Abstract
Background
Insufficient specificity of the high-risk human papillomavirus (hrHPV) assay in primary cervical cancer screening results in unnecessary referral. Additional assays to triage hrHPV-positive women are needed to improve molecular cervical cancer screening. DNA methylation is a promising biomarker in cervical cancer. We evaluated the clinical performance of potentially methylated genes as a triage assay for hrHPV-positive women.
Results
We conducted a retrospective hospital-based case–control study in Taiwan. Cervical scrapings were collected before colposcopy for hrHPV testing and quantitative methylation-specific PCR (QMSP) of 16 genes. Five genes, POU4F3, HS3ST2, AJAP1, PAX1, and SOX1, were prioritized for the clinical performance to triage hrHPV-positive women. Two hundred cervical scrapings were randomly classified into a training set (n = 111) and testing set (n = 89). All samples were tested for hrHPV using a Hybrid Capture II (HCII) assay. HrHPV-positive women were subjected to DNA methylation analysis by QMSP. In the training set, the receiver operating characteristic (ROC) curves defined the optimal methylation index (M-index) cutoff values for discriminating CIN3+ from CIN1/normal, which then were applied to the testing set. Among the five genes, POU4F3 revealed the highest area under the ROC curve (AUC) (0.86; 95 % CI, 0.78–0.95) in detecting CIN3+. In the testing set, POU4F3 revealed the best clinical performance in triage of hrHPV-positive women with a sensitivity of 74 % and specificity of 89 % for detecting CIN3+.
Conclusions
POU4F3 methylation analysis is a potential molecular tool for triage in detecting CIN3+ in hrHPV-positive women. The combined use of broad-spectrum HPV assay and POU4F3 methylation analysis as a new generation of molecular cervical cancer screening warrants further population-based study.
Electronic supplementary material
The online version of this article (doi:10.1186/s13148-015-0122-0) contains supplementary material, which is available to authorized users.
Keywords: DNA methylation, HrHPV test, QMSP, Biomarker, Cervical intraepithelial neoplasia (CIN), Cervical cancer screening
Background
Cervical cancer is a common medical problem in women, with 528,000 new cases and 266,000 deaths globally in 2012 indicating the need to develop and implement an effective cancer screening strategy [1]. The Papanicolaou (Pap) smear for cytological examination has been used for the detection of precancerous cellular abnormalities of cervical cells for decades and has lessened the disease burden by reducing the mortality and morbidity of cervical cancer [2]. The Pap smear or cytology test has high specificity for cervical intraepithelial neoplasia; however, it has many drawbacks such as suboptimal sensitivity [3] and moderate accuracy to detect relevant lesions and subjective diagnosis of cervical abnormalities with poor reproducibility [4]. Oncogenic high-risk human papillomavirus (hrHPV) infection is a well-known etiology of cervical cancer [5]. Because the duration of the initial hrHPV infection until the development of invasive cancer is long, assay of HPV DNA as a screening tool is appealing [6, 7]. However, HPV infection is transient in nature, and only few infected lesions further progress as invasive cancer [8]. Insufficient specificity of the HPV DNA assay results in a high false-positive rate and extra medical burden because of the consequent high colposcopy referral rate [6]. Findings of HPV-positive assay results also cause adverse psychosocial impact [9]. Therefore, an additional triage assay is required to improve HPV-based molecular cervical cancer screening [10, 11].
Persistent oncogenic hrHPV infection causes genetic and epigenetic changes [12]. Promoter hypermethylation-mediated silencing of tumor suppressor genes is common in cervical carcinogenesis [12, 13]. Because DNA methylation can be easily quantitated using molecular methods, it is gaining attraction as a molecular assay for detecting cervical cancer [14]. Several studies including our group have revealed that numerous aberrantly DNA-methylated cervical cancer-related genes could be potential biomarkers to improve cervical cancer detection [12, 15–17], to triage women with atypical squamous cells [18, 19] and low-grade squamous intraepithelial lesions (LSILs) in Pap smears [20]. Methylated genes could be potential markers for the triage of hrHPV-positive women [21–27]. However, the sensitivity and specificity are not satisfactory even if combining two or more genes [23, 24, 26], highlighting the need for novel methylation biomarkers.
Using methylomic approaches, many methylated candidate genes have been revealed, including ADRA1D, AJAP1, COL6A2, EDN3, EPO, HS3ST2, MAGI2, POU4F3, PTGDR, SOX8, SOX17, ST6GAL2, SYT9, ZNF614 [28], SOX1, and PAX1 [29]. The performance of these methylated genes to triage hrHPV-positive women remains unexplored.
