Abstract
Wnt and Netrin signaling regulate diverse essential functions. Using a genetic approach combined with temporal gene expression analysis, we found a regulatory link between the Wnt receptor MOM-5/Frizzled and the UNC-6/Netrin receptor UNC-5. These two receptors play key roles in guiding cell and axon migrations, including the migration of the C. elegans Distal Tip Cells (DTCs). DTCs migrate post-embryonically in three sequential phases: in the first phase along the Antero-Posterior (A/P) axis, in the second, along the Dorso-Ventral (D/V) axis, and in the third, along the A/P axis. Loss of MOM-5/Frizzled function causes third phase A/P polarity reversals of the migrating DTCs. We show that an over-expression of UNC-5 causes similar DTC A/P polarity reversals and that unc-5 deficits markedly suppress the A/P polarity reversals caused by mutations in mom-5/frizzled. This implicates MOM-5/Frizzled as a negative regulator of unc-5. We provide further evidence that small GTPases mediate MOM-5’s regulation of unc-5 such that one outcome of impaired function of small GTPases like CED-10/Rac and MIG-2/RhoG is an increase in unc-5 function. The work presented here demonstrates the existence of cross talk between components of the Netrin and Wnt signaling pathways and provides further insights into the way guidance signaling mechanisms are integrated to orchestrate directed cell migration.
Author Summary
Cells are exposed to a multitude of environmental cues that are often eliciting additive, overlapping, or even conflicting inputs. How the information from multiple extracellular cues is integrated within the cell to generate distinct patterning is largely unknown. Netrin and Wnt signaling pathways are critical to multiple developmental processes and play key roles in normal development, as well as in malignancies. The involvement of these two signaling pathways in establishing cellular polarity is key to their ability to determine organ shape and to regulate cell and axon migration. Here, we reveal a regulatory link between the Wnt Frizzled receptor, MOM-5, and the Netrin receptor UNC-5. We present evidence showing that MOM-5/Frizzled signals through small GTPases to negatively regulate the UNC-5 Netrin receptor. This regulatory link enables the integration of Netrin and Wnt signaling pathways and facilitates their orchestrated function in mediating polarity of cell migration.
Introduction
Cell migrations play a central role in both development and pathogenesis. However, the mechanisms underlying guided cell migration, and in particular the means by which extracellular information is integrated within the cell, are poorly understood. The C. elegans Distal Tip Cells (DTCs) provide an excellent model system to study various regulatory aspects of cell migration. In the hermaphrodite, a DTC is found at the extending tip of each of two elongating hermaphrodite gonad arms. These cells are born post-embryonically in the ventral mid-body and migrate along a stereotyped path involving 3 stages of polarized movements along A/P and D/V axes. In phase 1 the DTCs migrate away from each other along the A/P axis. In phase 2 the DTCs turn 90° and migrate along the D/V axis from the ventral to dorsal body wall muscles. In phase 3, the DTCs again turn 90° and migrate along the A/P axis back to the dorsal mid-body. This migration path determines the U shape of the two symmetrical gonad arms in wild-type animals (Fig 1A). Thus, by monitoring DTC movements in real time or gonad shapes in developed wild type and mutant hermaphrodites, insights may be gained into the regulation of sustained polarized migration over a single axis or transitions from one axis to another, which require the coordinated output of multiple guidance signaling pathways.
Fig 1. Over-expression of the UNC-6/Netrin receptor UNC-5 causes DTC phase 3 A/P polarity reversals.
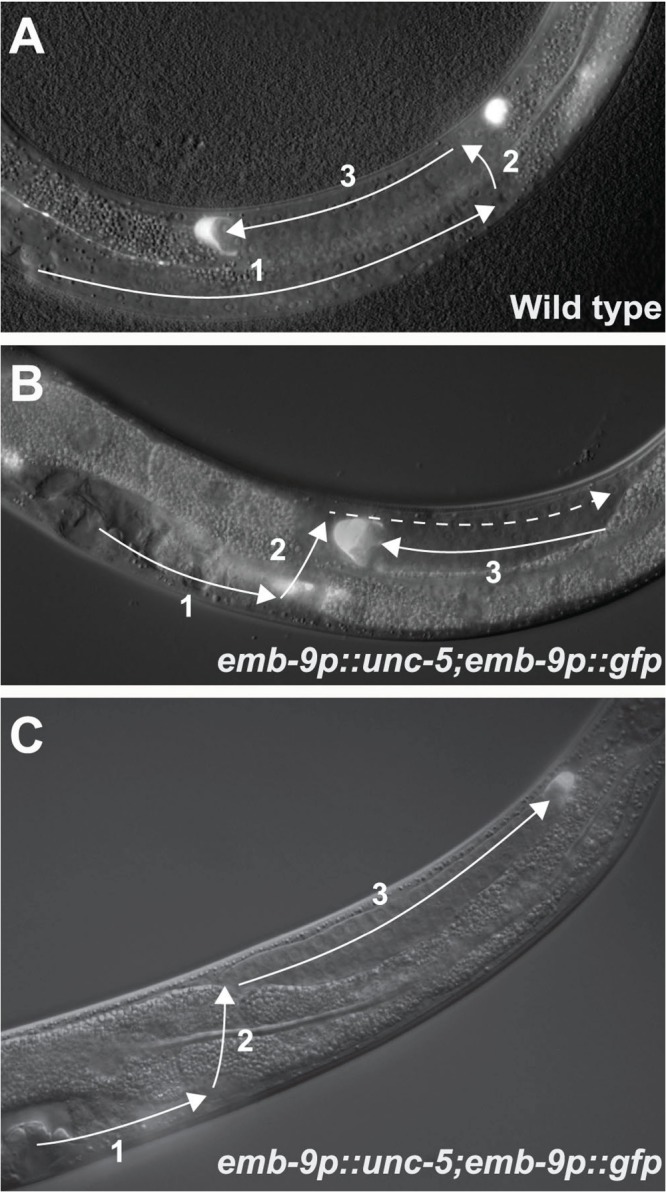
DIC images of posterior gonad arms in L4 stage hermaphrodites overlaid with florescence images of GFP labeled DTCs. A DTC is located at the tip of each gonad arm. The migratory route taken by the DTC is depicted (white arrows). In all panels anterior is left and dorsal is up. (A) In the wild type, anterior and posterior U-shaped gonad arms are formed by 3 sequential migratory phases (labeled 1, 2, 3 accordingly) of the DTCs. Only the posterior gonad arm and DTC (visualized by the gly-18p::gfp reporter) are shown. (B) In evIs129[emb-9p::unc-5(+); emb-9p::gfp] animals the anterior or posterior (shown) DTC or both frequently migrates precociously towards the dorsal side. The first migratory phase is then completed on the dorsal muscle band and with normal timing the third migratory phase is initiated, reorienting the DTC back to the mid-body (left edge of photo). Dashed line represents the gonad segment formed in phase 1 that overlaps the segment formed in phase 3. (C) In evIs129 animals, in addition to the precocious migration of the DTC towards the dorsal side, the anterior or posterior DTC (marked by the emb-9p::gfp reporter) or both frequently exhibits an A/P polarity reversal that fails to reorient back to the mid-body.
Several highly conserved genes have been found to be involved in the regulation of the different aspects of DTC migration [1]. We have discovered that UNC-6/Netrin and Wnt signaling redundantly regulate the guidance of these cells [2]. Functioning together, Netrin and Wnt signaling orchestrate the migratory transitions of the DTCs between the A/P and D/V axes [2]. Here we begin to reveal how migrating DTCs tune their response to these two signaling mechanisms at a molecular level to guide DTC migration.
The UNC-6/Netrin guidance cue and its receptors, UNC-5 and UNC-40/DCC are highly conserved in invertebrates and vertebrates and play central roles in cell and axon guidance. UNC-6 is expressed selectively at the ventral side of the developing animal [3]. This expression pattern predicts a D/V graded distribution of UNC-6 during all phases of DTC migration, which imparts to UNC-6 an ability to provide polarity information needed to guide migrations along the D/V axis of the body wall. Response to this polarity information is mediated by the UNC-5 and UNC-40 receptors.
unc-5 was found to be transcriptionally activated at the onset of phase 2 just prior to the first 90° turn of the DTC from the A/P to the D/V axis. It was also shown that multi-copy arrays of unc-5 designed to drive precocious over-expression of UNC-5 in the DTCs caused precocious ventral to dorsal migration of these cells. This precocious migration suggests that increased function of UNC-5 in the DTCs is both necessary and sufficient to drive the transition from the A/P to the D/V axis [4]. Nevertheless, the increase in unc-5 levels at the initiation of phase 2 is apparently transient as levels of an unc-5 transcriptional reporter appeared to decrease during phase 3 [4]. Here we show that interfering with the regulation of UNC-5 by over-expression causes A/P polarity reversals during the third migratory phase. This suggests that although an increase in UNC-5 levels at the beginning of phase 2 is required to induce reorientation to the D/V axis, a decrease in unc-5 function during or prior to transitioning back to the A/P axis is required for proper orientation of DTC phase 3 migration along that axis [2].
Our goal then became to identify the mechanism or mechanisms responsible for this phase 3 regulation of UNC-5. Loss-of-function (lf) mutations in several genes have been reported to induce phase 3 A/P DTC polarity reversals that are phenotypically similar to those induced by overexpressing UNC-5 in the DTCs [5–10]. Among these are genes with mammalian homologs encoding small GTPases (ced-10/rac and mig-2/rhoG) [6] and small GTPase co-regulators (such as ced-2/crkII, ced-5/dock180 and ced-12/elmo). These small GTPase regulators function together in a signaling pathway that activates CED-10/Rac [11] to regulate apoptotic cell engulfment and DTC migration [7–11]. Cabello et al., subsequently reported that this pathway is activated by MOM-5/Frizzled [5], implicating MOM-5/Frizzled in a non-canonical Wnt signaling pathway mediated by the activation of CED-10/Rac [5,12].
Our previous findings indicate that UNC-6/Netrin, Wnts, and small GTPases have shared functions in both A/P and D/V guidance, and that small GTPases can function as upstream regulators of guidance cue receptors [2,13]. We hypothesized, therefore, that MOM-5 functions through small GTPases to negatively regulate UNC-5 function in phase 3 migration. Here we show that unc-5 deficits markedly suppress the DTC phase 3 A/P polarity reversals caused by mutations in mom-5/frizzled, ced-12/elmo, ced-10/rac, and mig-2/rhoG. This suggests that MOM-5/Frizzled normally functions through small GTPases like CED-10 and MIG-2 to negatively regulate unc-5. We provide additional evidence to show that mom-5/frizzled function is not only necessary, but also sufficient to negatively regulate an unc-5-dependent DTC dorsal migration phenotype and to induce D/V axon guidance defects like those of the unc-5 null. Furthermore, consistent with its suggested role as a negative regulator of unc-5, we show that mom-5/frizzled expression is up-regulated in the DTCs when unc-5 down-regulation is apparently required to prevent DTC phase 3 A/P polarity reversals. We conclude that the UNC-5 receptor is a downstream negatively regulated target of the non-canonical Wnt signaling pathway triggered by MOM-5/Wnt activation and mediated by small GTPase signaling involving CED-12/ELMO, CED-10/Rac and MIG-2/RhoG. This reveals a previously unknown regulatory link between components of Wnt and Netrin signaling pathways and illuminates how guidance signaling mechanisms can be integrated to orchestrate directed cell migration.
Results
Over-expression of UNC-5 in the DTCs causes phase 3 A/P polarity reversals
Our previous studies demonstrated the involvement of unc-5 in A/P polarity [2,13]. We have also demonstrated that A/P polarity requires a fine balance between the functions of UNC-5 and Wnt signaling components [2]. These results led us to examine the consequences of over-expressing unc-5 in the DTCs. We analyzed DTC migrations in several transgenic lines that overexpress unc-5, including a line carrying evIs129[emb-9p::unc-5(+); emb-9p::gfp] (S1 Table), a transgenic multi-copy array that drives precocious over-expression of unc-5 in the DTCs and also labels the DTCs with GFP. As anticipated from the phenotypes of related transgenic animals [4], many evIs129 transgenic animals exhibited precocious migration of the DTCs from the ventral to the dorsal body wall muscles (Figs 1B and 2B, S2 Table). In some evIs129 animals, phase 3 migrations were executed with normal back-to-mid-body polarity (Figs 1B and 2A, S2 Table). However, in many evIs129 animals, one or both DTCs exhibited an early or mid phase 3 A/P polarity reversal. Instead of migrating to the mid-body, the DTCs of these animals turned and migrated away from mid-body toward the head (anterior DTC) or tail (posterior DTC) (Figs 1C and 2A, S2 Table).
