Abstract
This Editorial has the intention to stress the complex chemistry and biology of redox-active compounds, regarded as SOD mimics. It further aims to caution the researchers of the importance of being up-to-date with the present knowledge on such compounds and their cellular redox biology when coming up with their conclusions, based on the particular species involved in their studies. Antioxid. Redox Signal. 20, 2323–2325.
The Forum on SOD therapeutics was primarily designed to address the most updated understanding of the role of such compounds. These are mainly redox-active metal-based compounds, designed to mimic SOD enzymes in vivo. If they are to mimic SOD enzymes, they must have the ability to easily accept electrons from one molecule of superoxide, O2•−, and give it back to another O2•−, performing such reactions at the reduction potential close to the reduction potential of SOD enzymes. That immediately teaches us that good SOD mimics have the potential to be as good antioxidants/reductants as they are pro-oxidants. This is an extremely important fact to be considered, in particular, with mechanistic studies. Keeping in mind that O2•− is neither a strong antioxidant/reductant nor a strong pro-oxidant and that numerous biomolecules react with it, one can easily imagine that SOD mimics will react with numerous targets in vitro and in vivo. To further support such likelihood, it must be also kept in mind that small-molecular-weight SOD mimics lack the limitations imposed by the tertiary structure of the SOD proteins, and thus nonselectively interact with various redox-active biomolecules. Thus, if compounds are of reduction potential similar to that of enzyme, which good SOD mimics must have to readily exchange electrons with superoxide, a wide range of reactions are indeed possible. Keeping further in mind that the very potent SOD mimics, cationic Mn porphyrins, with excellent electrostatics for O2•−, which contributes ∼2 log-units to the catalysis of O2•− dismutation, have also superior electrostatics for the reactions with other anionic species, both small and large and, in particular, deprotonated thiols. In turn, this further supports the likelihood of the wide range of the reactivities of SOD mimics. We and others reported thus far on such reactivities (2, 4, 8, 9). Yet, these reports have still made only modest impact on the field of Redox Biology and Drug Design. We hope that this Forum may further point to the very complex redox chemistry and biology of these compounds from a mechanistic point of view (2). We are far away from fully comprehending the full spectrum of their actions and therapeutic effects they may cause. For example, the anionic Mn porphyrin, Mn(III) meso-tetrakis(4-carboxylatophenyl)porphyrin, MnTBAP3−, is convincingly shown not to be an SOD mimic (1). Yet, it is often used as an SOD mimic to support the conclusion on the involvement of O2•− such as most recently in (6). However, the wealth of data, ours included, suggests the impact of this compound on NO/HNO/ONOO− biology (1). Due to complex chemistry and biology of redox-active drugs, it is an oversimplification to assign the experimental data to simply superoxide or peroxide or peroxynitrite, even if the compounds do react with such species with high rate constants, which are obtained in aqueous cell-free systems. The genetic approaches are needed in addition to pharmacological strategies, as well as information on localization of these compounds. The information on the colocalization with reactive species and their concentrations would greatly contribute to an understanding of their real action(s). We have recently provided ample evidence that Mn porphyrins (in accord with their ability to act as pro-oxidants) interact with thiols. If that interaction occurs in the presence of peroxides, it produces an impact on the oxidation/S-glutathionylation of signaling proteins (such as NF-κB) and components of the mitochondrial respiratory chain, suppressing ATP production (7, 8). Oxidation of cysteine of Keap1 and thus activation of Nrf2 may well be involved in the therapeutic effects as shown with another class of potent SOD mimics (cyclic polyamines). The action upon Nrf2/Keap1 further agrees with the data on natural redox-active compounds addressed by Forman et al. (5). We have provided evidence on the feasibility of such MnP/thiol/H2O2 interactions in aqueous solutions (2), supporting their role in the actions of metalloporphyrins. The same would be true for SOD proteins if sterics would have been less demanding. In turn, the reactions of SOD enzymes occur with targets other than O2•− at orders of magnitude at lower rate constants.