Results
Selection of potential candidate genes in hrHPV-positive women
We randomly collected cervical scrapings from 100 women including 20 normal, 20 CIN1, 20 CIN2, 20 CIN3/CIS, and 20 SCC/AC before treatment. Those samples from hrHPV-positive women, 67 out of 100, were subjected to quantitative methylation-specific PCR (QMSP) analysis of 14 genes, ADRA1D, AJAP1, COL6A2, EDN3, EPO, HS3ST2, MAGI2, POU4F3, PTGDR, SOX8, SOX17, ST6GAL2, SYT9, and ZNF614, and used the same cutoff values previously described [28] (Table 1). We selected candidate genes with a sensitivity of >85 % or specificity of >98 % in detecting CIN3+ in hrHPV-positive women for further validation. Three genes, POU4F3, HS3ST2, and AJAP1, fulfilled these criteria.
Table 1.
Sensitivities and specificities of candidate genes in hrHPV-positive women (N = 67) in the selection set
| Detection modality | Sensitivity (%) | Specificity (%) |
|---|---|---|
| ADRA1D | 61 | 97 |
| AJAP1 | 64 | 100 |
| COL6A2 | 42 | 91 |
| EDN3 | 58 | 97 |
| EPO | 67 | 97 |
| HS3ST2 | 88 | 82 |
| MAGI2 | 70 | 94 |
| POU4F3 | 88 | 82 |
| PTGDR | 67 | 97 |
| SOX8 | 46 | 91 |
| SOX17 | 64 | 94 |
| ST6GAL2 | 64 | 97 |
| SYT9 | 73 | 94 |
| ZNF614 | 58 | 97 |
Generation of methylation cutoff values for triage of hrHPV-positive women in the training set
We tested the clinical performance of POU4F3, HS3ST2, and AJAP1 methylation for the triage of hrHPV-positive women (Fig. 1). The independently enrolled 200 women were randomly classified into two groups with a training-to-testing ratio of 1:1 (Table 2). There was no significant difference in the age (P = 0.17) and diagnosis distribution in the training set and testing set. Methylation levels of POU4F3, HS3ST2, and AJAP1 in hrHPV-positive women increased with disease severity (Fig. 2a–c). The optimal methylation index (M-index) cutoff values for detecting CIN3+ were 38 for POU4F3 and 2 for HS3ST2 and AJAP1 as defined by receiver operating characteristic (ROC) curves. The areas under the ROC curves (AUC) were 0.86 (95 % CI, 0.78–0.95) for POU4F3, 0.82 (95 % CI, 0.71–0.92) for HS3ST2, and 0.71 (95 % CI, 0.59–0.83) for AJAP1 (Fig. 2d–f). Because we have previously discovered and tested SOX1 and PAX1 genes as potential biomarkers [29], we also included the data of these two genes in this study to compare their clinical performance. At the optimal M-index cutoff values, the sensitivities of POU4F3, HS3ST2, AJAP1, SOX1, and PAX1 in discriminating CIN3+ among hrHPV-positive women were 79, 67, 63, 78, and 70 %, respectively, whereas the specificities were 78, 89, 64, 71, and 89 %, respectively (Table 3).
Fig. 1.
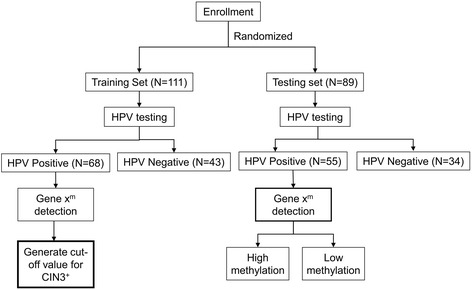
Work flow for analysis of clinical performance of candidate genes. A total of 200 women were enrolled and randomly assigned to a training set and a testing set. Methylation analysis of candidate genes using cervical scrapings of hrHPV-positive women under the training set was used for generating M-index cutoff values, which were then applied for analysis of the clinical performance of the candidate genes. Xm is the level of methylation of the candidate gene
Table 2.
Histopathology, mean age, and HPV percentage of the patients
| Variable | Training set | Testing set | |
|---|---|---|---|
| Age | Mean ± SD | 46.8 ± 13.5 | 44.2 ± 13.3 |
| No (%) | No (%) | ||
| Result of pathology | Normal | 34 (30.6) | 31 (34.8) |
| CIN1 | 31 (27.9) | 19 (21.3) | |
| CIN2 | 0 (0) | 0 (0) | |
| CIN3/CIS | 28 (25.2) | 27 (30.3) | |
| SCC/AC | 18 (16.2) | 12 (13.5) | |
| HPV | Negative | 43 (38.7) | 34 (38.2) |
| Positive | 68 (61.3) | 55 (61.8) | |
| Total | 111 | 89 |
CIN cervical intraepithelial neoplasia, CIN1 CIN grade 1, CIN2 CIN grade 2, CIN3 CIN3 grade 3, CIS carcinoma in situ, SCC squamous cell carcinoma, AC adenocarcinoma, HPV human papillomavirus
Fig. 2.