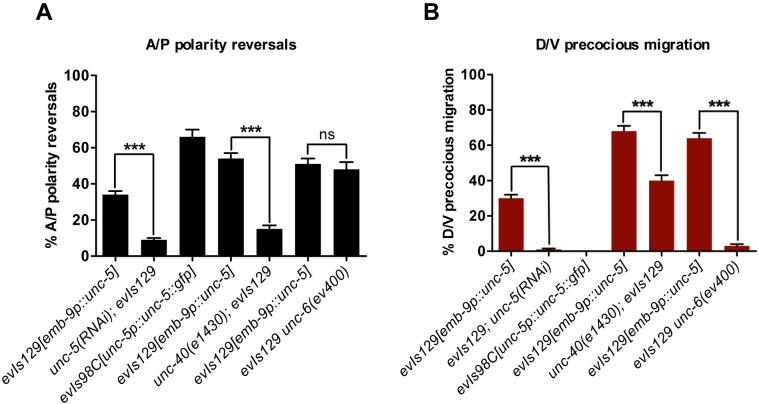
Fig 2. The evIs129[emb-9p::unc-5] phase 3 A/P polarity reversals are dependent on unc-5 and unc-40, but independent of unc-6/netrin.
(A) Quantification of phase 3 A/P polarity reversals in evIs98C (a multi-copy transgenic array of unc-5(+) regulated by its own promoter) or evIs129 (another multi-copy transgenic array of unc-5(+) driven by the emb-9 promoter) versus evIs129 treated with unc-5(RNAi) or in the background of unc-40 or unc-6 null alleles. Bars represent the percentage of posterior DTC phase 3 polarity reversals. The corresponding raw data are presented in S2 Table. Error bars indicate standard error of the sample proportion. Comparisons of the phase 3 A/P reversals were made between each corresponding pair as indicated by the connecting lines. ***P <0.00001; ns = not significant (P≥0.01). (B) Quantification of phase 2 D/V precocious migrations in the strains presented in panel (A). Bars represent the percentage of posterior DTCs that migrate precociously to the dorsal side as determined by the shape of the gonad arms. The corresponding raw data are presented in S2 Table. Error bars indicate standard error of the sample proportion. Comparisons of the D/V precocious migration were made between each corresponding pair as indicated by the connecting lines. ***P <0.00001; ns = not significant (P≥0.01). The corresponding control for RNAi was grown on empty vector RNAi feeding bacteria.
To determine if the DTC phase 3 A/P polarity reversals induced by evIs129 depend on unc-5, we reduced unc-5 levels in evIs129 animals using unc-5(RNAi). unc-5(RNAi) suppressed the defects observed in evIs129; both A/P polarity reversals (Fig 2A and S2 Table) and precocious ventral to dorsal migrations (Fig 2B and S2 Table). This demonstrates that unc-5 over-expression and, by implication, unc-5 over-activity, causes both the A/P polarity defects and the precocious D/V migration observed in evIs129 animals.
We also analyzed DTC migrations when unc-5(+) over-expression was driven by its own promoter as in the evIs98C[unc-5p::unc-5(+)::gfp] multi-copy array (S1 Table). This array is not expected to induce precocious dorsal migration of the DTCs because the endogenous promoter is only up-regulated at the phase 1 to 2 transition [4]. However, we predicted that this array could possibly over-express unc-5 at the phase 2 to 3 transition to an extent that would still induce phase 3 reversals. As anticipated, this array selectively induced phase 3 A/P polarity reversals (Fig 2A and 2B, S2 Table). These data demonstrate that phase 3 reversals occur independently of precocious D/V migration. Furthermore, they suggest that UNC-5 must be tightly regulated at initiation of both the 2nd and 3rd phases of migration to allow for normal transitions of the DTCs from one migration axis to another, and to determine the direction taken along the subsequent axis of migration.
DTC phase 3 A/P polarity reversals induced by UNC-5 overexpression depends on UNC-40/DCC but not on UNC-6/Netrin
To further illustrate the function of over-expressed unc-5 in determining A/P polarity, we examined whether unc-40 or unc-6 is required for the A/P polarity reversals observed in evIs129. We found that unc-40 deficits, but not unc-6 deficits, suppressed the DTC phase 3 A/P polarity defects caused by unc-5 over-expressing arrays (Fig 2A and S2 Table). The DTC results parallel the results we obtained previously from a related analysis performed on axonal A/P polarity reversals induced by overexpressing unc-40 in the touch receptor neurons [13]. These A/P polarity reversals were also unaffected by unc-6 mutations but were dependent on unc-5. Thus, higher than normal levels of unc-5 and/or unc-40 activity may induce DTC and ALM axon A/P polarity reversals independent of UNC-6/Netrin function, but dependent on the respective Netrin receptor, suggesting that these two receptors function together in A/P guidance as they do in D/V guidance [14–16]. This is unlike the precocious D/V migration phenotype of evIs129, which was markedly suppressed by unc-6 mutations but more refractory to the loss of unc-40 (Fig 2B and S2 Table).
MOM-5/Frizzled negatively regulates unc-5 to allow normal polarity of DTC phase 3 migration
Recently, we showed that UNC-5, like some Wnts and Wnt receptors, functions redundantly with specific Wnts while opposing the function of others to regulate phase 3 A/P polarity of the DTCs [2]. It has also been reported that mom-5/frizzled mutants exhibit DTC phase 3 A/P polarity reversals [5]. These polarity reversals are similar to the A/P polarity reversals observed when UNC-5 is over-expressed in the DTCs (Fig 1C). This evidence led us to examine the hypothesis that MOM-5/Frizzled negatively regulates unc-5 prior to or at the onset of phase 3 migration, thereby allowing normal back-to-mid-body polarity. We tested this hypothesis by reducing unc-5 function in mom-5 null mutants to determine if this would rescue the A/P polarity reversals associated with eliminating mom-5 function. We used unc-5(RNAi) to reduce UNC-5’s function in two mom-5 mutant alleles: a severe lf allele, mom-5(gk812), which is a null for DTC migration (see below), and the putative null allele, mom-5(zu193) [5]. In both mom-5 mutant strains, reducing unc-5 function caused a marked suppression of the mom-5 mutant A/P polarity reversals. Reversals decreased in the posterior DTC from a frequency of 65–85% down to 20–25% (Fig 3A and S3 Table). Significant suppression was also observed for the anterior DTC (S3 Table). Similarly, A/P polarity reversals induced by mom-5(RNAi) were markedly reduced for the posterior DTC in unc-5 mutant strains (e53 or ev489 alleles) compared to the wild type N2 strain (Fig 3A and S3 Table). This suppression indicates that the A/P polarity reversals caused by depleting mom-5 function are dependent on unc-5 function. A likely explanation for the above results is that MOM-5 normally negatively regulates unc-5 at the onset of phase 3 in order to allow for the back to mid-body reorientation of the DTCs.
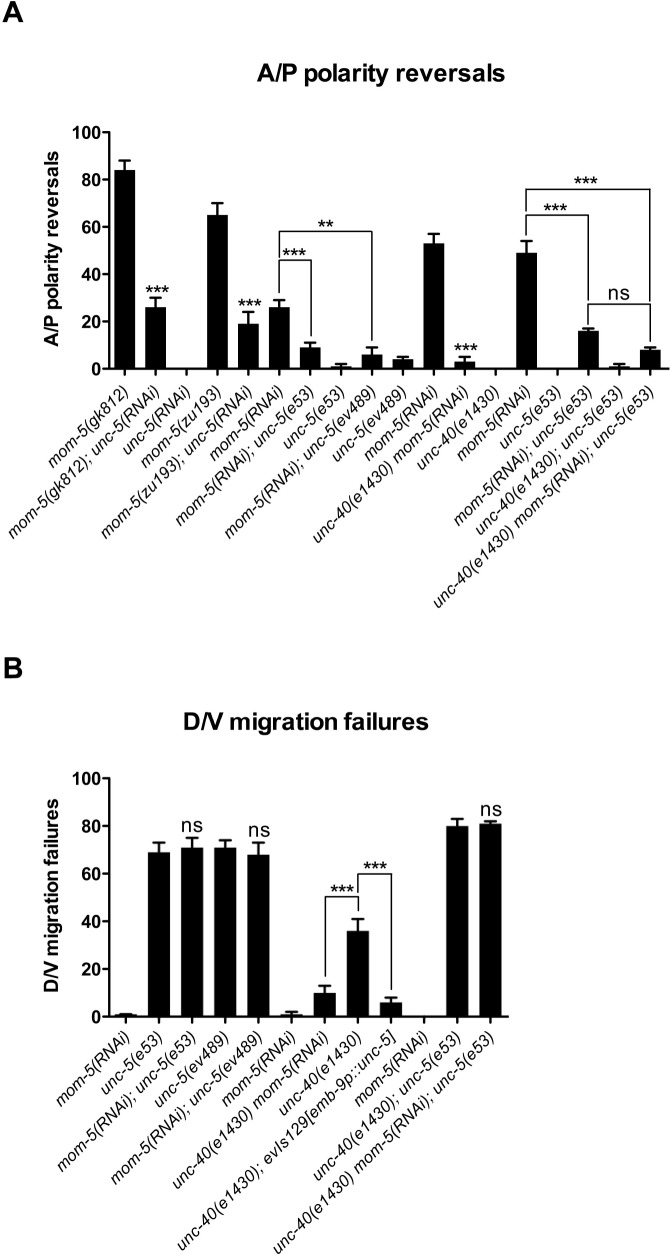
Fig 3. mom-5 mutant phase 3 A/P polarity reversals are rescued by impairing unc-5 or unc-40 function or both, while impairing mom-5 can rescue unc-40 D/V defects in a manner that is strictly dependent on unc-5 function.
(A) Percent posterior DTC phase 3 A/P polarity reversals in mom-5(gk812) or mom-5(zu193) alleles treated or not with unc-5(RNAi) plus unc-5 and unc-40 single and unc-40; unc-5 double mutants treated or not with mom-5(RNAi). (B) Percent posterior DTC phase 2 D/V migration failures in unc-5 and unc-40 single and unc-40; unc-5 double mutants treated or not with mom-5(RNAi). Also shown are the effects of evIs129[emb-9p::unc-5] on DTC phase 2 migration failures of unc-40(e1430). The corresponding raw data including the data for the anterior DTC are presented in S3 Table. Error bars indicate the standard error of the sample proportion. Comparisons of A/P polarity reversals were made between mom-5 mutant animals treated or not with unc-5(RNAi), or between mom-5(RNAi) of N2 compared to mom-5(RNAi) of Netrin receptor mutants. Comparisons of D/V defects were made between Netrin receptor mutants treated or not with mom-5(RNAi) and unc-40(e1430) in the absence or presence of evIs129. ***P <0.00001; **P<0.001; ns = not significant (P≥0.01). The corresponding control for RNAi was grown on empty vector RNAi feeding bacteria.
It should be pointed out that the use of null alleles is key to this analysis for two main reasons: First, it reduces the likelihood of sheer competition between separate MOM-5 and UNC-5 pathways on a common downstream target (one pathway activating and the other inhibiting the target) as the root cause of the observed suppression. Second, if two genes are found to function in the same pathway with one effectively inhibiting the other, null alleles help determine the hierarchy of gene action. However, mom-5 mutants are maternally rescued embryonic lethals, so homozygotes must be derived from heterozygous mothers and could therefore harbor maternally inherited wild type mom-5 function that might still contribute to DTC phase 3 migration. To examine the likelihood of this possibility, we treated wild type and mom-5 mutants with mom-5 RNAi to further deplete any possible maternal product and found that mom-5(RNAi) induced significant phase 3 A/P polarity defects in the wild type, but failed to increase the penetrance of mom-5(gk812) null mutant phase 3 defects (S1A Fig). This suggests that mom-5(gk812) is a true null for mom-5 function in DTC phase 3 migration.
