells in vitro. Co-culture of WT or CD11a−/− T-cells with EAg-pulsed DCs induced the generation of similar amounts of INF-γ, IL-4 and IL-10 whereas IL-17A production was reduced approximately 2-fold in co-cultures with CD11a−/− T-cells. Short term (20-22 hr) trafficking studies demonstrated that while both WT and CD11a−/− T-cells migrated equally well into the spleen, liver, lungs, small intestine, cecum and colon, trafficking of CD11a−/− T-cells to the MLNs was reduced by 50% when compared to WT T-cells. When the observation period was extended from 3-7 days post transfer, we observed approximately 2-3 fold more WT T-cells within the MLNs and colon than CD11a−/− T-cells whereas T cell proliferation (as measured by CSFE dilution) was comparable in both populations. Taken together, our data suggest that LFA-1 is not required for EAg-induced activation of CD4+ T-cells in vitro or in vivo but is required for trafficking of T-cells to the MLNs and homing of colitogenic effector cells to the colon where they initiate chronic gut inflammation.
Keywords: endothelial cells, dendritic cells, enteric antigens, inflammatory bowel disease, leukocyte homing, T cell proliferation
INTRODUCTION
There is an accumulating literature suggesting that the inflammatory bowel diseases (IBD; Crohn's disease; ulcerative colitis) arise from a complex interaction among genetic, immune and environmental factors1-3. It is thought that the presence of one or more genetic defects predisposes individuals to a dysregulated immune response to components of the normal gut flora4-7. Indeed, the role of commensal bacteria in the pathogenesis of experimental IBD has been well-documented in a number of different animal models of chronic gut inflammation2,3,8,9. It is well-known that luminal enteric antigens continuously gain access to the intestinal interstitium where they are endocytosed by interstitial dendritic cells (DCs) and transported to the gut-draining mesenteric lymph nodes (MLNs)10-13. It is becoming increasingly clear that naïve T-cells migrate from the blood to the MLNs where, in the absence of appropriate regulatory mechanisms, they interact with enteric antigen-loaded DCs resulting in their priming, polarization and expansion to yield large numbers of colitogenic effector (Th1/Th17) cells6,7,10,11,13-17. These effector cells then exit the lymphoid tissue via the efferent lymphatics, enter the systemic circulation and home to the gut where they initiate intestinal inflammation.
Trafficking of naïve and effector T-cells to lymphoid and nonlymphoid tissues is a complex process that is mediated by a sequence of adhesion and signaling steps that include tethering of T-cells to the high endothelial venules (HEV) within MLNs or post-capillary venules within the small and large bowel; rolling along the endothelial cell surface, activation-induced firm adhesion of the T-cells to the endothelium and finally extravasation of these lymphocytes into lymphoid or nonlymphoid tissue18-22. One of the T-cell-associated adhesion molecules that has been shown to play an important role in promoting T-cell recruitment to lymphoid and nonlymphoid tissue is lymphocyte function-associated antigen-1 (LFA-1). LFA-1 is a heterodimeric β2 integrin composed of an alpha chain (CD11a, αL) and a beta chain (CD18, β2) and is expressed on virtually all leukocytes including T- and B-cells, granulocytes and macrophages20-22. At least two LFA-1 ligands associated with HEVs and post capillary venules have been identified in mice including ICAM-1 and-223-31 as well as junctional adhesion molecule-1 (JAM-1; CD166)32. Short term homing experiments have demonstrated that LFA-1 is important for trafficking of T-cells to MLNs of immune-competent mice 24,33. Once naïve T-cells enter the MLNs, they may encounter their cognate antigens presented on the surface of DC-associated major histocompatability complex class II thereby initiating their firm adhesion to the DC and promoting T-cell priming, polarization and proliferation. It has been proposed that LFA-1 may also function as a co-stimulatory molecule for T-cell activation24,34-39. In fact, it has been shown that blocking the interaction of LFA-1 with ICAM-1 expressed on DCs inhibits Th1 polarization and cytokine production and in some cases may skew polarization to a Th2 response29,38,40,41. Following priming and polarization, effector T-cells exit the MLNs and enter the systemic circulation via the efferent nodal lymphatics and home to the gut. Again, T-cell-associated LFA-1 as well as other integrins and selectins have been shown to play important roles in mediating the homing of effector cells to nonlymphoid tissue such as the small and large intestine19,21,22,42,43. Upon entering the gut interstitium, these effector cells re-encounter their cognate antigens presented on the surface of a wider range of antigen presenting cells becoming rapidly activated to produce a variety of pro-inflammatory cytokines and mediators. In the absence of appropriate regulatory mechanisms, unfettered T cell activation initiates chronic intestinal inflammation.
We have previously demonstrated that adoptive transfer of naïve, LFA-1 deficient (CD11a−/−) T-cells into lymphopenic recipients fails to induce chronic colitis whereas transfer of wild type T-cells induces chronic and unrelenting colonic inflammation42,43. Failure to induce disease was associated with large and significant reductions in the numbers of CD4+ T-cells within the MLNs and colon suggesting that LFA-1 may be critical for T-cell migration to and/or activation within the MLNs and colon. As a first step toward determining whether the lack of disease is due to defects in T-cell priming/activation and/or trafficking to MLNs and colon, we undertook a series of in vitro and in vivo studies to differentiate between these two possibilities. We present evidence demonstrating that LFA-1 is not required for enteric antigen-induced activation of CD4+ T-cells in vitro or in vivo but is important for the trafficking of these T-cells to the MLNs where these cells become primed and polarized to yield colitogenic effector cells. Our data also suggest that T-cell-associated LFA-1 may play an important role in homing of effector cells to colon where they initiate chronic gut inflammation.
MATERIALS AND METHODS
Preparation of enteric antigens
Enteric antigens (EAg) were prepared from cecal luminal contents of mice using a minor modification of the method described by Gad and co-workers44-46. Ceca of healthy WT (C57Bl/6) mice were removed and placed in a 90 mm Petri dish containing PBS (Gibco, Grand Island, NY, USA) and 10 μg/ml DNase-1 (Roche, Basel, Switzerland). The luminal contents were expelled, collected, homogenized and then disrupted by ultra-sonication on ice. Whole bacteria and debris were removed by centrifugation, and the supernatant was filter-sterilized using a 0.2-μ Acrodisk filter (Pall, Ann Arbor, MI, USA). Protein content of the sterilized extract was determined using Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Richmond, CA, USA). Aliquots of the enteric luminal extract were stored at −80°C.