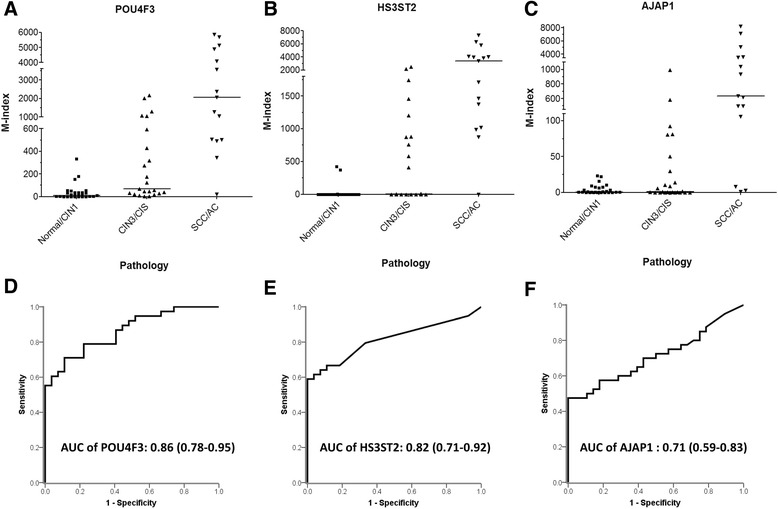
ROC curves of genes for M-index to trade off performance in detecting CIN3+. Methylation index levels of POU4F3 (a), HS3ST2 (b), and AJAP1 (c) in cervical scrapings such as normal and tumors graded as normal/CIN1, CIN3/CIS, or SCC/AC diagnosed by proven histopathology in hrHPV-positive samples. Each dot in the figure represents the M-index level of an individual woman. Analysis of ROC curve of POU4F3 (d), HS3ST2 (e), and AJAP1 (f). The AUC of the ROC curve of an individual candidate gene was calculated to diagnose CIN3+ lesions
Table 3.
Performance of methylation biomarkers to detect CIN3+ in hrHPV-positive women at training and testing sets
| Gene name | ||||||
|---|---|---|---|---|---|---|
| POU4F3 | HS3ST2 | AJAP1 | SOX1 | PAX1 | ||
| M-index cutoff value | 38 | 2 | 2 | 4 | 4 | |
| Training set (N = 68) | Sensitivity (%) | 79 | 67 | 63 | 78 | 70 |
| Specificity (%) | 78 | 89 | 64 | 71 | 89 | |
| PPV (%) | 83 | 90 | 71 | 80 | 90 | |
| NPV (%) | 72 | 65 | 55 | 69 | 68 | |
| Testing set (N = 55) | Sensitivity (%) | 74 | 55 | 80 | 63 | 60 |
| Specificity (%) | 89 | 100 | 74 | 74 | 100 | |
| PPV (%) | 93 | 100 | 85 | 82 | 100 | |
| NPV (%) | 64 | 56 | 67 | 52 | 58 | |
PPV positive predictive value, NPV negative predictive value, CIN3 + including CIN3/CIS, SCC/AC
Validation of the clinical performance of methylated genes in the testing set
Cervical scrapings of 55 hrHPV-positive women out of 89 women were analyzed further in the testing set for DNA methylation levels (Fig. 1). The testing set validated that POU4F3 methylation analysis conferred the best clinical performance among five potential candidates with 74 % sensitivity and 89 % specificity (Table 3). When stratified by histology, POU4F3 and AJAP1 methylation testing did not miss any invasive cancer patients (Table 4). AJAP1 methylation had better performance in detecting CIN3/CIS lesions than POU4F3 (70.8 vs. 62.5 %). However, more CIN1 lesions were detected using AJAP1.
Table 4.
