Abstract
Background
With changing patterns and increasing use of medical abortion in the United States, it is important to have accurate statistics on the use of this method regularly available. This study assesses the accuracy of medical abortion data reported annually to the Centers for Disease Control and Prevention (CDC) and describes trends over time in the use of medical abortion relative to other methods.
Study Design
This analysis included data reported to CDC for 2001–2008. Year-specific analyses included all states that monitored medical abortion for a given year, while trend analyses were restricted to states that monitored medical abortion continuously from 2001 to 2008. Data quality and completeness were assessed by (a) examining abortions reported with an unspecified method type within the gestational age limit for medical abortion (med-eligible abortions) and (b) comparing the percentage of all abortions and med-eligible abortions reported to CDC as medical abortions with estimates based on published mifepristone sales data for the United States from 2001 to 2007.
Results
During 2001–2008, the percentage of med-eligible abortions reported to CDC with an unspecified method type remained low (1.0%–2.2%); CDC data and mifepristone sales estimates for 2001–2007 demonstrated strong agreement [all abortions: intraclass correlation coefficient (ICC)=0.983; med-eligible abortions: ICC=0.988]. During 2001–2008, the percentage of abortions reported to CDC as medical abortions increased (p<.001 for all abortions and for med-eligible abortions). Among states that reported medical abortions for 2008, 15% of all abortions and 23% of med-eligible abortions were reported as medical abortions.
Conclusion
CDC’s Abortion Surveillance System provides an important annual data source that accurately describes the use of medical abortion relative to other methods in the United States.
Keywords: Early medical abortion, Mifepristone, Surveillance
1. Introduction
In September 2000, the US Food and Drug Administration (FDA) approved the use of mifepristone in combination with misoprostol for medical abortion. Because medical abortion does not require specialized surgical skills or equipment and may be offered earlier in gestation than is typical for curettage procedures, it was anticipated that FDA approval would lead to the availability of abortion services through a wider range of providers and at earlier gestational ages [1,2]. During the initial years following FDA approval, use of mifepristone increased sharply, although subsequent increases were more gradual [3]. However, mifepristone use may be rising again [3], and in 2008, medical abortion accounted for more than 20% of US abortions performed up through the completion of 8 weeks’ gestation [4,5].
With changing patterns and increasing use of medical abortion, it is important to ensure that accurate statistics on the use of this method are regularly available. The Centers for Disease Control and Prevention (CDC) compiles abortion data reported by state health departments every year, including information on the methods used for abortion [4]. However, while most states legally require medical providers to submit a report for all the abortions they perform, there is no federal mandate. Thus, a few states do not have an abortion reporting system [6]. Moreover, because states establish their own reporting requirements, some states do not collect all the information CDC requests, and states also vary considerably in the degree to which reporting requirements are enforced [4,7]. Completeness of reporting may be especially problematic for capturing information on medical abortion. While the majority of medical abortions are provided at specialized abortion clinics with large caseloads [5], a higher percentage of the abortions provided in physician’s offices and small-caseload facilities are medical abortions [5,8]. Because it may be particularly difficult to identify providers who perform only a small number of abortions and identifying new providers in the wider medical community requires active surveillance efforts [8], medical abortions may be disproportionately undercounted even when reporting is technically required by law.
Through its periodic census of known abortion providers, the Guttmacher Institute provides an additional source of abortion data [5]. While this census generates the most complete national estimate of the total number of abortions performed in the United States [3,9], it is conducted only once every 3 or 4 years. Moreover, it does not obtain information on medical abortions from many of the providers surveyed and also may have difficulties identifying small-caseload providers [5,10]. Given these limitations, an additional source of data ideally should be used to evaluate the quality of CDC surveillance for medical abortion. Because the sole distributor of mifepristone in the United States only sells this medication to licensed physicians, who must sign and return a prescriber’s agreement [11], sales data from this company are not limited by the difficulties of identifying smaller providers within the wider medical community. Previously published estimates based on mifepristone sales data are available annually through 2007 [3].
Given the importance of having accurate estimates of the use of medical abortion in the United States regularly available, this study has the following objectives: (a) to assess the quality and completeness of CDC’s annual estimates of the use of medical abortion and (b) to evaluate trends in the use of medical abortion relative to other methods, both among all abortions performed and among abortions performed within the gestational age limit for medical abortion.
