Abstract
Background and Purpose
Since patients with phenylketonuria (PKU) have to follow a lifelong restriction of natural protein to lower phenylalanine-intake, they never eat fish. This diet may lead to a chronic deficit of omega-3 and omega-6 fatty acids with the risk of early atherosclerotic changes. The aim of the study was to analyse the fatty acid profile of PKU patients and to correlate the results with surrogate markers of early atherosclerotic changes [enhanced carotid intima media thickness (CIMT) and ß-stiffness index] and platelet activation.
Methods
In 43 PKU patients and in 58 healthy controls we prospectively examined the fatty acid profile, CIMT, ß-stiffness index and platelet activation (flow cytometric determination of markers of platelet activation). CIMT was measured bilaterally by ultrasound. CIMT mean was defined as the mean value of the sum of CIMT left and CIMT right.
Results
Despite of lower HDL-cholesterol and higher triglyceride concentrations in the PKU group, there was no significant difference in the omega-6 or omega-3 fatty acid profile, CIMT, ß-stiffness index between both groups. Platelet activation was not enhanced in the PKU group.
Conclusions
Fish-free diet does not induce early atherosclerotic changes or enhanced platelet activation in PKU patients.
Introduction
Classical phenylketonuria is an inherited disease characterised by the absence or deficiency of the enzyme phenylalanine hydroxylase, resulting in an increase in blood phenylalanine concentration [1]. The aim of treatment is to lower blood phenylalanine concentration to prevent mental retardation [1]. The controlled low-phenylalanine diet is started in the first weeks of life and should be adhered to lifelong [2,3]. The low-phenylalanine diet consists of vegan-vegetarian fare (no meat, fish, milk, cheese, eggs, nuts, bread, or soya products) plus a synthetic, phenylalanine-free “medical food-formula” containing essential and nonessential amino acids, vitamins, trace elements, and minerals but without fat supplement [1,2]). As these patients never consume fish, this may result in a chronically omega-3 and omega-6 fatty acids deficiency with the risk of early atherosclerotic changes. Several epidemiological studies [4–10] show an association between cardiovascular disease and the consumed amount of omega-3 or omega-6 fatty acids. The atherothrombotic risk of adult PKU patients has not been investigated so far. Hence we analysed early atherosclerotic changes (surrogate markers as carotid media thickness and ß-stiffness index) in PKU patients in comparison to a healthy control group. Since platelets play a critical role in atherogenesis and its complications, we also investigated platelet activation in these patients, especially in the light of reports that platelets of vegetarians and vegans show hyperaggregability to agonists [11, 12, 13].
Methods
Patients
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee Charite-Universitaetsmedizin Berlin. All participants provided written informed consent. Written informed consent was obtained and stored. The ethics approval number was EA2/089/05.
Forty-three PKU patients and 58 healthy controls were prospectively enrolled in the study. The inclusion of PKU patients was restricted to those with a lifelong phenylalanine-reduced diet i.e. patients diagnosed by newborn screening. In Germany screening for PKU has been initiated in 1970, therefore the maximum age of the patients investigated was 38 years. Compliance to the diet was controlled by measuring plasma phenylalanine and tyrosine levels of the PKU patients. The PKU patients were seen in regular intervals at our outpatient clinic.
Ultrasound evaluation
Intima-Media thickness (IMT)
Ultrasonographic scanning was performed using an ultrasonic phase-locked echotracking system, which was equipped with a high-resolution real-time 8-MHz linear 2D scanner (Sytem Five, GE-Vingmed, Solingen Germany). The intima-media thickness (CIMT) was measured bilaterally at a defined location, 30 mm proximal to the bifurcation at the far wall of the common carotid artery (ACC) provided that this point was free of calcification. If there was an atherosclerotic calcified plaque the measurement was done 25 mm proximal to the bifurcation. CIMTright and CIMTleft were determined and the average CIMTmean was calculated. The investigator who performed the CIMT analysis was blinded to the results of the platelet measurements and the diet protocol of the patients.
β-Stiffness-Index
Several parameters have been proposed as indexes of the elastic properties of the arteries. In the present study we determined the arterial stiffness of both carotid arteries and calculated the average of the right and left β-stiffness index. The stiffness-index is based on the assumption that an exponential relation exists between relative pressure and strain [14]. Carotid stiffness index was calculated using the following formula: β = (natural logarithmic systolic blood pressure–natural logarithmic diastolic blood pressure)/ (systolic diameter–diastolic diameter)/diastolic diameter [15,16].