Clinical performance of methylation biomarker in hrHPV-positive women of testing set stratified by histology
| Total number of detectable | 53 | 52 | 54 | |
| Detection modality | Gene name | |||
| POU4F3 | HS3ST2 | AJAP1 | ||
| Methylation positive/total number (%) | ||||
| Result of pathology | Normal | 0/5 (0 %) | 0/5 (0 %) | 0/5 (0 %) |
| CIN1 | 2/13 (15.4 %) | 0/14 (0 %) | 5/14 (35.7 %) | |
| CIN3/CIS | 15/24 (62.5 %) | 8/22 (36.3 %) | 17/24 (70.8 %) | |
| SCC/AC | 11/11 (100 %) | 10/11 (90.9 %) | 11/11 (100 %) | |
| Total | 28 | 18 | 33 | |
CIN cervical intraepithelial neoplasia, CIN1 CIN grade 1, CIN3 CIN3 grade 3, CIS carcinoma in situ, SCC squamous cell carcinoma, AC adenocarcinoma
Discussion
Previous studies support the concept that DNA methylation could be a potential molecular biomarker for detection of cervical lesions [12, 15, 16, 28, 30]. An ideal methylation biomarker should have better specificity than HPV testing and better sensitivity than cytology when applied as a primary screening tool. Recent studies proposed the alternative role of methylation biomarkers as a triage method for hrHPV-positive women [22–24, 26, 27]. More high-risk HPV genotype detection may have a better chance to include more women at risk for triage in the primary screening. In addition, the distribution of HPV type varies across continents because 16, 31, 33, and 18 are prevalent in Europe, and 16, 58, 52 and 18 are prevalent in the Asia–Pacific region [31, 32]. We used the Hybrid Capture II (HCII) assay for hrHPV testing, which assays 13 high-risk genotypes simultaneously [7, 32, 33]. The present study demonstrated that DNA methylation analysis as a triage for hrHPV-positive women is feasible. The POU4F3 methylation analysis confers the best clinical performance when combined with the HCII assay. In this study, the primary objective was to use broad-spectrum hrHPV testing capable of detecting more susceptible women for further triage with POU4F3 methylation to achieve a better sensitivity. Further hrHPV subtype analysis may clarify type-specific correlation with POU4F3 methylation, which may be useful in estimating the impact of molecular screening strategy using HPV detection followed by methylation triage in post-vaccination era.
POU4F3 is located on chromosome 5q32 and plays various biological functions, such as regulation of transcription, cellular and metabolic processes, organ development, cellular differentiation, nervous system development, neurogenesis, and generation of neurons [34]. The function of POU4F3 in cancer biology remains largely unknown. POU4F3 hypermethylation in cervical cancer and glioma suggests its suppressor role in cancer [28, 34]. This study supports the concept that POU4F3 could be a potential triage biomarker for hrHPV-positive women.
In the present study, a single gene, POU4F3, has a specificity of 89 % in detecting CIN3+ in hrHPV-positive women with limited compromise in sensitivity (79 to 74 %), which is better than the specificities previously published using FAM19A4 (67 %) [27], or a panel of two genes CADM1–M18/MAL-M1 (71–83 %) [22, 23, 35, 36], or a panel of at least two out of five methylated biomarkers (77 %) [26], or a panel with four methylated biomarkers (69 %) [24] or comparable to the specificity of JAM (88 %) [37]. We propose a scenario for the combination of HPV assay and POU4F3 methylation analysis for cervical cancer screening (Fig. 3). However, it requires further independent validation together with additional standalone biomarker. Women without hrHPV infection undergo follow-up 3 to 5 years later [38]. Women with hrHPV infection will undertake POU4F3 methylation analysis. Women having positive POU4F3 methylation are referred for colposcopy. Because POU4F3 methylation analysis did not miss any invasive cancer, POU4F3 methylation-negative, hrHPV-positive women may repeat HPV assay and DNA methylation analysis 1 year later. This strategy may substantially reduce the referral rate. However, a longitudinal follow-up study is needed to clarify the natural history of those infected with hrHPV, but without POU4F3 hypermethylation to determine if a longer interval between screenings is also safe. The high negative predictive value in hrHPV-negative women is well documented, which means a longer screening interval is safe. However, determining POU4F3 methylation in HPV-negative women to assess POU4F3 as independent from HPV as a marker for cervical neoplasia/CIN/CIN3/cancer may also be a consideration. Because HPV assay and methylation analysis can be conducted in the same self-collected cervical sample, the application of this approach may improve the participation of women for screening [39, 40], especially those in low-resource areas. The application of a DNA methylation analysis using self-collected vaginal samples warrants further evaluation. In addition, this is a retrospective hospital-based study, which did not follow up the participants. Population-based studies in different geographical and ethnic backgrounds are needed to validate these results.
Fig. 3.
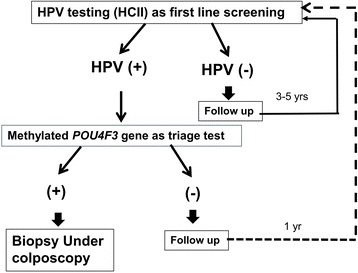
Proposed cervical cancer screening strategy using hrHPV assay and POU4F3 methylation analysis as a triage test. In this proposed scenario, HCII hrHPV DNA assay is used as the primary screening test, where women without hrHPV infection undergo follow-up 3 to 5 years later. Samples from women with hrHPV infection undergo POU4F3 methylation analysis, where women having positive POU4F3 methylation are referred for colposcopy. Additionally, women with a positive hrHPV assay but negative POU4F3 methylation may repeat HPV assay and DNA methylation analysis 1 year later
In the present study, we adapted histopathologically diagnosed CIN3+ as the end point because CIN2 is equivocal in nature with a tendency to regress to normal instead of progressing to CIN3+, where the likelihood of CIN2 progression to invasive cancer is only 5 % [41]. Further, diagnosis of CIN2 is much less reproducible than CIN3 because of the difference in the natural history of CIN2 from that of CIN3 [42]. However, CIN3 has a higher tendency to progress to invasive cancer because it is an immediate precursor with a similar virological profile and has better reproducibility [31]. Therefore, it is more appropriate to adapt CIN3+ as a surrogate end point for early diagnosis of cervical cancer.