2. Methods
2.1. Data source
Since 1969, CDC’s Abortion Surveillance System has documented the number and characteristics of women obtaining legal induced abortions in the United States. Each year, CDC requests states to provide aggregate abortion counts according to maternal demographics (age, race/ethnicity, residence, and previous live births and abortions), gestational age at the time of abortion, and the method used to complete the abortion. Additional details of CDC’s Abortion Surveillance System have been published elsewhere [4]. This analysis uses data from 2001 to 2008. Because this is public health surveillance data with no personal identifiers, this study was determined to be exempt from review by CDC’s Institutional Review Board.
2.2. Definitions
CDC compiles aggregate data on abortion methods by week of gestation using the following categories: curettage, medical, intrauterine instillation, other and unknown. CDC defines early medical abortion as the administration of medication or medications (typically mifepristone followed by misoprostol) to induce an abortion at ≤8 weeks’ gestation [4]. CDC collects information only on the estimated number of weeks (not days) of gestation and acknowledges the conventional use of completed weeks of gestation to describe pregnancy duration. CDC’s category “≤8 weeks’ gestation” thus includes abortions up through 8 weeks and 6 days, which closely corresponds to the gestational age limit of 63 days for the early medical abortion protocol endorsed by the American College of Obstetricians and Gynecologists [12]. For this analysis, we defined an abortion that is eligible for medical abortion on the basis of gestational age (med-eligible abortion) as an abortion performed at ≤8 weeks’ gestation. Also for this analysis, we defined an abortion with an unspecified method type as an abortion reported to CDC as having been completed through an “other” or an “unknown” method.
2.3. Analysis
This analysis evaluated the reported use of early medical abortion from 2001 — the first full year following FDA approval of mifepristone — through 2008. Only states and other reporting areas (New York City and the District of Columbia) that specifically monitored the use of medical abortion were included (N=30–41, for a given year). Most reporting areas met this inclusion criterion by having medical abortion as a specific category on their reporting form. However, we retained Minnesota in our analyses for 2001–2006 despite the fact that it did not have medical abortion on its reporting form until 2007: whereas most states without medical abortion on their forms reported many abortions at ≤8 weeks’ gestation in the “other” or the “unknown” category, Minnesota supplied detailed notes in a text field that allowed >90% of these abortions to be reassigned to the medical abortion category. Measures of reported use included the percentage of all abortions that were performed by early medical abortion and the percentage of med-eligible abortions that were performed by early medical abortion.
While states that did not have medical abortion on their reporting form tended to report a high percentage of abortions at ≤8 weeks’ gestation in the “other” or “unknown” categories, this pattern also was observed to varying degrees among states that did have medical abortion on their reporting form. Because this pattern suggests that even states with medical abortion on their reporting form may misclassify and therefore underascertain the use of early medical abortion, we used med-eligible abortions reported with an unspecified method type as an indicator of the quality and completeness of reporting for early medical abortion. For this analysis, we used two different measures: (a) abortions reported with an unspecified method type as a percentage of all med-eligible abortions and (b) the number of unspecified abortions relative to the number of early medical abortions. This second measure was used to control for greater opportunities for misclassification as the percentage of abortions performed medically increased.
To assess the quality and completeness of medical abortion reporting, we also compared CDC’s annual estimates of the percentage of abortions that were performed by early medical abortion with estimates based on published mifepristone sales data from 2001 to 2007 [3]. Because the estimates based on mifepristone sales data were published before the availability of estimates of the total number of abortions in the United States for 2006 and 2007, for these years, the authors [3] calculated the percentage of abortions that were performed medically assuming that the downward trend in the total number of abortions from 2000 to 2005 [13] would continue through 2007. However, because data subsequently published for 2006 and 2007 indicated that the total number of abortions leveled off [5], we recalculated the contribution of mifepristone abortions for these years. For this calculation, we used the number of mifepristone abortions initially reported (numerator) [3] and the more recently published total number of abortions in the United States (denominator) [5]. Finally, to evaluate the robustness of CDC estimates relative to estimates based on mifepristone sales data, we considered alternative scenarios for each year from 2001 to 2007 to assess the impact of reclassifying varying percentages of med-eligible abortions reported to CDC with an unspecified method type as early medical abortions. We hypothesized that med-eligible abortions reported with an unspecified method type were more likely to be medical than surgical abortions because of the tendency for some states to report abortions with an unspecified method type in a cluster at ≤8 weeks’ gestation.