Platelet analysis
For flow cytometric analysis 0.5 ml of blood was collected into a polypropylene syringe with 1.0 ml of a fixative containing methacrolein (CyfixII) [17]. Evaluation of surface expression of platelet membrane glycoproteins (CD62P, CD63, CD40L, PAC-1) was performed with specific monoclonal antibodies and two-color whole blood flow cytometry, as described in detail elsewhere [18]. Specific monoclonal antibody binding was expressed as mean immunofluorescence intensity (CD63, CD40L) or percentage of positive platelets of total platelet population (CD62P, PAC-1) and was used as a quantitative measurement of surface glycoprotein expression. The platelet assay used in the present study obtains reproducible results without significant artifactual platelet activation and has proved suitable for platelet analysis in a variety of clinical settings [19–21]. To establish reference values, control samples obtained from healthy individuals were always processed simultaneously with patient samples.
Determination of fatty acid profile
Fatty acid and lipid profiles were measured in red cell membranes according to a modified procedure of Morrison et al [22] and Eder et al [23]. Lipid extraction was performed by the hexan-isopropanol extraction technique [24].
Statistical analyses
Results of flow cytometric parameters are reported as median (interquartile range) unless otherwise indicated. Differences between groups were evaluated either by using the t-test for parameters showing normal distribution or by appropriate unpaired nonparametric tests (Mann Whitney U test) for parameters not normally distributed. A value of p<0.05 was regarded as significant (SPSS, Windows, version 10.0).
Results
Demographic data of the patients
Between April 2005 and November 2005 we consecutively enrolled 43 PKU patients and 58 healthy controls. The basic demographic and clinical characteristics are given in Table 1. The PKU group comprised less women (48.8%) than the control group (65.5%) and the body mass index of the PKU patients was significantly greater (24.30 ± 0.69 kg/m² versus 21.96 ± 0.31 kg/m²; p = 0.009). HDL-cholesterol was significantly lower in the PKU group compared to the controls (51.8 ± 2.43 mg/dl versus 63.11 ± 2.23 mg/dl; p = 0.003), but still in a normal range, whereas there was no difference in total cholesterol and LDL-cholesterol. The remaining routine clinical and laboratory parameters were not statistically different between both groups. Compliance to the diet was controlled by measuring plasma phenylalanine and tyrosine levels of the PKU patients. In our patient group plasma phenylalanine was 15.5 ± 0.6 mg/dl and tyrosine 0.9 ± 0.08 mg/dl respectively.
Table 1. Baseline characteristics of the patients and controls.
| Patients | PKU n = 43 | Controls n = 58 | p-value |
|---|---|---|---|
| Age, years | 28.1 ± 0.96 | 26.2 ± 0.5 | 0.434 |
| Female gender, n (%) | 21 (48.8) | 39 (67.2) | 0.064 |
| Smokers, n (%) | 8 (18.6) | 14 (24.2) | 0.546 |
| Body mass index, kg/m2 | 24.3 ± 0.7 | 21.9 ± 0.3 | 0.009 |
| Platelets, nl-1 | 255 ± 10.2 | 239 ± 6.7 | 0.167 |
| Leukocytes, nl-1 | 6.8 ± 0.3 | 5.9 ± 0.2 | 0.031 |
| Cholesterol, mg/dl | 160 ± 4.8 | 168 ± 4.3 | 0.167 |
| LDL-cholesterol, mg/dl | 84.3 ± 4.2 | 89.9 ± 3.8 | 0.374 |
| HDL-cholesterol, mg/dl | 51.8 ± 2.4 | 63.1 ± 2.2 | 0.003 |
| Triglycerides, mg/dl | 108.7± 8.9 | 88.2± 5.3 | 0.017 |
Results are shown as mean ± SEM or n (%)
Intima media thickness and β-stiffness index
The surrogate markers CIMT and β-stiffness index were not significantly different between the PKU patients [(CIMT: 0.43 ± 0.02 mm; β = 4.57 ± 0.47) and the healthy controls (CIMT: 0.48 ± 0.01 mm; β = 4.18 ± 0.59) pCIMT = 0.88 and pβ = 0.70] (Fig 1).
