Abstract
Objective
To increase hand sanitizer usage among healthcare workers by developing and implementing a low-cost intervention using RFID and wireless mesh networks to provide real-time alarms for increasing hand hygiene compliance during opportune moments in an open layout Intensive Care Unit (ICU).
Method
A wireless, RFID based system was developed and deployed in the ICU. The ICU beds were divded into an intervention arm (n=10) and a control arm (n=14). Passive RFID tags were issued to the doctors, nurses and support staff of the ICU. Long range RFID readers were positioned strategically. Sensors were placed beneath the hand sanitizers to record sanitizer usage. The system would alert the HCWs by flashing a light if an opportune moment for hand sanitization was detected.
Results
A significant increase in hand sanitizer use was noted in the intervention arm. Usage was highest during the early part of the workday and decreased as the day progressed. Hand wash events per person hour was highest among the ancilliary staff followed by the doctors and nurses.
Conclusion
Real-time feedback has potential to increase hand hygiene compliance among HCWs. The system demonstrates the possibility of automating compliance monitoring in an ICU with an open layout.
Keywords: RFID, hand hygiene compliance, low-cost, healthcare associated infections, behavioral change, real-time feedback
Background
Healthcare associated infections (HAIs) have been regarded as the most frequent adverse event occurring in healthcare in a report by the World Health Organization (WHO) WHO [1]. According to the Centers for Disease Control and Prevention (CDC) one in every 10–20 patients in hospitals in the United States are subjected to HAIs [2]. In a study by Burke in 2003, it has been shown that 25% of HAIs usually occur in the Intensive Care Units (ICU) [3]. The global burden of disease report on HAIs by the WHO, reports that the prevalence of HAIs in developed countries is between 5.1% and 11.6% [1]. In the United States in 2007, the cost of HAIs for inpatients was estimated between $35.7 to $45 billion [4]. In United Kingdom alone the cost to treat HAIs is $ 1.6–18 billion [5].
Surveillance and reporting mechanisms for HAIs in developing countries are largely insufficient and therefore data are difficult to come by [1]. A systematic review on the prevalence of HAIs in Africa reported an overall prevalence between 2.5% to 14.8%, which is twice the average prevalence of European nations (7%) [6]. One study conducted by Mehta, et al in the ICUs of seven Indian cities reported an overall HAI rate of 4.4% [7]. A study conducted by Sathpathy, et al in 2013, reports that the cost to both the hospital as well as the patient increased between 2–4 times due to HAIs. This coupled with the fact that an attendant, usually a family member, accompanies the patient during the hospital stay, also results in an additional loss of income [8]. This is especially worrisome considering the low per capita income of Indian families [9].
Contaminated hands of the healthcare workers can increase the risk of patients developing HAIs. Evidence based guidelines have emphasized that the main reason for cross transmission of infections in any healthcare setting is poor hand hygiene compliance by the healthcare worker (HCW) [10]. Proper hand hygiene compliance therefore could considerably reduce the risk of HAIs resulting in decreased rate of morbidity and mortality.
One of the major challenges to hand hygiene compliance is the behavioral change of the healthcare workers [3]. Despite educational efforts made to increase awareness, the observance of standard hand hygiene protocol is very poor in HCWs including physicians [3].
Previous attempts at hand hygiene compliance and infection control protocol monitoring include, direct observational surveys [11, 12], self reporting by healthcare workers [13–15], monitoring hand hygiene product usage [16] and electronic monitoring systems [17, 18].
Among electronic monitoring systems, Radio Frequency Identification (RFID) based systems have been studied to some extent [19–21]. There are also commercially available products that utilize RFID technology. However there are shortfalls where some systems require a change in HCW workflow, some do not provide real-time prompts to the HCW to perform hand hygiene, many systems utilize battery operated active tags that are prone to battery discharge and are cumbersome to carry around and finally designs such as wristbands are unacceptable to providers in an intensive care unit. Also, most importantly, all the studies as well as commercially available products have been implemented in ICUs and other high dependency areas that are private rooms. While most ICUs in the industrialized nations are built as private rooms, most Indian ICUs have been designed with an open layout where nothing more than a plastic screen separates adjacent beds. This layout of the ICU poses unique challenges while deploying wirelessly communicating systems. The absence of a strong physical barrier causes cross interference and overlapping of RFID signals among adjacent spaces.