Year-specific estimates of the percentage of abortions that were performed by early medical abortion included all reporting areas that monitored the use of medical abortion for a given year. Trend analyses only included areas that monitored the use of medical abortion every year during 2001–2008. We used the Spearman rank order correlation coefficient to evaluate changes across years in med-eligible abortions reported to CDC with an unspecified method type and the percentage of abortions that were performed by early medical abortion. We used the intraclass correlation coefficient (ICC) for absolute agreement with a two-way random-effects model to evaluate the agreement between CDC and mifepristone sales estimates of the percentage of abortions performed by early medical abortion. All statistical tests were completed in SPSS version 19 (IBM, Armonk, NY).
3. Results
For 2001, 30 reporting areas systematically collected and provided information to CDC on the use of medical abortion. From 2001 to 2008, the number of areas that reported medical abortion increased to 41, although only 28 states provided information on the use of medical abortion every year during this period.
Among the 28 continuously reporting areas, abortions reported with an unspecified method type constituted a very small percentage of all med-eligible abortions (1.0%–2.2%), and this percentage did not change during 2001–2008 (rs= −0.24; p=.57; Fig. 1). However, due to the increasing percentage of abortions that were performed medically (see below) the number of med-eligible abortions reported with an unspecified method type decreased relative to the number of early medical abortions (rs=−0.85; p<.01; Fig. 1).
Fig. 1.
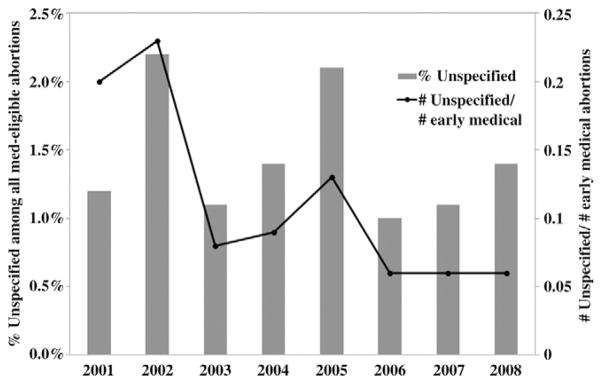
Abortions reported with an unspecified method type among med-eligible abortions in 28 continuously reporting areas, 2001–2008.
Annual CDC estimates of the percentage of all abortions and med-eligible abortions that were performed medically differed by no more than one percentage point from the mifepristone sales estimates in any given year (Table 1). Thus, CDC and mifepristone sales estimates of early medical abortion demonstrated strong absolute agreement (all abortions: ICC= 0.983; med-eligible abortions: ICC=0.988). The degree of agreement between the two estimates for all abortions and for med-eligible abortions was greatest when we assumed that 40% of med-eligible abortions reported to CDC with an unspecified method type actually were early medical abortions (all abortions: ICC=1.00; med-eligible abortions: ICC= 0.995). Assuming that >40% of med-eligible abortions reported to CDC with an unspecified method type actually were medical abortions did not result in greater agreement (Table 1).
Table 1.