If indeed a mom-5 deficit causes unc-5 over-activity one would expect unc-40 mutations to suppress the DTC A/P polarity reversals caused by a mom-5 deficit, just as these mutations suppress the A/P polarity reversals caused by overexpressing UNC-5 (Fig 2A and S2 Table). We found that an unc-40 null mutation suppressed the A/P polarity reversals induced by mom-5(RNAi) (Fig 3A and S3 Table). This suppression was as pronounced as when both unc-5 and unc-40 were depleted simultaneously compared to either single mutant (Fig 3A and S3 Table), suggesting that unc-5 and unc-40 function together in the same pathway to regulate the phase 3 A/P polarity of the DTCs.
A mom-5 deficit rescues DTC phase 2 D/V migration failures of an unc-40 null in an unc-5 dependent manner
Although unc-5 mutations markedly suppressed the A/P polarity reversals of mom-5 mutants, mom-5(RNAi) had no effect on the frequency of phase 2 D/V migration failures of unc-5(e53) or unc-5(ev489) (Fig 3B and S3 Table). However, mom-5(RNAi) rescued the posterior DTC phase 2 D/V defects of an unc-40 null mutant, but not the phase 2 D/V defects of an unc-40; unc-5 double null (Fig 3B and S3 Table). Thus, the rescue by a mom-5 deficit of unc-40 mutant phase 2 D/V guidance is strictly dependent on unc-5 function. Notably, unc-5 over-expression in the DTCs also rescued unc-40 null D/V phase 2 defects (compare unc-40(e1430) to unc-40(e1430); evIs129 in Fig 3B and S3 Table). These results support a model in which a mom-5 deficit causes an increase in unc-5 function during phase 2 migration, and that this increase in unc-5 function rescues the unc-40 null phase 2 D/V migration failures.
ced-12/elmo, ced-10/rac, and mig-2/rhoG negatively regulate unc-5 to allow normal polarity of DTC phase 3 migration
It has also been reported that mutants of mig-2/rhoG, ced-10/rac, ced-2/crk-II, ced-5/dock180 and ced-12/elmo all exhibit DTC phase 3 A/P reversals [5–9,17]. Published work by Cabello et al [5] supports the notion that MOM-5/Frizzled functions upstream of CED-10/Rac to prevent DTC phase 3 polarity reversals [5]. Furthermore, we and others reported the involvement of small GTPases as putative upstream regulators of guidance cue receptors [13,18,19]. Together, these reports raise the possibility that the activation of CED-10/Rac by CED-2,-5, and -12 negatively regulates unc-5 at the onset of phase 3 to allow for normal back-to-mid-body DTC orientation. In this case reducing unc-5 activity should suppress the DTC phase 3 A/P polarity reversals observed in mutants of ced-10/rac, mig-2/rhoG and ced-12/elmo.
ced-12 encodes the C. elegans homolog of vertebrate ELMO, which functions with CED-2/CrkII and CED-5/DOCK180 as an effective Guanine Nucleotide Exchange Factor (GEF) for CED-10/Rac [7–9,11]. We generated double mutants carrying either of two different alleles of ced-12 (e.g. k149 and n3261), plus either of two different alleles of unc-5 (e.g. ev489 and e53). In all double mutant combinations, an unc-5 mutation partially but markedly suppressed the posterior A/P polarity reversals caused by a ced-12 mutation (Fig 4A and S4 Table). Furthermore, unc-5(RNAi) reproduced this suppression (Fig 4A and S4 Table). These results are consistent with the possibility that a normal role of CED-12/ELMO, like the proposed role of MOM-5/Frizzled, is to negatively regulate the UNC-5 receptor at the initiation of or during phase 3 to allow normal polarity establishment and back-to-mid-body orientation of the DTCs.
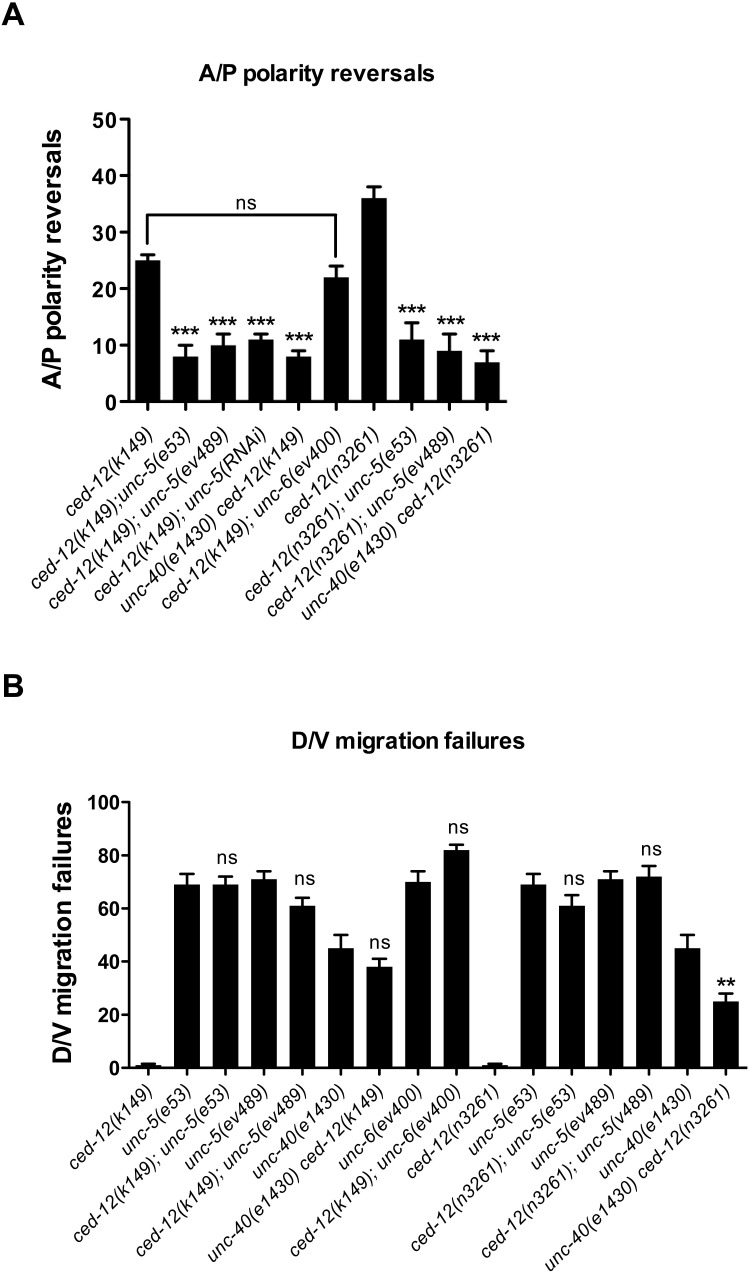
Fig 4. ced-12 mutant phase 3 A/P polarity reversals are dependent on unc-5 and unc-40, but independent of unc-6/netrin.
(A) The frequency of posterior DTC phase 3 A/P polarity reversals in single ced-12(k149) or ced-12(n3261) mutants versus double mutants of ced-12 with mutants of various UNC-6/Netrin signaling components. (B) The frequency of posterior DTC phase 2 D/V migration failures of unc-5, unc-40, and unc-6 mutants and double mutants of these with ced-12 mutations. The corresponding raw data—including the data for the anterior DTC—are presented in S4 Table. Error bars indicate the standard error of the sample proportion. Comparisons of A/P polarity reversals or D/V migration failures were made between paired single and double deficits. ***P <0.00001 **P<0.001; ns = not significant (P≥0.01).
If indeed over-activity of unc-5 is responsible for the A/P polarity reversals observed in ced-12 mutants, then these polarity reversals should, like over-expressed unc-5, depend on unc-40 but not on unc-6. To test this we generated double mutants of ced-12 with unc-40 and with unc-6 null alleles. The unc-40 null mutation suppressed the A/P polarity reversals of ced-12 mutants, whereas the unc-6 null mutation had no significant effect on these A/P polarity reversals (Fig 4A and S4 Table). These results provide further support to the hypothesis that unc-5 is effectively overactive in ced-12 mutants and that the normal function of CED-12/ELMO is to negatively regulate UNC-5 during phase 3 DTC migration. The observation that both mom-5(RNAi) and ced-12(k149) enhance the A/P polarity reversals of evIs129 (S2 Fig) is consistent with this model.
Similar to what we observed for mom-5 mutations, ced-12 mutations had no effect on the phase 2 D/V migration failures of posterior DTCs in the unc-5 mutants (Fig 4B and S4 Table) and even enhanced the anterior DTC phase 2 failures of unc-5(ev489) and unc-40(e1430) (S4 Table, see below). However, similar to what we observed for mom-5(RNAi), ced-12 mutations partially rescued the phase 2 D/V defects of the posterior DTCs in an unc-40 null mutant (Fig 4B and S4 Table). This supports a model in which a ced-12 deficit causes an increase in unc-5 function that can partially bypass the need for unc-40, just as unc-5 over-expression by multi-copy transgene arrays can partially bypass the need for unc-40 to mediate phase 2 D/V guidance. This result supports a scenario in which CED-12/ELMO, like MOM-5, functions as a negative regulator of UNC-5 expression, function, or localization.
CED-10/Rac is activated by a complex of CED-12/ELMO with CED-2/CrkII and CED-5/DOCK180 [7–9,11] and is expressed in the DTCs [6]. unc-5(RNAi) significantly suppressed A/P polarity reversals of the posterior DTC in a ced-10(n3417) severe lf mutant and the putative null mutant, ced-10(t1875) (Fig 5 and S5 Table). This suggests that ced-10/rac also functions as a negative regulator of unc-5 to determine normal polarity of DTC phase 3 migration.
Fig 5. ced-10 mutant phase 3 A/P polarity reversals are dependent on unc-5.
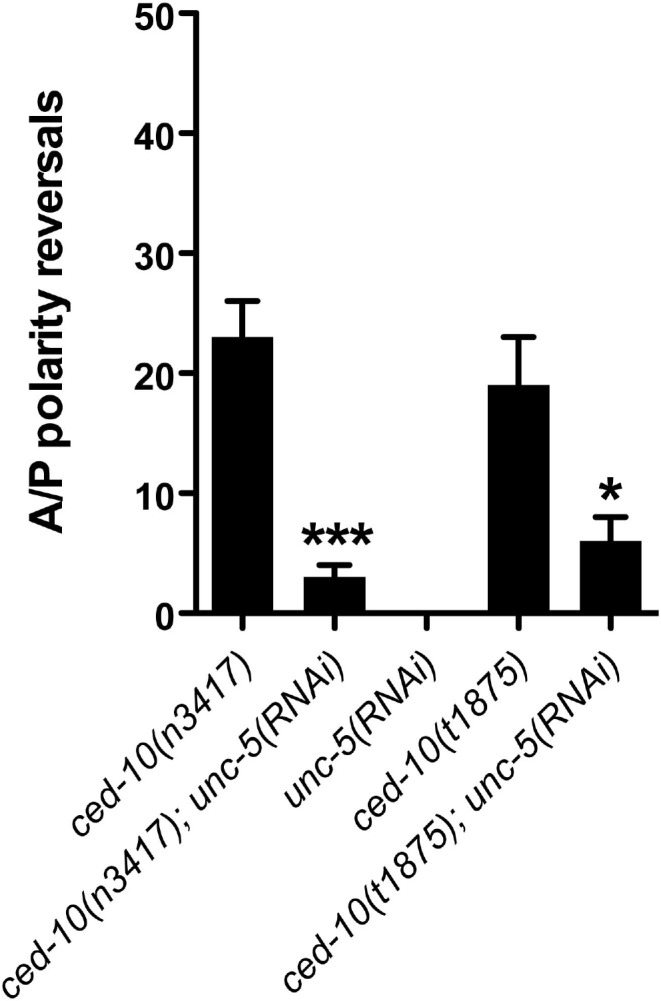
The frequency of posterior DTC phase 3 A/P polarity reversals in ced-10(t1875) or ced-10(n3417) alleles treated or not with unc-5(RNAi). The corresponding raw data including the data for the anterior DTC are presented in S5 Table. Error bars indicate the standard error of the sample proportion. Comparisons of A/P polarity reversals were made between control (empty vector) and unc-5(RNAi) treated animals. ***P <0.00001 *P<0.01; ns = not significant (P≥0.01).