Direct observation studies are the gold standard in monitoring healthcare personnel-patient interactions, but are also labor intensive and time consuming. Self-reporting has been shown to over-estimate hand washing rates [22] and both observation and self-report are vulnerable to Social Desirability biases. Other monitoring strategies have included Radio Frequency Identification (RFID) [17, 20] and video monitoring [23] technology. However, to-date, perhaps the reason for not implementing them in mainstream practice is due to high cost associated with development, maintenance and reliability.
This study was designed to combine these approaches into an intervention that is technology driven, low cost, sustainable and easily scalable.
The study objectives were to increase hand sanitizer usage among healthcare workers by developing and implementing a low-cost intervention using RFID and wireless mesh networks to provide real-time alarms for increasing hand hygiene compliance during opportune moments in an open layout Intensive Care Unit of a tertiary care teaching hospital in India.
The primary outcome under consideration is an increase in hand sanitizer use. This would be based on the amount of the liquid/gel based hand rub that would be consumed between the intervention and control groups during the course of the study.
Methods
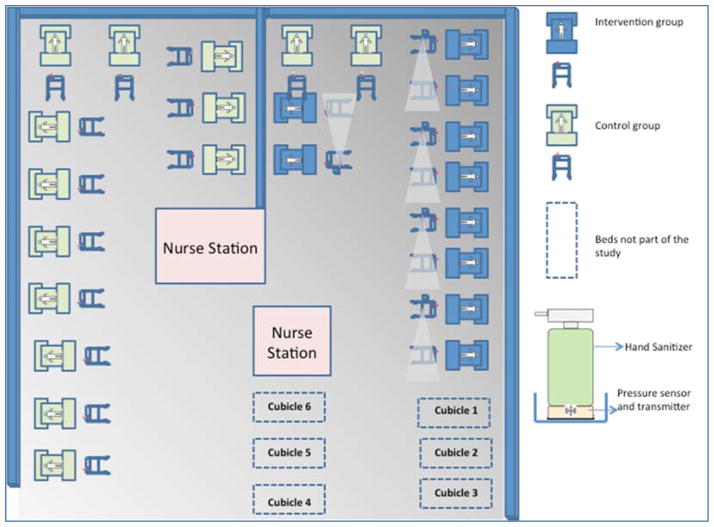
This was an interventional, quasi-experimental study design. Ethical Approval was obtained from the Institutional Ethics Committee. Informed consent was obtained from all participants included in the study. The study was carried out in the 30 bedded Level III, closed ICU of St. John’s Hospital located in the city of Bangalore in India. The ICU has an open layout (Fig 2) where a physical curtain that goes all around the patient’s bed is the only partition separating the beds from one another. There are 24 beds present in this open area. There are also 6 private rooms used for isolation of infected patients. The intervention was carried out in the open area consisting of 24 beds. Ten beds were assigned to the intervention group while the remaining 14 beds were used as control.
Fig. 2.
Layout of the ICU showing the placement of the RFID modules on alternate beds in the intervention arm.
The study participants were the permanent staff of the ICU consisting of doctors, nurses and ancillary staff/nursing aides. The ancillary staff are the support staff concerned with transportation of patients, laboratory samples and replacing spent items at the patient’s bedside. External consultants, interns, medical post-graduate students, student nurses, technicians, visitors and janitorial staff were excluded as they were a migrating population and tracking them would be difficult during this pilot phase.
Baseline workflow analysis
The investigators observed and charted workflow of events that occurred around a patient’s bed-space. It was noted that nurses spent the most amount of time in patient care related activities while the nursing aides spent the least amount of time. It was also observed that while doctors spent majority of their time either examining the patient or performing procedures, nurses spent a substantial amount of time on documentation.
Requirements specification
The system was designed based on the following requirements:
capability to detect the presence of HCWs around a designated area around the patient’s bed-space
capability of detecting movement of HCWs around the patient
should provide real-time visual indication/feedback to the HCW to use the hand sanitizer at opportune moments
should detect whether or not the hand sanitizer was used
It should be capable of wirelessly transmitting data to a software application for data analysis
The application should be capable of receiving data with zero or minimal data loss
The application must be capable of processing data and generate compliance reports
The application must be capable of tracking compliance at the level of an individual
Specifications related to dimensions of the ICU, distance between beds and dimensions of the container that held the hand sanitizer were measured.