Percentage of abortions reported to CDC as early medical abortions, and comparison with mifepristone sales estimates under different assumptions for med-eligible abortions reported to CDC with unspecified procedures
| All abortions
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Early medical abortion (%)based on
|
Percentage point difference in CDC and mifepristone estimates based on the percentage of unspecified CDC abortions reclassified as early medical abortions
|
|||||||
| Numbers reported to CDC | Mifepristone sales data | 0% Medical | 10% Medical | 20% Medical | 30% Medical | 40% Medical | 50% Medical | 60% Medical | |
| 2001 | 3 | 4 | −1 | −1 | 0 | 0 | 0 | 0 | 0 |
| 2002 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 2003 | 8 | 9 | −1 | −1 | 0 | 0 | 0 | 0 | 0 |
| 2004 | 11 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2005 | 11 | 12 | −1 | −1 | −1 | −1 | 0 | 0 | 0 |
| 2006 | 12 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2007 | 13 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ICC | 0.983 | 0.983 | 0.994 | 0.994 | 1.000 | 1.000 | 0.994 | ||
| Med-eligible abortions
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Early medical abortion (%)based on
|
Percentage point difference in CDC and mifepristone estimates based on the percentage of unspecified CDC abortions reclassified as early medical abortions
|
|||||||
| Numbers reported to CDC | Mifepristone sales data | 0% Medical | 10% Medical | 20% Medical | 30% Medical | 40% Medical | 50% Medical | 60% Medical | |
| 2001 | 6 | 7 | −1 | −1 | −1 | −1 | −1 | −1 | −1 |
| 2002 | 9 | 10 | −1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 2003 | 14 | 14 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 2004 | 17 | 18 | −1 | −1 | −1 | −1 | 0 | 0 | 0 |
| 2005 | 18 | 19 | −1 | −1 | −1 | −1 | −1 | 0 | 0 |
| 2006 | 18 | 19 | −1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2007 | 21 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ICC | 0.988 | 0.992 | 0.992 | 0.992 | 0.995 | 0.992 | 0.992 | ||
Among the 41 areas that reported medical abortion data to CDC for 2008, 15% of all abortions and 23% of med-eligible abortions were reported as early medical abortions. After applying the assumption that 40% of med-eligible abortions reported to CDC with an unspecified method type were medical abortions, the estimate for all abortions remained at 15% and the estimate for med-eligible abortions increased only one percentage point, to 24%.
Among the 28 continuously reporting areas that provided CDC data on medical abortion every year during 2001–2008, the percentage of all abortions and med-eligible abortions that were performed by early medical abortion increased over time (all abortions: rs=0.99, p<.001; med-eligible abortions: rs=1.00, p<.001; Fig. 2). Annual estimates among the continuously reporting areas changed by no more than one percentage point if it was assumed that a certain percentage (10%–60%) of med-eligible abortions reported to CDC with an unspecified method type were early medical abortions.
Fig. 2.
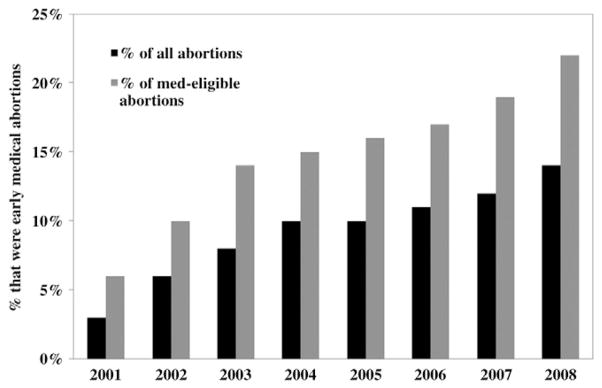
Percentage of abortions that were early medical abortions among 28 continuously reporting areas, 2001–2008.
During 2001–2003, the percentage of all abortion and med-eligible abortions that were performed by early medical abortion increased. In 2004 and 2005, the contribution of medical abortion leveled off but began increasing again in 2006 (Fig. 2).
4. Discussion
This analysis points to the strength of CDC’s Abortion Surveillance System for monitoring the percentage of abortions that are performed medically in the United States. Annual early medical abortion estimates based on surveillance data reported to CDC were essentially identical to national estimates based on mifepristone sales data. Moreover, the quality and completeness of CDC’s annual estimates have improved over time: the number of areas reporting the use of medical abortion to CDC increased from 30 in 2001 to 41 in 2008. Additionally, abortions reported with an unspecified method type accounted for a very small percentage of all med-eligible abortions, and the number of med-eligible abortions reported with an unspecified method type decreased relative to the number of early medical abortions over the study period.
This study also suggests that the percentage of abortions completed by early medical abortion has begun to increase more rapidly in recent years. The current analysis shows that the use of early medical abortion increased quickly in the initial years following FDA approval of mifepristone and then plateaued during 2004–2006; however, during 2007–2008, more pronounced increases were observed once more. Hence, while early medical abortion currently accounts for 23% of med-eligible abortions, further increases are possible. Full integration of early medical abortion into US abortion practices could resemble the pattern observed in many European countries, where increases in the use of mifepristone took place over many years as health care systems adapted to new regulations and protocols [14]. Because CDC data are available annually, they represent an important source of information for monitoring trends and determining whether a similar pattern might occur in the United States.
