Abstract
Traumatic brain injury (TBI) provokes inflammatory responses, including a dramatic rise in brain macrophages in the area of injury. The pathway(s) responsible for macrophage infiltration of the traumatically injured brain and the effects of macrophages on functional outcomes are not well understood. C-C-chemokine receptor 2 (CCR2) is known for directing monocytes to inflamed tissues. To assess the role of macrophages and CCR2 in TBI, we determined outcomes in CCR2-deficient (Ccr2−/−) mice in a controlled cortical impact model. We quantified brain myeloid cell numbers post-TBI by flow cytometry and found that Ccr2−/− mice had greatly reduced macrophage numbers (∼80–90% reduction) early post-TBI, compared with wild-type mice. Motor, locomotor, and cognitive outcomes were assessed. Lack of Ccr2 improved locomotor activity with less hyperactivity in open field testing, but did not affect anxiety levels or motor coordination on the rotarod three weeks after TBI. Importantly, Ccr2−/− mice demonstrated greater spatial learning and memory, compared with wild-type mice eight weeks after TBI. Although there was no difference in the volume of tissue loss, Ccr2−/− mice had significantly increased neuronal density in the CA1-CA3 regions of the hippocampus after TBI, compared with wild-type mice. These data demonstrate that Ccr2 directs the majority of macrophage homing to the brain early after TBI and indicates that Ccr2 may facilitate harmful responses. Lack of Ccr2 improves functional recovery and neuronal survival. These results suggest that therapeutic blockade of CCR2-dependent responses may improve outcomes following TBI.
Key words: : brain injury, CCR2, chemotaxis, inflammation, macrophage
Introduction
Traumatic brain injury (TBI) is defined as head injury caused by blunt or penetrating external physical trauma.1,2 TBI is a worldwide problem and the leading cause of morbidity and mortality among children and adolescents in the US.1 Further, TBI is the signature injury of the wars in Iraq and Afghanistan, where it accounts for approximately 20% of major injuries.3 Life-long motor, cognitive, and psychosocial disabilities can result from TBI, and recent studies suggest that the consequences of TBI may worsen, rather than improve, over time.4–6 The long term consequences of TBI due to sports injuries are increasingly being recognized as clinically significant.7
Following the primary mechanical injury to the brain, secondary injuries can worsen brain damage both immediately and over a period of years. Secondary injuries may reflect inflammation, glutamate excitotoxicity, apoptosis, and diffuse axonal injury.1 The delay in secondary injury creates a window of opportunity for treatment.8 To date, however, there are no proven effective pharmacological treatments for TBI in humans.5 Since multiple pathways are involved in damage to the brain, more than one agent may be required to protect from injury, and several avenues for therapeutics should be explored.1,5,8
C-C-chemokine receptor 2 (CCR2) is a G-protein–coupled surface receptor that is highly expressed on pro-inflammatory monocytes in both humans and mice. In humans, it is expressed on classically activated monocytes that resemble Ly6Chi monocytes in mice.9 CCR2 plays a critical role in recruiting peripheral monocytes from the bone marrow to sites of inflamed tissues.9 CCR2 expression is also observed on other immune cells, including activated memory T cells, B cells, NK cells, basophils, and immature dendritic cells.10,11 In the CNS, under homeostatic and pathological conditions, CCR2 expression is likely restricted to macrophages and/or peripherally-derived leukocytes,12,13 although there is some evidence suggesting that CCR2 also may be expressed on microglia, astrocytes, and neuronal cells.14–17 Multiple ligands for CCR2 have been described. The most potent activator of CCR2 signaling is CCL2, also known as monocyte chemotactic protein 1 (MCP-1), but CCR2 also binds to CCL7 (MCP-3), CCL8 (MCP-2), CCL12 (MCP-5), and CCL13 (MCP 4).9,18
CCR2 contributes to immune defense against several pathogens.9 CCR2 also promotes fracture healing and angiogenesis, and it reduces amyloid plaque formation in animal models for Alzheimer's disease, presumably due to beneficial functions of macrophages such as phagocytosis of plaque material.19–22 CCR2, however, plays a harmful role in models of experimental autoimmune encephalitis (EAE), arthritis, atherosclerosis, ischemia-reperfusion injury, multiple tumor models, pancreatic islet transplantation, experimental diabetes, stroke, and cranial irradiation injury.9,23–28
In humans, TBI induces in the cerebrospinal fluid a rapid and significant rise in CCL2 protein, a ligand for CCR2, and CCL2 remains elevated for several days.29 CCL2 upregulation also is detected in mouse brain tissue after TBI.29–31 Further, CCL2-deficient mice have improved long-term neurological scores in simple motor tasks following TBI.29 Although it is promising that a deficiency in a single chemokine ligand for CCR2 improves outcomes in TBI, other CCR2 ligands also are upregulated following murine TBI, including CCL12, and these may partially compensate for the lack of CCL2.31 Although the mechanism has yet to be defined, inhibition of CCL2 also has been observed to promote pro-inflammatory cytokine production from astrocytes.29,32 Taken together, these data suggest that to maximize blockade of macrophage infiltration and inflammation, it may be advantageous to target the receptor, CCR2, for which drugs have recently been generated.33
We therefore investigated TBI outcomes in CCR2-deficient (Ccr2−/−) mice, using a controlled cortical impact model of experimental TBI. Our study shows that CCR2 is required for 80–90% of macrophage infiltration from the periphery in the first few days following TBI. We also demonstrate that Ccr2−/− mice perform significantly better in cognitive tests, including the open field test and Morris water maze test. Finally, we show that Ccr2−/− mice have reduced neuronal loss following TBI. These data are the first to show in the context of TBI that CCR2 controls CD45hi CD11b+macrophage infiltration from the periphery, and that a reduction in peripheral macrophages is associated with improved long-term cognition. CCR2 may be a therapeutic target to improve outcomes following TBI.
Methods
Animals
Male animals that were 10–14 weeks of age were subjected to TBI and used for flow cytometry studies. Animals that were 12–16 weeks of age were subjected to TBI for behavior and histology studies. Ccr2−/− mice backcrossed onto a C57BL/6 background for nine generations34 were originally from Jackson Laboratories (Bar Harbor, ME) and were bred in the AALAC-approved transgenic animal facility of the San Francisco VA Medical Center. C57BL/6 mice were from the same source as the Ccr2−/− mice (Jackson Laboratories) and housed in the same room at the San Francisco VA Medical Center.
Surgery
Controlled cortical impact (CCI) surgery or sham surgery was performed on anesthetized animals under a protocol approved by the San Francisco VA Medical Center Animal Care Committee. Briefly, bupivacaine was administered subcutaneously above the skull. Under general anesthesia produced by isoflurane, an incision was made followed by a 2.5 mm circular craniectomy. TBI was inflicted by a 2-mm circular, flat pneumatic piston traveling at 3 m/s, penetrating 1.5 mm, for 150 ms (Amscien Instruments, Richmond, VA, with extensive modifications by H&R Machine, Capay, CA). Target brain coordinates for the center of injury were 1.5 mm lateral, 2.3 mm posterior to the bregma point. Sham animals received all surgical procedures without piston impact.
Brain and blood leukocyte isolation
Brain leukocytes were harvested similarly to previously published methods.35,36 Briefly, following perfusion, brain tissues were cut along the sagittal midline to separate the contralateral and ipsilateral hemispheres. Tissues were pooled from two animals for each sample. Tissue was mechanically disassociated through a 100 μm nylon cell strainer (BD Biosciences, San Jose, CA) and washed in ice-cold GKN buffer (8 g/L NaCl, 0.4 g/L KCl, 1.41 g/L Na2HPO4, 0.6 g/L NaH2PO4, and 2g/L D(+) glucose, pH 7.4). Cells were re-suspended in NOSE buffer (4 g/L MgCl2, 2.55 g/L CaCl2, 3.73 g/L KCl, 8.95 g/L NaCl, pH 6–7) supplemented with 200 U/mL DNase I (Sigma-Aldrich, St. Louis, MO and 0.2 mg/mL collagenase type I (Worthington, Lakewood, NJ) at 37°C in a CO2 incubator for 30 min with shaking every 5–10 min, and then washed with GKN buffer. Leukocytes were separated on a discontinuous isotonic Percoll (90% Percoll, 10% 1.5M NaCl, GE Biosciences, Piscataway, NJ) gradient where cells were suspended in 20 mL of a 1.03 g/mL Percoll solution in GKN buffer, and underlayed with 10 mL of a Percoll solution of 1.095 g/mL in phosphate buffered saline. Cells were spun at 900 g for 20 min with no brake. The buffy layer was isolated for further study.