Design and development of the hand hygiene system
The hand hygiene system was designed and conceived by the study team. Development was outsourced to a vendor, NestingBits Technologies based in the same city as the study site.
Components of the hand hygiene system
The system consisted of the following components (a) passive RFID tags that were issued to the HCWs, (b) RFID reader for identifying the tags that come within reading range of the RFID reader and (c) the hand sanitizer module which consisted of a pressure sensor (to detect hand sanitizer use), a movement detector, a flashing Light Emitting Diode (LED) and a wireless transmitter. The system underwent extensive pilot testing before implementation in the ICU.
Implementation
The ICU had an open layout with only a curtain separating most of the patient spaces. Every patient space consisted of the bed along with equipment such as ventilator and dialysis machine. The foot end of each bed also had a trolley that contained the hand sanitizer bottle, all emergency bundles, medications and space for the patient record. This trolley was used for installing the RFID reader and hand sanitizer module (Figures 1(a) and 1(b)).
Fig. 1.
Fig 1(a) Patient trolley with the RFID reader (1) and sanitizer module (2)
Fig 1(b) Close-up of the hand sanitizer module with the glowing LED
RFID readers with a long read range in excess of 2 meters under standard conditions were used. The reader operated in the ultra high frequency (UHF) band of 902–928 MHz (FCC- Federal Communications Commission).
ZigBee wireless transmission protocol was used for transferring data from the hand sanitizer modules. ZigBee is a specification for a suite of high level communication protocols that uses small, low-power digital radios based on the Institute of Electrical and Electronics Engineers (IEEE) 802.15.4™ standard for wireless personal area networks [24]. A wireless mesh network [25] was created to facilitate transfer of data between the RF readers and hand sanitizer modules to a primary/master module that would transfer the data to a software application.
One RF reader covered the distance between two ICU beds. We therefore placed them on alternate patient trolleys (Fig 2). The hand sanitizer module was placed on each patient trolley in the intervention group. The sanitizer module with the motion sensor was placed on the side of the trolley that corresponded to the right hand side of the patient as by a process of observation it was noted that the likelihood of HCWs approaching the patient was predominantly towards the patient’s right. The motion sensor could detect movement up to one meter away from the trolley in a single direction. All HCWs in the ICU were issued RFID tags that had a unique identity number. The system was also designed in mind to protect privacy of HCWs in that the tracking was only confined to the patient areas while areas such as toilets, staff lounge and nursing stations were not included in the tracking network topology.
Lastly, the strength of the RFID readers was adjusted in order to counter the effect of overlapping frequencies from adjacent beds due to the open layout of the ICU.
System Workflow
The system was configured to capture events such as a ‘Hit’ or a ‘Miss’. Whenever a HCW carrying the RFID approached the patient’s bed-space, the system triggered a series of events. Firstly, it would detect the presence of the RFID carried by the HCW. Secondly, any movement that occurred near the patient trolley would be simultaneously recorded by the motion sensor. Finally, when these two events occurred in tandem, the LED would be triggered to emit flashes of light for 10 seconds that would illuminate the entire hand sanitizer unit thereby providing a visual cue to the HCW to use the sanitizer. When the HCW used the sanitizer, the LED would stop flashing. The system would register a ‘Hit’ whenever the sanitizer was used per the sequence of events described above, else it would register a ‘Miss’ (Fig 3).
Fig. 3.