Another strength of CDC’s abortion surveillance data is that information on use of different abortion methods can be reported by gestational age. Given the potential advantages of using mifepristone as an adjuvant in abortion by labor induction and in dilation and evacuation procedures [15–17], it may increasingly be used in second-trimester abortions. If so, mifepristone sales data will overestimate the use of early medical abortion. By combining information on gestational age and abortion method, CDC data can be used to identify abortions for which mifepristone was used for purposes other than early medical abortion, thereby more accurately estimating the percentage of early abortions that are performed by early medical abortion.
One reason for the close correspondence of estimates based on data reported to CDC and mifepristone sales data may be that early medical abortion is not being offered by as wide of a range of providers as was anticipated initially. Although identifying small-caseload providers in the wider medical community is challenging [8], existing data suggest that the number of abortions provided in physician’s offices and small-caseload facilities decreased from 2005 to 2008 [5] and that early medical abortion continues to be offered primarily by obstetrician-gynecologists [3]. Several factors may limit the number and range of providers who offer early medical abortion. First, many states recently have passed laws requiring facilities that offer abortion services to meet the building standards of an ambulatory surgical center [18,19]. Because these regulations may apply to facilities offering medical as well as surgical abortions, it may be financially impossible for private offices and small-caseload providers to offer any type of abortion services. Similarly, the cost and availability of liability insurance may prevent many family physicians from offering medical abortion: they may be refused coverage for abortion, or they may be charged the same liability insurance premiums for providing medical abortions as for providing surgical abortion procedures [20]. Finally, it was anticipated that the use of telemedicine (i.e., the delivery of health care services at a distance using information and communication technology) and the provision of early medical abortion by advanced practice clinicians (i.e., physician assistants, nurse practitioners and certified nurse-midwives) would increase the availability of abortion services in remote areas with relatively few physicians and surgical facilities [21,22]. However, most states have laws preventing advanced practice clinicians from providing abortion services, and these laws have been applied to medical as well as surgical abortions [23]. Additionally, a number of states have begun to pass laws prohibiting physicians from using telemedicine to provide medical abortions remotely [18].
This analysis is not without limitations. First, although mifepristone sales data were used as a comparison for CDC data, mifepristone also may be used for second-trimester procedures [15–17]. Mifepristone sales data, therefore, may overestimate the use of early medical abortion. Conversely, methotrexate offers another option for early medical abortion [24] that would have been included in the CDC estimates, but not the estimates based on mifepristone sales data. Similarly, misoprostol alone, although not as effective as the combination of mifepristone and misoprostol and not recommended where mifepristone is available, can be used for early medical abortion [24,25]. Nonetheless, in spite of these alternatives, prior to FDA approval of mifepristone, the percentage of abortions reported to CDC as medical abortions remained very low (≤1%) [26]. Finally, use of estimates based on mifepristone sales data as a comparison for CDC data assumes that providers are using mifepristone units in the year of purchase and that providers comply with the evidence-based (200-mg mifepristone dose) medical abortion protocol. Although these assumptions appear valid [3,27], given the increasing number of states requiring providers to comply with the current FDA-approved protocol (600-mg mifepristone dose) [18], a certain percentage of early medical abortions will be performed with this higher dose of mifepristone until a relabeling of this product is approved.
Another limitation is that the gestational age cutoff of ≤8 weeks misclassifies a small proportion of early medical abortions performed at 63 days’ gestation. The dramatic decrease in medical abortion after 8 weeks’ gestation that has been documented in CDC data [4] suggests that this gestational age cutoff is appropriate for the purpose of surveillance. However, given research showing continuing efficacy of mifepristone and misoprostol between 9 and 13 weeks’ gestation [28–33], this cutoff will need to be reconsidered upon any indication of changes in abortion practice patterns in the United States. CDC will continue to monitor patterns in reported use of medical abortion by gestational age and adjust its classification of abortions as necessary.