Flow cytometry and antibodies
Fc receptors were blocked with 10% rat serum (Sigma) and cells were stained with fluorescent antibodies. Leukocyte analysis used a combination of the following antibodies: anti-CD45 (clone Ly5) allophycocyanin (eBioscience, San Diego, CA), anti-CD11b (clone M1/70) PE (Invitrogen, Grand Island, NY), or PE-Cy5 (eBioscience), anti-Ly6G (clone 1A8) PE-Cy7 (BD Biosciences). SYTOX Blue (Invitrogen) was used to gate out dead cells. Cells were sorted on a FACSAria (BD Biosciences), and data were analyzed by using FlowJo Software (Treestar, Ashland, OR, version 9.6). All data represent mean±standard error of the mean (SEM), and animal group sizes for each day are as follows for wild-type TBI mice: day 1 (n=14), day 4 (n=8), day 7 (n=4), day 14 (n=8). Animal group sizes for Ccr2−/− TBI mice are as follows: day 1 (n=5), day 4 (n=6), day 7 (n=5), day 14 (n=6). Sham group sizes for all time points were two to four mice.
Histology, tissue volume assessment, and stereology
Brains were perfused with saline, followed by 3.7% formaldehyde. After a 2-h fixation period, brains were incubated in 30% sucrose overnight and frozen in tissue-freezing medium (Sakura, Inc., Torrance, CA). For F4/80 staining, 5-μm sections were quenched for endogenous peroxidases and blocked with streptavidin and biotin (Vector Labs, Burlingame, CA), then immunostained with an anti-F4/80 antibody diluted at 1:200 (Clone BM8, eBioscience), followed by biotinylated rabbit anti-rat antibody diluted at 1:100 (Vector Labs), and biotin was visualized by using the Vectastain ABC elite kit (Vector Labs). Sections were counterstained with hematoxylin. Three animals per group and at least five sections per animal were analyzed. All sections were imaged through use of an Olympus BX-51 microscope.
For assessment of tissue loss volume, nine weeks after surgery, euthanized mice were perfused with saline, after which harvested brains were fixed overnight, followed by a 30% sucrose overnight incubation. Frozen tissues were sectioned as free-floating sections. Mounted sections were counterstained with cresyl violet (FD Neurotechnologies, Ellicott City, MD). Beginning approximately 1.82 mm posterior from the bregma point, cortical and hippocampal areas were traced using OsteoII software (Bioquant, TN). For each animal, 40-μm thick sections (with each analyzed section equally spaced at 0.24 mm apart) that spanned across a total distance of 1 mm were analyzed. Contralateral (left hemisphere) and ipsilateral (right hemisphere) substructure (cortex or hippocampus) volumes were calculated using Cavalieri's formula, volume=∑A×ISF×t, where A is the area, ISF is the inverse of the sampling fraction, and t is section thickness. Volume of tissue loss for each substructure was calculated by subtracting the right hemisphere substructure volume from the left hemisphere substructure volume. Animal group sizes for sham groups were five to six mice, for wild-type TBI group (n=5), and for the Ccr2−/− TBI group (n=9).
For CA volume assessment and stereological counting of neurons, multiple 40-μm thick sections (each equally spaced at 0.24 mm apart) near the lesion site encompassing the hippocampus were assessed. For each section, the cumulative CA region (CA1-CA3) was traced and the volume calculated by using StereoInvestigator software (MicroBrightField, Williston, VT). Cresyl violet stained neurons in the traced region were counted by random sampling under high power magnification (600×) using StereoInvestigator software. Five animals per group were analyzed.
Behavioral studies
Three weeks after TBI, mice were assessed for motor coordination and balance through use of rotarod tests.37–40 Specifically, mice (n=20 per group) were placed on a rotating rod that accelerated 5 rpm every 15 sec with a maximum speed of 40 rpm, and the length of time that the mouse remained on the rod (fall latency) was recorded and averaged for five consecutive trials with 5-min intervals between trials. The trials were repeated on three separate days.
Following rotarod testing, basic locomotor activity and exploratory behavior were determined by the open field test.37,38,40,41 Individual mice (n=20 per group) were placed in a brightly lit, square Plexiglas enclosure (40×40 inches) surrounded by automated infrared photocells that interfaced with a computer (Hamilton & Kinder) to record data. Beam breaks generated by movement were monitored, allowing measurements of the number of basic movements and total path length traveled during the exploration.40 For analysis of the exploratory behavior, the arena was divided on a zone map consisting of a center region and a peripheral region. Time spent in the center versus peripheral zones was used as an indicator of anxiety-like behavior. On each of three consecutive days, open field activity was recorded for 10 min.
Spatial learning and memory were evaluated by using the Morris water maze,35,37–41 starting at eight weeks after TBI or sham surgery.38,40–44 Mice (n=12 per group) were trained to locate a platform in opaque water in a circular pool (135 cm diameter) during two successive daily sessions (three trials per session, 60 sec per trial) with a 1-h intersession interval. The starting positions of the animals were altered and counterbalanced to one of four pre-determined quadrants to rule out spatial preference. During the cued platform test on days 1 and 2, a submerged platform that was marked by a visible flag mounted on the platform was placed in a different quadrant in every session. On days 3 to 5, mice were then trained to locate a hidden platform (no flag) where the platform remained stationary. Latency, path length, swim speeds, and percent time spent swimming in a zone near the walls of the maze (zone of 13.5 cm from the maze wall), the latter of which is known as thigmotaxis, were recorded and derived from the use of a video tracking system (Noldus EthoVision, Noldus, Leesburg, VA). On the sixth day, a one-minute probe trial during which the platform was removed from the pool was performed to test memory retention.
Statistical analysis of behavior studies
Data were analyzed with Statview 5.0.1 software (SAS Institute Inc, Cary, NC). and Prism software (GraphPad Software, Inc, La Jolla, CA version 6.0). Data were expressed as mean±SEM (some error bars were too small to be visible). For analyses of rotarod tests, open field tests, and the non-probe trial days of the Morris water maze tests, statistical significance was evaluated by using a two-way repeated measure analyses of variance (RANOVA). For analysis of the percent of time spent in each quadrant during the probe trial of the Morris water maze, three paired Student's t-tests within each group were performed to determine statistical differences between the time spent in the target quadrant versus each of the other quadrants, as previously analyzed.40,44,45 For analysis of crossing frequency and latency to first crossing of the platform location during the probe trial, two-way analyses of variance (ANOVA) was conducted. Post hoc paired comparisons using the Bonferroni test were used when appropriate.
Statistical analysis of flow cytometry and histological assessments
Two-way ANOVA for main effects of injury, genotype, and interaction was conducted for each time point in our flow cytometric analyses of macrophage and neutrophil numbers by using Prism software. Repeated measures analysis was not conducted, since separate groups of mice were sacrificed at each time point. For statistical analysis of tissue loss volume, hippocampal volume, and neuronal density, two-way ANOVA for main effects of injury, genotype, and interaction was performed.
Results
The early recruitment of brain wound macrophages post-TBI is dependent on CCR2
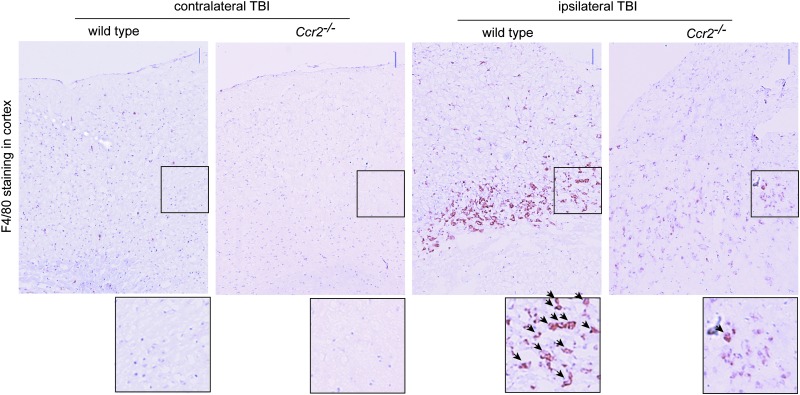
As part of the inflammatory cascade that occurs after TBI, macrophages and neutrophils accumulate in the brain in humans and rodents.46–48 To determine the role of CCR2 in monocyte recruitment, we performed TBI in Ccr2−/− mice and analyzed the presence of macrophages in the brain. Immunohistochemistry for F4/80 antigen, a marker on both macrophages and microglia, was performed at day 4, a time point where we observed peak macrophage infiltration in this model.48 Four days after TBI, wild-type mice showed a large increase in F4/80+cells in and around the area of injury, and this response was markedly reduced in Ccr2−/− mice, although a few F4/80+cells were present (Fig. 1).
FIG. 1.
