Abstract
Background
Hemodialysis (HD) patients have a high risk of infections. The uremic milieu has a negative impact on several immune responses. Online hemodiafiltration (HDF) may reduce the risk of infections by ameliorating the uremic milieu through enhanced clearance of middle molecules. Since there are few data on infectious outcomes in HDF, we compared the effects of HDF with low-flux HD on the incidence and type of infections.
Patients and Methods
We used data of the 714 HD patients (age 64 ±14, 62% men, 25% Diabetes Mellitus, 7% catheters) participating in the CONvective TRAnsport STudy (CONTRAST), a randomized controlled trial evaluating the effect of HDF as compared to low-flux HD. The events were adjudicated by an independent event committee. The risk of infectious events was compared with Cox regression for repeated events and Cox proportional hazard models. The distributions of types of infection were compared between the groups.
Results
Thirty one percent of the patients suffered from one or more infections leading to hospitalization during the study (median follow-up 1.96 years). The risk for infections during the entire follow-up did not differ significantly between treatment arms (HDF 198 and HD 169 infections in 800 and 798 person-years respectively, hazard ratio HDF vs. HD 1.09 (0.88–1.34), P = 0.42. No difference was found in the occurrence of the first infectious event (either fatal, non-fatal or type specific). Of all infections, respiratory infections (25% in HDF, 28% in HD) were most common, followed by skin/musculoskeletal infections (21% in HDF, 13% in HD).
Conclusions
HDF as compared to HD did not result in a reduced risk of infections, larger studies are needed to confirm our findings.
Trial Registration
ClinicalTrials.gov NCT00205556
Introduction
Infections are an important cause for morbidity and mortality in hemodialysis (HD) patients [1]. The uremic environment affects both the innate and the adaptive immune response by dysfunction of neutrophilic polymorphonuclear leukocytes [2] and monocytes, a depletion of dendritic cells, naïve and central memory T cells and B cells [3]. In addition, frequent damaging of the skin in case of a graft or arterio-venous fistula or the presence of a central venous catheter facilitates the entry of micro-organisms into the body. Patients with a catheter as a vascular access have the largest infectious risk, followed by patients with grafts [4–6]. Several patient related factors contribute to the susceptibility to infection, including older age, hypo-albuminemia, co-morbidity, personal hygiene and underlying chronic infectious disease [5,7,8].
Theoretically, amelioration of the uremic milieu by improving clearance of middle molecules by convection might reduce this risk of infections. The HEMO study however, comparing low-flux with high-flux HD, did not show a decrease in the risk of infections for patients treated with high flux membranes [5]. Online hemodiafiltration (HDF), a therapy that achieves markedly better clearance of middle molecules than high-flux HD, might reduce the incidence of infections. However, removal of middle molecules might also lead to a depletion of immune effector molecules, pro-inflammatory cytokines, or other mediators relevant for immune function. In addition, despite tight monitoring and quality control, the infusion of large amounts of substitution fluid, when contaminated, might impose a larger risk for infections. So far the effect of HDF on the incidence of infectious episodes as compared to low-flux HD has hardly been studied. Therefore, we performed a secondary analysis of data of the CONvective TRAnsport STudy (CONTRAST) to evaluate the effect of HDF as compared to low-flux HD on the incidence and causes of infection related hospitalizations and mortality.