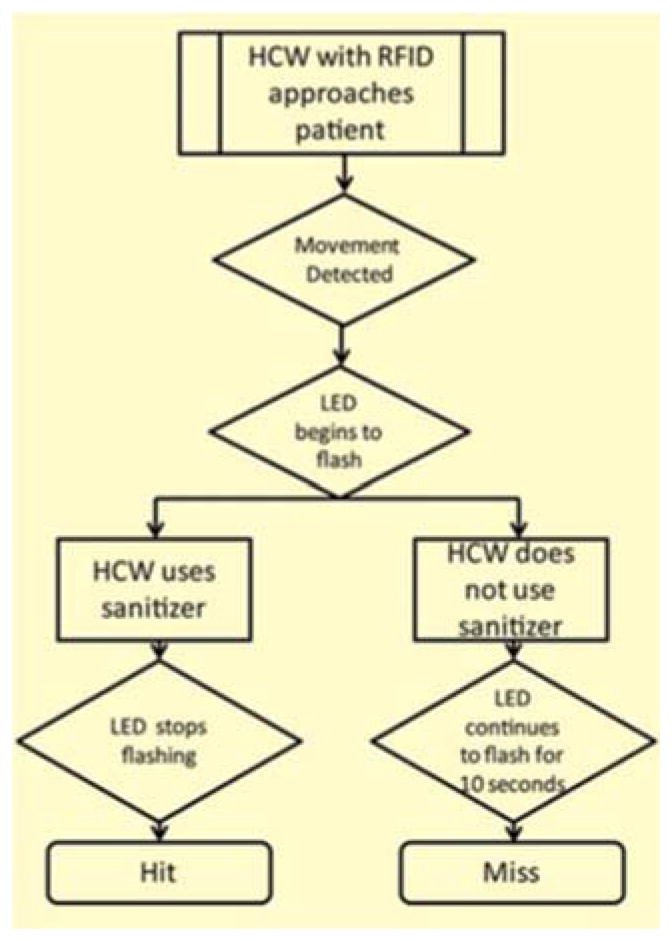
Workflow describing the occurrence of “Hit” and “Miss” events
Costs
The development of the system was outsourced to a technology start-up group. The solution consisting of 5 long-range RFID readers, 10 sanitizer modules which housed the pressure sensor and motion detectors and the ZigBee module, 100 passive RFID tags, a master-unit that received data wirelessly from the sanitizer modules, development of software that kept track of hand hygiene associated events, other information technology components for hosting and maintenance of the software application was developed with a budget slightly under Twenty Thousand US Dollars. If this system were to be mass-produced, the costs can be further substantially reduced.
Results
A total of 94 RFID cards were issued of which 64 were issued to the nurses, 20 to the doctors and the 10 to the ancillary staff (Table 1).
Table 1.
HCW Subgroups
| HCW Type | F | M | Total |
|---|---|---|---|
| Doctor | 7 | 13 | 20 |
| Nurse | 64 | 0 | 64 |
| Ancillary Staff | 5 | 5 | 10 |
| Total | 76 | 18 | 94 |
The period of observation for the intervention was from November 2013 through April 2014. A further four months of observation was performed after uninstalling the intervention to observe the sustainability of the effect of the intervention over time. There were 47,434 usable events recorded during this period. The system was able to track between 36 to 42 staff members on any given day. Each staff member contributed an average of 124 observations (IQR 6–613) during the observation period.
It was also observed that hand sanitizer usage was significantly higher in the intervention group (p value <0.05) with a median hand sanitizer use of 9,250 ml (IQR 8,125 – 10,375 ml) and 7,035 (IQR 6,500 – 8,375 ml) in the intervention and control groups respectively (Table 2).
Table 2.
Sanitizer usage data between the intervention and control groups
| mean | Sd | 25% | 75% | Data(n) | |
|---|---|---|---|---|---|
| Control | 8535.714 | 2885.403 | 7625 | 10000 | 14 |
| Intervention | 10850 | 1972.731 | 10000 | 11500 | 10 |
A consistent increase in sanitizer use was observed in the intervention group both during and four subsequent months after the intervention (Fig 4).
Fig. 4.
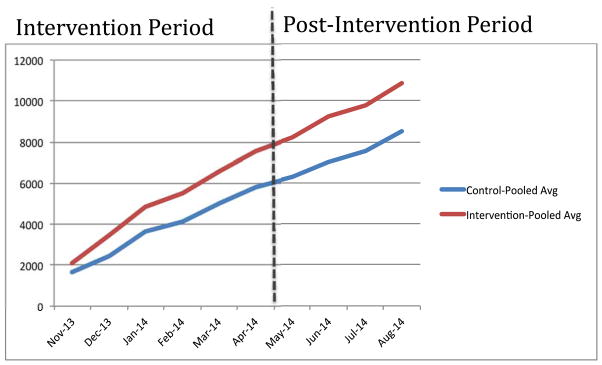
Pooled averages of sanitizer use (in ml) between the two groups during the study period.
Number of hand wash events was analyzed across different groups of HCWs and over different shifts. It was noted that number of hand wash events decreased as the day progressed. Hand wash events were highest during the first shift (7 AM to 1 PM) while it was the least during the night shift (9 PM to 7 AM). Hand wash events per person hour was highest among the nursing aides, followed by the doctors and finally by the nurses (Table 3).