In conclusion, this analysis suggests that CDC’s Abortion Surveillance System provides a valuable measure of the use of early medical abortion relative to other methods for abortion in the United States. Importantly, CDC data are available annually and can capture rapid changes in usage patterns. Even during the initial years following FDA approval of mifepristone, CDC estimates of the percentage of abortions that were performed medically closely matched estimates from other sources, and the quality and completeness of CDC surveillance data appear to have improved over time. The most recent CDC data suggest that the percentage of abortions that are performed medically is rising. Because CDC abortion surveillance data are available annually, they are an important source of information for determining whether the recent increase is part of a sustained trend.
Acknowledgments
The authors thank Jessica Reno for her assistance with this manuscript.
There was no external funding used to support this study.
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Jordan B, Shields WC. Happy anniversary mifepristone: a decade of promise and challenges. Contraception. 2010;82:219–20. doi: 10.1016/j.contraception.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra HD. Mifepristone in the United States: status and future. Guttmacher Rep Public Policy. 2002;5:4–7. [Google Scholar]
- 3.Finer LB, Wei J. Effect of mifepristone on abortion access in the United States. Obstet Gynecol. 2009;114:623–30. doi: 10.1097/AOG.0b013e3181b2a74d. [DOI] [PubMed] [Google Scholar]
- 4.Pazol K, Zane SB, Parker WY, Hall LR, Berg CJ, Cook DA. Abortion surveillance — United States, 2008. MMWR Surveill Summ. 2011;60:1–41. [PubMed] [Google Scholar]
- 5.Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health. 2011;43:41–50. doi: 10.1363/4304111. [DOI] [PubMed] [Google Scholar]
- 6.Guttmacher Institute. [Accessed 5/17/2012];State policies in brief: abortion reporting requirements. at: http://www.guttmacher.org/statecenter/spibs/spib_ARR.pdf.
- 7.Saul R. Abortion reporting in the United States: an examination of the federal-state partnership. Fam Plann Perspect. 1998;30:244–7. [PubMed] [Google Scholar]
- 8.Yunzal-Butler C, Sackoff J, Li W. Medication abortions among New York City residents, 2001–2008. Perspect Sex Reprod Health. 2011;43:218–23. doi: 10.1363/4321811. [DOI] [PubMed] [Google Scholar]
- 9.Ventura SJ, Abma JC, Mosher WD, Henshaw SK. National vital statistics reports. 15. Vol. 56. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2008. Estimated pregnancy rates by outcome for the United States, 1990–2004. [PubMed] [Google Scholar]
- 10.Henshaw SK, Van Vort J. Abortion services in the United States, 1991 and 1992. Fam Plann Perspect. 1994;26:100–6. 12. [PubMed] [Google Scholar]
- 11.Winikoff B, Hassoun D, Bracken H. Introduction and provision of medical abortion: a tale of two countries in which technology is necessary but not sufficient. Contraception. 2011;83:322–9. doi: 10.1016/j.contraception.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Committee. ACOG practice bulletin: clinical management guidelines for obstetrician–gynecologists. Obstet Gynecol. 2005;106:871–82. doi: 10.1097/00006250-200510000-00051. [DOI] [PubMed] [Google Scholar]
- 13.Jones RK, Zolna MR, Henshaw SK, Finer LB. Abortion in the United States: incidence and access to services, 2005. Perspect Sex Reprod Health. 2008;40:6–16. doi: 10.1363/4000608. [DOI] [PubMed] [Google Scholar]
- 14.Jones RK, Henshaw SK. Mifepristone for early medical abortion: experiences in France, Great Britain and Sweden. Perspect Sex Reprod Health. 2002;34:154–61. [PubMed] [Google Scholar]
- 15.Hammond C. Recent advances in second-trimester abortion: an evidence-based review. Am J Obstet Gynecol. 2009;200:347–56. doi: 10.1016/j.ajog.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Kapp N, von Hertzen H. Medical methods to induce abortion in the second trimester. In: Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG, Creinin MD, editors. Management of unintended and abnormal pregnancy: comprehensive abortion care. West Sussex: Blackwell Publishing; 2009. pp. 178–92. [Google Scholar]
- 17.Borgatta L, Kapp N Society of Family Planning. Clinical guidelines. Labor induction abortion in the second trimester. Contraception. 2011;84:4–18. doi: 10.1016/j.contraception.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Guttmacher Institute. [Accessed 5/17/2012];Medication abortion. 2011 at: http://www.guttmacher.org/statecenter/updates/index.html#MedicationAb.