Fig 1. Schematic representation of carotid intima media thickness (CIMT) and β-stiffness index in PKU patients (group I) and controls (group II).
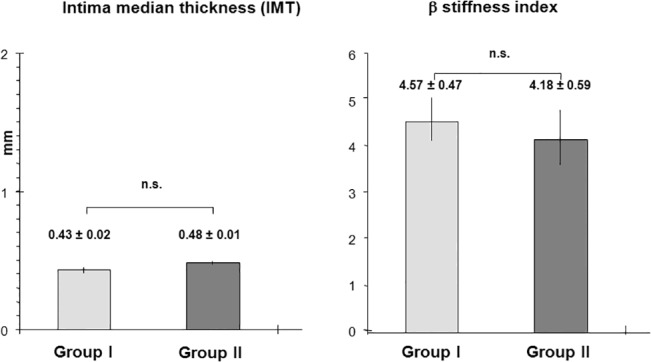
Bar-graphs showing the mean value and SEM. There was no significant difference in CIMT [(0.43 ± 0.02 mm) versus (0.48 ± 0.01mm), pCIMT = 0.88] and β-stiffness index [(4.57 ± 0.47) versus 4.18 ± 0.59) pß = 0.7].
Platelet markers
Surface expression of activation dependent platelet membrane glycoproteins (CD62P, PAC-1, CD63 and CD40L) was not enhanced in the PKU group compared to the control group: CD62 P (percentage of positive platelets) as [median; interquartile range] in the PKU group versus control group: [(2.0; 1.6–2.7) versus (2.1; 1.57–2.8), p = 0.86] and PAC-1 (percentage of positive platelets) as [median; interquartile range]: [(0.46; 0.32–0.68) versus (0.55; 0.42–0.71), p = 0.29] (Fig 2A).
Fig 2. Schematic expression of platelet activation markers CD62P, PAC-1, CD63 and CD40 L in PKU patients (group I) versus controls (group II).
Histobars representing a) percentage of positive platelets as [median; interquartile range]: CD62P: [2.0; 1.6–2.7] versus [2.1; 1.57–2.8], p = 0.86 and PAC-1: [0.46; 0.32–0.68] versus [0.55; 0.42–0.71], p = 0.29; b) Mean immunofluorescence intensity (MFI); [median; interquartile range]: CD63 [12.5; 11.0–14.9] versus [13.23; 11.61–15.25], p = 0.3; and CD40L [16.31; 13.10–18.7] versus [16.5; 13.5–19.3], p = 0.62.
CD63 (mean immunofluorescence intensity) as [median; interquartile range]: [12.5; 11.0–14.9] versus [13.23; 11.61–15.25], p = 0.3 and CD40L (mean immunofluorescence intensity) as [median; interquartile range]: [16.31; 13.1–18.7] versus [16.5; 13.5–19.3], p = 0.62 (Fig 2B).
Fatty acid profile
The level of omega-6 fatty acids and surprisingly also of the omega-3 fatty acids in red cell membranes did not differ significantly between the PKU and the control group (Table 2). The content of omega -3 fatty acids was 8.22 ± 24 amount % in PKU patients and 8.41 ± 24 amount % in the control group. We also found no significant difference when we further discriminated the omega-3 fatty acids in the following chains 18:3, 20:5, 22:5 and 22:6. (Table 2). The concentration of omega-6 fatty acids was 26.39 ± 1.29 amount % in the PKU group and 26.61 ± 1.08 amount % in the control group. Concerning the content of the omega-6 fatty acid chains 20:3 and 20:4 there was also no significant difference between both groups. The omega-3/omega-6 ratio was also not significant different between both groups (1:3.3 versus controls: 1:3.26; p = 0.54).