C-C-chemokine receptor 2-deficient (Ccr2−/−) mice show reduced presence of F4/80+cells near the injury site after traumatic brain injury (TBI). Brain sections from wild-type and Ccr2−/− mice 4 d after TBI were immunostained for F4/80 antigen (brown staining), a marker on macrophages and microglia. Sections were counterstained with hematoxylin (purple). Areas of the contralateral and ipsilateral cortex were imaged. Enlarged images of selected regions (boxed) are shown below. Arrows in insets point out F4/80+cells. n=3 animals per group. Scale bars indicate 50 μm. Color image is available online at www.liebertpub.com/neu
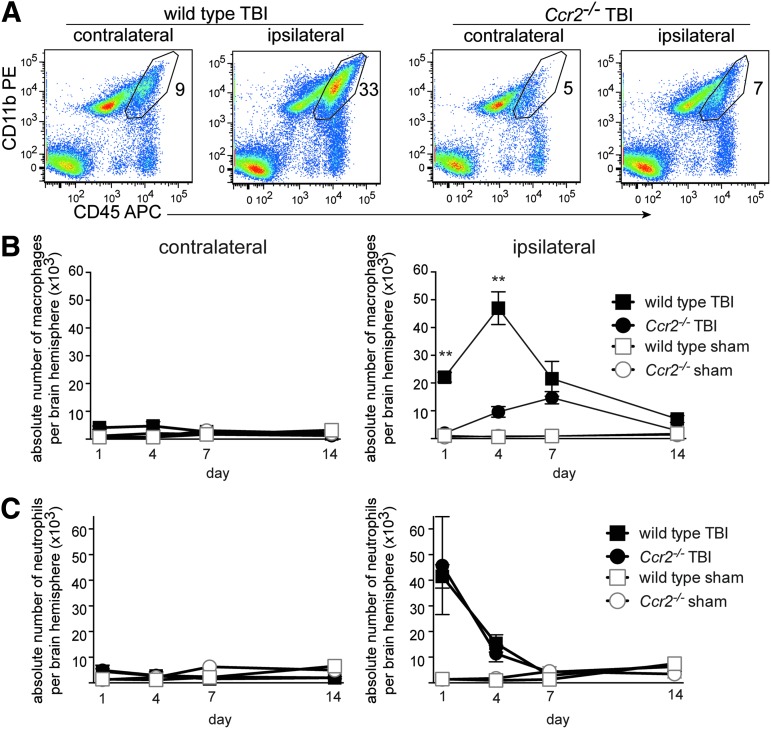
We quantified brain macrophages after TBI in Ccr2−/− mice and wild-type mice by flow cytometry for leukocyte subsets, comparing contralateral and ipsilateral brain hemispheres. By flow cytometry, macrophages can be distinguished from microglia in homeostatic and activated conditions by their level of CD45 staining.12,13,36,49 Macrophages are CD45hi CD11b+ and microglia are CD45lo CD11b+. An antibody against Ly6G was also used to analyze and gate out neutrophils, which are CD45hi CD11b+Ly6G+, as described previously.48 The proportion and number of macrophages in wild-type brains increased greatly in the ipsilateral hemisphere 4 d after TBI, compared with the contralateral hemisphere (Fig. 2A). In contrast, the infiltration of macrophages in Ccr2−/− mice showed reduced proportions of macrophages particularly in the ipsilateral side (Fig. 2A).
FIG. 2.
Early macrophage infiltration is markedly reduced in Ccr2−/− mice after traumatic brain injury (TBI). (A) Representative flow cytometry plots of brain cell preparations isolated from contralateral and ipsilateral brain hemispheres 4 d after TBI. Gated cells represent the proportion of CD45hi CD11b+cells identified as macrophages in the brain cell preparation. Ly6G+CD45hiCD11b+granulocyte cells were excluded from these plots (n=6–8 animals per group). (B) Absolute number of brain macrophages following TBI in contralateral and ipsilateral hemispheres in wild-type and Ccr2−/− mice sacrificed at 1, 4, 7, and 14 d after surgery (mean±standard error of the mean) was assessed. Two-way ANOVA for main effects of genotype and TBI was conducted at each time point. The main effect of genotype was significant at days 1 and 4, F(1,20)=13.9, **p=0.001, and F(1,18)=15.1,**p=0.001, respectively. The effect of TBI on mean macrophage number was also significant at days 1 and 4, F(1,20)=16.4, p=0.0006, and F(1,18)=33.4, p<0.0001. A significant interaction effect was found on days 1 and 4, F(1,20)=12.4, p=0.002, and F(1,19)=15.5, p=0.001), indicating the effect of injury on mean macrophage number was greater among wild-type mice than among Ccr2−/− mice. However, on days 7 and 14, there were neither significant interaction effects nor main effects of genotype, although there were significant main effects of TBI, F(1,9)=12.6, p=0.006, and F(1,14)=5.16, p=0.039, for day 7 and day 14, respectively). Group sizes for wild-type TBI groups in B and C are as follows: day 1, n=14; day 4, n=8; day 7, n=4; and day 14, n=8). Group sizes for Ccr2−/− TBI mice are: day 1, n=5; day 4, n=6; day 7, n=5; day 14, n=6. Sham group sizes for all time points are 2 to 4. (C) Absolute number of neutrophils in the brain following TBI in contralateral and ipsilateral hemispheres was assessed. Although significant effects of TBI were found on days 1 and 4, F(1,18)=10.8, p=0.004, and F(1,18)=13.9, p=0.002, respectively, there were no significant effects of interaction or genotype at any time point. Color image is available online at www.liebertpub.com/neu
Quantification of the absolute numbers of macrophages in each brain hemisphere over time (days 1–14) was performed. Two-way ANOVA for the main effects of TBI and genotype was conducted for each time point at which mice were sacrificed. There was a significant interaction effect for both day 1 and day 4 time points, F(1,20)=12.4, p=0.002 and F(1,18)=15.5, p=0.001, respectively, indicating that the effect of injury on mean macrophage number was greater among wild-type mice than among Ccr2−/− mice. Mean macrophage numbers were significantly higher among wild-type mice than among Ccr2−/− mice, F(1,20)=13.9, p=0.001 and F(1,18)=15.1, p=0.001, for day 1 and day 4, respectively. Similarly, TBI mice had significantly higher mean macrophage numbers than sham mice at those time points, F(1,20)=16.4, p=0.0006 and F(1,18)=33.4, p<0.0001, for day 1 and day 4, respectively. Main effects ANOVA for day 7 and day 14 revealed that only TBI was associated with mean macrophage number at those time points, F(1,9)=12.6, p=0.006 and F(1,14)=5.16, p=0.039, for day 7 and day 14, respectively. There was no effect of Ccr2−/− genotype nor was there an interaction effect between injury and genotype, indicating that only TBI and not genotype had an effect on macrophage numbers at those later time points. In a model of experimental autoimmune uveitis, lack of CCR2 led to a compensating increase in neutrophil infiltration.50 In our model of TBI, neutrophil numbers were unaffected by CCR2 deficiency as there was no main effect of genotype nor interaction at any time point (Fig. 2C). Overall, these data demonstrate that the early rapid rise in macrophages following TBI is largely dependent on CCR2.
CCR2 does not affect recovery of gross motor function
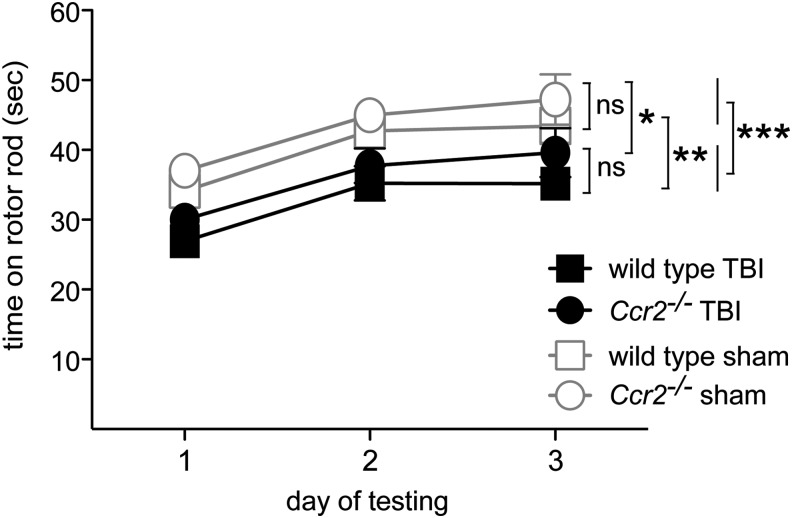
Similar to deficits found in humans after TBI, mice after CCI TBI demonstrate functional impairments as detected by the rotarod test, open field tests, and a Morris water maze.51–53 We used these behavioral tests to assess effects of CCR2 on the functional consequences of TBI. We first tested motor ability and motor learning by using the rotarod assay three weeks after TBI. Four groups of animals were tested: 1) wild-type TBI; 2) Ccr2−/− TBI; 3) wild-type sham; and 4) Ccr2−/− sham. All four groups of mice improved their performance over three days of repeated testing, showing motor learning during training (day effect: F[2,152]=44.4, p<0.001). The TBI groups, however, were impaired in their ability to stay on the rotating rod, compared with the sham-operated groups (TBI effect: F[1,76]=13, p<0.001; Fig. 3). CCR2 gene deletion did not affect the overall performance on the rotarod (genotype effect: F[1,76]=2.4, p>0.12). There was also no interaction between TBI and Ccr2 gene deletion (p>0.9). Thus, in our model TBI impaired motor function but CCR2 deficiency did not alter outcomes of rotarod tests. The lack of effect on motor function allowed us to test other behavioral changes with the assurance that deficiencies would not reflect motor disabilities.