Table 3.
HCW type and shift based statistics
| Staff type | Shift type | N | Number of obs | Obs/staff! (Median, IQR) | Hand wash events | Hand wash events/person_hours | p value |
|---|---|---|---|---|---|---|---|
| Nursing | AM shift, 7 hrs. | 55 | 18,933 | 225(62,460) | 2,775 | 7.2 | |
| PM shift, 7 hrs. | 53 | 14,580 | 94(53,334) | 1,720 | 4.6 | p=0.000 (Fisher’s exact) | |
| Night,10 hrs. | 48 | 9,421 | 46(13,202) | 809 | 1.68 | ||
| Medical | AM shift, 7 hrs. | 13 | 721 | 22(3,46) | 154 | 1.69 | |
| PM shift, 7 hrs. | 9 | 276 | 2(2,3) | 18 | 0.28 | p=0.000 (Fisher’s exact) | |
| Night,10 hrs. | 5 | 27 | 6(2,8) | 2 | 0.2 | ||
| Ancillary Staff | AM shift, 7 hrs. | 4 | 3,119 | 26(10,101) | 786 | 4.6 | |
| PM shift, 7 hrs. | 5 | 2,423 | 26(10,90) | 504 | 2.88 | p=0.000 (Fisher’s exact) | |
| Night,10 hrs. | 1 | 2,082 | 19(9,81) | 317 | 1.5 |
Discussion
Traditional observation based methods for monitoring hand hygiene compliance are cumbersome and expensive. Manual observations are also prone to the Hawthorne Effect [26].
Although there are several studies that have used RFID technology in the past for similar experiments, this study is unique in many ways in that the system was deployed in an open-layout ICU. The reading distance of the RFID readers was in excess of 2 meters which meant that fewer readers had to be placed which also meant fewer deployment issues. The tags used for detection in most of the studies researched used active tags that needed to be powered by batteries [19–21]. All studies where RFID was used were conducted in private rooms. Only one study by Filho et al used ZigBee which is a wireless transmission protocol. In the studies conducted by Boudjema and Sahud, the data was transferred using Ethernet and USB respectively. None of these studies The sustenance of the effect of the intervention over time has also largely not been studied till date.
Our study clearly demonstrates that the effect of the intervention is sustainable over time. The study also reinforces the feasibility of using automated systems to both monitor as well as increase compliance among HCWs.
The study gives insights into the frequency of hand sanitizer usage among different categories of healthcare staff. This has helped identify individuals with low compliance and to apply remedial measures.
It was also observed during the course of the study that hand sanitizer use also increased among the HCWs in the control group, although to a lesser extent than the intervention group. A possible explanation for this phenomenon could be ascribed to the fact that the nurse’s duty rostering was scheduled such that they would be posted to different beds between the intervention and control groups throughout their work-week. The behavioral change that caused an increase in the frequency of using the hand rub in the intervention arm was carried over to the control arm as well. It is also worth noting that the pooled averages (Fig 4) show a steady increase in sanitizer use in the beds that belong to the control group, which can be ascribed to the intervention than merely a case of more use or increase in the number of encounters between the healthcare workers with the patients.
The most important challenge faced in this study was the open layout of the ICU. This posed challenges as there was minimal physical barrier separating the beds, which sometimes caused an overlap of radiofrequencies between adjacent beds. Therefore, triangulation of the exact position of the HCW posed a challenge initially.
Study Limitations
One limitation of this system is that it cannot accurately assign compliance when multiple individuals wearing RFID tags enter a single patient’s bed space. Whenever such an incident is detected, the system would first identify the tags in the order in which it was detected. As the second step, it would assign hit or missed events to the individuals in the same order as the tag detection based on data received from the sanitizer module. For instance if the system detects two RFID tags and two hand hygiene events occurring one after the other, the first “Hit” would be assigned to the ID that was detected first and the second “Hit” would be assigned to the ID that was detected next. There is also a possibility that the system could assign false misses to the staff. This is especially true when a staff enters a patient’s zone but does not actually touch the patient or the patient’s surroundings. This also being the reason as to why we did not calculate individuals’ compliance rates. Although this could be seen as a limitation of the system, it must be remembered that even a marginal increase in compliance may go a long way in preventing HAIs. This is especially true in settings where the HCWs are often overworked and understaffed and also where the hospitalization expenses are borne by the patient as an out-of-pocket expense.