Preparation of bone marrow and spleen dendritic cells
Bone marrow-derived dendritic cells (bmDCs) were prepared as previously described47 with minor modifications. Bone marrow was removed from the femurs and tibia of WT C57Bl/6 mice and cultured for 7 days in L-glutamine and Phenol Red containing RPMI-1640 medium supplemented with 5% FBS (HyClone Laboratories, South Logan, UT, USA), 10mM HEPES (pH 7.4), 50 μM 2-mercaptoethanol, 20 ηg/ml recombinant murine GM-CSF (R&D Systems, Minneapolis, MN, USA) and antibiotics47. The CD11c+ population was enriched using magnetic separation (Miltenyi). Splenic DCs (sDCs) were isolated using a minor modification of the method described previously48. Spleens from WT mice were removed, injected with 1 ml of 100 U/ml Collagenase D (Roche) in HBSS, cut into 1-2 mm pieces, and incubated for 90 min at 37°C in HBSS containing 400 U/ml Collagenase D. The fragmented tissue was then passed through a 70 μm strainer, washed by centrifugation and erythrocytes removed using hypotonic lysis. The resulting splenocyte preparation was then depleted of B220+ cells (B-cells) and CD3+ cells (T-cells) using magnetic negative selection. The CD11c+ population was purified by cell sorting using the BD FACS Aria IIu (BD Biosciences, San Jose, CA, USA).
T-cell Proliferation in vitro
DCs (bmDCs or sDCs) were incubated overnight (16 hrs) with PBS or different concentrations of EAgs. The cells were then washed and irradiated with 2000 rad44-46. CD4+CD25− T-cells were isolated from WT or CD11a−/− mice using the Dynal Mouse Negative isolation kits for CD4+ T-cells followed by positive selection to remove CD25+ cells (Invitrogen, Carlsbad, CA, USA). T-cell proliferation was quantified as described previously45,46. 104 bmDCs or sDCs were incubated with 105 CD11a−/− or WT CD4+CD25− T-cells for 6 days in 96-well round-bottom culture plates (Corning, Corning, NY, USA). One μCi of [3H]thymidine (PerkinElmer, Boston, MA, USA) was added to each well on day 5 and incubation was continued for an additional 24 hrs. Cells were then harvested using the Brandel 200A cell harvester (Brandel, Gaithersburg, MD, USA) and radioactivity quantified for each well. For some experiments, a time-course for T-cell activation was performed. For some experiments, WT or CD11a−/− CD4+CD25− T-cells were activated by addition of CD3 and CD28 monoclonal antibodies (mAbs) as previously described49. Briefly, 1×105 cells were added into wells of round-bottom 96-well immunologic plates (Corning) coated with 0.5 mg/well anti-CD3 mAb (eBioscience) and incubated for 4 days in presence of 1 ug/ml anti-CD28 mAb (eBioscience). Control T-cells were incubated in the absence of the mAbs. T-cell proliferation was quantified as described above.
Cytokine Determinations
Cytokine levels in the culture media were quantified following co-culture of CD4+CD25− T-cells with EAg-pulsed bmDCs as described above. Following the 6 day incubation period, culture supernatants were collected and stored at −80°C until analyzed. IFN-γ, IL-4, IL-10 and IL- 17A levels in the supernatants were determined by sandwich ELISA using commercial kits for murine cytokine ELISA (eBioscience, San Diego, CA, USA) as described by the manufacturer.
T-cell Trafficking to Lymphoid and Nonlymphoid Tissues using 51Cr-labeled T-cells
CD4+ T-cells were isolated from the spleens of CD11a−/− and WT mice as described above and labeled with 51Cr (Na chromate) using a minor modification of the method described by Siegmund et. al.50. Cell suspensions were adjusted to a concentration of 20×106 cells/ml in RPMI-1640 medium (Gibco) supplemented with 5% FBS (Hyclone), 10mM HEPES (Fisher Scientific), and an antibiotic/antimicotic solution (Mediatech). Twenty μCi of 51Cr were added for every ml of cell suspension. Cells were incubated at 37°C for 1 hour with occasional mixing. Cells were then washed twice by centrifugation to remove unbound 51Cr twice using RPMI media. The cells were suspended in calcium- and magnesium-free HBSS at a concentration of 1×108 cells/ml. Mice were anesthetized (using ketamine/xylazine) and injected (iv) with 107 T-cells (in 0.1 ml). At 20-22 hrs following injection of T-cells, mice were euthanized and the entire blood volume was exchanged with prewarmed (37°C) PBS to remove all cells within the vasculature. Lymphoid and nonlymphoid tissues were removed, blotted with absorbent paper to remove excess fluid, weighed and the amount of radioactivity was quantified. Data were expressed as percent (%) of recovered radioactivity as described by Siegmund et. al.50.
Labeling of T-cells with 5-(and-6)-carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) and Lymphocyte Analysis
T-cells were labeled with CFSE using a modification of the method described by Parish et. al.51. A cell suspension containing equal numbers of congenically marked CD11a−/− (CD45.2) and WT (CD45.1) CD4+ T-cells (107 cells/ml each in FACS buffer) was incubated with 5 uM CFSE for 10 min at 37°C with gentle mixing. Five volumes of ice cold FACS buffer (PBS supplemented with 4% fetal calf serum) were then added, and cells were put on ice for 5 min. The T-cells were washed twice with FACS by centrifugation, resuspended in a small volume of HBSS and adjusted to concentration of 2×108 cells / ml. One hundred μl of cell suspension containing 1×107 cells of each population was then injected (iv) into anesthetized RAG−/− recipients and animals were allowed to recover from the anesthetic. At 3 and 7 days following T-cell transfer, mice were euthanized and mononuclear leukocytes were isolated from the colonic lamina propria and MLNs of the recipient mice using our previously published methods42,52,53. Colons were removed, cut into 0.5-1.0 cm pieces and washed in PBS. Epithelial cells were removed from the colonic tissue by three subsequent 15 min at 37°C in RPMI-1640 supplemented with 2 g/l D-glucose, 2mM Na2-EDTA, and 2mM dithithreitol. The colonic tissue was then washed with RPMI-1640 media supplemented with 4% FBS (Cellect) to remove residual EDTA and DTT. Tissue was then minced with the razor blade in a sterile 60 mm Petri dish and then incubated for 40 min at 37°C in RPMI-1640 media supplemented with 4% FBS and 200 U/ml collagenase type VIII. The leukocyte-containing supernatants were collected, pooled, filtered through a 70 μm strainer and centrifuged for 10 min at 4°C at 400 × g. Mononuclear cells were further enriched by centrifugation over 40%/70% Percoll gradient. The cell pellet was washed and resuspended in FACS buffer and cell viability determined. Mononuclear leukocytes were prepared from the MLNs and spleens as we previously described54. Cells were stained for CD4, CD11a, CD44, CD45.1, and CD45.2 and analyzed for these surface markers as well as for CFSE fluorescence using the FACScalibur (BD Biosciences).