FIG. 3.
Ccr2−/− and wild-type animals show similar motor function deficiency in rotarod tests three weeks after TBI. Average fall latencies during a 3-d rotarod test of wild-type and Ccr2−/− mice after TBI or sham surgery±standard error of the mean (n=20/group). All mice exhibited motor learning as evidenced by progressively increasing latency on the rod over 3 d. Although TBI induced impairment in the rotarod performance, compared with sham controls (***p<0.0001; Ccr2−/−, *p<0.05; wild-type, **p<0.01), there was no significant difference in latency between the wild-type and Ccr2−/− mice with TBI (post hoc test, p>0.2).
Ccr2−/− mice show improved locomotor behavior after TBI
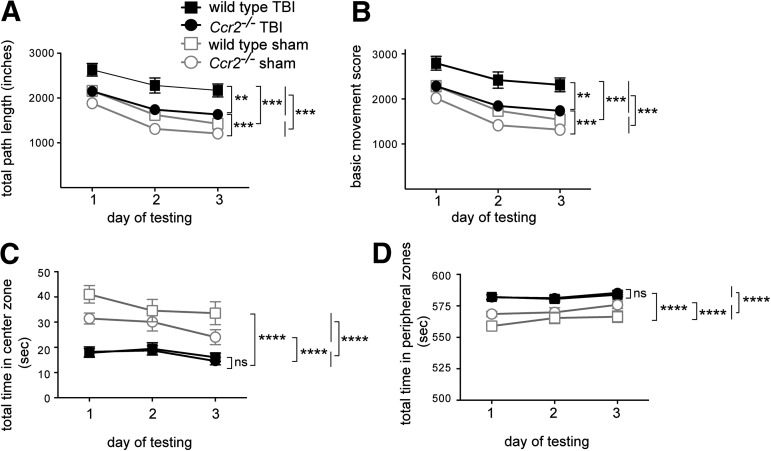
To examine the role of CCR2 in the long-term recovery of locomotor behavior and anxiety after TBI, open field tests were performed. Three weeks after TBI, animals demonstrated an increase in locomotor activity levels, compared with sham controls. Hyperactivity is a symptom described after TBI in both humans and mice.54,55 In accord with this, wild-type mice showed significant increases in path length, F(1,76)=31.9, p<0.0001 (Fig. 4A) and in basic movement, F(1,76)=29.4, p<0.0001 (Fig. 4B). Deficiency in CCR2 notably attenuated hyperactivity following TBI as assessed both by path length, F(1,76)=19.8, p<0.0001; post-hoc analysis, p<0.005, and basic movement, F(1,76)=18.9, p<0.01; post-hoc analysis p<0.005 (Fig. 4B). Thus, by these measures, CCR2 deficiency improved outcomes of locomotor activity after TBI.
FIG. 4.
Deficiency in CCR2 partially rescues traumatic brain injury (TBI)-induced hyperactivity in the novel open field test. The open field test was performed over three consecutive days to test mice three weeks post-TBI (mean±SEM, n=20/group). (A) TBI-induced a hyperactive phenotype as shown by increased path length, compared with sham controls (***p<0.0001; post hoc test, **p<0.005). Lack of CCR2 improved hyperactivity levels in TBI mice, and to a lesser degree in sham-operated mice, compared with wild-type mice (post hoc test, **p<0.001). (B) TBI increased basic movement scores, compared with sham controls (***p<0.0001; post hoc test, **p<0.005). CCR2 deficiency improved hyperactivity after TBI, compared with wild-type mice (**p<0.005). (C) TBI-induced anxiety in mice as shown by reduced time spent in the center zone of the open field, compared with sham controls (****p<0.0001). CCR2 deficiency did not affect the time spent in the center zone after TBI, compared with wild-type TBI mice (p>0.05). (D) TBI-induced anxiety in mice as shown by increased time spent in the peripheral zones of the open field (****p<0.0001). CCR2 deficiency did not affect the time spent in the peripheral zones after TBI (p>0.05).
Animals subjected to TBI have increased anxiety levels
Because the environment in the open field is new to the animal, it concurrently evokes both anxiety and exploration; a decrease in time spent in the center of the open field suggests an increase in anxiety.56,57 TBI induced both wild-type and Ccr2−/− mice to spend significantly less time exploring in the center zone of the open field, compared with sham controls (wild-type: F[1,19]=28.5, p<0.0001; Ccr2−/−: F[1,19]=31.6, p<0.0001; Fig. 4C). Consistent with this, TBI caused both genotypes of mice to spend significantly more time exploring the peripheral zones that is, zones in the corners and zones adjacent to walls (wild-type: F[1,19]=28.5, p<0.0001; Ccr2−/−: F[1,19]=31.5, p<0.0001; Fig. 4D). There was, however, no difference between wild-type and Ccr2−/− mice in anxiety levels after TBI (center zone: F[1,114]=0.14, p>0.05; peripheral zones: F[1,114]=0.14, p>0.05; Fig. 4D).
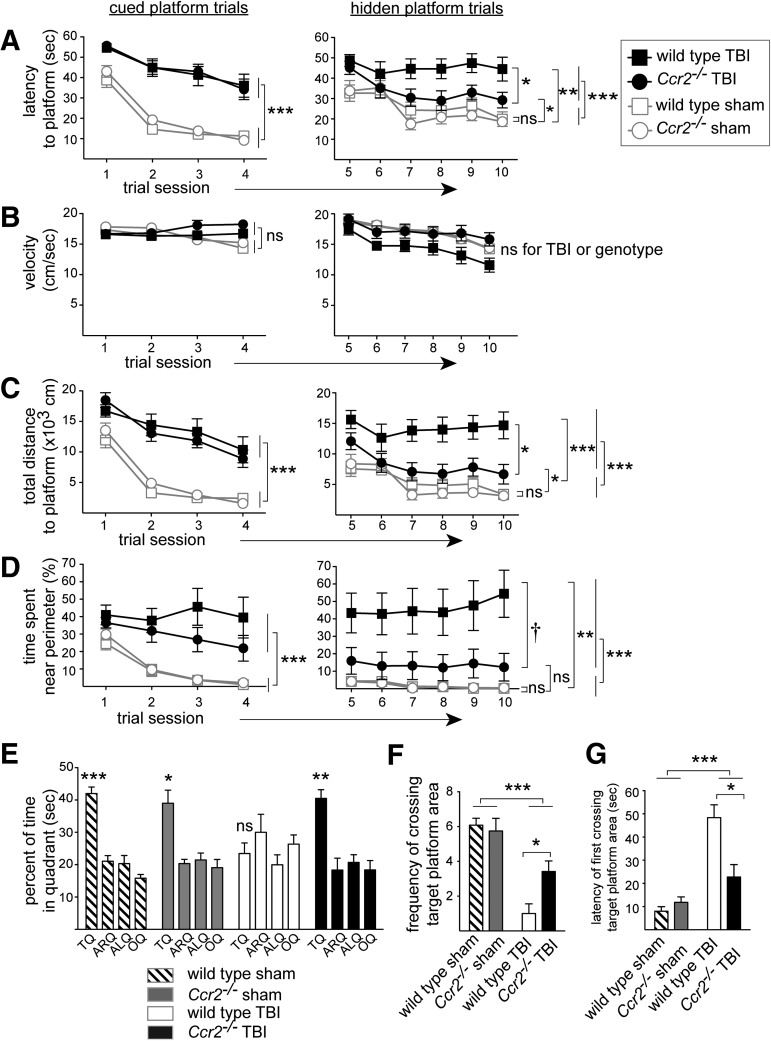
Ccr2−/− mice have improved long-term spatial memory and learning after TBI
We assessed learning and memory by using Morris water maze tests eight weeks after TBI. All groups learned to locate a cued or a hidden platform as evidenced by progressively shortened latencies to find the platform (cued: F[3,129]=61.6, p<0.0001; hidden: F[5,215]=8.9, p<0.0001; Fig. 5A). TBI groups exhibited prolonged latency times to locate the cued or hidden platform (cued: F[1,43]=133.3, p<0.0001; hidden: F[1,43]=24.3, p<0.0001; Fig. 5A). TBI groups also showed extended path lengths to find the cued or hidden platform (cued: F[1,43]=117.1, p<0.0001; hidden: F[1,43]=15.8, p<0.0005; not shown), and TBI increased the total distance of the animal (proximity) from the cued or hidden platform (cued: F[1,43]=85.6, p<0.0001, hidden: F[1,43]=22.9, p<0.0001; Fig. 5C). TBI groups also exhibited characteristics of thigmotaxic behavior as evidenced by spending significantly more time swimming near the perimeter of the pool (cued: F[1,43]=13.7, p<0.001, hidden: F[1,43]=27.4, p<0.0001; Fig. 5D).
FIG. 5.