We also tested the effects of unc-5 loss of function on the A/P polarity reversals of mig-2/rhoG mutants. mig-2/rhoG functions partially redundantly with ced-10/rac in other contexts [6,20,21], was implicated as an upstream regulator of CED-12/ELMO in apoptotic cell engulfment [11] and is expressed in the DTCs [22] throughout their migration (S3 Fig). Two alleles of unc-5 (e53 and ev489) and unc-5(RNAi) significantly suppressed the posterior DTC A/P polarity reversals of mig-2(mu28) null allele (Fig 6A and S6 Table). Similar to what we observed with ced-12 mutant alleles and mom-5(RNAi), the penetrance of D/V phase 2 migration failures in unc-5; mig-2 double mutants were not significantly different from those of unc-5 mutants (Fig 6B). However, mig-2(mu28) selectively enhanced the defects of the unc-5(ev489) allele in the anterior DTC (S6 Table). This recurring anterior enhancement is not entirely surprising given that the A/P polarity of anterior and posterior DTC phase 3 migrations have different requirements for Wnt signaling. Some Wnts function redundantly with UNC-5 in the context of anterior but not posterior DTC migration and thus these Wnts could utilize MIG-2, CED-12, or both to regulate phase 3 A/P polarity in anterior DTC migration [2].
Fig 6. unc-5 mutations suppress mig-2(mu28) mutant DTC phase 3 A/P polarity reversals, but mig-2(mu28) does not suppress unc-5 mutant phase 2 D/V migration failures.
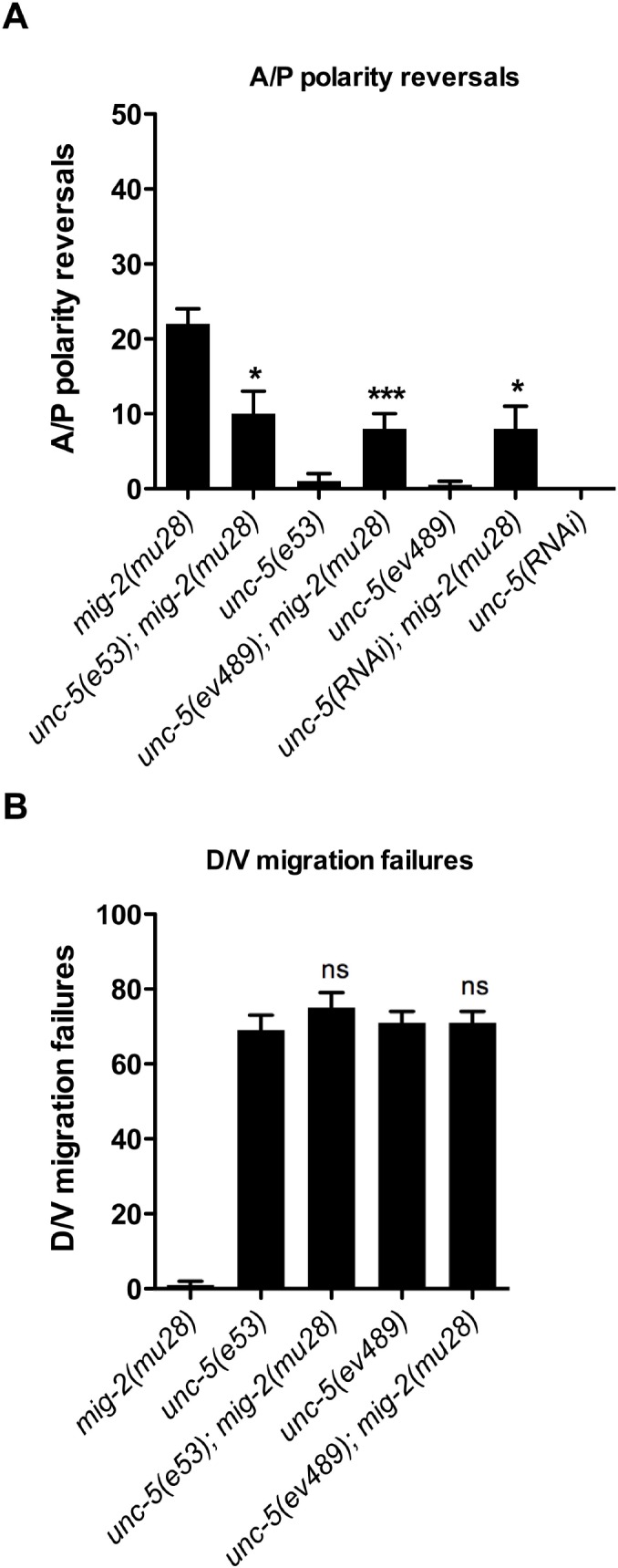
(A) The frequency of posterior DTC phase 3 A/P polarity reversals in single mig-2(mu28) mutants versus double mutants of mig-2(mu28) with various unc-5 null alleles or unc-5(RNAi). (B) The frequency of posterior DTC phase 2 D/V migration failures in double mutants of mig-2(mu28) with unc-5 (e53 and ev489 alleles) versus the respective unc-5 single mutants. The corresponding raw data, including the data for the anterior DTC, are presented in S6 Table. Error bars indicate the standard error of the sample proportion. Comparisons of A/P polarity reversals or D/V migration failures were made between paired single and double deficits. ***P <0.00001 *P<0.01; ns = not significant (P≥0.01).
A mig-2 gain-of-function mutation induces DTC D/V phase 2 failures that are rescued by a transgene array expressing unc-5(+) in the DTCs
In further support for the proposed role of small GTPases as negative regulators of the UNC-5 receptor, mig-2(gm103), a constitutively active gain-of-function (gf) allele of mig-2/rhoG [22], causes defects typical of unc-5 mutants. mig-2(gm103) worms are severely uncoordinated and display DTC migration defects, which include DTC phase 2 D/V migration defects (Fig 7C and S6 Table). The unc-5 transgene evIs129 can rescue the DTC migration defects of mig-2(gm103) animals (Fig 7D). This rescue demonstrates that the DTC defects of this mig-2(gf) allele arise from a reduction or loss of unc-5 function (S6 Table). The suppression of the DTC phenotype of the mig-2(gm103) allele by unc-5(+) overexpression leads to the same conclusion obtained by the suppression of a mig-2(lf) by an unc-5 deficit, namely, MIG-2/RhoG (like MOM-5/Frizzled, CED-12/ELMO, and CED-10/Rac) negatively regulates unc-5 to allow normal polarity of DTC phase 3 migration.
Fig 7. The constitutively active mig-2(gm103) gain-of-function allele displays unc-5 mutant-like phase 2 D/V migration failures suppressed by over-expressing unc-5(+) in the DTCs.
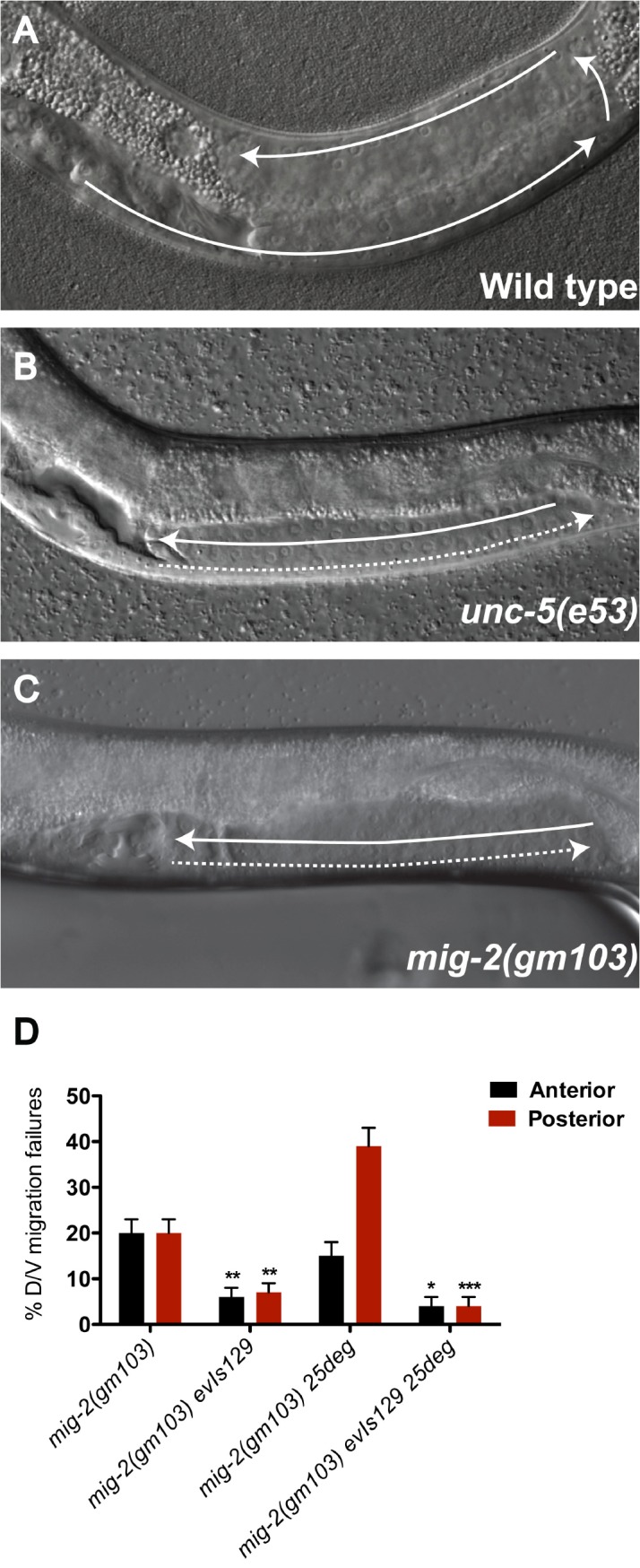
(A-C) DIC images of posterior gonad arms in L4 stage hermaphrodites. In mig-2(gm103) the anterior or posterior (shown) DTC or both (C), like unc-5(e53) (B), fails to execute the phase 2 D/V migratory phase and generates ventralized gonad arms. (D) Phase 2 failures of mig-2(gm103) are significantly suppressed by evIs129, which over-expresses unc-5 in the DTCs. The corresponding raw data, including the data for the anterior DTC, are presented in S6 Table. Error bars indicate the standard error of the sample proportion. Comparisons of phase2 D/V migration failures were made between paired mig-2(gm103) and mig-2(gm103) evIs129 doubles. ***P <0.00001; **P<0.001; *P<0.01.
Ectopic expression of mom-5(+) is sufficient to negatively regulate unc-5 in the DTCs and in neurons
The data shown in Fig 3 suggest that MOM-5 is necessary to negatively regulate unc-5 in the DTCs during their phase 3 migration. To test whether over-expressing mom-5 is sufficient to negatively regulate unc-5 in the DTCs, we used the gly-18 promoter to drive expression of a mom-5 transgene in these cells [23]. The gly-18 promoter drives expression of a GFP reporter in the DTCs beginning prior to their first phase migration and continuing to the adult stage (S4A Fig and Fig 1A). Since mom-5 expression in DTCs normally appears near the beginning of phase 2 migration (see below), the gly-18p::mom-5 transgene array is likely to cause precocious mom-5 expression during phase 1. A measure of unc-5 function during DTC phase 1 migration is provided by precocious over-expression of unc-5 in these cells (as induced by the evIs129 array). This precocious over-expression causes precocious ventral to dorsal DTC migration (Fig 2 and S2 Table) that is highly sensitive to the levels of unc-5 [4]. By creating a line carrying both arrays, we found that gly-18p::mom-5 expression suppressed the evIs129-induced unc-5-dependent precocious DTC migrations (S5 Fig). This suppression indicates that mom-5 expression in the DTCs during phase 1 migration is sufficient to negatively regulate unc-5 in these cells.
As a control, we found that mom-5 expressed from the gly-18p::mom-5 array is functional since it can largely rescue the phase 3 migration of mom-5 mutant DTCs (S1B Fig). While gly-18p::mom-5 was sufficient to rescue mom-5 mutant phase 3 polarity defects and suppress unc-5 induced precocious migration during phase 1, it was not sufficient to induce phase 2 failures. Apparently the gly-18p::mom-5 array establishes an unc-5 activity level in wild-type animals that is above the threshold for phase 2 initiation, but below the threshold for precocious unc-5 dependent ventral to dorsal migration. The apparent difference between phase 1 and phase 2 thresholds is predicted by our previous finding that unc-5 dependent ventral to dorsal migrations are greatly facilitated at the beginning of phase 2 compared to phase 1 [4], thus the above results are not unexpected.