Statistics
Data are presented as mean ± SEM (standard error of mean). Statistical significance between 2 groups was evaluated using a paired T-test whereas a one way analysis of variance followed followed by Dunnett's post hoc test was used for comparing 3 or more groups. A probability value of p < 0.05 was considered significant.
RESULTS
LFA-1 is not Required for Polyclonal- or Enteric Antigen-Induced Activation of CD4+ T-cells in vitro
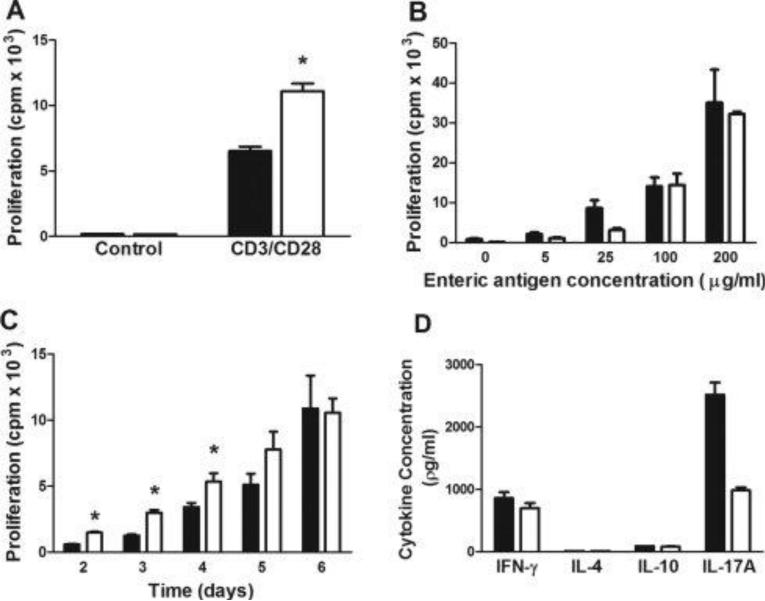
Previous studies have suggested that T-cell-associated LFA-1 is important for optimal T-cell activation in vitro and in vivo using T-cells obtained from CD18−/− DO11.10 transgenic mice24,37. Because CD18−/− T-cells are deficient in all β2 intergrins including LFA-1 and Mac-1 (CD11b/CD18), we wished to re-evaluate more specifically the role of LFA-1 in T-cell activation using polyclonal or enteric antigen (EAg)-induced proliferation of CD4+ T-cells in vitro. To do this, we activated WT or CD11a−/− CD4+CD25− T-cells with CD3 and CD28 mAbs and quantified T-cell proliferation following a 96 hr incubation period. Surprisingly, we found that polyclonal activation induced a significantly greater proliferative response in CD11a−/− than in WT T-cells (Figure 1A). In order to more closely mimic the immunological interactions thought to occur during induction of chronic colitis following transfer of CD4+ T-cells into RAG−/− recipients, we co-cultured WT or CD11a−/− CD4+CD25− T-cells with bmDCs pulsed with different concentrations of EAg. We observed a concentration-dependent increase in proliferation that was quantitatively very similar between the two T-cell populations (Fig. 1B). When shorter incubation times were used (2-4 days), we observed significant increases in proliferation of CD11a−/− T-cells when compared to WT T-cells (Fig. 1C). The ability of EAgs to induce T-cell proliferation was not specific for bmDCs as we found that the WT and CD11a−/− T-cells proliferated, in a similar and concentration-dependent manner when co-cultured with EAg-loaded sDCs for 6 days (data not shown). Co-culture of WT or CD11a−/− T-cells with EAg-pulsed bmDCs resulted in the production of comparable amounts of IFN-γ protein in WT vs. CD11a−/− cultures however, IL-17A generation was reduced by approximately 60% when CD11a−/− T cells were used (Fig. 1D). Production of IL-4 and IL-10 by either group of T-cells was very low or undetectable (Fig. 1D). Taken together, our in vitro data suggest that while LFA-1 is not critical for EAg-induced priming, polarization and expansion of IFN-γ producing T-cells, it may be important for maximal production of IL-17A generation (Fig. 1D).
Figure 1. Activation of wild type or CD11a−/− T-cells in vitro.
(A). Wild type (WT; black bars) or CD11a−/− (white bars) CD4+CD25− T-cells were incubated with plate-bound CD3 and soluble CD28 mAbs for 4 days and T-cell proliferation was quantified using incorporation of [3H]thymidine as described in the Methods section. (B) WT or CD11a−/− CD4+CD25− T-cells were co-cultured with enteric antigen (EAg)-pulsed, bone marrow-derived DCs (bmDCs) 6 days and T-cell proliferation quantified as described in (A). (C) Time-dependent proliferation of WT or CD11a−/− CD4+CD25− T-cells induced by EAg-pulsed bmDCs. (D) Protein levels of different cytokines produced following co-culture of EAg-pulsed bmDCs with WT or CD11a−/− CD4+CD25− T-cells for 6 days as described in B. Cytokines were quantified in the supernatants using ELISA. Black bars represent WT cells obtained from WT mice whereas white bars are cells taken from CD11a−/− mice. Data represent the mean±SEM from 3-4 independent experiments and *p<0.05 vs. WT T-cells.
LFA-1 is Required for the Short Term Trafficking of T cells to the MLNs in vivo
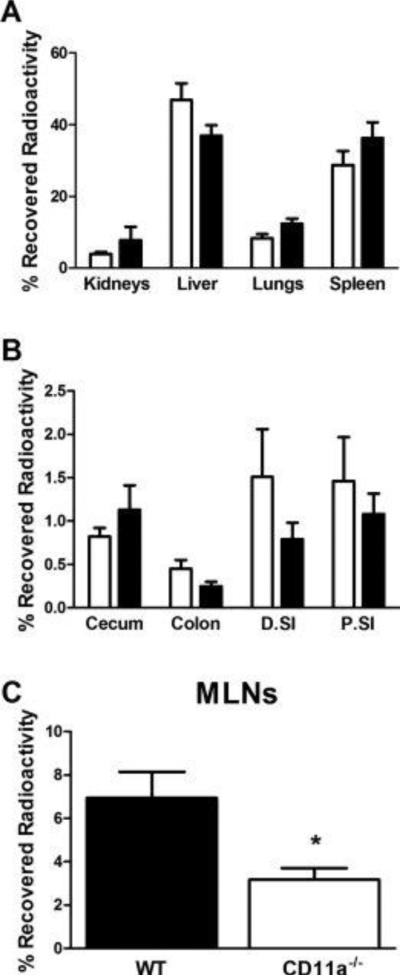
It is well known that adoptive transfer of naïve CD4+ T cells into RAG−/− mice induces a chronic and unrelenting colitis53. Because previous studies have demonstrated that LFA-1 is important for trafficking of T-cells to the MLNs in immuno-competent WT mice24,33, we wished to ascertain whether T-cell-associated LFA-1 plays an important role in the migration of intravascular T-cells to the MLNs as well as nonlymphoid tissues in lymphopenic RAG−/− recipients at a time prior to T cell proliferation post-transfer (i.e. 20-22 hrs). We observed large and equivalent accumulation of 51Cr-labeled WT and CD11a−/− T-cells within the liver, spleen, lungs and kidneys (Fig. 2A). The amount of T-cell-associated 51Cr within these tissues accounted for >80% of the total recovered radioactivity. Few, if any T-cells were detected in the brain, heart, thymus or stomach (data not shown). Evaluation of T-cell trafficking to the gastrointestinal tract and draining MLNs revealed that while similar numbers of WT and CD11a−/− T-cells migrated to the small bowel, cecum and colon (Fig. 2B), trafficking of CD11a−/− T-cells to the MLNs was reduced by ~50% when compared to WT T-cells (p<0.05; Fig. 2C).