Table 2. Fatty acid profile.
| Patients | PKU n = 43 | Controls n = 58 | p-value |
|---|---|---|---|
| Omega-3 fatty acids, amount % | 8.22 ± 0.24 | 8.41 ± 0.22 | 0.52 |
| α-Linolenic acid (ALA), 18:3 | 0.20 ± 0.03 | 0.19 ± 0.02 | 0.995 |
| Eicosapentaenoic acid (EPA), 20:5 | 0.59 ± 0.04 | 0.62 ± 0.04 | 0.686 |
| Docosapentapentaenoic acid (DPA), 22:5 | 1.98 ± 0.14 | 1.95 ± 0.12 | 0.816 |
| Dococohexaenoic acid (DHA), 22:6 | 4.20 ± 0.15 | 4.50 ± 0.17 | 0.167 |
| Omega-6 fatty acids, amount % | 26.39 ± 1.29 | 26.61 ± 1.08 | 0.94 |
| Dihomo-gamma-linoleic acid, 20:3 | 1.42 ± 0.08 | 1.34 ± 0.07 | 0.331 |
| Arachidonic acid, 20:4 | 11.43 ± 0.64 | 11.38 ± 0.07 | 0.959 |
| Omega-3/omega-6 fatty acid ratio | 1:3.3 ± 0.19 | 1:3.26 ± 0.17 | 0.54 |
Results are shown as mean ± SEM; p value of < 0.05 was regarded as significant *.
Fatty acid profile were measured in red cell membranes.
Discussion
This is the first study to analyse the risk for early atherosclerotic changes (surrogate marker CIMT and β-stiffness index) and platelet activation in adult PKU patients. Although PKU patients keeping to a life-long phenylalanine-restricted diet, never eat fish, we did not find early atherosclerotic changes or enhanced platelet activation. The total amount of omega-3 and omega-6 fatty acids and likewise also the ratio of omega-3 /omega-6 fatty acids did not differ significantly between the PKU group and the controls. These results were not due to lack of compliance of diet since phenylalanine blood levels were always within recommended limits, respectively 0.7–20 mg/dl. Surprisingly the PKU patients showed even no deficiency of the omega-3 fatty acid chains as 18:3, 20:5 and 22:5 although the intake of these fatty acids is mostly by fatty fish. It seems that PKU patients compensate successfully the lack of omega-3 fatty acid intake caused by fish-free diet by the intake of special vegetable oils as for instance canola oil or flax seed oil. On the other hand one might argue that there was no difference because the intake of fatty fish is also low in the healthy control group.
Our findings are in contrast to Mosely [25] who in 2002 found significant lower levels of omega-3 fatty acids in adult PKU patients. In contrast to earlier (before 2002) nutritional recommendations, PKU patients today are encouraged to balance their diet by vegetable fats rich in omega-3 fatty acids. As a result of the predominantly vegetable fat intake by PKU patients, we observed significantly lower levels of saturated fatty acids compared to the control group.
Pioneering studies in Greenland Eskimos almost 30 years ago suggested that ingestion of omega-3 fatty acids (mostly present in fatty fish) conveys protection from cardiovascular disease [4]. This initial observation has set off of a number of epidemiological studies to examine this hypothesis, but data from randomized trials are limited [26, 27] and case-control studies on fish intake and fatal CAD are sparse [5, 26, 27, 28]. Results from prospective cohort studies have been inconsistent. Some studies showed an inverse association between fish intake [8, 9, 10] and CAD mortality whereas others did not [29–30].
Possible mechanisms underlying the cardioprotective effects of the consumption of fish include the reduction in serum triglycerides, a decrease in platelet aggregability and the presence of antiarrhythmic effects [31]. But it is still not clear whether omega-3 fatty acids have a direct effect on the pathogenesis of atherosclerosis. The addition of omega-3 fatty acids to standard diets demonstrated no protective effect on the progression of atherosclerosis in carotid arteries [31, 32]. A large study including 551 patients, failed to show that supplementation of 8 g/d of omega-3 fatty acids prevent restenosis following angioplasty [33]. Consequently, at present, we have no evidence that the intake of omega-3 fatty acids induce regression of atherosclerosis although the beneficial effect in reducing mortality after a first myocardial infarction has been demonstrated in many studies [34]. One might speculate that although omega-3 fatty acids have been demonstrated to prevent cardiac death in patients after myocardial infarction, they exhibit no direct effect on the early stages of atherosclerosis. The latter could explain why there was no difference in carotid intima-media thickness and β-stiffness-index between both groups.