Methods
General methods
The CONvective TRAnsport STudy (CONTRAST) (ISRCTN38365125, NCT00205556) is a randomized controlled trial, conducted in twenty-nine dialysis centers in The Netherlands (n = 26), Canada (n = 2), and Norway (n = 1), that compared the effects of low-flux HD and online post-dilution HDF on all-cause mortality and cardiovascular morbidity and mortality [9,10]. Patients were eligible if treated with HD 2 or 3 times a week, for at least 2 months, with a single pool Kt/Vurea ≥ 1.2. Exclusion criteria were age < 18 years, treatment by HDF or high-flux HD in the 6 months preceding randomization, severe incompliance defined as non-adherence to the dialysis prescription, a life expectancy < 3 months due to causes other than kidney disease, and participation in another clinical intervention study evaluating cardiovascular outcome. All patients were randomized centrally by a computer-based randomization service (Julius Center University Medical Center, Utrecht, The Netherlands) into a 1:1 ratio for treatment with online hemodiafiltration or continuation of low-flux hemodialysis, stratified per participating center (permuted blocks). Because of the nature of the intervention, it was not possible to blind the patients, the local study nurses, or the investigators to the treatment assignment. The laboratory samples were measured in routine clinical care; hence, personnel were unaware of treatment assignment. The event adjudication committee was blinded to the treatment assignment. Patients were enrolled from June 2004 until January 1st 2010 by nephrologists and research nurses in participating centers. Follow-up ended December 31st 2010. The study was conducted in accordance with the Declaration of Helsinki and centrally approved by the Medical Ethical committee VU University medical Center, Amsterdam, The Netherlands, for all the participating hospitals on the 31st of July 2003, an amendment was approved on 28th of June 2007. Local approval was obtained from the Medical Ethical Committees of all participating centers, which are listed in the acknowledgements. The authors confirm that all ongoing and related trials for this intervention are registered. Written informed consent was obtained from all patients prior to randomization. The study was registrated in the trial registries after the first patients were enrolled, immediately by the time the investigators knew trial registration was obligatory. The assessment of difference in risk of infections between dialysis modalities was one of the predefined secondary outcomes [10].
Dialysis procedures
Treatment times were fixed during follow-up in both treatment arms, unless single pool Kt/Vurea was below 1.2. Online HDF was performed in the post-dilution mode; target volume was 6 L/h. For HDF, synthetic high-flux dialyzers were used (FX80: 24%, FX100: 12% and Optiflux F200NR: 9% [Fresenius Medical Care, Bad Homburg, Germany]; Polyflux 170H: 20% and Polyflux 210H: 30% [Gambro AB, Lund, Sweden] or other dialyzers: 4%, based on data of 3 month visit). HD patients were treated with synthetic low-flux dialyzers (F6HPS: 4%, F8HPS: 46% and Optiflux 18NR: 11% [Fresenius]; Polyflux 14L: 4%, Polyflux 17L: 25% and Polyflux 21L: 4% [Gambro], or other: 6%, based on data of 3 month visit). All patients were treated with ultra pure dialysis fluids, defined as less than 0.1 colony forming units per mL and less than 0.03 endotoxin units per mL. Routine patient care was performed according to national and international Quality of Care Guidelines.
Data collection
At baseline standardized forms were used to collect demographical, clinical and laboratory data. Type of vascular access, duration of dialysis (dialysis vintage), and medical history (presence of diabetes mellitus (DM) and previous cardiovascular disease (CVD)), were also recorded. CVD was defined as a history of angina pectoris, myocardial infarction, prior coronary revascularization, stroke or transient ischemic attack and/or peripheral vascular disease. Dialysis vintage was determined as the sum of time patients were treated with HD or peritoneal dialysis (PD) before inclusion in CONTRAST.
At each three monthly visit, data on clinical events (including infections), dialysis treatment, medication, and laboratory values were recorded. Infectious events were registered for all patients before drop out due to transplantation, switch to PD, move to another clinic or stop for other reasons. All infectious events were adjudicated by an independent Endpoint Adjudication Committee, whose members reviewed source documentation and were not aware of the treatment assignments. Infections were adjudicated as categorized definite or probable when patients were admitted to the hospital with a clinical picture of an infection, and with laboratory results suggesting an infection (leukocytosis, elevated CRP) or when infection was proven by culture. Infections were also adjudicated when these occurred during an admission for another cause. Only definite and probable infections were used for this analysis, possible infections (when a patient was admitted with only a clinical picture of infection, but without elevated inflammation parameters or a positive culture result) were considered as no infections. A report of two or more infections within a timeframe of 14 days was counted as one infection. If the second infection was fatal, that infection was used in the analysis. Infections were grouped as graft or fistula infection, catheter sepsis, sepsis, respiratory, urinary and ‘other infections’. Those that were categorized as ‘other infection’ have been subdivided into gastro-intestinal, skin/musculoskeletal, cardiac and miscellaneous infections retrospectively. Written diagnoses from the adjudication committee were used for this categorization.