Figure 2. Short-term trafficking of T-cells to different tissues in RAG-1−/− mice.
51Cr-labeled WT or CD11a−/− CD4+ T-cells were injected (iv; 107 cells per mouse) into RAG-1−/− recipients. At 20-22 hrs following transfer, animals were euthanized and the entire blood volume was exchanged with pre-warmed (37°C) PBS to remove all cells within the vasculature. Lymphoid and nonlymphoid tissues were removed, blotted dry, weighed and the amount of radioactivity was quantified and expressed as percent (%) of recovered activity. (A) T-cell trafficking to the kidneys, liver, lungs and spleen. (B) T-cell trafficking to the cecum, colon, proximal small intestine (P.SI) and distal small intestine (D.SI). (C) T-cell trafficking to the MLNs. Black bars represent WT cells obtained from WT mice whereas white bars are cells taken from CD11a−/− mice. Data represent the mean±SEM for N=6-7 mice per group and *p<0.05 vs. WT T-cells.
LFA-1 is Required for T-cell Trafficking to the MLNs and Colon but is not Required for T-cell activation in vivo
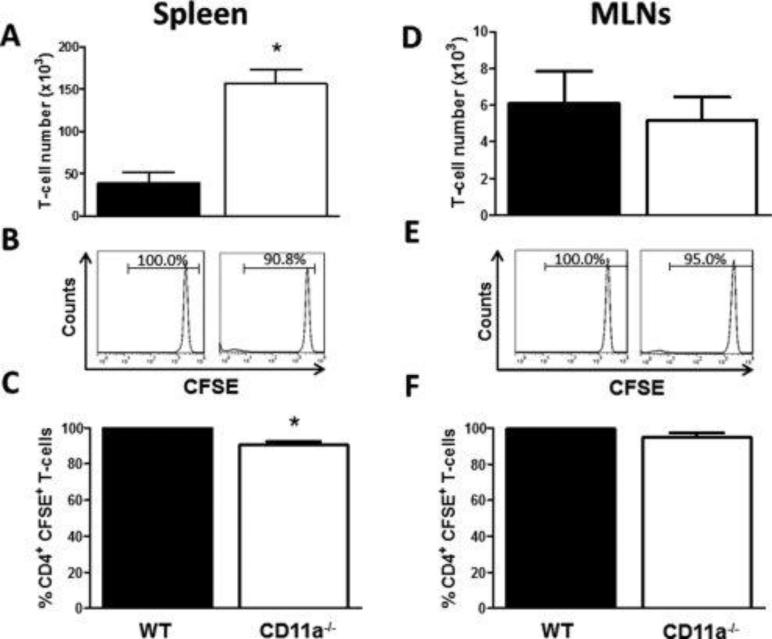
Data obtained from our short-term homing experiments suggest that within the first 20-22 hrs, LFA-1 is not required for T-cell trafficking to the small or large bowel but is important for trafficking of these lymphocytes to the MLNs. We next wished to ascertain whether LFA-1 plays an important role in T-cell trafficking and activation in vivo at longer periods of time. To do this, we co-transferred congenically marked CSFE-labeled WT (CD45.1) and CD11a−/− (CD45.2) CD4+T-cells into RAG−/− mice and quantified cell number for each population as well as CFSE dilution as an index of cell proliferation within the different tissues. At 3 days post T-cell transfer we observed large and significantly greater numbers of CD11a−/− vs. WT T-cells (3-fold) within the spleen (Fig. 3A). In addition, we found a small but significant decrease (9%) in the percentage of CD11a−/− T-cells that were CFSE+ compared to their WT CFSE+ counterparts (p<0.05) suggesting a slight increase in proliferation of LFA-1-deficient T-cells compared to WT T-cells at 3 days following transfer (Fig. 3B and 3C). In contrast to the spleen, we observed similar numbers of WT vs. CD11a−/− within the MLNs at 3 days post transfer (Fig. 3D) with 95-100% of both populations remaining CSFE+ suggesting that neither T-cell population had undergone extensive proliferation at this time period (Fig. 3E and 3F). Very few T-cells could be obtained from the colons of mice at 3 days following transfer of WT and CD11a−/− T-cells (data not shown). The ability to quantify small but significant numbers of T cells in the colon at 20-22 hrs but not at 3 days is most likely due to the increased sensitivity of measuring 51Cr-labeled T cells within the tissue compared to cytometric flow determination of cells isolated from the colonic lamina propria.
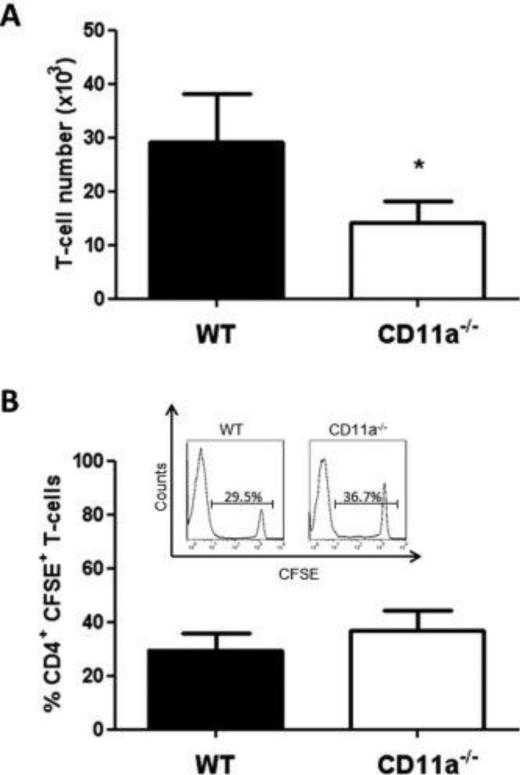
Figure 3. Accumulation and proliferation of wild type and CD11a−/− T-cells within the spleen and MLNs at 3 days following transfer into RAG-1−/− mice.