Conclusions
POU4F3 methylation testing is a potential molecular biomarker for the triage of hrHPV-positive women for CIN3+ lesions. We envision an era of molecular screening for cervical cancer.
Methods
Patients
We conducted a retrospective case–control study using hospital-based patient samples in the Tri-Service General Hospital, Taiwan, from December 2009 to November 2010. Patients aged ≥20 years referred for a colposcopy and cervical biopsy and who were managed with conization or surgery after biopsy revealing CIN3+ were enrolled in this study. Cervical scrapings for laboratory analysis were collected in sterile phosphate-buffered saline before biopsy using a cervical brush and were stored at 4 °C until DNA extraction for HPV testing using a HCII hrHPV DNA assay (Digene, Silver Spring, MD, USA) and quantitative DNA methylation analysis of potential candidate genes using QMSP. Healthy women undergoing routine Pap screening were selected as controls, only when their Pap smears showed normal pattern. Women with positive or suspicious Pap smears were excluded from control. Before the study, all the subjects were informed about the study and were enrolled after obtaining documented full consent. Final diagnosis regarding different stages of cancer was performed by tissue-proven histopathological examination, except in healthy control women. Exclusion criteria applied in this study were compromised quality of Pap smears, patients previously vaccinated with anti-HPV vaccine, cervical neoplasia or existence of other malignancies, surgery related to the uterine cervix, an immunocompromised state, genital warts, or pregnancy. Further, all specimens were delinked from clinical information after numbering each of them until data analysis. In this study, all the women were tested for HPV infection and only samples from hrHPV-positive women underwent DNA methylation analysis. Cervical scrapings of 67 hrHPV-positive women among 100 recruited women underwent DNA methylation analysis to prioritize candidate genes for further analysis of clinical performance. Cervical scrapings from 200 women recruited for analysis of clinical performance were randomly classified using a random number table as a 1:1 ratio into a training set (n = 111) and a testing set (n = 89). The training set included 46 women with histopathologically confirmed CIN3+ and 65 women with normal/CIN1. Cervical scrapings from 68 hrHPV-positive women of the 111 underwent DNA methylation analysis. Methylation levels in the training set were used to generate optimal M-index cutoff values of candidate genes that can distinguish relevant cancerous cases from control. The clinical performance of candidate genes was validated using the optimal cutoff values in the testing set. The testing set comprised 89 women including 39 women with CIN3+ and 50 women with normal/CIN1. Of the 89 women, cervical scrapings from 55 hrHPV-positive women were analyzed further for quantitative assay of DNA methylation. This study was approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center.
Extraction of DNA followed by bisulfite modification
Genomic DNA was extracted as previously described using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations [28]. Those samples with a DNA yield as low as 500 ng or more (>500 ng) as measured by NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE, USA) were considered for further analysis in this study. Bisulfite modification of genomic DNA samples was performed using a CpGenome DNA Modification Kit (Millipore, Temecula, CA, USA) according to the manufacturer’s recommendations, and the samples were dissolved in 70 μL of nuclease-free water [29]. Bisulfite-converted DNA was stored at −80 °C until further use.
Methylation assays of potential candidate genes
QMSP used for analysis of the methylation status of the candidate genes was based on the principle of fluorescence-based real-time PCR. TaqMan-based QMSP amplification was performed on the bisulfite-treated DNA [43]. The type II collagen gene (COL2A) was used as an internal reference. In vitro methylated genomic DNA treated with CpG methyltransferase (M.SssI; New England Biolabs, Beverly, MA, USA) was used as a positive control. While prioritizing potential candidate methylated genes for further performance analysis, QMSP was performed in a TaqMan probe system using an Applied Biosystems 7900HT Fast Real-Time PCR System in a total volume of 20 μL reaction mixture containing 2 μL of bisulfite template DNA, 250 nM of each primer, 225 nM TaqMan probe, and 10 μL of FastStart Universal Probe Master (ROX) (Roche Diagnostics, Roche Applied Science, Mannheim, Germany) [28]. 6-Carboxy-fluorescein was used to label the 5′ end of probes, while a quencher dye (MGB by Applied Biosystems, or BHQ1 by TIB) was used to label the 3′ end of the probes (Additional file 1: Table S1). However, for analysis of clinical performance of candidate genes, QMSP for AJAP1, HS3ST2, and POU4F3 and multiplex QMSP for PAX1 and SOX1 were performed in a TaqMan probe system using a LightCycler 480 Real-Time PCR System (Roche Diagnostics, Roche Applied Science) [29]. Briefly, the total reaction volume of 20 μL contained 2 μL of modified template DNA, 1 μL of 20× Custom TaqMan reagent, and 10 μL LightCycler 480 Probes Master (Roche Diagnostics, Roche Applied Science). A mixture of primers and probes was used for PAX1 and SOX1. The reactions were conducted using an initial incubation at 95 °C for 10 min, followed by 50 cycles of 95 °C for 10 s, and annealing and extension for 40 s at 60 °C (using the thermal cycler protocol in the standard mode). The level of DNA methylation was measured in terms of M-index [30]. Results showing the very high Cp values of COL2A (>36) were defined as detection failures.