A prevalent phenotype observed in over 30 transgenic lines carrying the gly-18p::mom-5 array was a locomotion defect. All of these transgenic lines segregated paralyzed Uncs resembling the unc-5 null uncoordinated phenotype. These Uncs exhibited motor axon D/V guidance defects like those of the unc-5 null (Fig 8). These defects were specific to the motor neurons as guidance of axons such as the ALM axons along the A/P axis was normal in these transgenic lines (Fig 8D). This suggests that while an attempt to over-express mom-5 in the DTCs was not sufficient to induce phase 2 migration failures, ectopic expression of mom-5 in other tissues was sufficient to cause D/V axon migration defects and an uncoordinated phenotype similar to that of the unc-5 null. We detect low expression of a gly-18 transcriptional reporter in the ventral nerve cord as presented in Supplemental S4B Fig, which could account for this phenotype. It should be noted that mom-5 expression is not normally detected in the motor neurons, and mom-5 null mutants are not Unc. The expression of gly-18p::mom-5 in the nervous system is therefore likely ectopic.
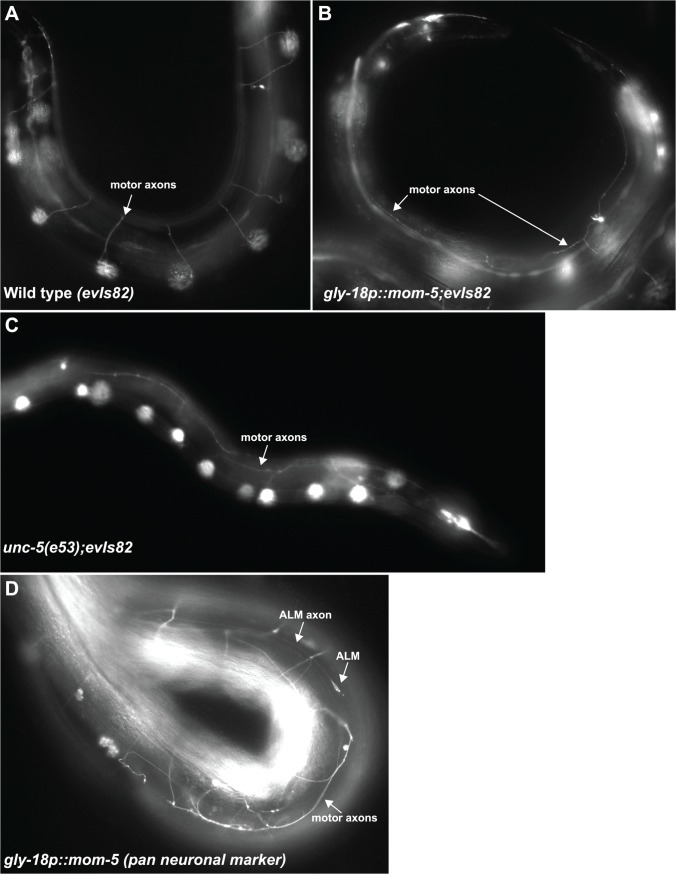
Fig 8. Expression of mom-5 driven by the gly-18 promoter induces unc-5 null-like D/V axon guidance defects of the motor axons.
Fluorescence micrographs of hermaphrodites carrying a gly-18p::mom-5 transgenic array. Anterior is left and dorsal is up. (A) In evIs82[unc-129p::gfp] gfp is expressed in the DA and DB motor neurons, which like other sets of motor neurons, normally extend their axons from the ventral to the dorsal side. The unc-5 receptor mediates the repulsion of these axons away from the ventral cord in response to the UNC-6/Netrin secreted from ventral neuroblasts. (B) In gly-18p::mom-5; evIs82 the DA and DB motor neuron axons fail to extend to the dorsal side but instead extend in an abnormal lateral position, similar to unc-5(e53) null mutant animals (C). (D) In gly-18p::mom-5 animals carrying the pan neuronal reporter rab-3p::gfp motor axons are misguided while ALM mechanosensory neuron guidance along the A/P axis is normal.
mom-5 expression in DTCs is up-regulated prior to the phase 2 to phase 3 transition
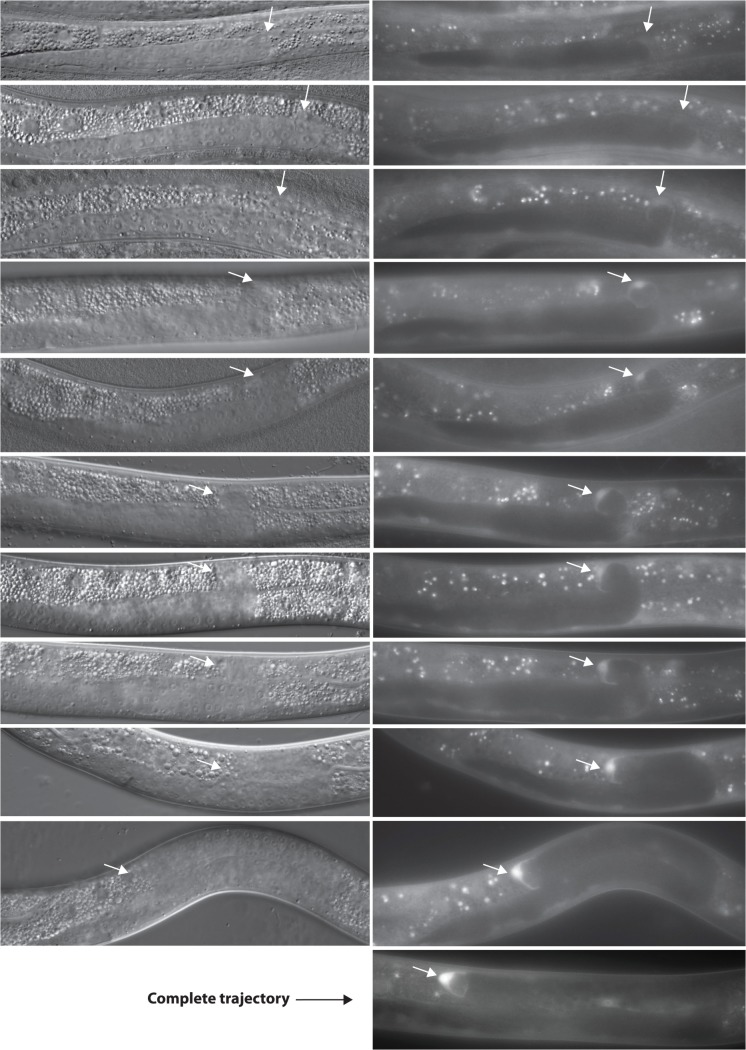
Our results suggest that unc-5 function needs to be down-regulated by MOM-5 by the time of the second DTC turn to allow proper DTC phase 3 polarity. In principle, this could be caused, among other things, by a rise in mom-5 function at or prior to the time of that turn. We therefore examined the expression of a mom-5 transcriptional GFP reporter generated as a single copy MosSCI insertion. The reporter is undetectable throughout phase 1 (S6 Fig) and begins to be faintly visible in the DTCs during phase 2 immediately after the first turn (Fig 9, top panel) then continues to increase in intensity as the turn occurs and into phase 3 (Fig 9), resulting in an overall 10-fold increase (S7 Fig and S7 Table). Thus, the expression of MOM-5 visibly increases when UNC-5 activity appears to be negatively regulated. These results are entirely consistent with the model favored by all of the above genetic data, which suggests that MOM-5 targets unc-5 for inhibition in the DTCs at or prior to the beginning of phase 3 migration and this inhibition is required for normal DTC A/P polarity during phase 3.
Fig 9. mom-5 is transcriptionally activated prior to the onset of phase 3.
DIC and fluorescence micrographs of hermaphrodites carrying an integrated mom-5p::gfp single copy transcriptional reporter (evIs462). Anterior is left and dorsal is up. Arrows mark the DTC. Sequential stages of DTC migration are shown starting at the onset of phase 2 to the end of phase 3. A single gonad arm is shown. Similar patterns of expression were observed both at the anterior and the posterior DTCs (shown). Gain and exposure time were kept constant for all images presented here. Additional images taken by confocal microscopy were quantified for different phases of DTC migration and the data are presented in S6 Fig.
Discussion
Exploring the molecular mechanisms that regulate transitions in cell migration from one migratory axis to another can help elucidate how integration of multiple signaling pathways orchestrate polarity of migrating cells, and thus could have broad implications for many aspects of development and tumor progression. In order to identify and characterize these mechanisms, we study the guided migrations of the C. elegans DTCs, which occur in 3 sequential linear phases along the A/P axis or the D/V axis. We originally discovered a role for UNC-6/Netrin signaling through UNC-5 and UNC-40/DCC receptors in the execution of the DTC phase 2 D/V migration [4,15]. Several reports have suggested that Wnt signaling regulates the A/P polarity of DTC phase 3 migration [5,24,25]. The effect of Netrin signaling on D/V migration and of Wnt signaling on A/P migrations is consistent with the D/V and A/P graded distributions of these guidance cues as these distributions can provide instructive polarity information along these respective axes. In contrast to this graded guidance cue determination of polarity, we recently found that UNC-6/Netrin and Wnt signaling have roles in guiding DTC migrations along axes orthogonal to the axes of their gradation [2]. These findings raise the possibility of cross-talk between Netrin and Wnt signaling mechanisms, which is the subject of the studies reported here. A study of unc-5 transcriptional reporters showed that these reporters are undetectable during DTC phase 1 migration and become highly visible precisely at the time of the first turn [4]. Furthermore, transgenic multicopy arrays of unc-5 designed to cause precocious unc-5 over-expression in the DTCs during phase 1, were found to induce precocious ventral to dorsal DTC migration. This demonstrates that the apparent up-regulation of unc-5 expression at the time of the first turn is largely causal for that turn [4]. Here we show that transgenic multi-copy arrays of unc-5 also induce DTC phase 3 polarity defects that are suppressed by unc-5(RNAi). This suggests that some form of down-regulation of unc-5 activity in phase 3 is necessary for proper A/P polarity of the DTCs. Together, these results suggest that UNC-5 activity levels must be tightly regulated not only for the transition from phase 1 A/P to the phase 2 D/V axis, but also for proper back-to-midbody polarity of phase 3 migrations along the A/P axis.
The effect of multi-copy arrays of unc-5 on DTC phase 3 A/P polarity is reminiscent of the effect of unc-40 multi-copy arrays designed to over-express unc-40 in the touch receptor neurons. We found that the latter arrays caused unc-5-dependent A/P polarity reversals of touch receptor axons [13]. Thus, in two different cell types, over-expression of an UNC-6/Netrin receptor causes polarity reversals for migrations (of cells or axons) that occur along the A/P axis. This raises the possibility that normal polarized migration requires signaling pathways that modulate the activity of these receptors at appropriate times during the migration process.
To identify a signaling pathway that regulates unc-5 during DTC phase 3 migration, we considered the function of the small GTPases MIG-2/RhoG, CED-10/Rac and the co-activator CED-12/ELMO, which usually functions in a complex with CED-2/CRKII and CED-5/DOCK180 [11]. This small GTPase signaling pathway is conserved in vertebrates [11,26–28] and has been shown to be required for apoptotic cell engulfment and guided DTC migrations in C. elegans [6–8,10,11,17]. Cabello et al reported that this small GTPase signaling pathway is activated by a signal from the MOM-5/Frizzled Wnt receptor [5].