Congenically-marked, CFSE-labeled WT (45.1; black bars) and CD11a−/− (45.2; white bars) CD4+ T-cells were co-injected (iv; 107 cells each) into RAG-1−/− recipients. At 3 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the indicated tissue, stained for CD4, CD45.1 and CD45.2 and analyzed by flow cytometry. (A) T-cell numbers within the spleen. (B) Representative histograms showing CFSE fluorescence of WT and CD11a−/− CD4+ T-cells. (C) Quantification of WT and CD11a−/− T-cells T-cell CFSE fluorescence. (D) T-cell numbers within the MLNs. (E) Representative histograms showing CFSE fluorescence of WT and CD11a−/− CD4+ T-cells. (F) Quantification of WT and CD11a−/− T-cell CFSE fluorescence. Black bars represent WT cells obtained from WT mice whereas white bars are cells taken from CD11a−/− mice. Data represent the mean±SEM for N=3 mice per group and *p<0.05 vs. WT T-cells.
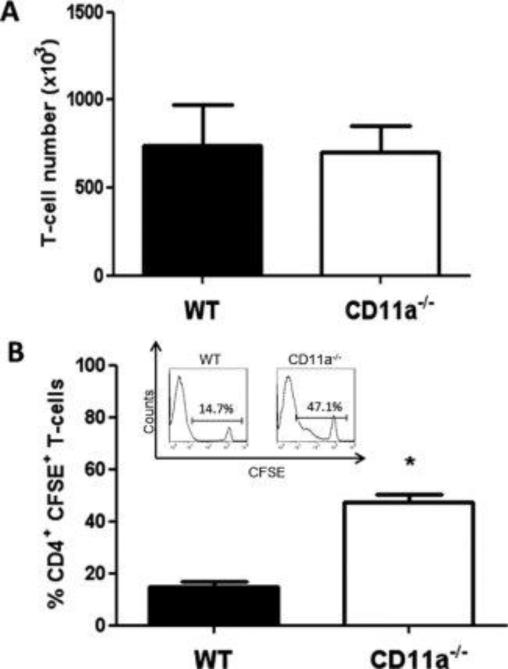
When we extended the observation period to 7 days, we observed large and equivalent numbers of WT and CD11a−/− T-cells within the spleens of the RAG−/− recipients (Fig. 4A), however, WT T-cells appeared to have undergone significantly more proliferation (3-fold) than CD11a−/− T-cells as only 15% of the WT and 47% of the CD11a−/− T-cells remained CFSE+ (Fig. 4B). In addition, we found greater numbers of WT T-cells (2-fold) within the MLNs when compared to their CD11a−/− counterparts at 7 days post transfer (Fig. 5A). Despite the significant increase in WT T-cell numbers in the MLNs, the percentages of CFSE+ WT and CD11a−/− T-cells were very similar (~30-35%) suggesting that both WT and CD11a−/− T-cells had undergone at least 1-2 population doublings within this lymphoid tissue (Fig. 5B). Although there was a trend for increased accumulation of WT vs CD11a−/− T-cells within the colon at 7 days following T-cell transfer, the differences were not significantly different (Fig. 6A). Interestingly, the vast majority of colonic WT and CD11a−/− T-cells had undergone extensive proliferation, with only 5-11% of each population of T-cells remaining CFSE+ at 7 days post transfer (Fig. 6B). Analysis of the CD4+CFSE negative population of CD11a−/− T-cells in different at 7 days post-transfer was not decreased compared to WT CD4+CFSE negative population indicating that CD11a deficiency does not interfere with the activation of these T-cells in vivo (Figs. 7 and 8).
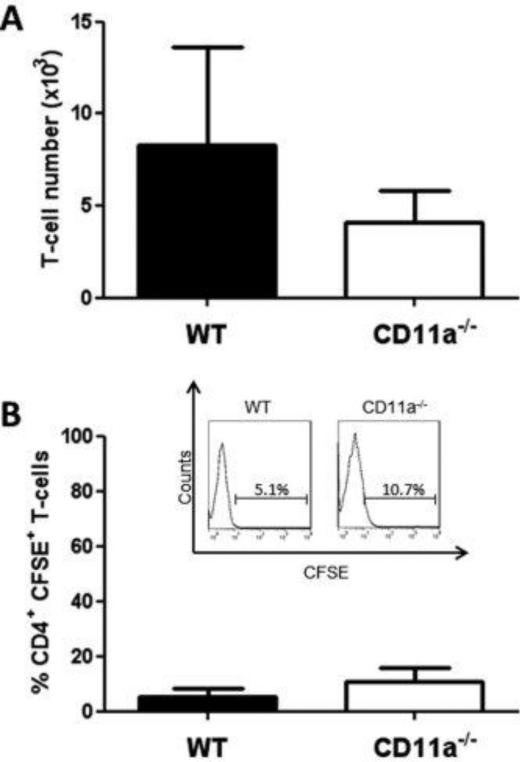
Figure 4. Accumulation and proliferation of WT and CD11a−/− T-cells within the spleen at 7 days following transfer into RAG-1−/− mice.
Congenically-marked, CFSE-labeled WT (45.1) and CD11a−/− (45.2) CD4+ T-cells were co-injected (iv; 107 cells each) into RAG-1−/− recipients. At 7 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the spleen, stained for CD4, CD45.1 and CD45.2 and analyzed by flow cytometry. (A) T-cell numbers within the spleen. (B) Quantification of CFSE fluorescence in WT and CD11a−/− T-cells. Insert shows representative histogram of CFSE fluorescence for each T-cell population. Data represent the mean±SEM for N=6 mice per group and *p<0.05 vs. WT T-cells.
Figure 5. Accumulation and proliferation of WT and CD11a−/− T-cells within the MLNs at 7 days following transfer into RAG-1−/− mice.