HPV DNA assay
The HCII hrHPV DNA assay (Digene) was used as the primary assay in this study following the manufacturer’s protocol to detect hrHPV infection. This HCII assay can detect 13 high-risk HPV subtypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. Samples with a ratio of relative light units (RLU)/cutoff value higher than 1.0 were recorded as positive.
Statistical analysis
ROC curves for each of the candidate genes were calculated using the data from the training set. Optimal M-index cutoff values of the candidate genes were generated from ROC curves and were used to further analyze the clinical performance of the candidate methylated genes in the testing set. Sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) of AJAP1, HS3ST2, POU4F3, PAX1, and SOX1 for detecting CIN3+ were calculated. IBM SPSS Statistics for Windows, version 20.0 (Armonk, NY, USA) was used for all statistical analyses.
Acknowledgements
This work was supported in part by the following grants: grant numbers NSC 102-2628-B-038-010-MY3 from the Ministry of Science and Technology; 103TMU-SHH-11, TMU103-AE1-B06, TMUTOP103005-1, and 104TMU-SHH-07 from Taipei Medical University; and TMU-NDMC-104-05 from Taipei Medical University and National Defense Medical Center. This work was also supported by the Teh-Tzer Study Group for Human Medical Research Foundation. PBP is thankful to Taiwan International Graduate program (TIGP) for the PhD Fellowship.
Abbreviations
- ADRA1D
adrenoceptor alpha 1D
- AJAP1
adherens junctions-associated protein 1
- AUC
area under the receiver operating characteristic curve
- CIN
cervical intraepithelial neoplasia
- CIN3+
CIN grade 3 or worse
- COL6A2
collagen, type VI, alpha 2
- EDN3
endothelin 3
- EPO
erythropoietin
- HrHPV
high-risk human papillomavirus
- HS3ST2
heparan sulfate (glucosamine) 3-O-sulfotransferase 2
- MAGI2
membrane-associated guanylate kinase, WW and PDZ domain-containing 2
- PAX1
paired box gene 1
- POU4F3
POU class 4 homeobox 3
- PTGDR
prostaglandin DP receptor
- QMSP
quantitative methylation-specific PCR
- SOX1
sex-determining region Y box 1
- SOX17
SRY-box 17
- SOX8
SRY (sex-determining region Y)-box 8
- ST6GAL2
ST6 betagalactosamide alpha-2,6-sialyltranferase 2
- SYT9
synaptotagmin IX
- ZNF614
zinc finger protein 614
Additional file
QMSP primers and probes in this study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PBP and HCL conceived and designed the experiments. PBP, HCW, YCC, YWH, and RLH performed the experiments. PBP drafted the manuscript. PBP, HCL, YPL, and PHS reviewed and edited the manuscript. CCC and HCL recruited and collected the clinical samples. PBP and YPL analyzed and interpreted the data. All the authors read and approved the final manuscript.
Contributor Information
Par Bahadur Pun, Email: punpb05aiims@gmail.com.
Yu-Ping Liao, Email: lypingg@gmail.com.
Po-Hsuan Su, Email: pohsuan@ms8.hinet.net.
Hui-Chen Wang, Email: egg-0420@yahoo.com.tw.
Yu-Chih Chen, Email: ycc.oliver@msa.hinet.net.
Yaw-Wen Hsu, Email: fri13wen@gmail.com.
Rui-Lan Huang, Email: gyntsgh@gmail.com.
Cheng-Chang Chang, Email: sundoor66@gmail.com.
Hung-Cheng Lai, Email: hclai@s.tmu.edu.tw, Email: hclai30656@gmail.com.
