Abstract
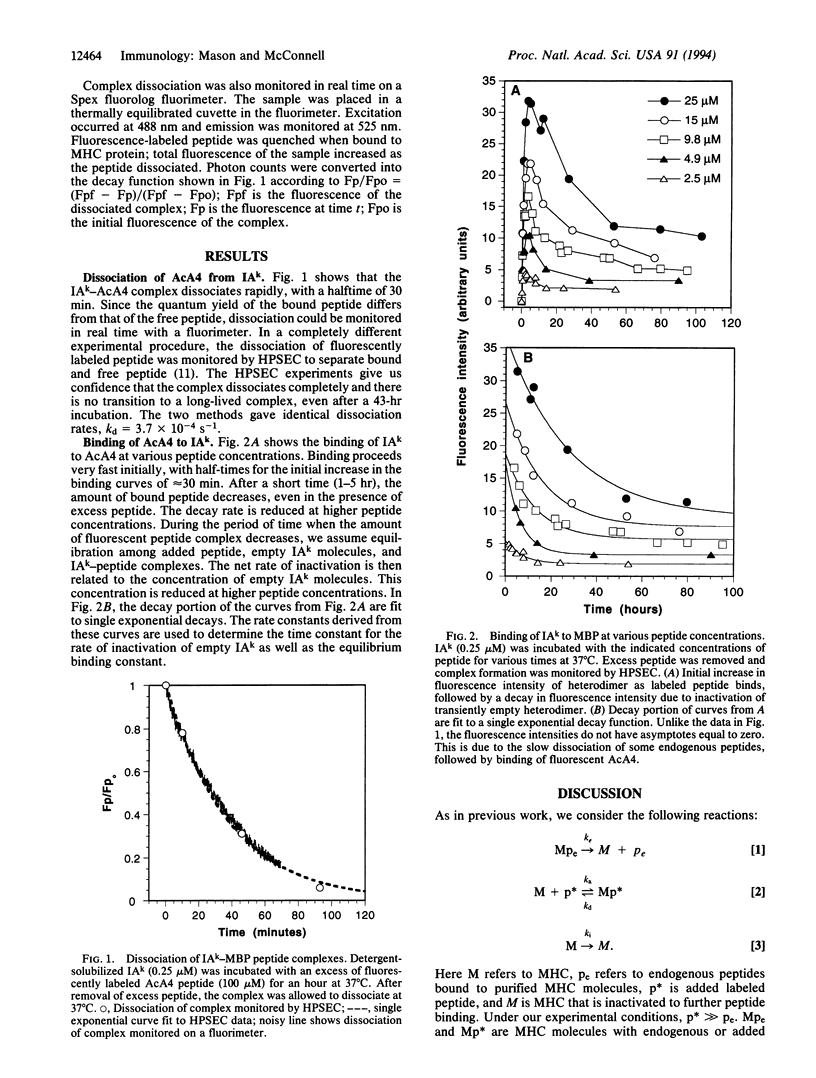
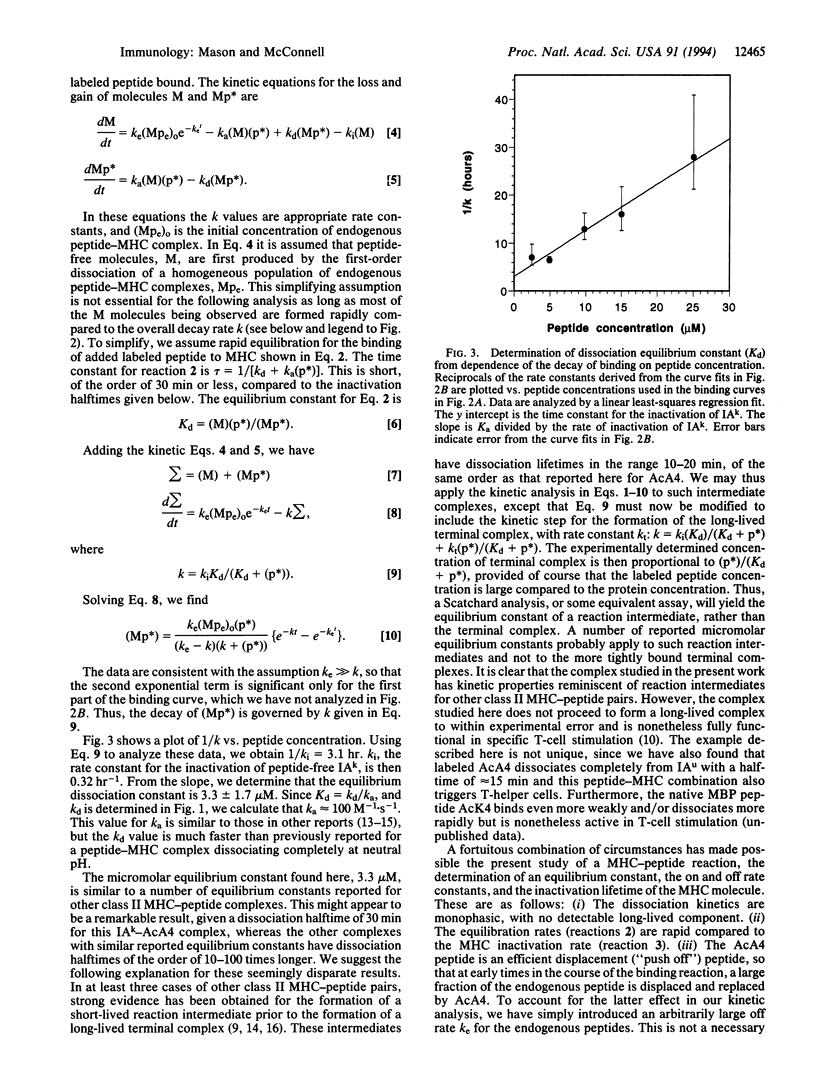
Kinetic rate constants and the equilibrium dissociation constant have been determined for the reaction between an affinity-purified class II major histocompatibility complex molecule IAk and a myelin basic protein analogue peptide, fluorescein-labeled Ac(1-14)A4C15. Under the experimental conditions used, the lifetime of the peptide-free IAk molecule with respect to inactivation is 3.1 hr. The equilibrium dissociation constant, 3.3 +/- 1.7 microM, is determined from measurements of the kinetics of peptide inhibition of IAk inactivation. The measured peptide dissociation halftime is relatively short, 30 min, and the deduced association rate is 100 M-1.s-1. The rate constants and the equilibrium constant are similar to those characteristic of kinetic intermediates in reactions of peptides and class II proteins that lead to long-lived terminal complexes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Adams S., Freeman J., Roof R. W., Lambert L., Unanue E. R. Enhanced immunogenicity of a T cell immunogenic peptide by modifications of its N and C termini. Int Immunol. 1989;1(2):141–150. doi: 10.1093/intimm/1.2.141. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Beeson C., McConnell H. M. Kinetic intermediates in the reactions between peptides and proteins of major histocompatibility complex class II. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8842–8845. doi: 10.1073/pnas.91.19.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Davis C. B., Mitchell D. J., Wraith D. C., Todd J. A., Zamvil S. S., McDevitt H. O., Steinman L., Jones P. P. Polymorphic residues on the I-A beta chain modulate the stimulation of T cell clones specific for the N-terminal peptide of the autoantigen myelin basic protein. J Immunol. 1989 Oct 1;143(7):2083–2093. [PubMed] [Google Scholar]