One other limitation is the fact that due to the orientation of the motion sensor in only one direction, a healthcare worker approaching in any other direction, although detected by the RFID reader, goes unnoticed by the software as it relies on a combination of RFID detection along with motion detection to allocate a “hit” or a “missed” event. This can however be easily corrected on the software.
While studies have indicated that good hand hygiene compliance can decrease HAIs among patients in the ICU, we did not study the prevalence of HAIs during the study period for reasons that are out of scope of this paper.
Study Strength
This system with further refinement can obviate the need to monitor compliance by manual observation. The fact that cumulative sanitizer usage increased over a period of nine months clearly demonstrates that the effect of this intervention can be sustained over time. The system was also developed using locally sourced skilled resources thereby keeping the development cost low. It is easy to install and maintain as in no major engineering or infrastructural changes need to be undertaken to deploy the solution. Since the design of the system is modular it can be easily configured and scaled and adapted to suit the requirements of various healthcare settings. Since the system does not interfere with the HCWs workflow, we also found the acceptance levels to be high and which was vital for designing an efficient system. The results of this study indicate that real-time feedback has the potential to increase sanitizer use among HCWs and to sustain the effect of the intervention over time. The system also demonstrates the usability in an open-layout ICU. In order to apply this intervention in other care settings, workflows specific to the environment must be studied and the system needs to be appropriately customized.
It is not necessary that the feedback system be used continuously to increase sanitizer use. It can be deployed for an initial duration where the practice of using the sanitizer reaches optimum levels and can be gradually weaned based on the performance data. However, the system for tracking compliance-only (without the feedback) can continue to operate which can identify early warning signs of non-compliance at which point, the feedback system can be re-implemented to improve compliance. Finally, in order for the effect of the intervention to be sustainable over time, it is suggested that the type of feedback received by the healthcare workers be changed intermittently in the form of changing the frequency of the glowing LEDs, changing the color of the LEDs or both.
Acknowledgments
Research reported in this publication was supported by National Institutes of Health (NIH) Research Training Grant # R25 TW009343 funded by the Fogarty International Center, Office of Behavioral and Social Sciences Research as well as the University of California Global Health Institute, USA.
The authors would like to acknowledge all the staff of the ICU at St. John’s for volunteering to participate in this study. The authors would also like to thank Dr. Savitha Nagaraj who is part of the infection control committee of the hospital for her valuable inputs in the study design.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of California Global Health Institute.
Contributor Information
Kedar Radhakrishna, Email: kedar@sjri.res.in, Division of Medical Informatics, St. John’s Research Institute, 100 Feet Road, Koramangala, Bangalore- 560034. Karnataka, India, Ph: +91-80-4946-7072/+91-994-549-8642.
Abijeet Waghmare, Email: abijeet@sjri.res.in, Division of Medical Informatics, St. John’s Research Institute, 100 Feet Road, Koramangala, Bangalore- 560034. Karnataka, India.
Maria Ekstrand, Email: Maria.Ekstrand@ucsf.edu, Div of Prevention Science, Dept of Medicine, University of California, 50 Beale Street, San Francisco CA 94143, USA.
Tony Raj, Email: tonyraj@sjri.res.in, Division of Medical Informatics, St. John’s Research Institute, 100 Feet Road, Koramangala, Bangalore- 560034. Karnataka, India.
Sumithra Selvam, Email: sumithras@sjri.res.in, Division of Epidemiology and Biostatistics, St. John’s Research Institute, 100 Feet Road, Koramangala, Bangalore- 560034. Karnataka, India.
Sai Madhukar Sreerama, Email: madhukars@sjri.res.in, Division of Medical Informatics, St. John’s Research Institute, 100 Feet Road, Koramangala, Bangalore- 560034. Karnataka, India.
Sriram Sampath, Email: sriram.sampath123@gmail.com, Department of Critical Care Medicine, St. John’s Medical College, Sarjapur Road, Bangalore-560034. Karnataka, India.
References
- 1.WHO. The burden of health care-associated infection worldwide. [cited 2013; Available from: http://www.who.int/gpsc/country_work/burden_hcai/en/
- 2.Yokoe DS, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S12–21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 3.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651–6. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 4.DS The Direct Medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. 2009 Available from: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf.