Congenically-marked, CFSE-labeled WT (45.1) and CD11a−/− (45.2) CD4+ T-cells were co-injected (iv; 107 cells each) into RAG-1−/− recipients. At 7 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the MLNs, stained for CD4, CD45.1 and CD45.2 and analyzed by flow cytometry. (A) T-cell numbers within the MLNs. (B) Quantification of WT and CD11a−/− T-cells T-cell CFSE fluorescence. Insert shows representative histogram of CFSE fluorescence for each T-cell population. Data represent the mean±SEM for N=6 mice per group and *p<0.05 vs. WT T-cells. (B) Quantification of CFSE fluorescence in WT and CD11a−/− T-cells. Insert shows representative histogram of CFSE fluorescence for each T-cell population. Data represent the mean±SEM for N=6 mice per group and *p<0.05 vs. WT T-cells.
Figure 6. Accumulation and proliferation of WT and CD11a−/− T-cells within the colon at 7 days following transfer into RAG-1−/− mice.
Congenically-marked, CFSE-labeled WT (45.1) and CD11a−/− (45.2) CD4+ T-cells were co-injected (iv; 107 cells each) into RAG-1−/−recipients. At 7 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the colon, stained for CD4, CD45.1 and CD45.2 and analyzed by flow cytometry. (A) T-cell numbers within the spleen. (B) Quantification of CFSE fluorescence in WT and CD11a−/− T-cells. Insert shows representative histogram of CFSE fluorescence for each T-cell population. Data represent the mean±SEM for N=6 mice per group and *p<0.05 vs. WT T-cells.
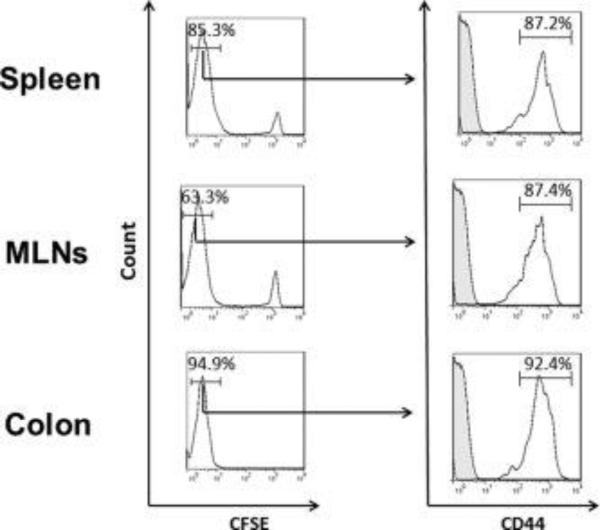
Figure 7. Expression of CD44 on CFSE negative WT CD4+ T-cells at 7 days following transfer into RAG-1−/− mice.
CFSE-labeled WT (CD45.1) CD4+ T-cells were injected (iv; 107 cells) into RAG-1−/− recipients. At 7 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the different tissues, stained for CD45.1 and CD44 and analyzed using flow cytometry. Representative histograms showing CD44 expression on CFSE negative T cells are presented. Data represent the mean±SEM for N=3 mice per group.
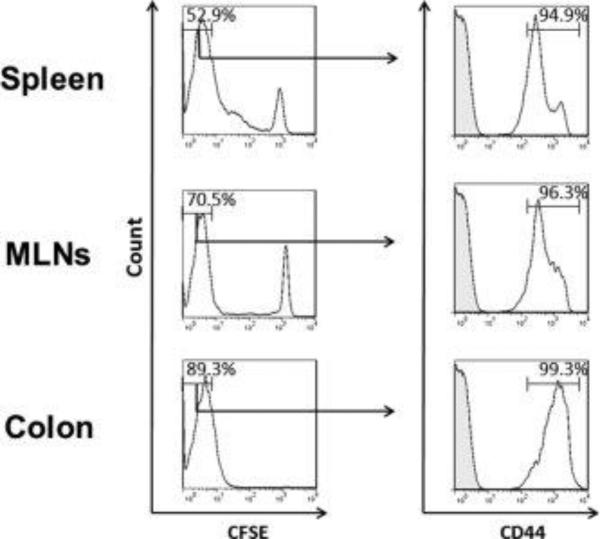
Figure 8. Expression of CD44 on CFSE negative CD11a−/− CD4+ T-cells at 7 days following transfer into RAG-1−/− mice.
CFSE-labeled CD11a−/− (CD45.2) CD4+ T-cells were injected (iv; 107 cells) into RAG-1−/− recipients. At 7 days following T-cell transfer, animals were sacrificed, T-cells were isolated from the different tissues, stained for CD45.1 and CD44 and analyzed using flow cytometry. Representative histograms showing CD44 expression on CFSE negative T cells are presented. Data represent the mean±SEM for N=3 mice per group.
DISCUSSION
Previous studies from our laboratory have demonstrated that T-cell associated LFA-1 is critical for induction of chronic colitis in RAG−/− recipients42,43. The failure of LFA-1-deficient T-cells to induce disease was associated with significant reductions in the numbers of CD4+ T-cells within the MLNs and colon suggesting that LFA-1 may be required for T-cell migration to and/or activation within the MLNs and colon. In order to determine whether the lack of disease was due to defects in T-cell priming and/or trafficking to MLNs and colon, we performed a series of studies to differentiate between these two possibilities. Although previous studies reported that LFA-1 is an important co-stimulatory molecule for T-cell activation in vitro and in vivo24,34-39 we found that proliferation of LFA-1-deficient (CD11a−/−) T cells proliferation was not reduced in response to either CD3/CD28 mAbs or EAg-pulsed DCs (Fig. 1A-C). Indeed, we observed increased not decreased proliferation of LFA-1-deficient T cells when induced by CD3/CD28 mAbs for 96 hrs or by EAg-pulsed DCs with the first 4 days of culture (Fig. 1A-C). The reasons for these differences are not entirely clear however, consideration of the specific T-cell populations used for the different studies as well as the methods used to activate these lymphocytes in vitro (and in vivo) may provide possible explanations. For example, we used CD11a−/− T-cells to insure that these lymphocytes were deficient in only LFA-1 (CD11a/CD18) while other investigators used CD18−/− mice as a source of LFA-1 deficient lymphocytes24,37. In addition to LFA-1, CD18−/− T-cells also lack expression of Mac-1 (CD11b/CD18) which is present on T-cells as well55-59. Thus, it is difficult to ascertain whether the defect in T-cell activation observed using CD18−/− T-cells is due to the absence of LFA-1 and/or Mac-1. Another possible explanation is that our EAg-based assay targets only those T-cells with the ability to recognize enteric antigens, which is estimated to be 3-5% of murine CD4+ T-cells45.