Data concerning the relation between platelet function and omega-3 and omega-6 fatty acid levels are controversial. Dyerberg and Bang [4] first reported on the decreased platelet function in Greenlanders. Since that seminal observation, data have been aquired to provide support for several mechanisms whereby omega-3 fatty acids help balance cellular-signalling networks to more antithrombotic, anti-inflammatory set points. Omega-3 fatty acids competewith and antagonize the metabolism of omega-6 fatty acids and eicosanoids [35]. Eicosanoids derived from omega-3 fatty acids are less efficient amplifiers of the cell-signalling process in contrast to those derived from the omega-6 fatty acid, arachidonic acid. Despite this reasonable mechanism of omega-3 fatty acids, intervention studies with omega-3 polyunsaturated fatty acids gave conflicting results. Mori [36] showed significant reduction of platelet aggregation to agonists as collagen and platelet activating factor (PAF) after supplementation with fish and /or fish oil. Prisco [37] demonstrated also decreased collagen-induced platelet aggregation in patients receiving omega-3 fatty acid supplementation. Braden [38] reported a modest platelet inhibitory effect of fish oil in vivo in a canine model of coronary thrombosis. However there exist also other studies [39, 40, 41] showing no effect of omega-3 supplementation on platelet aggregation and thromboxan production.
Platelets of vegetarians or vegans are said to be more sensitive to proaggregatory agonists than those of omnivores [42]. Suboptimal taurine status [42] besides deficiency of omega-3 fatty acids in vegans is reported to be responsible for the increased aggregability of platelets. Thus platelet activation in PKU patients could occur independently from the lipid status. Yet, the expression of platelet activation markers (CD62P, PAC-1, CD40L and CD63) in our PKU patients was not enhanced in comparison to the control group. But PKU patients on phenylalanine-restricted diet are strictly speaking no real vegans, because their diet include the intake of synthetic, phenylalanine-free “medical food-formula” containing essential and non-essential amino acids, including taurine, vitamins, trace elements, and minerals.
Our data can be seen in the light of the Origin-trial [43] investigating the addition of n-3 fatty acids vs placebo on the overall cardiovascular outcome in a high-risk patient group. This trial demonstrated no positive effect of the addition of n-3 fatty acids on the overall cardiovascular outcome. Our results may help to further clarify the role of fish oil and its effect on cardiovascular outcome. By means of this specific group of patients who besides the nutritional difference of never eating fish are well comparable to the normal population, we could demonstrate that the lack of fish oil has no negative effect on early atherosclerotic changes and platelet activation, despite the higher BMI, triglyceride and the lower HDL cholesterol concentrations. Hence these data challenge some traditional beliefs that i.e the lack of unsaturated fatty acids as they are contained in fish meals may have a negative effect on early atherosclerotic changes. The PKU group is the only group of patients who never eats fish and has the great advantage that we can monitor compliance of diet by measuring the phenylalanine blood concentrations. In all other studies one has to rely on the uncertainty of anamnestic statements to eating habits.
Conclusion
According to our results, fish free diet does not induce early atherosclerotic changes and platelet activation in PKU patients. Surprisingly omega-3 fatty acid level were not different in both groups implicating that PKU patients compensate their lack of fish intake by vegetable fats rich in omega-3 fatty acids. Further interventional studies with omega-3 supplementation have to evaluate the long-term effect on atherosclerotic surrogate markers and platelet function in these patients.
Acknowledgments
The authors want to thank Bernd Tomandl for his help in preparing the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported in part by grants from the Charité Research Fund (Anschubfinanzierung), by the DFG - Klinische Forschergruppe (KFO274) Platelet-Basic Mechanisms and Translational Medicine and “Deutsche Interessengemeinschaft Phenylketonurie und verwandte Stoffwechselstörungen e.V. (DIG-PKU)”.