- 5.Voss A. Healthcare associated infections. BMJ. 2009;339:b3721. doi: 10.1136/bmj.b3721. [DOI] [PubMed] [Google Scholar]
- 6.Bagheri Nejad S, et al. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89(10):757–65. doi: 10.2471/BLT.11.088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta A, et al. Device-associated nosocomial infection rates in intensive care units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67(2):168–74. doi: 10.1016/j.jhin.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Satpathy SCA, Gupta S, Kapil A. Study of hospital associated infections (HAI) at tertiary hospital in India; economic implication In developing countries. Antimicrobial Resistance and Infection Control. 2013;2:261. [Google Scholar]
- 9.(CIA), C.I.A. The World Fact Book. 2013 [cited 2013. [Google Scholar]
- 10.Pratt RJ, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007;65(Suppl 1):S1–64. doi: 10.1016/S0195-6701(07)60002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Mortel TF, Apostolopoulou E, Petrikkos G. A comparison of the hand hygiene knowledge, beliefs, and practices of Greek nursing and medical students. Am J Infect Control. 2010;38(1):75–7. doi: 10.1016/j.ajic.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 12.WHO. WHO Guidelines for Hand Hygiene in Healthcare. World Alliance for Patient Safety; 2005. [Google Scholar]
- 13.Larson EL, Aiello AE, Cimiotti JP. Assessing nurses’ hand hygiene practices by direct observation or self-report. J Nurs Meas. 2004;12(1):77–85. [PubMed] [Google Scholar]
- 14.Moret L, Tequi B, Lombrail P. Should self-assessment methods be used to measure compliance with handwashing recommendations? A study carried out in a French university hospital. Am J Infect Control. 2004;32(7):384–90. doi: 10.1016/j.ajic.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tibballs J. Teaching hospital medical staff to handwash. Med J Aust. 1996;164(7):395–8. doi: 10.5694/j.1326-5377.1996.tb124899.x. [DOI] [PubMed] [Google Scholar]
- 16.Pittet D, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–12. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 17.Chang YT, et al. A novel method for inferring RFID tag reader recordings into clinical events. Int J Med Inform. 2011;80(12):872–80. doi: 10.1016/j.ijmedinf.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Swoboda SM, et al. Electronic monitoring and voice prompts improve hand hygiene and decrease nosocomial infections in an intermediate care unit. Crit Care Med. 2004;32(2):358–63. doi: 10.1097/01.CCM.0000108866.48795.0F. [DOI] [PubMed] [Google Scholar]
- 19.Boudjema S, et al. MediHandTrace (R): a tool for measuring and understanding hand hygiene adherence. Clin Microbiol Infect. 2014;20(1):22–8. doi: 10.1111/1469-0691.12471. [DOI] [PubMed] [Google Scholar]
- 20.Filho MA, et al. Comparison of human and electronic observation for the measurement of compliance with hand hygiene. Am J Infect Control. 2014 doi: 10.1016/j.ajic.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Sahud AG, et al. An electronic hand hygiene surveillance device: a pilot study exploring surrogate markers for hand hygiene compliance. Infect Control Hosp Epidemiol. 2010;31(6):634–9. doi: 10.1086/652527. [DOI] [PubMed] [Google Scholar]
- 22.Stone AA, et al. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24(2):182–99. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 23.Armellino D, HE, Schilling ME, Senicola W, Eichorn A, Dlugacz Y. Using High-Technology to Enforce Low-Technology Safety Measures: The Use of Third-party Remote Video Auditing and Real-time Feedback in Healthcare. Clinical Infectious Diseases. 2011;54(1) doi: 10.1093/cid/cis201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alliance Z. ZigBee Standards. [cited 2014 30-09-2014]; Available from: http://www.zigbee.org/About/AboutTechnology/Standards.aspx.
- 25.World P. Definition of: wireless mesh network. 2014 [cited 2014 30/09/2014]; Available from: http://www.pcmag.com/encyclopedia/term/54776/wireless-mesh-network.
- 26.Marilynn Wood JCK. Basic Steps in Planning Nursing Research: From Question to Proposal. 7. Jones and Bartlett Publishers LLC; 2011. [Google Scholar]