Ccr2−/− mice demonstrate improved spatial memory and learning following traumatic brain injury (TBI). The Morris water maze was used to assess cognitive function eight weeks following TBI (n=12/group). Animals were trained to locate a cued platform for 2 d (A–D, left column), and a hidden platform for 3 d (A–D, right column). Cued platform graphs show statistical results for the effect of TBI. Hidden platform graphs show statistical results for the effect of TBI and post-hoc analyses, and when a genotype effect achieved significance in a repeated measure analyses of variance with post-hoc analyses. Mean±standard error of the mean is shown. (A) TBI prolonged the time (latency) to reach the platform (***p<0.001). For the hidden platform trials, the Ccr2−/− TBI group performed better than the wild-type TBI group by finding the hidden platform quicker (post hoc test, *p<0.05). (B) Swim velocity for all animal groups were statistically identical. (C) TBI increased the animals' total distance from the platform during the trials (***p<0.001). For the hidden platform trials, the Ccr2−/− TBI group performed better than the wild-type TBI group by being closer to the hidden platform at all times (post hoc test, *p<0.05). (D) TBI induced thigmotaxic behavior as shown by increased time spent near the perimeter during the platform search (***p<0.001). For the hidden platform trials, the Ccr2−/− TBI group showed decreased thigmotaxic behavior, compared with the wild-type TBI group, as indicated by reduced time spent near the perimeter (post hoc test, †p=0.05), indicating that Ccr2−/− mice had improved spatial learning and more productive search strategies post-TBI, compared with wild-type mice. (E) Ccr2−/− mice had improved memory retention as assessed during a probe trial of the Morris water maze. The percent of time spent searching in each quadrant of the pool is shown. Sham groups and the Ccr2−/− TBI group preferentially searched in the target quadrant, while the wild-type TBI group failed this test (most stringent student's t-test result: wild-type sham, ***p<0.001;, Ccr2−/− sham, *p<0.05; wild-type TBI, p>0.05; Ccr2−/− TBI, **p<0.01). (F) TBI impaired memory retention as assessed by the animals' crossing frequency over the area of the target platform during a probe trial (***p<0.0001). Ccr2−/− TBI mice crossed the target platform area more frequently, compared with wild-type TBI mice (post hoc test, *p<0.05). (G) TBI delayed the first encounter to the target platform position during the probe trial (***p<0.0001). Ccr2−/− TBI mice came across the target platform location much sooner than wild-type TBI mice (post hoc test, *p<0.05).
In these tests, genetic deficiency in CCR2 improved spatial learning post-TBI. During the hidden platform trials, Ccr2−/− mice demonstrated significantly improved latency times, post hoc: p<0.05 (Fig. 5A), and total distance to platform, post hoc, p<0.05 (Fig. 5C), compared with wild-type mice, after TBI. All groups of mice had similar swim speeds during cued platform trials indicating that the prolonged time of TBI mice and/or wild-type TBI mice to find the platform following TBI did not reflect a decreased capacity to swim (TBI effect: F[1,43]=2.5, p>0.1; genotype effect: F[1,43]=3.2, p>0.08). Although wild-type mice after TBI showed a modest decrease in average swim velocity during the hidden platform trials, this difference did not achieve significance (TBI effect: F[1,43]=3, p>0.05; genotype effect: F[1,43]=3.9, p>0.05). The impaired performance during the hidden platform trials by the wild-type TBI mice, compared with Ccr2−/− TBI mice, is at least partially attributable to their more pronounced thigmotaxic behavior (Fig. 5D), leading to unproductive searches and poor learning in the wild-type TBI group (post hoc, p=0.053).
Genetic deficiency in CCR2 also improved spatial memory retention following TBI, as determined by probe trial analysis (Fig. 5E–5G). For each group of mice during the probe trial, we assessed their preference for searching in the target quadrant. The sham control groups, and the Ccr2−/− TBI mice group, all showed a significant preference to search in the target quadrant, but the wild-type TBI group failed to show a preference for the target quadrant. The most stringent p-value of three student's t-test assessing difference from the time spent in the target quadrant within each group is reported: wild-type sham, p<0.001; Ccr2−/− sham, p<0.05; wild-type TBI, p>0.3 (not significant); Ccr2−/− TBI, p<0.01; Fig. 5E). One-way ANOVA between groups also demonstrated a significant difference in time spent in the target quadrant during the probe trial (p=0.0004). Consistent with their preference in searching in the target quadrant during the probe trial, Ccr2−/− TBI mice also crossed the target platform location more frequently (p<0.05; Fig. 5F) and earlier (latency of first occurrence, p<0.05; Fig. 5G), compared with wild-type TBI mice. Thus, a deficiency in CCR2, which greatly reduced the early macrophage response in TBI, also improved spatial learning and memory after TBI. These findings suggest that the CCR2-dependent inflammatory response is detrimental to long-term functional cognitive outcomes after TBI.
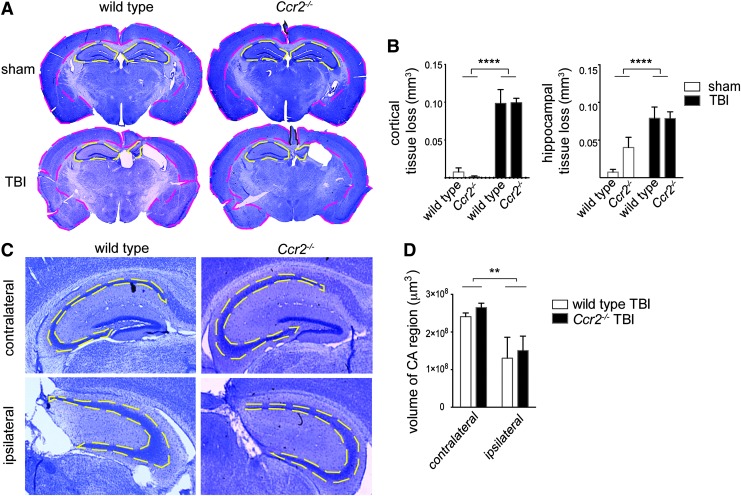
Lack of CCR2 does not affect tissue loss or hippocampal volume after TBI
To assess the effects of CCR2 deficiency on gross lesion volume after TBI, brain sections from Ccr2−/− and wild-type mice nine weeks post-TBI were stained with cresyl violet. Substructure (cortex or hippocampus) areas were traced (Fig. 6A) in multiple sections per brain, volumes were calculated, and differences in volume between the contralateral (left) and ipsilateral (right) substructures were used to estimate the extent of tissue loss. Two-way ANOVA determined that TBI induced moderate tissue loss in the cortical and hippocampal regions, F(1,21)=113.3, p<0.0001 and F(1,21)=23.6, p<0.0001, respectively (Fig. 6B). No significant difference in tissue loss could be detected between wild-type and CCR2-deficient mice post-TBI (interaction and genotype effects, p>0.05). Additionally, the collective CA region (CA1-CA3) was traced in multiple sections in TBI groups and the volume was determined (Fig. 6C and 6D). Although an effect of TBI was found, F(1,16)=10.5, p=0.005, consistent with our analysis on tissue loss, we did not detect a significant effect of CCR2 deficiency on hippocampal CA region volume. Thus, tissue loss and hippocampal volume detected by standard methods did not correlate with genotype-associated changes in cognitive function in our model.
FIG. 6.
Ccr2−/− and wild-type mice have similar levels of tissue loss and hippocampal volume after traumatic brain injury (TBI). (A) Brains from wild-type and Ccr2−/− animals nine weeks after sham or TBI surgery were sectioned and stained with cresyl violet. Representative low-power images shown (20×) are from approximately 1.82 mm posterior from the bregma point. Contralateral (left) and ipsilateral (right) cortical (pink dashed lines) and hippocampal areas (yellow dashed lines) were traced in multiple sections per animal to estimate area. Area assessments were used to calculate the volume of each substructure (cortex or hippocampus). The tissue loss per brain substructure was calculated as a difference in volume between left and right substructures. (B) Quantification of tissue loss in sham and TBI animals of wild-type and Ccr2−/− mice. TBI animals demonstrated significant tissue loss in the cortex and hippocampus, F(1,21)=113.3, ****p<0.0001, and F(1, 21)=23.6, ****p<0.0001, however, there was no genotype effect or interaction effect. Lesion volume was calculated as (contralateral area − ipsilateral area)×0.24 mm3. Groups sizes for sham controls were n=5–6, for wild-type TBI mice n=5, and for Ccr2−/− TBI mice n=9. (C) The volume of the collective CA (CA1-CA3) adjacent to the TBI lesion in wild-type and Ccr2−/− mice was assessed nine weeks post-TBI (mean±standard error of the mean). Images are representative cresyl violet stained brain sections of the hippocampus at approximately−2.30 mm posterior of the bregma. Yellow dashed lines indicate the CA region. (D) Ccr2−/− and wild-type mice have similar volumes of CA1-CA3 after TBI. TBI decreased the volume of the CA region on the ipsilateral side, compared with the contralateral side, F(1,16)=10.5, **p=0.005, but there was no effect of genotype or interaction (p>0.05). n=5 animals per group.