When components of this non-canonical Wnt pathway (MOM-5 –CED-2,-5,-12 –CED-10) are functionally deficient, DTC phase 3 A/P polarity reversals are induced [5]. Here we report that unc-5 multi-copy arrays overexpressing UNC-5 in the DTCs [4] induce phase 3 A/P reversals that largely mimic the DTC migration defects of mom-5, ced-12, ced-10 and mig-2 mutants. These results raise the possibility that this pathway is involved in the negative regulation of unc-5 during phase 3 migration, which, as shown here, is necessary for normal DTC phase 3 A/P polarity. We examined this possibility by determining whether mom-5, ced-12, ced-10 and mig-2 mutant DTC migration defects depend on unc-5 function. We found that impairing unc-5’s function significantly suppressed phase 3 A/P polarity reversals found in mutants of mom-5 and of all the small GTPase signaling molecules tested here (ced-12/elmo, ced-10/rac and mig-2/rhoG). Furthermore, the A/P polarity reversals induced in ced-12 mutants displayed the same molecular requirements as the A/P polarity reversals induced by over-expressing unc-5, as they were dependent on unc-40/DCC, but were independent of unc-6/netrin function.
Eliminating unc-40 function could also suppress the phase 3 A/P polarity reversals caused by mom-5 deficits. This suppression was incomplete and similar in extent to the suppression caused by eliminating unc-5 function or both unc-5 and unc-40 function. These results suggest that although unc-5 and unc-40 may function redundantly to some extent to regulate phase 2 D/V migrations, they function largely interdependently to regulate phase 3 A/P migrations. Furthermore, although unc-5 and unc-40 are major targets for MOM-5/Frizzled and small GTPase regulation, other MOM-5/Frizzled targets likely exist.
The ability of unc-5 deficits to suppress phase 3 A/P polarity reversals caused by mutations of the non-canonical Wnt pathway involving mom-5/frizzled, ced-10, and ced-12 is consistent, in theory, with either of two different interpretations. One, that the MOM-5-CED-10 pathway normally negatively regulates unc-5 function, the other, that the MOM-5-CED-10 pathway functions in parallel to UNC-5, with each receptor mediating an opposite outcome (as implicated from their phenotypes i.e. a mom-5 deficit phenocopies unc-5 over-expression/activation) and the balance between their outputs is required for normal DTC polarity. Thus when one of the two pathways is compromised, a defect arises, but when both pathways are compromised, the required balance is restored. By this model, a deficit in unc-5 function could suppress a deficit in mom-5 function simply by restoring the balance between the output of these two pathways.
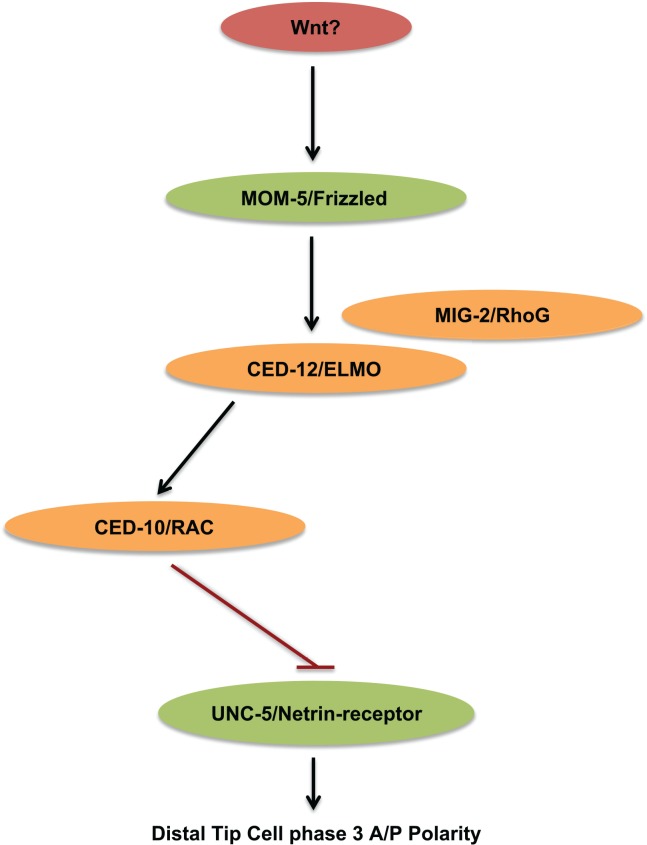
These models make different predictions about the effects of alterations in one pathway on the outcome of the other. If mom-5 and unc-5 act in parallel pathways and the balance between their outputs is required for normal DTC polarity, then one would expect the suppression to be reciprocal that is, a mom-5 deficit is expected to suppress the DTC phase 2 failures of an unc-5 null mutant just as an unc-5 deficit suppresses the phase 3 A/P polarity defects of a mom-5 null mutant. However, if mom-5 functions to negatively regulate unc-5 by functioning in the same pathway, we would expect a mom-5 deficit to cause an increase in unc-5 activity. One read out we have for unc-5 activity is its ability to induce and mediate DTC phase 2 ventral to dorsal migration. We found that a transgenic array designed to induce unc-5 over-expression in the DTCs (evIs129) could partially rescue the phase 2 defects of an unc-40 null mutant. This rescue suggests that increased unc-5 activity can largely compensate for the loss of unc-40 activity in phase 2 migration. We also found that mom-5 and ced-12 deficits rescued the DTC phase 2 D/V migration defects of an unc-40 null mutant while, in stark contrast, mom-5 deficits had no effect on the D/V defects in the unc5 null mutant or the unc-40; unc-5 double null mutant. This demonstrates that a mom-5 deficit can rescue unc-40 mutant phase 2 D/V failures, but can only do so in a manner that is strictly dependent on unc-5 activity. These results not only place mom-5 and ced-12 in the same pathway as unc-5, they are entirely consistent with a model in which mom-5 and ced-12 function upstream of unc-5 to negatively regulate its function (Fig 10). Furthermore, the effects of unc-5 and mom-5 deficits lack reciprocity; mom-5 deficit does not suppress any unc-5 mutant DTC migration defects, though unc-5 deficits suppress mom-5 mutant DTC migration defects. These results argue against the parallel pathway model and favor a model by which the Wnt receptor MOM-5/Frizzled functions through activated small GTPases to negatively regulate the UNC-5 Netrin receptor (Fig 10).
Fig 10. MOM-5/Frizzled through small GTPases functions to negatively regulate the UNC-5 receptor for UNC-6/Netrin.
Here, we provide a target for the non-canonical Wnt signaling pathway mediated by the MOM-5/Frizzled receptor, CED-12/ELMO, and CED-10/Rac [5], placing MOM-5 and small GTPases upstream of UNC-5 to negatively regulate unc-5 and determine the polarity of phase 3 DTC migration. MIG-2/RhoG had been shown to function as an activator of CED-12/ELMO by recruiting the ELMO/DOCK180 complex to the plasma membrane during engulfment of apoptotic cells [11]. It is possible that also in the DTCs MIG-2/RhoG functions upstream of CED-12/ELMO to regulate CED-10/Rac. Ligand in red, small GTPase signaling molecules in orange, and receptors in Green.
The above results suggest that mom-5, ced-12, ced-10, and mig-2 are necessary to negatively regulate unc-5 in the DTCs during phase 3 migration. However, another question of interest is whether a gain of function in any of these genes is sufficient to negatively regulate unc-5. As one example of sufficiency, we found that a mig-2 gain-of-function mutation causes an unc-5 mutant-like Unc phenotype as well as DTC phase 2 D/V migration failures. Using a transgene array to drive unc-5(+) expression in the DTCs, we were able to rescue the D/V migration defects, supporting the predicted negative regulation of unc-5 by increased MIG-2 function. We also found that a transgene array (gly-18p::mom-5) predicted to ectopically express mom-5 in the DTCs during phase 1 and possibly in the motor neurons was able to suppress an unc-5 induced precocious dorsal migration during phase 1. This array was also able to induce an unc-5 mutant-like motor axon D/V guidance defects and corresponding uncoordinated phenotype. This suggests that mom-5 function, like mig-2 function, is also sufficient to negatively regulate unc-5 in the DTCs and possibly in neurons. These gain-of-function results complement the loss-of-function studies suggesting that unc-5 is a target of negative regulation by mig-2 and mom-5 function. These results further support a model by which MOM-5/Frizzled signal through MIG-2/RhoG and CED-10/Rac to negatively regulate unc-5 during phase 3 migration to prevent DTC phase 3 polarity reversals.
The rescue of the unc-40 null D/V defects by impairing mom-5 function demonstrates that mom-5 is functional during phase 2. The fact that mom-5 mutations do not normally affect phase 2 DTC migrations (during which unc-5 expression is already up-regulated), suggests this effect is functionally negligible for phase 2. Instead, mom-5 becomes functionally relevant for phase 3 to ensure that high levels of unc-5 function are restricted to phase 2. These effects of MOM-5/Frizzled on UNC-5 function during phase 2 and phase 3 suggest that MOM-5 expression and/or activity levels should begin to increase possibly as early as phase 2 and continue to increase in phase 3 of DTC migration to allow for more robust inhibition of unc-5. In full accordance with mom-5’s demonstrated role as a negative regulator of unc-5 function in the execution of phase 2 and in determining the A/P polarity of phase 3 migrations, we found that mom-5 expression is developmentally up-regulated beginning in phase 2 and further increases in phase 3. This regulation of mom-5 is temporally correlated, therefore, with its proposed role as a negative regulator of unc-5.
We performed several experiments to explore further the mode of regulation imposed by MOM-5/Frizzled on unc-5. We examined relative levels of a GFP-tagged UNC-5 translational reporter in the wild type compared to mom-5(RNAi) or to ced-12 null alleles, but observed no obvious changes in GFP abundance or localization within the DTCs in animals compromised for mom-5 or ced-12 function. A caveat to these experiments is that the UNC-5::GFP transgenic line overexpresses UNC-5, so a further increase in UNC-5 levels might be masked by this over-expression. However, an increase in UNC-5 activity need not be mediated by an increase in UNC-5 protein expression. There are numerous post-translational means by which MOM-5 could in principle regulate UNC-5’s activity, such as by effects on protein modification like phosphorylation [29,30] or membrane localization. Small GTPases are well known to carry diverse functions. Some of the ascribed functions of small GTPases are to regulate actin dynamics in response to membrane receptors [12,31], however GTPases are also implicated in membrane remodeling and endocytic trafficking of membranal proteins [32–37]. Our use of null alleles places the small GTPases upstream of the UNC-5 receptor and implicate an upstream regulatory role that could potentially align with their proposed function in trafficking. There is some precedence for the latter possibility since both small GTPases and Wnt signals have been found in vertebrates to regulate endocytic recycling of cell adhesion receptors [33–36,38] while in C. elegans CED-12/ELMO and CED-10/Rac were also found to be involved in endoctyic recycling in the intestinal epithelium [37]. These results raise the possibility that the MOM-5—CED-2,-5,-12—CED-10 signaling pathway, possibly in response to one or more A/P graded external cues, is required to regulate UNC-5 by altering endocytic trafficking of this receptor in the DTCs in a manner that allows normal phase 3 DTC polarity. It should prove interesting to further explore this possibility in the future.
Complex networks of Wnt signaling regulate multiple polarized migrations in C. elegans. At times, like in the case of the C. elegans Q neuroblast migration, which migrate in opposite directions with left-right asymmetry, the Wnt signaling pathway that is activated is determined intrinsically [39]. While in other cases a fine balance between various Wnt signaling pathways is crucial for normal patterning [40,41]. We previously found that DTC phase 3 A/P polarity is highly sensitive to the balance between opposing Wnt signaling pathways [2]. Similarly, a balance between various Wnt signals has been found to determine the mirror image division polarities of the vulval precursor cells (VPC) [40], as well as various neuronal migrations [41] suggesting this balance is a common theme in polarity determination in spite of the differences in specific Wnt signals involved in different cell types. It would be interesting to further explore how the mom-5/unc-5 pathway interplays with other Wnt signaling pathways to determine the A/P polarity of the DTCs and consequently generate the mirror image symmetry of the hermaphrodite gonad.
unc-5 activity levels seem crucial for the normal manifestation of the different phases of DTC migration. Depending on the context, overexpression as presented here, or loss of function of unc-5 (in certain Wnt signaling deficient strains) [2], can contribute to A/P polarity reversals. This is very typical of Wnt signaling components. We speculate the UNC-5 might function as a shared module between Wnt signaling and Netrin signaling such that its activity levels relay the readout of both signaling pathways. The regulatory link between MOM-5/Frizzled and UNC-5 presented in this study illuminates how the joint action of Wnt and Netrin signaling is coordinated such that the two signaling pathways manifest an efficient, orchestrated, regulation of polarity formation. We further show that small GTPases can mediate cross talk between these two signaling pathways and propose that small GTPases can function to link other signaling pathways in order to integrate the information from multiple extracellular cues and generate a specific desired outcome in a coordinated manner. The proposed regulatory role of small GTPases presented here also suggests that an outcome of the deregulated activity of small GTPases, which frequently occurs in tumor progression, can consequently result in deregulation of the Netrin receptors. Netrin receptor functions have been implicated in tumorigenic processes such as angiogenesis [42,43], apoptosis, and cell invasion [44–46], thus suggesting UNC-5 or UNC-40/DCC as targets for cancer therapy that might be less deleterious to normal cells than targeting small GTPases. Taken together, the data presented in this study identifies small GTPases as regulatory links allowing for cross talk between Wnt and Netrin signaling pathways, which play central roles in normal development and human disease.