References
- 1.GlOBOCAN . GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. [Google Scholar]
- 2.Cannistra SA, Niloff JM. Cancer of the uterine cervix. New Engl J Med. 1996;334(16):1030–7. doi: 10.1056/NEJM199604183341606. [DOI] [PubMed] [Google Scholar]
- 3.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 4.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 5.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA. 2002;288(14):1749–57. doi: 10.1001/jama.288.14.1749. [DOI] [PubMed] [Google Scholar]
- 7.Kim JJ. Practice-based evidence for primary HPV testing in the United States. J Natl Cancer Inst. 2014;106(8). [DOI] [PubMed]
- 8.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 9.McCaffery K, Waller J, Forrest S, Cadman L, Szarewski A, Wardle J. Testing positive for human papillomavirus in routine cervical screening: examination of psychosocial impact. BJOG. 2004;111(12):1437–43. doi: 10.1111/j.1471-0528.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 10.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100(7):492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 11.Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VMH, Hesselink AT, Rozendaal L, et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer. 2012;130(3):602–10. doi: 10.1002/ijc.26056. [DOI] [PubMed] [Google Scholar]
- 12.Steenbergen RDM, Snijders PJF, Heideman DAM, Meijer CJLM. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer. 2014;14(6):395–405. doi: 10.1038/nrc3728. [DOI] [PubMed] [Google Scholar]
- 13.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen LS, Hansen LL. PCR-based methods for detecting single-locus DNA methylation biomarkers in cancer diagnostics, prognostics, and response to treatment. Clin Chem. 2009;55(8):1471–83. doi: 10.1373/clinchem.2008.121962. [DOI] [PubMed] [Google Scholar]
- 15.Lai HC, Lin YW, Huang TH, Yan P, Huang RL, Wang HC, et al. Identification of novel DNA methylation markers in cervical cancer. Int J Cancer. 2008;123(1):161–7. doi: 10.1002/ijc.23519. [DOI] [PubMed] [Google Scholar]
- 16.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112(2):293–9. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai HC, Lin YW, Huang RL, Chung MT, Wang HC, Liao YP, et al. Quantitative DNA methylation analysis detects cervical intraepithelial neoplasms type 3 and worse. Cancer. 2010;116(18):4266–74. doi: 10.1002/cncr.25252. [DOI] [PubMed] [Google Scholar]
- 18.Chao TK, Ke FY, Liao YP, Wang HC, Yu CP, Lai HC. Triage of cervical cytological diagnoses of atypical squamous cells by DNA methylation of paired boxed gene 1 (PAX1) Diagn Cytopathol. 2013;41(1):41–6. doi: 10.1002/dc.21758. [DOI] [PubMed] [Google Scholar]
- 19.Liou Y-L, Zhang Y, Liu Y, Cao L, Qin C-Z, Zhang T-L, et al. Comparison of HPV genotyping and methylated ZNF582 as triage for women with equivocal liquid-based cytology results. Clin Epigen. 2015;7(1):50. doi: 10.1186/s13148-015-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Chen TC, Chang TC, Cheng YM, Chen CH, Chu TY, et al. Methylated ZNF582 gene as a marker for triage of women with Pap smear reporting low-grade squamous intraepithelial lesions—a Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol Oncol. 2014;135(1):64–8. doi: 10.1016/j.ygyno.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 21.van der Meide WF, Snellenberg S, Meijer CJ, Baalbergen A, Helmerhorst TJ, van der Sluis WB, et al. Promoter methylation analysis of WNT/beta-catenin signaling pathway regulators to detect adenocarcinoma or its precursor lesion of the cervix. Gynecol Oncol. 2011;123(1):116–22. doi: 10.1016/j.ygyno.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Overmeer RM, Louwers JA, Meijer CJ, van Kemenade FJ, Hesselink AT, Daalmeijer NF, et al. Combined CADM1 and MAL promoter methylation analysis to detect (pre-)malignant cervical lesions in high-risk HPV-positive women. Int J Cancer. 2011;129(9):2218–25. doi: 10.1002/ijc.25890. [DOI] [PubMed] [Google Scholar]
- 23.Hesselink AT, Heideman DA, Steenbergen RD, Coupe VM, Overmeer RM, Rijkaart D, et al. Combined promoter methylation analysis of CADM1 and MAL: an objective triage tool for high-risk human papillomavirus DNA-positive women. Clin Cancer Res. 2011;17(8):2459–65. doi: 10.1158/1078-0432.CCR-10-2548. [DOI] [PubMed] [Google Scholar]
- 24.Eijsink JJ, Lendvai A, Deregowski V, Klip HG, Verpooten G, Dehaspe L, et al. A four-gene methylation marker panel as triage test in high-risk human papillomavirus positive patients. Int J Cancer. 2012;130(8):1861–9. doi: 10.1002/ijc.26326. [DOI] [PubMed] [Google Scholar]