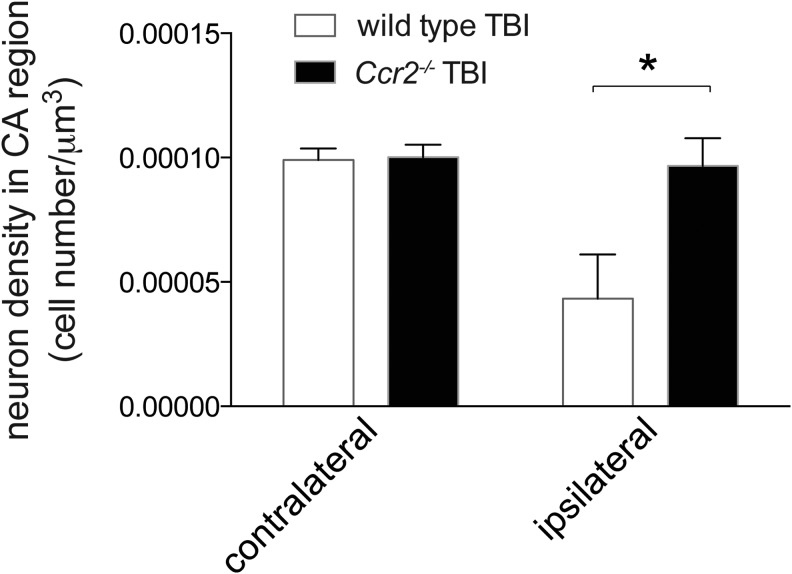
CCR2 deficiency preserves neuronal density in the hippocampus following TBI
Ccr2−/− mice possessed improved memory function after TBI. Because the hippocampus is critical for memory, we assessed neuronal density in the residual hippocampus of the collective CA1-CA3 regions adjacent to the lesion cavity in the same areas used to determine CA volume in Figure 6C. The remaining neurons were stereologically counted and compared with the corresponding contralateral regions. In wild-type mice, an average of 57% loss in neuronal density was seen on the ipsilateral side after TBI, compared with contralateral neuronal counts (contralateral neuronal density 1×10−4±5×10−6 [mean±SEM]; ipsilateral neuronal density 4.3×10−5±1.8×10−5; Fig. 7). Remarkably, in Ccr2−/− mice, the neuronal density in the CA regions were comparable in the ipsilateral and contralateral hemispheres (contralateral neuronal density 1×10−4±5×10−6; ipsilateral neuronal density 1×10−4±1.1×10−5; Fig. 7). Two-way ANOVA demonstrated a significant interaction effect, F(1,16)=5.7, p=0.03. Both injury and genotype effects were significant, F(1,16)=7.3, p=0.01 and F(1,16)=6.2, p=0.02, respectively. These data indicate that injury had a greater effect on neuronal density in wild-type mice than in Ccr2−/− mice. These data suggest that CCR2-dependent cells can significantly affect and lead to a diminished number of surviving neurons during secondary injury following TBI. A lack of CCR2 limits deficits in neuronal density and improves function.
FIG. 7.
Ccr2−/− preserves neuronal density in the hippocampal CA region after traumatic brain injury (TBI). Stereological assessment of neuronal density in the hippocampal CA1-CA3 regions adjacent to the TBI-induced cavity in contralateral and ipsilateral sides nine weeks after TBI was performed. CCR2-deficient mice had preserved neuronal density in the ipsilateral side, compared with wild-type mice after TBI. Two-way analysis of variance results show a significant interaction (p=0.03). There were significant effects of genotype and TBI on neuronal density, F(1,16)=6.2, *p=0.02, and F(1,16)=7.3, p=0.01, respectively). These data indicate that injury had a greater effect on wild-type mice than Ccr2−/− mice. n=5 animals per group.
A summary of behavior, tissue volume, and neuronal density assessments from this study is presented in Table 1.
Table 1.
Summary of Behavior, Tissue Loss, and Neuronal Density Outcomes
| Method | Animal group sizes | Outcomes | |
|---|---|---|---|
| 1 | Rotarod | n=20/group | TBI impaired rotarod performance three weeks after injury. CCR2 deficiency had no effect on rotarod performance. |
| 2 | Open field | n=20/group | TBI induced hyperactivity and anxiety three weeks after injury. CCR2 deficiency partially rescued hyperactivity, but had no effect on anxiety after TBI. |
| 3 | Morris water maze | n=12/group | TBI impaired spatial memory and learning eight weeks after injury. CCR2 deficiency improved spatial memory and learning after TBI. |
| 4 | Volume of tissue loss |
n=5/ wild type group n=6 Ccr2−/− sham animals n=9 Ccr2−/− TBI animals |
TBI resulted in tissue loss in the cortex and hippocampus of the ipsilateral side. CCR2 deficiency had no effect on tissue loss. |
| 5 | CA1-CA3 volume and stereological counting of neuronal density | n=5/group | TBI caused decreases in CA volume and neuronal density nine weeks after TBI. CCR2 did not affect CA volume. However, CCR2 deficiency preserved neuronal density in the hippocampus after TBI. |
TBI, traumatic brain injury; CCR2, C-C-chemokine receptor 2; Ccr2−/−, C-C-chemokine receptor 2–deficient.
Discussion
We demonstrate that CCR2 is required for the early rise in brain macrophages following TBI. In behavior studies, lack of CCR2 does not affect gross motor function, motor learning or balancing, as assessed by swimming ability in the Morris water maze test or rotarod testing. Lack of CCR2, however, significantly affects hyperactivity levels and long-term cognitive function after TBI. Mice lacking CCR2 show less hyperactivity in open field tests after TBI. More importantly, Ccr2−/− mice demonstrate improved spatial learning and memory retention of learned spatial information associated with increased neuronal density in the CA region of the hippocampus, compared with wild-type animals, after TBI. Remarkably, in Ccr2−/− mice the neuronal density in the ipsilateral TBI hippocampus (CA1-CA3) was comparable to the contralateral side. Sustaining neuronal numbers in the hippocampus may be one mechanism by which CCR2 deficiency improves memory function after TBI.
We did not detect a difference in the extent of tissue loss or hippocampal volume (CA1-CA3 substructures) between wild-type and Ccr2−/− mice nine weeks after TBI. This is in contrast to Ccl2−/− mice, in which lesion size was reduced four weeks after TBI, but not earlier, by similar detection methods.29 This may reflect differences in the models of TBI that were used. In our model of moderate CCI, a much greater severity of tissue damage is induced by mechanical force, which would decrease the sensitivity to detect small differences in loss of tissue. It is also possible that tissue loss continues to be altered over time. Techniques with greater sensitivity, such as in vivo imaging, might detect differences in the volume of tissue loss over time.
Our findings are in accord with previous studies indicating that CCR2 is detrimental in other models of CNS injury, including experimental autoimmune encephalitis, stroke, and cranial irradiation.23,24,27,28,58,59 Our studies also complement a previous report showing that CCL2-deficient mice have improved function following TBI, as assessed by a combined score for motor function, alertness and behavior.29 In addition, our studies show significant long-term improvement in cognitive function after TBI in the absence of CCR2. In contrast to our findings, others have shown a beneficial role for CCR2 in a model where CCR2-dependent macrophages were critical for nerve regeneration following axotomy.60 It is possible that CCR2 plays different roles depending on the site and mechanism of injury. A greater understanding of the mechanisms, cellular subsets, timing, and environmental cues should clarify our understanding of this molecule and of the cells on which it is expressed.
CCR2 in the CNS has been demonstrated on macrophages and T cells.12,13 Other studies, however, provide evidence of CCR2 expression in microglia,14,17 as well as in neuronal cells, including as dorsal root ganglion neurons,37,38 and neural precursor cells.15,16,61 Thus the response to TBI by glial and/or neuronal cells may be altered in in Ccr2−/− mice.