In HDF patients, infusion volumes (liters per treatment) were reported as the mean value of three consecutive treatment sessions. Convection volumes (liters per treatment) were calculated by the sum of the intradialytic weight loss and the substitution volume. Patients with a urinary production of less than 100 mL per day were considered anuric.
Routine laboratory values were measured in the different participating hospitals using standard techniques.
Outcome
The primary study outcome was the risk of hospitalizations due to infection during the follow-up period. The secondary outcomes were mortality due to an infectious cause, the risk for the 1st fatal or non-fatal infection and the risk for 1st cause specific infection. Furthermore we studied the distribution of cause specific infections on the event level (enabling the analysis of more than one infectious event per patient).
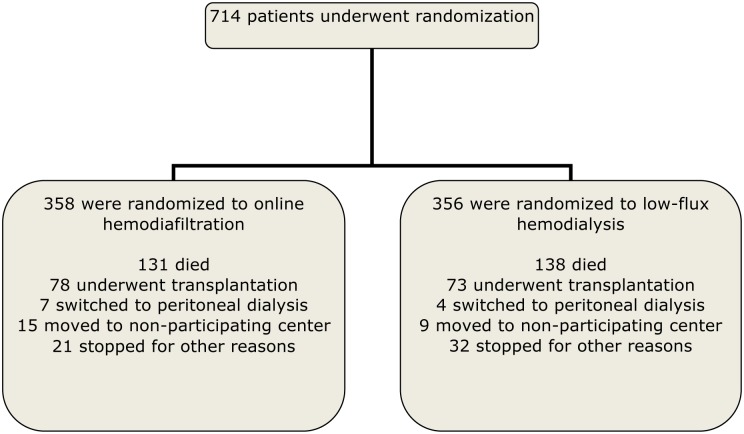
All infectious events that occurred after randomisation up to censoring were used in our analyses. Censoring could be due to death, due to end of study or due to dropping out. Dropping out means that a participant either stopped because of a renal transplantation (n = 151), switch to PD (n = 11), transfer to another hospital which did not participate in CONTRAST (n = 24) or due to other reasons (n = 53).
Data analysis
Data were reported as means with standard deviations, medians with interquartile (IQR) ranges, or proportions when appropriate.
The difference in risk for hospitalization due to infections between patients treated with HDF and HD during the follow-up was evaluated with a Cox proportional hazards model for repeated events, which is a Prentice-Williams-Peterson (PWP) conditional model [11]. In this model patients have a number of follow-up periods, depending on the number of events. The model takes intra-patient risk for infections into account. Furthermore we added interaction-terms to this model to explore whether the difference in risk between HDF and HD treated patients was different for certain predefined subgroups, notable age, sex, presence of CVD, presence of DM, presence of RKF and dialysis vintage. Furthermore, we explored whether the risk of infections was affected by the magnitude of the delivered convection volumes during the trial, with using the HD group as a reference group. In this last model adjustments were made for determinants of convection volume and mortality. Finally, since the distribution of vascular access type was somewhat different between patients from the Netherlands and Canada, we evaluated if there was an interaction between the treatment and the country of residence and performed additional analyses on these countries separately, with an adjustment for vascular access type.
The difference in risk for 1st infectious events (fatal- and nonfatal and cause specific infections) between HDF and HD was analyzed with Cox proportional hazard models. The distribution of infectious events from different causes was compared on the event level. The analyses were conducted in SPSS software (version 18.0; SPSS Inc. Headquarters, Chicago, Illinois, US) and in R (version 2.9.2; 2009 The R Foundation for Statistical Computing).
Results
Patient characteristics at baseline are shown in Table 1. The participant flow chart is shown in Fig 1. Thirty one percent of the patients suffered from one or more infections leading to hospitalization during the study (median follow-up 1.96 years). The treatment effect on risk of infectious events is depicted in Table 2. HDF did not reduce the risk of infections: hazard ratio (HR) HDF versus HD 1.09 (0.88–1.34), P = 0.42. In addition, no statistically significant differences were found between the two treatment arms for the first occurring infection (Table 2).