References
- 1. Scriver CR, Kaufmann S. Hyperphenylalaninemia.Phenyl hydroxylase deficiency In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc.eds The metabolic and molecular bases of inherited disease, 8th edn. New York: Mc Graw Hill; 2001: 1667–1724. [Google Scholar]
- 2. Hanley WB. Adult phenylketonuria. Am J Med.2004;17:590–595. [DOI] [PubMed] [Google Scholar]
- 3. National Institute of Health Consensus Development Conference Statement. Phenylketonuria. National Institutes of Health Consensus Development Panel. Pediatrics 2001;108:972–982. [DOI] [PubMed] [Google Scholar]
- 4. Bang HO, Dyerberg J, Hjorne N.The composition of food consumed by Greenland Eskimos. Acta Med Scan. 1976; 200 (1):69–73. [DOI] [PubMed] [Google Scholar]
- 5. Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. [DOI] [PubMed] [Google Scholar]
- 6. Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet. 1991; 66:205–216. [DOI] [PubMed] [Google Scholar]
- 7. Kromhout D, Feskens EJ, Bowles CH. The protective effect of a small amount of fish on coronary heart disease mortaility in an elderly population. Int J Epidemiol. 1995; 24:340–345. [DOI] [PubMed] [Google Scholar]
- 8. Oomen CM, Feskens EJ, Raesaenen L, Fidanza F, Nissinen AM, Menotti A, et al. Fish consumption and coronary heart disease mortality in Finland, Italy and The Netherlands. Am J Epidemiol 2000; 151:999–1006. [DOI] [PubMed] [Google Scholar]
- 9. Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001; 154 (9):809–816. [DOI] [PubMed] [Google Scholar]
- 10. Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed:the Cardiovascular Health Study. Circulation 2003; 107:1372–1377. [DOI] [PubMed] [Google Scholar]
- 11. Li D, Sinclair A, Mann N, Turner A, Ball M, Kelly F, et al. The association of diet and thrombotic risk factors in healthy male vegetarians and meat eaters. EurJ.Clin Nutr. 1999; 53:612–619. [DOI] [PubMed] [Google Scholar]
- 12. Chetty N, Bradlow BA. The effects of a vegetarian diet on platelet function and fatty acids. Thromb Res 1983; 30:619–624. [DOI] [PubMed] [Google Scholar]
- 13. Mezzano D, Munoz X, Martinez C, Cuevas A, Panes O, Aranda E, et al. Vegetarians and cardiovascular risk factors:hemostasis, inflammatory markers and plasma homocysteine. Thromb Haemost 1999; 81:913–917. [PubMed] [Google Scholar]
- 14. Iannuzzi A, Licenziati MR, Acampora C, Renis M, Agrusta M, Romano L, et al. Carotid artery stiffness in obese children with metabolic syndrome. Am J Cardiol 2006; 297:528–531. [DOI] [PubMed] [Google Scholar]
- 15. Hirai T, Sasayama S, Kawasaki T, Yagi S.Stiffness of systemic arteries in patients with myocardial infarction: a non-invasive method to predict the severity of coronary atherosclerosis. Circulation 1989; 80:78–87. [DOI] [PubMed] [Google Scholar]
- 16. Emoto M, Nishizawa Y, Kawagishi T, Maekawa K, Hiura Y, Kanda H, et al. Stiffness indexes beta of the common carotid and femoral arteries are associated with insulin resistance in NIDDM. Diabetes Care 1998; 21:1178–1182. [DOI] [PubMed] [Google Scholar]
- 17. Ruf A, Pick M, Deutsch V, Patscheke H, Goldfarb A, Rachmilewitz EA, et al. The procoagulant activity of red cells from patients with β-thalassemia major correlates with in-vivo platelet activation. Br J Haematol 1997; 98:51–56. [DOI] [PubMed] [Google Scholar]
- 18. Gawaz M, Neumann FJ, Schomig A.Evaluation of platelet membrane glycoproteins in coronary artery disease: consequences for diagnosis and therapy. Circulation 1999; 99:E1–E11. [DOI] [PubMed] [Google Scholar]
- 19. Fateh-Moghadam S, Bocksch W, Ruf A, Dickfeld T, Schartl M, Pogátsa-Murray G, et al. Changes in surface expression of platelet membrane glycoproteins and progression of heart transplant vasculopathy. Circulation 2000;102:890–897. [DOI] [PubMed] [Google Scholar]
- 20. Fateh-Moghadam S, Li Z, Ersel S, Reuter T, Htun P, Plöckinger U, et al. Platelet degranulation is associated with progression of intima-media thickness of the common carotid artery in patients with diabetes mellitus type 2. Arterioscler Thromb Vasc Biol 2005; 25: 1299–1203. [DOI] [PubMed] [Google Scholar]
- 21. Fateh-Moghadam, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, et al. Hyperresponsiveness of platelets in ischemic stroke.Thromb Haemost 2007; 97:974–978. [PubMed] [Google Scholar]