Prior studies of lung inflammation following nematode infestation have shown that macrophages, specifically M2 macrophages, can expand without recruitment of peripheral blood monocytes.62 In our studies, by the use of flow cytometry, we have shown that CCR2 is required for the rapid expansion of macrophages, arguing that these cells are recruited from outside the CNS rather than expanded from within. Macrophage infiltration of the TBI brain from the periphery is consistent with studies in parabiotic mice, which demonstrated that CNS-macrophages during inflammation come from the periphery and are distinct from microglia.59,63
We and others have shown that macrophages are a major component of the inflammatory immune response to TBI.29,48,64,65 The macrophage inflammatory response can be beneficial to sterilize wounds, to clear necrotic debris and to promote wound repair.66 In some models of CNS injury, such as spinal cord injury, there is evidence that macrophages suppress inflammation and are critical for recovery.67 Similarly, in an EAE model, macrophages that suppress inflammation through the production of IL-10 and TGFβ were beneficial.68 Other reports, however, indicate that macrophages are harmful in CNS injury, in which macrophages can extend neuronal cell death and impair recovery.23,24,29,58,59,69,70
In vitro and in vivo studies have demonstrated that macrophages can differentiate into at least two major subsets: classically activated (M1) and alternatively activated (M2) macrophages.71–73 M2 macrophages can be further subtyped into M2a (a for alternative), M2b, and M2c subsets.74 M1 macrophages directly incite inflammation by releasing inflammatory cytokines and reactive oxygen and nitrogen species. In contrast, M2a and M2c macrophages, which both express Arginase-1 (Arg1) in the mouse, and M2b macrophages, which express 10, suppress inflammation and promote wound healing.66,74 In vitro studies have demonstrated that M1 macrophages are neurotoxic, while M2 macrophages promote regenerative neuronal growth.75 An in vivo study showed that injection of M2-polarized cells could enrich cognitive function.76 However, macrophage polarization is not always completely dichotomous and multiple in vivo studies demonstrate that macrophages may differentiate along a spectrum of phenotypes.9,66,71,77,78
As assessed by histology, the presence of macrophages/microglia early after TBI has been associated with the expression of tumor necrosis factor, IL-6, and IL-11,29,30,79,80 consistent with an M1 phenotype. However, we previously demonstrated in our TBI model that the early macrophage response involves at least two major subsets of macrophages.48 By using a reporter mouse for Arg1 expression, we showed that one day after TBI 20% of the macrophage population expresses Arg1. Gene expression profiling of the Arg1+ and Arg1- brain macrophage subsets following TBI showed that each preferentially expresses a combination of M2a and M1 genes, as well as their own unique profile of chemotaxis genes. Thus, macrophage populations induced by TBI cannot simply be categorized as M1 or M2 macrophages. Because CCR2 is required for 90% of the macrophages recruited on day 1 after TBI, both Arg1+ and Arg1− brain macrophage subsets must be affected at least partially by the absence of CCR2. It is nonetheless possible that one subset of macrophages is harmful in TBI, while another is protective. While our studies show that CCR2-dependent cells are harmful in TBI, it is likely that further subsetting of macrophages could allow more precise removal of harmful macrophages and enhancement of those that are beneficial.
A limitation to our study is that our Ccr2−/− and wild-type mice were not littermates, although the Ccr2−/− mice were completely backcrossed to source-matched C57BL/6 mice for nine generations and were housed in the same room and animal facility. However, our demonstration that CCR2-deficient mice had greater neuronal density in subregions CA1-CA3 of the hippocampus compared with wild-type post-TBI provides a rational explanation for the improved cognitive function in Ccr2−/− mice after TBI. Our studies used mice genetically deficient in CCR2, not mice in which CCR2 was conditionally depleted, thus we cannot rule out that effects of CCR2 depletion on development may have altered responses to injury indirectly. This should be addressed in future studies by blocking CCR2 at the time of injury.
As a therapeutic target, CCR2 has advantages over CCL2. First, blocking CCR2 would interrupt monocyte recruitment by other monocyte-recruiting chemokines that bind this receptor including CCL7 and CCL12. CCL12 is transcriptionally upregulated to high levels in brain tissue after TBI, in addition to CCL2.31 Second, CCR2 has multiple binding sites for small molecule antagonists and several investigational drugs have been generated to successfully target this molecule.33
In summary, our studies demonstrate that CCR2 plays a critical role in the recruitment of the majority of peripheral macrophages to the lesion following TBI. Absence of CCR2 leads to improved functional cognitive outcomes and preserved neuronal density following TBI, suggesting that CCR2-induced macrophage recruitment following TBI is detrimental. These results suggest the possibility that blockade of CCR2 may be an effective treatment to lead to improvement in long-term cognitive functional outcomes post TBI.
Acknowledgments
The authors thank Ruby Gribi of the San Francisco VA Flow Cytometry core, Ivy Hsieh and Sherry Kamiya of the San Francisco VA Cell Imaging core for their dedication and contributions to this project. This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Career Development Award-2 to CLH. MCN is supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Award and by the Russell/Engelman Center for Arthritis Research. With tremendous gratitude, we acknowledge Dr. William E. Seaman for helpful discussions and an insightful review of this manuscript.
Author Disclosure Statement
The only financial disclosure to report is that Israel Charo is on the Scientific Advisory Board of ChemoCentryx (Mountain View, California). For the remaining authors, no other financial interests exist.
References
- 1.Potts M.B., Koh S.E., Whetstone W.D., Walker B.A., Yoneyama T., Claus C.P., Manvelyan H.M., and Noble-Haeusslein L.J. (2006). Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 3, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain K.K. (2008). Neuroprotection in traumatic brain injury. Drug Discov. Today 13, 1082–1089 [DOI] [PubMed] [Google Scholar]
- 3.Okie S. (2005). Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 [DOI] [PubMed] [Google Scholar]
- 4.Smith D.H., Johnson V.E., and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez C.R., Boehmer E.D., and Kovacs E.J. (2005). The aging innate immune system. Curr. Opin. Immunol. 17, 457–462 [DOI] [PubMed] [Google Scholar]
- 6.Smith C. (2013). Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 35–44 [DOI] [PubMed] [Google Scholar]
- 7.Jordan B.D. (2013). The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 9, 222–230 [DOI] [PubMed] [Google Scholar]
- 8.Kumar A. and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 26,1191–1201 [DOI] [PubMed] [Google Scholar]
- 9.Shi C. and Pamer E.G. (2011). Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose S. and Cho J. (2013). Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res. 36, 1039–1050 [DOI] [PubMed] [Google Scholar]
- 11.Bonecchi R., Galliera E., Borroni E.M., Corsi M.M., Locati M., and Mantovani A. (2009). Chemokines and chemokine receptors: an overview. Front. Biosci. 14, 540–551 [DOI] [PubMed] [Google Scholar]
- 12.Mizutani M., Pino P.A., Saederup N., Charo I.F., Ransohoff R.M., and Cardona A.E. (2012). The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188, 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saederup N., Cardona A.E., Croft K., Mizutani M., Cotleur A.C., Tsou C.L., Ransohoff R.M., and Charo I.F. (2010). Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PloS One 5, e13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Shi X.Q., Echeverry S., Mogil J.S., De Koninck Y., and Rivest S. (2007). Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 27, 12396–12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melik-Parsadaniantz S. and Rostene W. (2008). Chemokines and neuromodulation. J. Neuroimmunol. 198, 62–68 [DOI] [PubMed] [Google Scholar]
- 16.White F.A., Feldman P., and Miller R.J. (2009). Chemokine signaling and the management of neuropathic pain. Mol. Interv. 9, 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbadie C., Lindia J.A., Cumiskey A.M., Peterson L.B., Mudgett J.S., Bayne E.K., DeMartino J.A., MacIntyre D.E., and Forrest M.J. (2003). Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Nat. Acad. Sci. U.S.A. 100, 7947–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schall T.J. and Proudfoot A.E. (2011). Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat. Rev. Immunol. 11, 355–363 [DOI] [PubMed] [Google Scholar]
- 19.Xing Z., Lu C., Hu D., Yu Y.Y., Wang X., Colnot C., Nakamura M., Wu Y., Miclau T., and Marcucio R.S. (2010). Multiple roles for CCR2 during fracture healing. Dis. Model Mech. 3, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., and Luster A.D. (2007). CCR2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 [DOI] [PubMed] [Google Scholar]
- 21.Naert G. and Rivest S. (2011). CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 6208–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willenborg S., Lucas T., van Loo G., Knipper J.A., Krieg T., Haase I., Brachvogel B., Hammerschmidt M., Nagy A., Ferrara N., Pasparakis M., and Eming S.A. (2012). CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120, 613–625 [DOI] [PubMed] [Google Scholar]
- 23.Mahad D.J. and Ransohoff R.M. (2003). The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin. Immunol. 15, 23–32 [DOI] [PubMed] [Google Scholar]
- 24.Dimitrijevic O.B., Stamatovic S.M., Keep R.F., and Andjelkovic A.V. (2007). Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38, 1345–1353 [DOI] [PubMed] [Google Scholar]