Table 1. Baseline characteristics of the patients.
| Variable | HDF(n = 358) | HD(n = 356) |
|---|---|---|
| Age (year) | 64.1±14.0 | 64.0±13.4 |
| Male sex—no. (%) | 214 (60) | 231 (65) |
| Region | ||
| Netherlands-no. (%) | 300 (84) | 297 (83) |
| Canada- no. (%) | 51 (14) | 51(14) |
| Norway- no. (%) | 7 (2) | 8 (2) |
| History of cardiovascular disease—no. (%) | 151 (42) | 162 (46) |
| Diabetes mellitus—no. (%) | 92 (26) | 78 (22) |
| Body mass index after dialysis—kg/m2 | 25.2±5.0 | 25.6±4.6 |
| Dialysis vintage (year) | ||
| -Median (inter-quartile range) | 1.8 (1.0–3.7) | 2.1 (1.0–4.0) |
| Systolic blood pressure—mmHg | 147±21 | 148±22 |
| Diastolic blood pressure-mmHg | 75±12 | 76±12 |
| Vascular access | ||
| Arteriovenous fistula- no. (%) | 279 (78) | 288 (81) |
| Graft- no. (%) | 57 (16) | 43 (12) |
| Central catheter- no. (%) | 22 (6) | 25 (7) |
| Number of treatments/week | ||
| -3- no. (%) | 332 (93) | 338 (95) |
| -2- no. (%) | 26 (7) | 18 (5) |
| Duration of a dialysis session—min | 226±26 | 227±22 |
| Blood flow—mL/min | 302±39 | 299±41 |
| Dialysis single pool Kt/Vurea | 1.41±0.24. | 1.38±0.19 |
| Residual kidney function no.(%)* | 186 (52) | 190 (53) |
| Estimated glomerular filtration rate | ||
| -ml/min/1.73m2; median (inter-quartile range) | 0.32 (0–3.30) | 0.30 (0–3.35) |
| Hemoglobin—mmol/L | 7.4±0.82 | 7.3±0.73 |
| Phosphorus—mmol/L | 1.65±0.51 | 1.63±0.47 |
| Beta-2-microglobulin—mg/L | 30.7±14.3 | 32.3±13.6 |
| Albumin—g/L^ | 40.2±3.8 | 40.6±3.9 |
| Creatinine–μmol/L pre-dialysis | 842±260 | 879±250 |
Values are means ±SD, median (interquartile range) or number (percentage).
HDF = online hemodiafiltration; HD = hemodialysis;
~pre-dialysis
*residual kidney function if diuresis >100 ml/24h
^albumin concentrations measured with the bromcresolpurple method have been converted to the bromcresolgreen method
To convert hemoglobin in mmol/L to g/dL divide by 0.62; phosphorus in mmol/L to mg/dL, divide by 0.323; albumin in g/L to g/dL, divide by 10; creatinine in μmol/L to mg/dL divide by 88.4
Fig 1. Enrolment, randomization, and follow-up of study participants.
For infections, all patients were followed until drop out, death or the end of the study.
Table 2. The risk for infectious events in patients treated with HD and HDF.
| HDF (n = 358) | HD (n = 356) | |||||
|---|---|---|---|---|---|---|
| Event | No. of events | Person-years of FU | No. of events | Person-years of FU | HR HDF vs HD (95%CI) | P |
| All infections | 198 | 800 | 169 | 798 | 1.09 ¶ | 0.42 |
| (0.88–1.34) | ||||||
| Fatal infection* | 23 | 800 | 28 | 798 | 0.85 ^ | 0.56 |
| (0.49–1.47) | ||||||
| 1st Graft- or fistula infection | 11 | 787 | 11 | 788 | 0.99 ^ | 0.98 |
| (0.43–2.29) | ||||||
| 1st Catheter sepsis | 14 | 787 | 8 | 781 | 1.77 ^ | 0.20 |
| (0.74–4.22) | ||||||
| 1st Sepsis | 17 | 790 | 7 | 794 | 2.40 ^ | 0.05 |
| (1.00–5.79) | ||||||
| 1st Urinary tract infection | 10 | 792 | 12 | 781 | 0.82 ^ | 0.64 |
| (0.35–1.89) | ||||||
| 1st Respiratory infection | 37 | 744 | 38 | 756 | 0.98 ^ | 0.98 |
| (0.62–1.53) | ||||||
| 1st Other infection | 52 | 735 | 38 | 755 | 1.40 ^ | 0.11 |
| (0.92–2.13) | ||||||
| 1st non-fatal or fatal infection | 118 | 652 | 106 | 681 | 1.16 ^ | 0.27 |