Although LFA-1 has been reported to be required for optimal T-cell activation using polyclonal or antigen specific activation of T cells24,34,35,40,60, there are also several reports demonstrating that LFA-1 is either dispensable for T-cell activation36,55,56,60 or is required for low but now not high concentrations of antigen36. Using an in vitro model that more closely mimics those early immunological events that are thought to occur during the priming, polarization and expansion of naïve T-cells to yield colitogenic effector cells in lymphopenic mice, we failed to demonstrate a major co-stimulatory role for LFA-1. It could be argued that while EAg-induced activation of CD11a−/− deficient T-cells is virtually identical to WT T-cells, cytokine production may be very different. Indeed, it has been reported that blocking LFA-1/ICAM-1 interactions skews cytokine production in favor of a Th2 profile40. Data obtained in the current study suggest that this is not necessarily the case as we observed the production of similar levels of representative Th1, Th2 and regulatory cytokines by WT vs. CD11a−/− T-cells (Fig. 1D). Of note, we did observe significantly less IL-17 production by CD11a−/− T-cells when compared to WT T-cells (Fig. 1D) however, it is not clear that this could account for the remarkable differences we observe in induction of chronic colitis in vivo. In fact, T-cell-derived IL-17A may exert a protective response in the T-cell transfer of chronic colitis as transfer of IL-17A−/− T-cells into lymphopenic recipients exacerbates rather than attenuates colonic inflammation61.
In addition to our in vitro experiments, we also performed a series of in vivo studies to ascertain the role that LFA-1 plays in trafficking and activation of T-cells in RAG−/− recipients. We found that while WT and CD11a−/− T-cells trafficked equally well to virtually all nonlymphoid tissues within the first 20-22 hrs following transfer (Fig. 2A and 2B), we observed approximately a 50% reduction in CD11a−/− T-cell accumulation within the MLNs (Fig. 2C). These data agree well with previously published studies using WT mice as recipients24,60. These data also suggest that trafficking to this lymphoid tissue may represent an important first step in the EAg-dependent priming, polarization and expansion of T-cells to yield colitogenic effector cells. Indeed, chronic gut inflammation induced by adoptive transfer of T-cells is associated with the polarization and expansion of Th1 cells within the MLNs53. Another surprising observation that we made was that while CD11a−/− T-cell accumulation within the MLNs was reduced compared to WT T-cells at 20-22 hrs post transfer, similar numbers of CD11a−/− and WT T-cells were found within MLNs at 3 days post transfer and >95% of both populations were CFSE+ indicating that little proliferation had occurred at this time point (Fig. 3D-F). Although the reasons for these time-dependent differences in trafficking to the MLNs were not investigated in the current study, it may be that CD11a−/− T-cells are retained for a longer period of time within this lymphoid tissue during the first few days following transfer compared to WT T-cells.
When the observation period was extended to 7 days post transfer, we observed significantly more WT T-cells within the MLNs vs. their CD11a−/− counterparts (Fig. 5A and B); however, equal percentages of WT and CD11a−/− T-cells were found to be CFSE+ (~30-35%) suggesting that CD11a−/− T-cell proliferation was not impaired when compared to WT T-cells. These data differ from those of Kandula et. al.24 who demonstrated that DO11.10 T-cells, proliferated to a greater extent that did CD18−/−DO11.10 T-cells within the MLNs of OVA treated mice. The reasons for these differences are not readily apparent but may be due the use of CD18−/− vs CD11a−/− T-cells and/or the different in vivo models employed as described above. We did observe a nonsignificant trend for an increase in WT vs CD11a−/− T-cells within the colon at 7 days post transfer with only 5-10% of either population remained CFSE+ suggesting multiple population doublings by both WT and CD11a−/− T-cells at this time point (Fig. 6A and B). Importantly, flow cytometry data revealed that the large majority (~90%) of WT or CD11a−/− T-cells that were CSFE negative expressed the activation marker CD44 confirming immunological activation of both T cell populations in vivo (Fig. 7 and 8). Taken together, these data suggest that the decreased numbers of CD11a−/− T-cells within the colon were due to defects in trafficking rather than a decrement in their ability to proliferate. The absence of LFA-1 expression also appeared to also affect the accumulation of T-cells within the spleen in a time-dependent manner. Although no significant differences in accumulation were observed between WT vs. CD11a−/− T-cells at 20-22 hrs post transfer, significantly greater numbers of CD11a−/− T-cells found within the spleen at 3 but not 7 days following transfer (Fig. 3A and 4A). These data are somewhat similar to what has been observed in CD11a−/− mice showing increased numbers of CD4+ T-cells in spleens of these mice in the steady state33. Taken together with our MLNs and colon T-cell accumulation data, this observation is consistent with idea that LFA-1−/− T-cells may increase their number in spleen due to decreased trafficking to other lymphoid and nonlymphoid tissues. Data obtained in the current study also suggest that peripheral (i.e. splenic) LFA-1-deficient T-cells undergo a small but significant increase in proliferation at 3 days post transfer when compared to WT cells (Fig. 3B and 3C). These data contrast to those of Kandula et. al. who reported significantly less proliferation of splenic CD18−/−DO11.10 transgenic T-cells vs. their heterozygous controls at 3 days following OVA immunization in vivo24. Although the reasons for these differences are not clear at the present time, differences in the use of CD11a−/− vs transgenic, antigen-specific CD18−/− T-cells followed by immunization in lymphoid tissue other than MLNs may represent reasonable explanations. In contrast to these data, we observed large and equivalent numbers of WT and CD11a−/− T-cells within the spleens at 7 days post transfer with significantly more of the WT T-cells undergoing proliferation than their CD11a−/− counterparts (Fig. 4B). These data suggest that while LFA-1 deficient T-cells accumulate to a much greater extent within the spleen than do WT T cells at 3 days post transfer, WT T-cell proliferation increases 3 fold over that observed for CD11a−/− at 7 days resulting in the presence of similar numbers of T-cells within the spleen. These data agree well with those reported by Kandula et. al. who demonstrated enhanced proliferation of WT vs. CD18−/−DO11.10 transgenic T cells in the spleen at 7 days post transfer 24.
Taken together, our data suggest that LFA-1 is not required for CD3/CD28 and EAg-induced activation of T-cells in vitro or in vivo and may even function as a negative regulator of T-cell activation under certain stimulatory conditions. In addition, we provided evidence that clearly demonstrate that LFA-1 is important for the initial trafficking of these T-cells to the MLNs where these cells are primed and polarized to yield colitogenic effector cells. Our data also suggest that T-cell-associated LFA-1 also plays an important in homing of effector cells to colon where they initiate chronic gut inflammation.
Acknowledgements
Supported by a grant from the NIH (PO1-DK43785; Project 1, Core B (Animal Models Core) and Core C (Histopathology Core).