- 25.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T.I., Donahoe J.S., Courties G., Lee K.M., Kim J.I., Markmann J.F., Marinelli B., Panizzi P., Lee W.W., Iwamoto Y., Milstein S., Epstein-Barash H., Cantley W., Wong J., Cortez-Retamozo V., Newton A., Love K., Libby P., Pittet M.J., Swirski F.K., Koteliansky V., Langer R., Weissleder R., Anderson D.G., and Nahrendorf M. (2011). Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinones M.P., Estrada C.A., Kalkonde Y., Ahuja S.K., Kuziel W.A., Mack M., and Ahuja S.S. (2005). The complex role of the chemokine receptor CCR2 in collagen-induced arthritis: implications for therapeutic targeting of CCR2 in rheumatoid arthritis. J. Mol. Med. 83, 672–681 [DOI] [PubMed] [Google Scholar]
- 27.Belarbi K., Jopson T., Arellano C., Fike J.R., and Rosi S. (2013). CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 73, 1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond M.D., Taylor R.A., Mullen M.T., Ai Y., Aguila H.L., Mack M., Kasner S.E., McCullough L.D., and Sansing L.H. (2014). CCR2+Ly6Chi inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J. Neurosci. 34, 3901–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple B.D., Bye N., Rancan M., Ziebell J.M., and Morganti-Kossmann M.C. (2010). Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J. Cereb. Blood Flow Metab. 30, 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd E., Somera-Molina K., Van Eldik L.J., Watterson D., and Wainwright M. (2008). Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Israelsson C., Bengtsson H., Kylberg A., Kullander K., Lewen A., Hillered L., and Ebendal T. (2008). Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J. Neurotrauma 25, 959–974 [DOI] [PubMed] [Google Scholar]
- 32.Semple B.D., Frugier T., and Morganti-Kossmann M.C. (2010). CCL2 modulates cytokine production in cultured mouse astrocytes. J. Neuroinflammation 7, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zweemer A.J., Nederpelt I., Vrieling H., Hafith S., Doornbos M.L., de Vries H., Abt J., Gross R., Stamos D., Saunders J., Smit M.J., Ijzerman A.P., and Heitman L.H. (2013). Multiple Binding Sites for Small Molecule Antagonists at the Chemokine Receptor CCR2. Mol. Pharmacol. 84, 551–61 [DOI] [PubMed] [Google Scholar]
- 34.Boring L., Gosling J., Cleary M., and Charo I.F. (1998). Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Langrish C.L., McKenzie B., Joyce-Shaikh B., Stumhofer J.S., MCCLanahan T., Blumenschein W., Churakovsa T., Low J., Presta L., Hunter C.A., Kastelein R.A., and Cua D.J. (2006). Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation 116, 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedgwick J.D., Schwender S., Imrich H., Dorries R., Butcher G.W., and ter Meulen V. (1991). Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. U. S. A. 88, 7438–7442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong S.M., Liu Z., Fan Y., Neumann M., Won S.J., Lac D., Lum X., Weinstein P.R., and Liu J. (2007). Reduced hippocampal neurogenesis and skill reaching performance in adult Emx1 mutant mice. Exper. Neurol. 206, 24–32 [DOI] [PubMed] [Google Scholar]
- 38.Fan Y., Liu Z., Weinstein P.R., Fike J.R., and Liu J. (2007). Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur. J. Neurosci. 25, 38–46 [DOI] [PubMed] [Google Scholar]
- 39.Liu Z., Fan Y., Won S.J., Neumann M., Hu D., Zhou L., Weinstein P.R., and Liu J. (2007). Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 38, 146–152 [DOI] [PubMed] [Google Scholar]
- 40.Raber J., Fan Y., Matsumori Y., Liu Z., Weinstein P.R., Fike J.R., and Liu J. (2004). Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann. Neurol. 55, 381–389 [DOI] [PubMed] [Google Scholar]
- 41.Suh S.W., Aoyama K., Matsumori Y., Liu J., and Swanson R.A. (2005). Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 54, 1452–1458 [DOI] [PubMed] [Google Scholar]
- 42.Aoyama K., Suh S.W., Hamby A.M., Liu J., Chan W.Y., Chen Y., and Swanson R.A. (2006). Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 9, 119–126 [DOI] [PubMed] [Google Scholar]
- 43.Lam T.I., Bingham D., Chang T.J., Lee C.C., Shi J., Wang D., Massa S., Swanson R.A., and Liu J. (2013). Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabil. Neural Repair. 27, 889–899 [DOI] [PubMed] [Google Scholar]
- 44.Suh S.W., Aoyama K., Chen Y., Garnier P., Matsumori Y., Gum E., Liu J., and Swanson R.A. (2003). Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J. Neurosci. 23, 10681–10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumori Y., Hong S.M., Fan Y., Kayama T., Hsu C.Y., Weinstein P.R., and Liu J. (2006). Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol. Dis. 22, 187–198 [DOI] [PubMed] [Google Scholar]
- 46.Lenzlinger P.M., Morganti-Kossmann M.C., Laurer H.L., and McIntosh T.K. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 47.Morganti-Kossmann M.C., Rancan M., Stahel P.F., and Kossmann T. (2002). Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care 8, 101–105 [DOI] [PubMed] [Google Scholar]
- 48.Hsieh C.L., Kim C.C., Ryba B.E., Niemi E.C., Bando J.K., Locksley R.M., Liu J., Nakamura M.C., and Seaman W.E. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43, 2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greter M. and Merad M. (2013). Regulation of microglia development and homeostasis. Glia 61, 121–127 [DOI] [PubMed] [Google Scholar]
- 50.Sonoda K.H., Yoshimura T., Egashira K., Charo I.F., and Ishibashi T. (2011). Neutrophil-dominant experimental autoimmune uveitis in CC-chemokine receptor 2 knockout mice. Acta Ophthalmol. 89, e180–e188 [DOI] [PubMed] [Google Scholar]
- 51.Laurer H.L., Bareyre F.M., Lee V.M., Trojanowski J.Q., Longhi L., Hoover R., Saatman K.E., Raghupathi R., Hoshino S., Grady M.S., and McIntosh T.K. (2001). Mild head injury increasing the brain's vulnerability to a second concussive impact. J. Neurosurg. 95, 859–870 [DOI] [PubMed] [Google Scholar]
- 52.Manville J., Laurer H.L., Steudel W.I., and Mautes A.E. (2007). Changes in cortical and subcortical energy metabolism after repetitive and single controlled cortical impact injury in the mouse. J. Mol. Neurosci. 31, 95–100 [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto S.T., Longhi L., Saatman K.E., Conte V., Stocchetti N., and McIntosh T.K. (2004). Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 28, 365–378 [DOI] [PubMed] [Google Scholar]
- 54.Sinopoli K.J. and Dennis M. (2012). Inhibitory control after traumatic brain injury in children. Int. J. Dev. Neurosci. 30, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kane M.J., Angoa-Perez M., Briggs D.I., Viano D.C., Kreipke C.W., and Kuhn D.M. (2012). A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 203, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dulawa S.C., Grandy D.K., Low M.J., Paulus M.P., and Geyer M.A. (1999). Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 19, 9550–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prut L. and Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33 [DOI] [PubMed] [Google Scholar]
- 58.Izikson L., Klein R.S., Charo I.F., Weiner H.L., and Luster A.D. (2000). Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ajami B., Bennett J.L., Krieger C., McNagny K.M., and Rossi F.M. (2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 [DOI] [PubMed] [Google Scholar]
- 60.Niemi J.P., DeFrancesco-Lisowitz A., Roldan-Hernandez L., Lindborg J.A., Mandell D., and Zigmond R.E. (2013). A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci. 33, 16236–16248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran P.B., Banisadr G., Ren D., Chenn A., and Miller R.J. (2007). Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J. Comp. Neurol. 500, 1007–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., and Allen J.E. (2011). Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ajami B., Bennett J.L., Krieger C., Tetzlaff W., and Rossi F.M. (2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z., Artelt M., Burnet M., Trautmann K., and Schluesener H.J. (2006). Early infiltration of CD8+macrophages/microglia to lesions of rat traumatic brain injury. Neuroscience 141, 637–644 [DOI] [PubMed] [Google Scholar]
- 65.Jin X., Ishii H., Bai Z., Itokazu T., and Yamashita T. (2012). Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PloS One 7, e41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colton C.A. (2009). Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., Jung S., and Schwartz M. (2009). Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6, e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weber M.S., Prod'homme T., Youssef S., Dunn S.E., Rundle C.D., Lee L., Patarroyo J.C., Stuve O., Sobel R.A., Steinman L., and Zamvil S.S. (2007). Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 13, 935–943 [DOI] [PubMed] [Google Scholar]
- 69.Popovich P.G., Guan Z., Wei P., Huitinga I., van Rooijen N., and Stokes B.T. (1999). Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365 [DOI] [PubMed] [Google Scholar]
- 70.Donnelly D.J., Longbrake E.E., Shawler T.M., Kigerl K.A., Lai W., Tovar C.A., Ransohoff R.M., and Popovich P.G. (2011). Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+macrophages. J. Neurosci. 31, 9910–9922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chawla A. (2010). Control of macrophage activation and function by PPARs. Circ. Res. 106, 1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon S. and Taylor P. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 73.Geissmann F., Gordon S., Hume D.A., Mowat A.M., and Randolph G.J. (2010). Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol.10, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., and Locati M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 75.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derecki N.C., Quinnies K.M., and Kipnis J. (2011). Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 25, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daley J.M., Brancato S.K., Thomay A.A., Reichner J.S., and Albina J.E. (2010). The phenotype of murine wound macrophages. J. Leukoc. Biol. 87, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clausen F., Hanell A., Israelsson C., Hedin J., Ebendal T., Mir A.K., Gram H., and Marklund N. (2011). Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 34, 110–123 [DOI] [PubMed] [Google Scholar]
- 80.Ziebell J.M., Bye N., Semple B.D., Kossmann T., and Morganti-Kossmann M.C. (2011). Attenuated neurological deficit, cell death and lesion volume in Fas-mutant mice is associated with altered neuroinflammation following traumatic brain injury. Brain Rese. 1414, 94–105 [DOI] [PubMed] [Google Scholar]