| (0.89–1.51) | ||||||
HDF = Online hemodiafiltration; HD = Low-flux hemodialysis; No. = number; FU = follow-up; HR = hazard ratio
¶ analyzed with Cox for repeated events
^obtained through unadjusted Cox proportional hazards models, time to (first) infectious event
*On treatment analysis, so infectious death on treatment of HD or HDF or within 28 days after censoring due to transplantation, switch to PD, move to other clinical or stop for other reasons
No difference in the effect of HDF was found in subgroups of age (below or above the median of 67 yrs, P value for the interaction term = 0.44), sex (P = 0.49), DM (present/absent, P = 0.64), previous CVD (P = 0.14), RKF (present/absent, P = 0.42) and dialysis vintage (below or above the median of 2 yrs, P = 0.20), or country of residence (P value for the interaction term = 0.15). We found a trend towards a different effect of HDF in patients with high or low serum albumin levels (P for interaction = 0.05). The HR (HDF vs. HD) for infections was 1.31 (0.96–1.79, P = 0.09) in patients with a baseline serum albumin above 40.5 g/L (median), and was 0.86 (0.65–1.13, P = 0.29) in patients with a baseline serum albumin below the median.
Compared to HD, the incidence of infections did not differ across tertiles of convection. Lowest convection volume tertile HR 1.10 (0.84–1.45), P = 0.49, middle convection tertile HR 1.13 (0.85–1.50), P = 0.41, highest convection tertile HR 0.88 (0.64–1.20), P = 0.42 (all versus HD and adjusted for country of origin). This result remained unaltered after adjustment for age, sex, CVD, DM, hematocrit, serum albumin, treatment time, blood flow rate, vascular access and site.
Of all infections, respiratory infections (25% in HDF, 28% in HD) were most common, followed by skin/musculoskeletal infections (21% in HDF, 13% in HD) (Tables 3 and 4).
Table 3. Total number of infectious events by cause.
| HDF | HD | |||||
|---|---|---|---|---|---|---|
| Event | Total no. of events | In n patients | % of all infections | Total no. of events | In n patients | % of all infections |
| (95% CI) | (95% CI) | |||||
| Fatal infection* | 23 | 23 | 11.6 | 28 | 28 | 16.6 |
| (7.1–16.1) | (11.0–22.2) | |||||
| Graft- or fistula infection | 11 | 11 | 5.6 | 12 | 11 | 7.1 |
| (2.4–8.7) | (3.2–11.0) | |||||
| Catheter sepsis | 16 | 14 | 8.1 | 8 | 8 | 4.7 |
| (4.3–11.9) | (1.5–7.9) | |||||
| Sepsis | 18 | 17 | 9.1 | 7 | 7 | 4.1 |
| (5.1–13.1) | (1.1–7.1) | |||||
| Urinary tract infection | 12 | 10 | 6.1 | 15 | 12 | 8.9 |
| (2.7–9.4) | (4.6–13.2) | |||||
| Respiratory infection | 49 | 37 | 24.7 | 47 | 38 | 27.8 |
| (18.7–30.8) | (21.1–34.6) | |||||
| Other infection | 69 | 52 | 34.8 | 52 | 38 | 30.8 |
| (28.2–41.5) | (23.8–37.7) | |||||
| Total no. of infections | 198 | 118 | 100 | 169 | 106 | 100 |
HDF = Online hemodiafiltration; HD = Low-flux hemodialysis; no. = number
*On treatment analysis, so infectious death on treatment of HD or HDF or within 28 days after censoring due to transplantation, switch to PD, move to other clinic or stop for other reasons
Note: one patient may have more than one infectious event
Table 4. Distribution of other infections in online hemodiafiltration and hemodialysis.