- 25.Vasiljevic N, Scibior-Bentkowska D, Brentnall AR, Cuzick J, Lorincz AT. Credentialing of DNA methylation assays for human genes as diagnostic biomarkers of cervical intraepithelial neoplasia in high-risk HPV positive women. Gynecol Oncol. 2014;132(3):709–14. doi: 10.1016/j.ygyno.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansel A, Steinbach D, Greinke C, Schmitz M, Eiselt J, Scheungraber C, et al. A promising DNA methylation signature for the triage of high-risk human papillomavirus DNA-positive women. PLoS One. 2014;9(3):e91905. doi: 10.1371/journal.pone.0091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Strooper LMA, Meijer CJLM, Berkhof J, Hesselink AT, Snijders PJF, Steenbergen RDM, et al. Methylation analysis of the FAM19A4 gene in cervical scrapes is highly efficient in detecting cervical carcinomas and advanced CIN2/3 lesions. Cancer Prev Res. 2014;7(12):1251–7. doi: 10.1158/1940-6207.CAPR-14-0237. [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Huang RL, Huang YK, Liao YP, Su PH, Wang HC, et al. Methylomics analysis identifies epigenetically silenced genes and implies an activation of beta-catenin signaling in cervical cancer. Int J Cancer. 2014;135(1):117–27. doi: 10.1002/ijc.28658. [DOI] [PubMed] [Google Scholar]
- 29.Lai HC, Ou YC, Chen TC, Huang HJ, Cheng YM, Chen CH, et al. PAX1/SOX1 DNA methylation and cervical neoplasia detection: a Taiwanese Gynecologic Oncology Group (TGOG) study. Cancer Med. 2014;3(4):1062–74. doi: 10.1002/cam4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang RL, Chang CC, Su PH, Chen YC, Liao YP, Wang HC, et al. Methylomic analysis identifies frequent DNA methylation of zinc finger protein 582 (ZNF582) in cervical neoplasms. PLoS One. 2012;7(7):e41060. doi: 10.1371/journal.pone.0041060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 32.Luu HN, Dahlstrom KR, Mullen PD, VonVille HM, Scheurer ME. Comparison of the accuracy of Hybrid Capture II and polymerase chain reaction in detecting clinically important cervical dysplasia: a systematic review and meta-analysis. Cancer Med. 2013;2(3):367–90. doi: 10.1002/cam4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesselink AT, Bulkmans NWJ, Berkhof J, Lorincz AT, Meijer CJLM, Snijders PJF. Cross-sectional comparison of an automated hybrid capture 2 assay and the consensus GP5+/6+ PCR method in a population-based cervical screening program. J Clin Microbiol. 2006;44(10):3680–5. doi: 10.1128/JCM.02078-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Rauch TA, Zhong X, Bennett WP, Latif F, Krex D, et al. CpG island hypermethylation in human astrocytomas. Cancer Res. 2010;70(7):2718–27. doi: 10.1158/0008-5472.CAN-09-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Strooper LMA, Hesselink AT, Berkhof J, Meijer CJLM, Snijders PJF, Steenbergen RDM, et al. Combined CADM1/MAL methylation and cytology testing for colposcopy triage of high-risk HPV-positive women. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1933–7. doi: 10.1158/1055-9965.EPI-14-0347. [DOI] [PubMed] [Google Scholar]
- 36.Verhoef VMJ, van Kemenade FJ, Rozendaal L, Heideman DAM, Bosgraaf RP, Hesselink AT, et al. Follow-up of high-risk HPV positive women by combined cytology and bi-marker CADM1/MAL methylation analysis on cervical scrapes. Gynecol Oncol. 2015;137(1):55–9. doi: 10.1016/j.ygyno.2015.01.550. [DOI] [PubMed] [Google Scholar]
- 37.Boers A, Bosgraaf RP, van Leeuwen RW, Schuuring E, Heideman DAM, Massuger LFAG, et al. DNA methylation analysis in self-sampled brush material as a triage test in hrHPV-positive women. Br J Cancer. 2014;111(6):1095–101. doi: 10.1038/bjc.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tota JE, Ramana-Kumar AV, El-Khatib Z, Franco EL. The road ahead for cervical cancer prevention and control. Curr Oncol. 2014;21(2):e255–64. doi: 10.3747/co.21.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, Rozendaal L, Heideman DAM, Hesselink AT, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15(3):315–22. doi: 10.1016/S1470-2045(14)70019-1. [DOI] [PubMed] [Google Scholar]
- 40.Lorincz A, Castanon A, Wey Lim AW, Sasieni P. New strategies for human papillomavirus-based cervical screening. Womens Health. 2013;9(5):443–52. doi: 10.2217/whe.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–92. doi: 10.1097/00004347-199304000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Carreon JD, Sherman ME, Guillen D, Solomon D, Herrero R, Jeronimo J, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007;26(4):441–6. doi: 10.1097/pgp.0b013e31805152ab. [DOI] [PubMed] [Google Scholar]
- 43.Ogino S, Kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, Spiegelman D, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8(2):209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]