Materials and Methods
Nematode culture
Standard procedures were used for the culture, maintenance and genetic analysis of C. elegans [47]. All strains were grown at 20°C for analysis, unless indicated otherwise. Mutant strains and transgenic lines used in this study are listed in S1 Table. Strains not isolated in our laboratory were obtained from the Caenorhabditis Genetics Center (University of Minnesota), or as indicated in the Acknowledgments section. When necessary, double mutants were verified by PCR; primers are listed in S1 Table.
Transgenic lines
NW1501 evIs129 was generated by co-injecting PJJ442 (emb-9p::gfp, a kind gift from J.M Kramer) and pSU16 emb-9p::unc-5 [4] into N2 and integrated by γ-irradiation. This line was outcrossed 6 times prior to the analysis.
NW2354 evIs402 was generated by MosSCI single copy insertion into the oxTi444 locus on LGIII using the EG8080 universal MosSCI insertion strain [48]. Detailed protocol is posted on Wormbuilder web page: http://www.wormbuilder.org/test-page/protocol/.
The plasmid used for the MosSCI insertion (pZH304) was generated by cloning- 2.8kb of the mom-5 5’UTR fused to GFP- into the pCFJ350 vector to generate a mom-5 transcriptional reporter.
The gly-18p::mom-5 transgene was generated by PCR fusion of the gly-18 promoter amplified from the pAK93-1 (a kind gift from Aldis Krizus) and a mom-5 genomic fragment amplified from N2 worm lysates. Primers: gly-18A: agtgggcatctttaaaggtagaa;
gly-18A*: gaggatccccatctacaatga;
gly-18_mom-5: cgtatcaagtttttaaataattatttttaaaatttcagATGCATCGACATATTCTGATATTAT
mom-5C: atgcatcgacatattctgatattat; mom-5D: tcataactctaaattcgagacaaag;
mom-5R1:tccgattggctcacattcaca; mom-5R2: gaaatcgagggatgtgctcg
A mix of independently generated PCR fusion products was injected into N2 and VC1848 together with a mix of co-injection markers (Pmyo-2::mCherry (pCFJ90); Pmyo-3::mCherry (pCFJ104) with or without Prab-3::mCherry (pGH8) to generate multiple extrachromosomal array lines (selected lines are listed S1 Table).
RNA interference (RNAi)
unc-5(RNAi) constructs were generated by cloning a 574bp EcoRI fragment spanning nucleotides 563–1137 of unc-5 into the pPD129.36 L4440 vector [49]. In vitro transcribed RNA (Ambion MEGAscript kit) was then injected into young adult hermaphrodites by standard procedures. Bacterial strains for mom-5(RNAi) (gene name: T23D8.1 Source Bioscience Location: I-5A13) were obtained from the C. elegans RNAi library [50]. RNAi by feeding was carried out by standard procedures [51] and compared to the respective control strains grown on the RNAi feeding bacteria HT115(DE3) harboring an empty L4440 vector. In both cases F1 progeny of the RNAi treated worms were analyzed as L4 larvae or adults.
Generation of synchronized populations
Gravid adults were bleached using 1:5 NaoCl and 0.25N KOH and monitored. Once most embryos were released, the suspension was forced through a 23 gauge needle onto a 45–52μm Nitrex screen. The embryo preparation was then washed 4–5 times with M9 buffer, re-suspended in M9 buffer and incubated overnight at 20°C. Hatched L1 larvae were then plated for further analysis.
Microscopy
DTC migration patterns were scored by mounting 1mM levamisole-treated animals (L4 or adult stage) on 2% agarose pads for observation using Differential Interference Contrast (DIC) and fluorescence microscopy (Leica DMRA2 or DMRB microscope). Indicated strains carried the gly-18p::gfp transgene to mark the DTCs. gly-18p::gfp rarely affects D/V or A/P guidance of the DTC [2]. The A/P polarity reversal phenotype is highly dependent on the incubation temperature. A slight temperature drop below 20°C can result in significantly lower penetrance of the A/P reversals while temperatures above 20°C increase the penetrance of these defects considerably. Care was taken to analyze all comparable strains under the same growth conditions. Therefore, a control strain grown under the same conditions was included in each set of experiments. Data from several independently generated lines were analyzed and the data pooled.
Quantification of the mom-5p::gfp signal
Confocal microscopy (C2+ on a Nikon Eclipse NI-E, 60x 1.4NA) was used for the quantification of the mom5p::gfp signal in order to separate the DTC signal from intestinal mom-5p::gfp signal. Acquisition parameters were set to ensure that measurements fell within the linear range and the conditions by which the images were acquired were kept constant across samples. The mom5p::gfp fluorescence intensity in different developmental stages was measured as pixel intensity values sampling a fixed size circle drawn around the nucleus of the DTCs in a single plane which was chosen using DIC optics through the axial center of the nucleus.
Statistical analysis
Standard errors of the proportion (SE) were calculated assuming a binomial distribution of the observed proportion and the actual sample size. Statistical tests were carried out using a standard (two-tailed) comparison of two proportions (Z test). All P values represent the probability that the measured frequency of the phenotype is the same for the two strains being compared. A P-value of less than 0.01 is considered significant. All comparisons described as significant in the Results section were based on this criterion.
Supporting Information
(A) Red (anterior DTCs) and black (posterior DTCs) bars represent the percentage of phase 3 polarity reversals in mom-5(RNAi) treated animals and mom-5(gk812) maternally rescued homozygotes treated or not with mom-5(RNAi). The corresponding raw data are presented in S8 Table. Error bars indicate standard error of the sample proportion. ns = not significant (P≥0.01). mom-5(RNAi) was effected by feeding animals balanced for the maternal effect lethal gk812 allele. Controls were grown on empty vector feeding bacteria. The experiment was carried out at 25°C to maximize the efficacy of the RNAi. (B) Quantification of phase 3 A/P polarity reversals in mom-5(gk812) maternally rescued homozygotes and mom-5(gk812) homozygotes carrying the gly-18p::mom-5(+) array. Bars represent the percentage of phase 3 polarity reversals for anterior DTCs (red bars) and for posterior DTCs (black bars). Error bars indicate standard error of the sample proportion. ***P <0.00001.
(EPS)
Bars represent the percentage of phase 3 polarity reversals for anterior DTCs (red bars) and for posterior DTCs (black bars). The corresponding raw data are presented in S9 Table. Error bars indicate standard error of the sample proportion. *P<0.01; **P<0.001; ns = not significant (P≥0.01). mom-5(RNAi) was effected by feeding animals balanced for the maternal effect lethal gk812 allele. Controls for RNAi were grown on empty vector feeding bacteria. A slight temperature drop below 20 degrees can result in significantly lower penetrance of the A/P reversals while temperatures above 20 degrees increase the penetrance of these defects considerably. Care was taken to analyze all comparable strains under the same growth conditions; therefore, a control strain grown under the same conditions was included in each set of experiments.
(EPS)
Fluorescence photomicrographs of hermaphrodites carrying muIs27, an integrated mig-2p::mig-2::gfp transgene array [22]. Anterior is left and dorsal is up. Arrows mark the DTC. Asterisk marks the vulva. Developmental stage is indicated on the right. L1-L4 represent larval stages preceding the adult stage. In L1 and the adult stages, both DTCs are shown. In L2 and L4 only the posterior DTC is shown.
(EPS)
DIC (A) and fluorescence photomicrographs (A’) and an overlay of the two (A”) of L1/L2 hermaphrodites carrying dnIs13[gly-18p::gfp], an integrated gly-18 transcriptional transgenic reporter array. Anterior is left and dorsal is up. Arrows mark the DTCs. (B) Fluorescence photomicrographs of adult hermaphrodite carrying dnIs13[gly-18p::gfp]. Anterior is left and dorsal is up.
(EPS)
Bars represent the percentage of DTCs that migrate precociously to the dorsal side in synchronized populations analyzed 24 hours after plating of L1 larva grown at 25°C. Red fluorescence, RFP(+), indicates the presence of the gly-18p::mom-5 extrachromosomal transgenic array that was created by co-injecting a mix of mCherry markers (S1 Table). These segregated some RFP(-) hermaphrodites that lacked the gly-18p::mom-5 array, which served as the evIs129 control. Error bars indicate standard error of the sample proportion. ***P <0.00001; ns = not significant (P≥0.01).
(EPS)
Upper panels: DIC and fluorescence micrographs of hermaphrodites carrying an integrated mom-5p::gfp single copy insertion (evIs462). Anterior is left and dorsal is up. Arrows mark the DTC. Sequential stages of DTC migration are shown starting at the L1 stage to the L3 to L4 stage (end of phase 1). Except for L1 and L2 only a single gonad arm is shown. For evIs462 no GFP is visible in the DTCs (gain and exposure times are identical to those of images presented in Fig 9). Lower panels: DIC and fluorescence micrographs of hermaphrodites carrying an integrated unc-5p::unc-5gfp transgene array (evIs98C). DTC GFP expression (arrows) is first visible just before or during the first DTC turn.
(EPS)
The average pixel intensity of mom-5p::gfp expression in the DTC is represented in several stages throughout DTC migration. The corresponding one way ANOVA column statistics data are presented in S7 Table.
(EPS)
(DOCX)
1DTC migration patterns were analyzed by DIC and fluorescence optics of anterior and posterior DTCs in L4 larvae or adults. ***P<0.00001; nsP≥0.01 n = number of gonad arms scored (anterior and posterior combined). SE = standard error of the proportion. 2The penetrance of defects in the evIs129 transgenic line was highly temperature sensitive and variable. Care was taken to analyze the respective control for each experiment grown under the same conditions. 3Grown on empty vector RNAi feeding bacteria.
(DOCX)
1DTC migration patterns were analyzed for anterior and posterior DTCs by DIC and fluorescent optics in L4 larvae or adults. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. 2 mom-5(gk812) and mom-5(zu193) are maternal effect embryonic lethal mutations. Balanced heterozygotes were injected with unc-5(RNAi) and the mutant homozygous progeny were analyzed for DTC migration defects; gk812 were identified as non-gfp, zu193 identified as Unc (S1 Table). 3 unc-5(RNAi) was introduced by ds RNA injection into the different strains, the D/V migration defects in the unc-5(RNAi) set of experiments reflect the efficacy of the RNAi treatment, which due to the method of delivery can be variable from strain to strain. 4 mom5(RNAi) was administered by feeding to bypass the associated embryonic lethality. This also allowed comparison of the RNAi induced defect across all strains, all grown on the same RNAi feeding bacteria under the same growth conditions.
(DOCX)
1DTC migration patterns for anterior and posterior DTCs were analyzed by DIC and fluorescence optics in L4 larvae or adults. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. The A/P polarity reversal phenotype is highly temperature sensitive. Care was taken to analyze a respective control grown under the same incubation conditions for each set of experiments.
(DOCX)
1DTC migration patterns of anterior and posterior DTCs were analyzed by DIC and florescence optics in L4 larvae or adults. ***P<0.00001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. 2D/V guidance defects result from impairing unc-5 function and reflect the efficacy of the unc-5(RNAi) in the population. 2 ced-10(n3417) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were injected with unc-5(RNAi) and the ced-10(n3417) homozygous progeny (identified as non-dpy) were analyzed for DTC migration defects. 3 ced-10(t1875) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were injected with unc-5(RNAi) and the ced-10(t1875) homozygous progeny identified as GFP(-) were analyzed for DTC migration defects.