| HDF | HD | |||
|---|---|---|---|---|
| No. | % of all infections | No. | % of all infections | |
| Gastro-intestinal | 17 | 8.6 (4.7–12.5) | 13 | 7.7 (3.7–11.7) |
| Skin/musculoskeletal | 42 | 21.2 (15.5–26.9) | 22 | 13.0 (7.9–18.1) |
| Cardiac | 2 | 1.0 (0–2.4) | 1 | 0.6 (0–1.7) |
| Miscellaneous | 8 | 4.0 (1.3–6.8) | 16 | 9.5 (5.1–13.9) |
| Total | 69 | 52 | ||
HDF = online hemodiafiltration; HD = hemodialysis; No. number
Table 5 shows that the distribution of access types was different between patients treated in the Netherlands and in Canada. The risk for infections was neither changed by HDF in the Netherlands (HR 1.15 (0.91–1.45, P = 0.23) nor in Canada (HR 0.93 (0.55–1.56, P = 0.79). Since the proportion of patients treated with grafts in the Netherlands was larger in the HDF group, we adjusted for access type at baseline. The HR’s for the Netherlands and for Canada remained however comparable (1.13 (0.90–1.43, P = 0.29) and 0.93 (0.55–1.56, P = 0.78) respectively.
Table 5. Vascular access types at baseline in the Netherlands versus Canada.
| HDF | HD | |||
|---|---|---|---|---|
| The Netherlands | ||||
| Vascular access | No. | % | No. | % |
| AV-fistula | 245 | 82 | 256 | 86 |
| Graft | 49 | 16 | 34 | 11 |
| CVC | 4 | 1 | 5 | 2 |
| Canada | ||||
| AV-fistula | 29 | 57 | 28 | 55 |
| Graft | 7 | 14 | 5 | 10 |
| CVC | 15 | 29 | 18 | 35 |
HDF = online hemodiafiltration; HD = hemodialysis; No. number; AV = arterio-venous; CVC = central venous catheter
Discussion
In this large randomized controlled trial we found that 31% of the participants suffered from one or more infections leading to hospitalization during the study (median follow-up 1.96 years). Our results suggest that treatment with HDF does not reduce the risk of mortality and hospitalization due to infections as compared to HD. The most common infections in both treatment arms were respiratory infections and skin/musculoskeletal infections.
To our knowledge, this is the first study investigating the risk of infections in patients treated with HDF as compared to patients treated with low-flux HD. Our data indicate that alteration of the uremic milieu by convective clearance does not reduce the risk of infections. Our data are in line with the results of the HEMO study, which showed that high flux HD did not decrease the risk of infectious outcomes [5]. Also in the Turkish HDF study no difference in infectious related mortality was found between HDF and high-flux HD patients[12]. However, they only reported mortality from infections[12]. Our results are different from the results of the Spanish ESHOL study in which a reduced mortality by infections was found in the online HDF group as compared to the high-flux HD group[13]. However, they neither showed a difference in the number of infectious related hospitalizations between patients treated with HDF and HD. They did not report categories of infection[13].
In our study we found a trend towards a higher risk of sepsis in HDF patients. However, it may be a chance finding, since the number of patients in whom a sepsis or catheter sepsis occurred was small. Also, if we would take multiple testing into account, the association would be far from statistically significant. We do not have a theoretical explanation for the trend towards an increased number of sepsis in the HDF group. We neither have an explanation for a potentially different effect of HDF in patients with an albumin below or above the median, this might be a chance finding as well.
The impact of various dialysis modalities on immune function is speculative. There is no evidence that alteration of dialysis efficiency or improving clearance of middle molecules from the blood would have a beneficial effect on immune processes on the tissue level. HDF might enhance the clearance of deleterious molecules such as complement factor D, granulocyte inhibitory protein II (GIP II) and immunoglobulin free light chains, which have been shown to have an in vitro depressant effect on degranulation, chemotaxis and phagocytosis of mainly polymorphonuclear leukocytes [14]. Alternatively, HDF may also enhance the clearance of immune-active molecules such as cytokines, or other unknown molecules essential for immune function. The limited data on clearance of specific toxins by HDF and the in vitro and in vivo function of these toxins makes it very difficult to explain clinical outcomes. In addition, despite tight monitoring and quality control, the infusion of large amounts of substitution fluid, when contaminated, might impose a larger risk for infections. However we showed in a previous study that microbiological cultures of the substitution fluid were negative in 98% of 193 tested samples and endotoxin levels were below the reference quality level in 98% of 177 tested samples [15].