References
- 1.Bamias G, Nyce MR, De La Rue SA, et al. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Inflammatory Bowel Disease. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng T, Wang L, Schoeb TR, et al. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 6.Powrie F, Read S, Mottet C, et al. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98. [PubMed] [Google Scholar]
- 7.Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- 8.Koboziev I, Karlsson F, Zhang S, et al. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis. 2011;17:1229–1245. doi: 10.1002/ibd.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 10.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 13.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 16.Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 20.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 21.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 22.von Andrian UH, Kogen AN. Adhesion and Communication Between Lymphocytes and Endothelial Cells. 2004:101–137. [Google Scholar]
- 23.Ding Z, Xiong K, Issekutz TB. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J Leukoc Biol. 2001;69:458–466. [PubMed] [Google Scholar]
- 24.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–4451. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 25.Kavanaugh AF, Lightfoot E, Lipsky PE, et al. Role of CD11/CD18 in adhesion and transendothelial migration of T cells. Analysis utilizing CD18-deficient T cell clones. J Immunol. 1991;146:4149–4156. [PubMed] [Google Scholar]
- 26.Lehmann JC, Jablonski-Westrich D, Haubold U, et al. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol. 2003;171:2588–2593. doi: 10.4049/jimmunol.171.5.2588. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheimer-Marks N, Davis LS, Bogue DT, et al. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991;147:2913–2921. [PubMed] [Google Scholar]
- 28.Sligh JE, Jr., Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits HH, de Jong EC, Schuitemaker JH, et al. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J Immunol. 2002;168:1710–1716. doi: 10.4049/jimmunol.168.4.1710. [DOI] [PubMed] [Google Scholar]
- 30.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 31.Van Epps DE, Potter J, Vachula M, et al. Suppression of human lymphocyte chemotaxis and transendothelial migration by anti-LFA-1 antibody. J Immunol. 1989;143:3207–3210. [PubMed] [Google Scholar]
- 32.Ostermann G, Weber KS, Zernecke A, et al. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 33.Berlin-Rufenach C, Otto F, Mathies M, et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham C, Griffith J, Miller J. The dependence for leukocyte function-associated antigen-1/ICAM-1 interactions in T cell activation cannot be overcome by expression of high density TCR ligand. J Immunol. 1999;162:4399–4405. [PubMed] [Google Scholar]
- 35.Abraham C, Miller J. Molecular mechanisms of IL-2 gene regulation following costimulation through LFA-1. J Immunol. 2001;167:5193–5201. doi: 10.4049/jimmunol.167.9.5193. [DOI] [PubMed] [Google Scholar]
- 36.Bachmann MF, McKall-Faienza K, Schmits R, et al. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 37.Marski M, Kandula S, Turner JR, et al. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 38.Perez OD, Mitchell D, Jager GC, et al. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4:1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 39.Ragazzo JL, Ozaki ME, Karlsson L, et al. Costimulation via lymphocyte function-associated antigen 1 in the absence of CD28 ligation promotes anergy of naive CD4+ T cells. Proc Natl Acad Sci U S A. 2001;98:241–246. doi: 10.1073/pnas.011397798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon B, Bluestone JA. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]
- 41.Schmits R, Kundig TM, Baker DM, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostanin DV, Furr KL, Pavlick KP, et al. T cell-associated CD18 but not CD62L, ICAM-1, or PSGL-1 is required for the induction of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1706–G1714. doi: 10.1152/ajpgi.00573.2006. [DOI] [PubMed] [Google Scholar]
- 43.Pavlick KP, Ostanin DV, Furr KL, et al. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. Int Immunol. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 44.Cong Y, Weaver CT, Lazenby A, et al. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 45.Gad M, Pedersen AE, Kristensen NN, et al. Demonstration of strong enterobacterial reactivity of CD4+. Eur J Immunol. 2004;34:695–704. doi: 10.1002/eji.200324394. [DOI] [PubMed] [Google Scholar]
- 46.Gad M, Lundsgaard D, Kjellev S, et al. Reactivity of naive CD4+. Int Immunol. 2006;18:817–825. doi: 10.1093/intimm/dxl018. [DOI] [PubMed] [Google Scholar]
- 47.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inaba K, Swiggard WJ, Steinman RM, et al. Isolation of dendritic cells. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0307s25. Chapter 3:Unit. [DOI] [PubMed] [Google Scholar]
- 49.Schinnerling K, Moos V, Geelhaar A, et al. Regulatory T cells in patients with Whipple's disease. J Immunol. 2011;187:4061–4067. doi: 10.4049/jimmunol.1101349. [DOI] [PubMed] [Google Scholar]
- 50.Siegmund K, Hamann A. Use of labeled lymphocytes to analyze trafficking in vivo. 2005 [Google Scholar]
- 51.Parish CR, Glidden MH, Quah BJ, et al. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0409s84. Chapter 4:Unit4. [DOI] [PubMed] [Google Scholar]
- 52.Ostanin DV, Pavlick KP, Bharwani S, et al. T cell-induced inflammation of the small and large intestine in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G109–G119. doi: 10.1152/ajpgi.00214.2005. [DOI] [PubMed] [Google Scholar]
- 53.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takebayashi K, Koboziev I, Ostanin DV, et al. Role of the gut-associated and secondary lymphoid tissue in the induction of chronic colitis. Inflamm Bowel Dis. 2011;17:268–278. doi: 10.1002/ibd.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bullard DC, Hu X, Schoeb TR, et al. Critical requirement of CD11b (Mac-1) on T cells and accessory cells for development of experimental autoimmune encephalomyelitis. J Immunol. 2005;175:6327–6333. doi: 10.4049/jimmunol.175.10.6327. [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Prince JE, Rais M, et al. Developmental control of integrin expression regulates Th2 effector homing. J Immunol. 2008;180:4656–4667. doi: 10.4049/jimmunol.180.7.4656. [DOI] [PubMed] [Google Scholar]
- 57.Link A, Lenz M, Legner D, et al. Telmisartan inhibits beta2-integrin MAC-1 expression in human T-lymphocytes. J Hypertens. 2006;24:1891–1898. doi: 10.1097/01.hjh.0000242415.73406.17. [DOI] [PubMed] [Google Scholar]
- 58.Wagner C, Hansch GM, Stegmaier S, et al. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: activation-dependent up-regulation and regulatory function. Eur J Immunol. 2001;31:1173–1180. doi: 10.1002/1521-4141(200104)31:4<1173::aid-immu1173>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Wu H, Rodgers JR, Perrard XY, et al. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 60.Marski M, Ye AL, Abraham C. CD18 is required for intestinal T cell responses at multiple immune checkpoints. J Immunol. 2007;178:2104–2112. doi: 10.4049/jimmunol.178.4.2104. [DOI] [PubMed] [Google Scholar]
- 61.O'Connor W, Jr., Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]