- Fairchild P. J., Wildgoose R., Atherton E., Webb S., Wraith D. C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993 Sep;5(9):1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- Franco A., Southwood S., Arrhenius T., Kuchroo V. K., Grey H. M., Sette A., Ishioka G. Y. T cell receptor antagonist peptides are highly effective inhibitors of experimental allergic encephalomyelitis. Eur J Immunol. 1994 Apr;24(4):940–946. doi: 10.1002/eji.1830240424. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Stern L. J., Wiley D. C., Germain R. N. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 1994 Aug 25;370(6491):647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Wall M., Southwood S., Sidney J., Oseroff C., del Guericio M. F., Lamont A. G., Colón S. M., Arrhenius T., Gaeta F. C., Sette A. High affinity for class II molecules as a necessary but not sufficient characteristic of encephalitogenic determinants. Int Immunol. 1992 Jul;4(7):773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauben M. H., Joosten I., Schlief A., van der Zee R., Boog C. J., van Eden W. Inhibition of experimental autoimmune encephalomyelitis by MHC class II binding competitor peptides depends on the relative MHC binding affinity of the disease-inducing peptide. J Immunol. 1994 Apr 15;152(8):4211–4220. [PubMed] [Google Scholar]
- Witt S. N., McConnell H. M. A first-order reaction controls the binding of antigenic peptides to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8164–8168. doi: 10.1073/pnas.88.18.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]