The groups of respiratory infections (25–28%) and skin/musculoskeletal infections (13–21%) were the most common infections in our study. These infections occurred more often in the same patients as well. In the HEMO study the contribution of respiratory disease as the cause for an infection related hospitalization was 22% as well [5]. Infections from cardiac, peripheral vascular disease, DM, hepatobiliary disease, musculoskeletal and gastrointestinal causes, which were included in our category ‘other infections’, also contributed to 31% of infection related hospitalizations [5]. The contribution of septicaemia and bacteremia and infections due to vascular access complications was larger than in our study, despite a comparable use of catheters in only 8% of patients [5]. Considering respiratory infections, a study from the United States (US) retrospectively linked Medicare claims to Dialysis Morbidity and Mortality Study data sets and reported that over an average follow-up of 3.3 years 28.9% of the study population was hospitalized with a pneumonia [16], which was 11% in a median follow-up of 1.96 years in our study. Another study from the US also based on claim data of Medicare reported a septicaemia rate of 17.5 per 100 patient years in HD patients in 1999, which is probably due to a high use of catheters [17]. The risk of non vascular access related infections, septicaemia related hospitalizations and infections of vascular access reported from an analysis of 5 European DOPPS countries was comparable to our study [18]. It remains however difficult to compare studies from different continents and data that are collected retrospectively or prospectively.
The strengths of our study are the randomized design and the prospective collection of the data on infectious events, including the categories of infection. Furthermore, all infectious events were adjudicated and all infections during the on treatment follow-up were taken into account in the data-analyses. A limitation is the fact that this study was powered on mortality and not on infections, so the study is underpowered to detect smaller, clinically relevant, differences. So a beneficial or harmful effect of treatment with HDF cannot be ruled out completely based on our findings. As the 95% of the HR (1.09) was 0.88–1.34, the effect of online HDF may vary between a 12% reduction and a 34% increase in hospitalization for infections, both extremes being clinically relevant. Most important in this superiority trial, a small beneficial effect for HDF can still exist. Furthermore the lack of cause specific registration of fatal infections, the retrospective categorization of the category ‘other infections’ and the generalizability to non-European countries, with a different composition of vascular accesses might be limitations.
More and larger studies are needed to confirm our findings. These studies should be powered on the incidence on infections, to rule out that the lack of effect we found, is not due to a lack of power. These studies should be designed as inferiority trials, to explore the trend towards more sepsis in the HDF group. The FINESSE trial, comparing high-flux HD with HDF, will investigate episodes of septicaemia as a secondary safety outcome [19]. Furthermore, data from the HDF Pooling Project could confirm our findings [20].
In conclusion, as compared to HD, our data suggest that treatment with HDF does not reduce the risk of infectious episodes in patients with end stage renal disease.
Supporting Information
(DOC)
(PDF)
Acknowledgments
The authors are grateful to patients, nursing staff and local investigators (listed below) who participated in this project.
This study has been presented as a poster presentation at the Kidney Week of the American Society of Nephrolgy in San Diego, United States, October 2012.