- 22. Morrison WR, Smith LM.Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 1964; 5:600–608. [PubMed] [Google Scholar]
- 23. Eder K, Reichlmayr-Lais AM, Kirchgessner M. Studies on the extraction of phospholipids from erythrocyte membranes in rat. Clinica Chimica Acta 1993; 219:93–104. [DOI] [PubMed] [Google Scholar]
- 24. Hara A, Radin NS.Lipid extraction of tissue with a low-toxicity solvent. Analytical Biochemistry 1978; 90:420–426. [DOI] [PubMed] [Google Scholar]
- 25. Moseley K, Koch R, Moser AB. Lipid status and long-chain polyunsaturated fatty acid concentrations in adults and adolescents with phenylketonuria on phenylalanine-restricted diet. J Inherit Metab Dis.2002; 25:56–64. [DOI] [PubMed] [Google Scholar]
- 26. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989; 2:757–761. [DOI] [PubMed] [Google Scholar]
- 27. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart diease: a meta-analyis of randomized controlled trials. Am J Med. 2002; 112:298–304. [DOI] [PubMed] [Google Scholar]
- 28. Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995; 274:1363–1367. [DOI] [PubMed] [Google Scholar]
- 29. Fish consumption and mortality from coronary heart disease. N Engl J Med 1985; 313:820–824. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez BL, Sharp DS, Abbott RD, Burchfiel CM, Masaki K, Chyou PH, et al. Fish intake may limit the increase in risk of coronary heart disease morbidity and mortality among heavy smokers. The Honolulu Heart Program. Circulation 1996; 94:952–956. [DOI] [PubMed] [Google Scholar]
- 31. Angerer P, Kothny W, Störk S, Schacky von C. Effect of dietary supplementation with ω-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovascular Research 2002; 54:183–190. [DOI] [PubMed] [Google Scholar]
- 32. Sacks FM, Stone PH, Gibson M, Silverman D, Rosner B, Pasternak RC, et al. for the HARP RESEARCH GROUP.Controlled trial of fish oil for the regression of human atherosclerosis. J Am Coll Cardiol 1995; 25:1492–1498. [DOI] [PubMed] [Google Scholar]
- 33. Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer A, et al. Do fish oil prevent restenosis after coronary angioplasty? Circulation 1994; 90:2248–2257. [DOI] [PubMed] [Google Scholar]
- 34. Hooper L, Thompsen RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database of Syst. Rev. 2004; Oct 18; (: CD003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoene NW (2001). Vitamin E and omega-3 fatty acids: effectors of platelet responsiveness. Nutrition. 2001; 17:793–796. [DOI] [PubMed] [Google Scholar]
- 36. Mori TA, Beilin LJ, Burke V, Morris J, Ritchie J (1997) Interaction between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. ATVB. 1997; 17:279–286. [DOI] [PubMed] [Google Scholar]
- 37. Prisco D, Filippini M, Francalanci I, Paniccia R, Gensini GF, Serneri GG. Effect of n-3 fatty acid ethyl ester supplementation on fatty acid composition of the single platelet phospholipids and platelet functions. Metabolism. 1995; 44:562–569. [DOI] [PubMed] [Google Scholar]
- 38. Braden GA, Knapp HR, Fitzgerald DJ, Fitzgerald GA (1990) Dietary fish oil accelerates the response to coronary thrombolysis with tissue-type plasminogen activator. Evidence for a modest platelet inhibitory effect in vivo. Circulation. 1990; 82:178–187. [DOI] [PubMed] [Google Scholar]
- 39. Myrup B, Rossing P, Jensen T, Parving HH, Holmer G, Gram J, et al. Lack of effect of fish oil supplementation on coagulation and transcapillary escape rate of albuminin insulin-dependent diabetic patients with diabetic nephropathy. Scand J Clin Lab Invest. 2001; 61:435–356. [DOI] [PubMed] [Google Scholar]
- 40. Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ.Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res. 1999; 96:239–250. [DOI] [PubMed] [Google Scholar]
- 41. Nelson GJ, Schmidt PS, Bartolini GL, Kelley DS, Kyle D (1997) The effect of dietary docosahexaenoic acid on platelet function, platelet fatty acid composition and blood coagulation in humans. Lipids 32:1129–1136. [DOI] [PubMed] [Google Scholar]
- 42. McCarty MF 2004). Sub-optimal taurine status may promote platelet hyperaggregability in vegetarians. Medical Hypotheses 63:426–433. [DOI] [PubMed] [Google Scholar]
- 43. The ORIGIN Trial Investigators. N-3 Fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012; 367: 309–318. doi: 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.