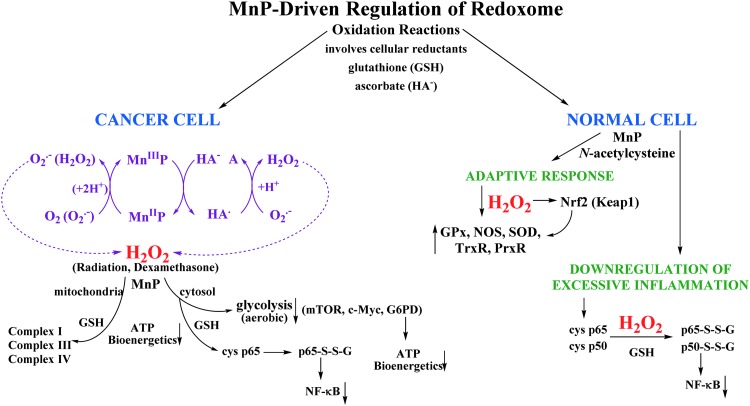
We have thus travelled a long way from identifying such redox-active drugs as SOD mimics to understand the key importance of their direct interactions with signaling protein thiols. In turn, we are offering here Figure 1 for the actions of cationic Mn N-substituted pyridylporphyrins and their impact on Redoxome (cellular redox-based pathways) in cancer and in normal cells. The figure is intended to serve as a challenge and work in progress that may inspire future research. We also want to further draw the attention of a reader to three important facts. First, the pro-oxidative actions of SOD mimics, that is, the oxidation or S-glutathionylation of protein thiols would eventually suppress the NF-κB-driven cycling inflammation and be demonstrated as their antioxidative therapeutic effects. If such suppression is overwhelming and permanent (similar to the action of steroids vs. ibuprofen), cells would undergo death. Second, the experimental data show the differential impact of MnPs on normal versus cancer cells, which we understand now to be due to their differential redox environment: the cancer cell appears to be under high oxidative stress and produces higher peroxide levels that redox-active drugs can employ, as shown in lymphoma and leukemia studies by the Tome's group, to modify protein thiols and greatly affect proliferative and apoptotic processes. Eventually, such action would kill a cancer cell (7, 8). At low levels of peroxide, the transient inhibition of NF-κB DNA binding would reduce secondary oxidative stress and, in turn, heal the normal cell, as indicated in Figure 1. Third, while it may be irrelevant what is the action of a compound as long as it produces satisfactory therapeutic effects (3), understanding the type of action is still of critical importance as it (i) guides us in drug development and (ii) allows us to correctly assign mechanistic issues in studies of diseases, therefore furthering our understanding of cell biology also.
FIG. 1.
Present understanding of the action(s) of MnP, with redox ability similar to SOD enzymes, upon the cellular redox-based pathways. Due to differential redox environment of cancer and normal cell, MnP would likely heal a normal cell (unless it is very sick and thus of similar redox environment as a cancer cell) and kill the cancer cell, peroxide most likely to be involved in its actions (2). MnP, Mn porphyrin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The Forum contains four reviews on the impact of metalloporphyrins on (i) immune responses by Delmastro-Greenwood et al.; (ii) central nervous system injuries by Sheng et al.; and (iii) single cell organisms, yeast, and bacteria, by Tovmasyan et al., which serve as simple but powerful screening methods to evaluate therapeutic potential of drug candidates. The fourth review, written by us (2), tried to address where we are at present in understanding compounds that are widely known as SOD mimics. Such compounds indeed mimic SOD enzymes in an aqueous solution, but are nonselective and thus suited to interact actively with numerous biotargets, some of which are yet to be addressed. Different classes of SOD mimics are covered in the review, ranging from metalloporphyrins, Mn(III) biliverdins, metallocorroles, Mn(II) cyclic polyamines, Mn(III) salens, nitroxides to metal salts. For all of those, and regardless of vast differences in structures, we have shown for the first time that thermodynamics governs their SOD-related actions (2).