Local Investigators
Canada: Georges-L Dumont Regional Hospital, Moncton—M Dorval; CHUM St Luc Hospital, Montréal—R Lévesque; The Netherlands: Academic Medical Center, Amsterdam—MG Koopman; Catharina Hospital, Eindhoven—CJAM Konings; Dialysis Clinic Noord, Beilen—WP Haanstra; Dianet Dialysis Centers, Utrecht—M Kooistra and B van Jaarsveld; Fransiscus Hospital, Roosendaal—T Noordzij; Gelderse Vallei Hospital, Ede—GW Feith; Groene Hart Hospital, Gouda—HG Peltenburg; Haga Hospital, The Hague—M van Buren; Isala Clinics, Zwolle—JJG Offerman; Jeroen Bosch Hospital, ‘s Hertogenbosch—EK Hoogeveen; Maasland Hospital, Sittard—F de Heer; Maasstad Hospital, Rotterdam—PJ van de Ven; Martini Hospital, Groningen—TK Kremer Hovinga; Medical Center Alkmaar, Alkmaar—WA Bax; Onze Lieve Vrouwe Gasthuis, Amsterdam—JO Groeneveld; Oosterschelde Hospital, Goes—ATJ Lavrijssen; Rijnland Hospital, Leiderdorp—AM Schrander-Van der Meer; Rijnstate Hospital, Arnhem—LJM Reichert; Slingeland Hospital, Doetinchem—J Huussen; St Elisabeth Hospital, Tilburg—PL Rensma; St Fransiscus Gasthuis, Rotterdam—Y Schrama; University Medical Center St Radboud, Nijmegen–HW van Hamersvelt; University Medical Center Utrecht, Utrecht—WH Boer; VieCuri Medical Center, Venlo—WH van Kuijk; VU University Medical Center, Amsterdam—MG Vervloet; Zeeuws-Vlaanderen Hospital, Terneuzen—IMPMJ Wauters. Norway: Haukeland University Hospital, Bergen—I Sekse.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CONTRAST is financially supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland, grant C02.2019) and unrestricted grants from Fresenius Medical Care (The Netherlands) and Gambro Lundia AB (Sweden). Additional support was received from the Dr. E.E. Twiss Fund, Roche Netherlands; the International Society of Nephrology/Baxter Extramural Grant Program; the Dutch Organization for Health Research and Development (ZonMW, grant 17088.2802). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Foley RN. Infectious complications in chronic dialysis patients. Perit Dial Int 2008. June;28 Suppl 3:S167–S171. [PubMed] [Google Scholar]
- 2. Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol 1993. March;3(9):1541–54. [DOI] [PubMed] [Google Scholar]
- 3. Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr 2012. January;22(1):149–56. 10.1053/j.jrn.2011.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 1998. May;9(5):869–76. [DOI] [PubMed] [Google Scholar]
- 5. Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol 2003. July;14(7):1863–70. [DOI] [PubMed] [Google Scholar]
- 6. Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access 2008. October;9(4):231–5. [PubMed] [Google Scholar]
- 7. Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, et al. The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 2005. June;20(6):1180–6. [DOI] [PubMed] [Google Scholar]
- 8. Kaplowitz LG, Comstock JA, Landwehr DM, Dalton HP, Mayhall CG. A prospective study of infections in hemodialysis patients: patient hygiene and other risk factors for infection. Infect Control Hosp Epidemiol 1988. December;9(12):534–41. [DOI] [PubMed] [Google Scholar]
- 9. Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of Online Hemodiafiltration on All-Cause Mortality and Cardiovascular Outcomes. J Am Soc Nephrol 2012. June;23(6):1087–96. 10.1681/ASN.2011121140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penne EL, Blankestijn PJ, Bots ML, van den Dorpel MA, Grooteman MP, Nube MJ, et al. Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients—the Dutch CONvective TRAnsport STudy (CONTRAST): rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr Control Trials Cardiovasc Med 2005. May 20;6(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. Springer; 2000. [Google Scholar]
- 12. Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013. January;28(1):192–202. 10.1093/ndt/gfs407 [DOI] [PubMed] [Google Scholar]
- 13. Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013. February;24(3):487–97. 10.1681/ASN.2012080875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanholder R, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, et al. Uremic toxicity: present state of the art. Int J Artif Organs 2001. October;24(10):695–725. [PubMed] [Google Scholar]
- 15. Penne EL, Visser L, van den Dorpel MA, van der Weerd NC, Mazairac AH, van Jaarsveld BC, et al. Microbiological quality and quality control of purified water and ultrapure dialysis fluids for online hemodiafiltration in routine clinical practice. Kidney Int 2009. July 15;76(6):665–72. 10.1038/ki.2009.245 [DOI] [PubMed] [Google Scholar]
- 16. Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney Int 2006. September;70(6):1135–41. [DOI] [PubMed] [Google Scholar]
- 17. Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol 2004. April;15(4):1038–45. [DOI] [PubMed] [Google Scholar]
- 18. Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004. January;19(1):108–20. [DOI] [PubMed] [Google Scholar]
- 19.FINESSE Trial. Filtration in the neuropathy of end stage kidney disease symptom evolution. Available: http://www.anzctr.org.au/trial_view.aspx?ID=308240. 2009.
- 20. Nephrol Dial Transplant 2015; 30 (suppl 3): iii306 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.