- 19.Joyce T. The supply-side economics of abortion. N Engl J Med. 2011;365:1466–9. doi: 10.1056/NEJMp1109889. [DOI] [PubMed] [Google Scholar]
- 20.Dehlendorf CE, Grumbach K. Medical liability insurance as a barrier to the provision of abortion services in family medicine. Am J Public Health. 2008;98:1770–4. doi: 10.2105/AJPH.2008.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman D, Grindlay K, Buchacker T, Lane K, Blanchard K. Effectiveness and acceptability of medical abortion provided through telemedicine. Obstet Gynecol. 2011;118:296–303. doi: 10.1097/AOG.0b013e318224d110. [DOI] [PubMed] [Google Scholar]
- 22.Yarnall J, Swica Y, Winikoff B. Non-physician clinicians can safely provide first trimester medical abortion. Reprod Health Matters. 2009;17:61–9. doi: 10.1016/S0968-8080(09)33445-X. [DOI] [PubMed] [Google Scholar]
- 23.Guttmacher Institute. [Accessed 3/17/2012];State policies in brief: an overview of abortion laws. 2012 at: http://www.guttmacher.org/statecenter/spibs/spib_OAL.pdf.
- 24.Creinin M, Gemzell-Danielsson K. Medical abortion early in pregnancy. In: Paul M, Lichtenberg ES, Borgatta L, Grimes DA, Stubblefield PG, Creinin MD, editors. Management of unintended and abnormal pregnancy: comprehensive abortion care. West Sussex: Blackwell Publishing; 2009. pp. 111–34. [Google Scholar]
- 25.Ngoc NT, Blum J, Raghavan S, et al. Comparing two early medical abortion regimens: mifepristone+misoprostol vs. misoprostol alone. Contraception. 2011;83:410–7. doi: 10.1016/j.contraception.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Elam-Evans LD, Strauss LT, Herndon J, et al. Abortion surveillance —United States, 2000. MMWR Surveill Summ. 2003;52:1–34. [PubMed] [Google Scholar]
- 27.Henshaw SK, Finer LB. The accessibility of abortion services in the United States, 2001. Perspect Sex Reprod Health. 2003;35:16–24. doi: 10.1363/3501603. [DOI] [PubMed] [Google Scholar]
- 28.Boersma AA, Meyboom-de Jong B, Kleiverda G. Mifepristone followed by home administration of buccal misoprostol for medical abortion up to 70 days of amenorrhoea in a general practice in Curacao. Eur J Contracept Reprod Health Care. 2011;16:61–6. doi: 10.3109/13625187.2011.555568. [DOI] [PubMed] [Google Scholar]
- 29.Bracken H, Ngoc NT, Schaff E, et al. Mifepristone followed in 24 hours to 48 hours by misoprostol for late first-trimester abortion. Obstet Gynecol. 2007;109:895–901. doi: 10.1097/01.AOG.0000259319.18958.76. [DOI] [PubMed] [Google Scholar]
- 30.Garbin O, Vayssiere C, Bettahar-Lebugle K, Nisand I. Consistency of medical abortion efficacy from 5 through 14 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2006;129:36–40. doi: 10.1016/j.ejogrb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Hamoda H, Ashok PW, Flett GM, Templeton A. A randomised controlled trial of mifepristone in combination with misoprostol administered sublingually or vaginally for medical abortion up to 13 weeks of gestation. BJOG. 2005;112:1102–8. doi: 10.1111/j.1471-0528.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamoda H, Ashok PW, Flett GM, Templeton A. Medical abortion at 9–13 weeks’ gestation: a review of 1076 consecutive cases. Contraception. 2005;71:327–32. doi: 10.1016/j.contraception.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Lokeland M, Iversen OE, Dahle GS, Nappen MH, Ertzeid L, Bjorge L. Medical abortion at 63 to 90 days of gestation. Obstet Gynecol. 2010;115:962–8. doi: 10.1097/AOG.0b013e3181da0c3e. [DOI] [PubMed] [Google Scholar]