It is critical to keep in mind that compounds, commonly regarded as SOD mimics, produce significant therapeutic effects in nearly any model of disease. Thus, such structure–activity relationships [between redox property of a compound and its SOD-like activity described by kcat (O2•−)] may be safely used to guide the development of redox-active therapeutics. How is that possible? Cellular metabolism is redox based, that is, it is governed by electron shuttling among biomolecules. Some degree of perturbation in the redox environment of a cell is invariably involved in nearly any disease. Metal complexes are the most redox-active compounds, and when designed as SOD mimics are neither strong pro- nor antioxidants. They are thus able to mildly modify electron shuttling within cells—to restore it to physiological levels—and do that most so with species that are at the moment present in the cell and in their vicinity, and at high-enough levels. The latter is very much true for the long-lasting, both major signaling and damaging species—H2O2 (2). This explains why the invariably favorable therapeutic impact of redox-active compounds echoes their SOD-like activities (2). In other words, the more potent an SOD mimic is, the higher the likelihood that it will be an efficacious therapeutic/redox modulator.
The Forum also contains three original articles, coming from St. Clair's, Fridovich-Keil's, Pervaiz's, and Clement's groups. The article by Kumar et al. relates to the MnSOD enzyme itself. MnSOD is frequently found overexpressed in tumors, where it presumably helps suppress cancer cell oxidative stress and thus promotes its survival and proliferation. Thus, the authors find MnSOD a valid therapeutic target and suggest its downregulation employing the PPARγ agonist. Indeed, those breast cancer patients who were treated for diabetes with PPARγ agonist had lower levels of MnSOD. Fridovich-Keil's group demonstrates the therapeutic relevance of Mn porphyrins in treating galactosemia, the untreatable and devastating disorder. The effects are at least in part related to the impact of Mn porphyrin on S-glutathionylation of protein thiols. The St. Clair's group showed that UV-induced activation of oncogenic signaling pathways, involving EGFR activation, is upregulated in MnSOD knockout mice and cells, but could be reverted by either MnSOD overexpression or Mn porphyrin-based MnSOD mimic (MnTnBuOE-2-PyP5+), supporting its mitochondrial localization and action.
In summary, those groups that use pharmacological and genetic approaches, along with direct measurements of mitochondrial distribution of true SOD mimics, may safely conclude that in their studies such compounds most likely mimic MnSOD. The chemistry of the redox-active compounds is complex and difficult to comprehend, even for the scientists with chemical backgrounds. Additional efforts are needed in order to fully understand and correctly discuss the chemistry and related biology of such compounds.
Abbreviation Used
- MnP
Mn porphyrin
Acknowledgments
This article was funded by NIH grant # P30 CA014236 - (Herbert K. Lyerly, PI).
References
- 1.Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, and Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med 46: 192–201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batinic-Haberle I, Tovmasyan A, Roberts ERH, Vujaskovic Z, Leong KW, and Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal 20: 2372–2415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day BJ. Antioxidant therapeutics: Pandora's box. Free Radic Biol Med 66: 58–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipovic MR, Koh ACW, Arbault S, Niketic V, Debus A, Schleicher U, Bogdan C, Guille M, Lemaitre F, Amatore C, and Ivanovic-Burmazovic I. Striking inflammation from both sides: manganese(II) pentaazamacrocyclic SOD mimics act also as nitric oxide dismutases: a single-cell study. Angew Chem Int Ed Engl 49: 4228–4232, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Forman HJ, Davies KJ, and Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66: 24–35, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue T. and Suzuki-Karasaki Y. Mitochondrial superoxide mediates mitochondrial and endoplasmic reticulum dysfunctions in TRAIL-induced apoptosis in Jurkat cells. Free Radic Biol Med 61: 273–284, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Jaramillo MC, Briehl MM, Batinic Haberle I, and Tome ME. Inhibition of the electron transport chain via the pro-oxidative activity of manganese porphyrin-based SOD mimetics modulates bioenergetics and enhances the response to chemotherapy. Free Radic Biol Med 65: S25, 2013 [Google Scholar]
- 8.Jaramillo MC, Briehl MM, Crapo JD, Batinic-Haberle I, and Tome ME. Manganese porphyrin, MnTE-2-PyP5+, acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic Biol Med 52: 1272–1284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okun Z. and Gross Z. Fine tuning the reactivity of corrole-based catalytic antioxidants. Inorg Chem 51: 8083–8090, 2012 [DOI] [PubMed] [Google Scholar]