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC and florescence optics in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. 2It should be noted that we refrained from comparing the penetrance of D/V migration failures when unc-5(RNAi) was used. unc-5(RNAi) was delivered by injection; the efficacy of the RNAi treatment can vary between the injected strains. The percent D/V defects in the unc-5(RNAi) set of experiments reflect the efficacy of the RNAi. 3 mig-2(gm103) evIs129/+ were used. Only the emb-9p::gfp positive animals were included in the analysis; these may have been either heterozygous or homozygous for evIs129.
(DOCX)
One way ANOVA column statistics.
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. nsP≥0.01. 2 mom-5(gk812) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were fed with mom-5(RNAi) and the mom-5 mutant homozygous progeny were analyzed for DTC migration defects; the balancer chromosome is marked with gfp hence gk812 homozygotes were identified as non-gfp (S1 Table).
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. **P<0.001; *P<0.01; nsP≥0.01 2RNAi was introduced by feeding; controls were grown on empty vector feeding bacteria. 3The penetrance of defects in the evIs129 transgenic line was highly temperature sensitive and variable. Care was taken to analyze the respective control for each experiment grown under the same conditions.
(DOCX)
Acknowledgments
The authors would like to thank Wendy Johnston and Aldis Krizus for providing the DE60 strain and the pAK93-1 plasmid. Some strains were provided by the Caenorhabditis Genetics Center. Thanks to Jim Kramer for providing the pJJ442 plasmid, Aldis Krizus for reagents and technical advice, Dan Strumpf and Wendy Johnston for critical reading and helpful comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Canadian Institutes of Health Research http://cihr-irsc.gc.ca/e/193.html Operating grants MOP 119505, MOP 13207 and MOP 77722 to JGC, the Canadian Institutes of Health Research http://cihr-irsc.gc.ca/e/193.html Group/team grant GMH 79044 to JGC, and by the March of Dimes http://www.marchofdimes.org/research-grants.aspx Operating grant MOD 1-FY08-448. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wong M-C, Schwarzbauer JE (2012) Gonad morphogenesis and distal tip cell migration in the Caenorhabditis elegans hermaphrodite. Wiley Interdiscip Rev Dev Biol 1: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy-Strumpf N, Culotti JG (2014) Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in C. elegans. PLoS Genet 10: e1004381 10.1371/journal.pgen.1004381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wadsworth WG, Bhatt H, Hedgecock EM (1996) Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16: 35–46. [DOI] [PubMed] [Google Scholar]
- 4. Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, et al. (2000) Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development 127: 585–594. [DOI] [PubMed] [Google Scholar]
- 5. Cabello J, Neukomm LJ, Günesdogan U, Burkart K, Charette SJ, et al. (2010) The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol 8: e1000297 10.1371/journal.pbio.1000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lundquist E a, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI (2001) Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development 128: 4475–4488. [DOI] [PubMed] [Google Scholar]
- 7. Gumienny T, Brugnera E (2001) CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107: 27–41. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Z, Caron E, Hartwieg E, Hall a, Horvitz HR (2001) The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell 1: 477–489. [DOI] [PubMed] [Google Scholar]
- 9. Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY (2001) C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 1: 491–502. [DOI] [PubMed] [Google Scholar]
- 10. Reddien P, Horvitz H (2000) CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol 2: 131–136. [DOI] [PubMed] [Google Scholar]
- 11. Côté J-F, Vuori K (2007) GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 17: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J (2013) Multiple functions of the noncanonical Wnt pathway. Trends Genet 29: 545–553. 10.1016/j.tig.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 13. Levy-Strumpf N, Culotti JG (2007) VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci 10: 161–168. [DOI] [PubMed] [Google Scholar]
- 14. Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, et al. (1996) UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195. [DOI] [PubMed] [Google Scholar]
- 15. Hedgecock EM, Culotti JG, Hall DH (1990) The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85. [DOI] [PubMed] [Google Scholar]
- 16. Rajasekharan S, Kennedy TE (2009) The netrin protein family. Genome Biol 10: 239 10.1186/gb-2009-10-9-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Y-C, Cheng T-W, Lee M-C, Weng N-Y (2002) Distinct Rac Activation Pathways Control Caenorhabditis elegans Cell Migration and Axon Outgrowth. Dev Biol 250: 145–155. [DOI] [PubMed] [Google Scholar]
- 18. Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G (2007) C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci 10: 169–176. [DOI] [PubMed] [Google Scholar]
- 19. Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, et al. (2009) C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development 136: 1201–1210. 10.1242/dev.026666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dalpé G, Zhang LW, Zheng H, Culotti JG (2004) Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development 131: 2073–2088. [DOI] [PubMed] [Google Scholar]
- 21. Kishore RS, Sundaram M V (2002) ced-10 Rac and mig-2 function redundantly and act with unc-73 trio to control the orientation of vulval cell divisions and migrations in Caenorhabditis elegans. Dev Biol 241: 339–348. [DOI] [PubMed] [Google Scholar]
- 22. Zipkin ID, Kindt RM, Kenyon CJ (1997) Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell 90: 883–894. [DOI] [PubMed] [Google Scholar]
- 23. Warren CE, Krizus a, Dennis JW (2001) Complementary expression patterns of six nonessential Caenorhabditis elegans core 2/I N-acetylglucosaminyltransferase homologues. Glycobiology 11: 979–988. [DOI] [PubMed] [Google Scholar]
- 24. Schwabiuk M, Coudiere L, Merz DC (2009) SDN-1/syndecan regulates growth factor signaling in distal tip cell migrations in C. elegans. Dev Biol 334: 235–242. 10.1016/j.ydbio.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 25. Nishiwaki K (1999) Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics 152: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geisbrecht ER, Haralalka S, Swanson SK, Florens L, Washburn MP, et al. (2008) Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev Biol 314: 137–149. 10.1016/j.ydbio.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu M, Ravichandran KS (2006) Dock180-ELMO cooperation in Rac activation. Methods Enzymol 406: 388–402. [DOI] [PubMed] [Google Scholar]
- 28. Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, et al. (1998) Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev 12: 3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Killeen M, Tong J, Krizus A, Steven R, Scott I, et al. (2002) UNC-5 Function Requires Phosphorylation of Cytoplasmic Tyrosine 482, but Its UNC-40-Independent Functions also Require a Region between the ZU-5 and Death Domains. Dev Biol 251: 348–366. [DOI] [PubMed] [Google Scholar]
- 30. Tong J, Killeen M, Steven R, Binns KL, Culotti J, et al. (2001) Netrin stimulates tyrosine phosphorylation of the UNC-5 family of netrin receptors and induces Shp2 binding to the RCM cytodomain. J Biol Chem 276: 40917–40925. [DOI] [PubMed] [Google Scholar]
- 31. Norris AD, Lundquist E a (2011) UNC-6/netrin and its receptors UNC-5 and UNC-40/DCC modulate growth cone protrusion in vivo in C. elegans. Development 138: 4433–4442. 10.1242/dev.068841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, et al. (2008) Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development 135: 2855–2864. 10.1242/dev.024133 [DOI] [PubMed] [Google Scholar]
- 33. Ridley a J (2001) Rho proteins: linking signaling with membrane trafficking. Traffic 2: 303–310. [DOI] [PubMed] [Google Scholar]
- 34. Ellis S, Mellor H (2000) Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol 10: 85–88. [DOI] [PubMed] [Google Scholar]
- 35. Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG (2008) Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 320: 365–369. 10.1126/science.1151250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ulrich F, Krieg M, Schötz E-M, Link V, Castanon I, et al. (2005) Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9: 555–564. [DOI] [PubMed] [Google Scholar]
- 37. Sun L, Liu O, Desai J, Karbassi F, Sylvain M-A, et al. (2012) CED-10/Rac1 regulates endocytic recycling through the RAB-5 GAP TBC-2. PLoS Genet 8: e1002785 10.1371/journal.pgen.1002785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, et al. (2008) Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134: 135–147. 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 39. Middelkoop TC, Korswagen HC (2014) Development and migration of the C. elegans Q neuroblasts and their descendants: 1–23. 10.1895/wormbook.1.173.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green JL, Inoue T, Sternberg PW (2008) Opposing Wnt pathways orient cell polarity during organogenesis. Cell 134: 646–656. 10.1016/j.cell.2008.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zinovyeva AY, Yamamoto Y, Sawa H, Forrester WC (2008) Complex network of Wnt signaling regulates neuronal migrations during Caenorhabditis elegans development. Genetics 179: 1357–1371. 10.1534/genetics.108.090290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freitas C, Larrivée B, Eichmann A (2008) Netrins and UNC5 receptors in angiogenesis. Angiogenesis 11: 23–29. 10.1007/s10456-008-9096-2 [DOI] [PubMed] [Google Scholar]
- 43. Mehlen P, Furne C (2005) Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol Life Sci 62: 2599–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimizu A, Nakayama H, Wang P, König C, Akino T, et al. (2013) Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem 288: 2210–2222. 10.1074/jbc.M112.397398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akino T, Han X, Nakayama H, McNeish B, Zurakowski D, et al. (2014) Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Cancer Res 74: 3716–3726. 10.1158/0008-5472.CAN-13-3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morrissey M a, Hagedorn EJ, Sherwood DR (2013) Cell invasion through basement membrane: The netrin receptor DCC guides the way. Worm 2: e26169 10.4161/worm.26169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, et al. (2014) Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat Methods 11: 529–534. 10.1038/nmeth.2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Timmons L, Fire a (1998) Specific interference by ingested dsRNA. Nature 395: 854 [DOI] [PubMed] [Google Scholar]
- 50. Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, et al. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330. [DOI] [PubMed] [Google Scholar]
- 51. Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2: RESEARCH0002.1–0002.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Red (anterior DTCs) and black (posterior DTCs) bars represent the percentage of phase 3 polarity reversals in mom-5(RNAi) treated animals and mom-5(gk812) maternally rescued homozygotes treated or not with mom-5(RNAi). The corresponding raw data are presented in S8 Table. Error bars indicate standard error of the sample proportion. ns = not significant (P≥0.01). mom-5(RNAi) was effected by feeding animals balanced for the maternal effect lethal gk812 allele. Controls were grown on empty vector feeding bacteria. The experiment was carried out at 25°C to maximize the efficacy of the RNAi. (B) Quantification of phase 3 A/P polarity reversals in mom-5(gk812) maternally rescued homozygotes and mom-5(gk812) homozygotes carrying the gly-18p::mom-5(+) array. Bars represent the percentage of phase 3 polarity reversals for anterior DTCs (red bars) and for posterior DTCs (black bars). Error bars indicate standard error of the sample proportion. ***P <0.00001.
(EPS)
Bars represent the percentage of phase 3 polarity reversals for anterior DTCs (red bars) and for posterior DTCs (black bars). The corresponding raw data are presented in S9 Table. Error bars indicate standard error of the sample proportion. *P<0.01; **P<0.001; ns = not significant (P≥0.01). mom-5(RNAi) was effected by feeding animals balanced for the maternal effect lethal gk812 allele. Controls for RNAi were grown on empty vector feeding bacteria. A slight temperature drop below 20 degrees can result in significantly lower penetrance of the A/P reversals while temperatures above 20 degrees increase the penetrance of these defects considerably. Care was taken to analyze all comparable strains under the same growth conditions; therefore, a control strain grown under the same conditions was included in each set of experiments.
(EPS)
Fluorescence photomicrographs of hermaphrodites carrying muIs27, an integrated mig-2p::mig-2::gfp transgene array [22]. Anterior is left and dorsal is up. Arrows mark the DTC. Asterisk marks the vulva. Developmental stage is indicated on the right. L1-L4 represent larval stages preceding the adult stage. In L1 and the adult stages, both DTCs are shown. In L2 and L4 only the posterior DTC is shown.
(EPS)
DIC (A) and fluorescence photomicrographs (A’) and an overlay of the two (A”) of L1/L2 hermaphrodites carrying dnIs13[gly-18p::gfp], an integrated gly-18 transcriptional transgenic reporter array. Anterior is left and dorsal is up. Arrows mark the DTCs. (B) Fluorescence photomicrographs of adult hermaphrodite carrying dnIs13[gly-18p::gfp]. Anterior is left and dorsal is up.
(EPS)
Bars represent the percentage of DTCs that migrate precociously to the dorsal side in synchronized populations analyzed 24 hours after plating of L1 larva grown at 25°C. Red fluorescence, RFP(+), indicates the presence of the gly-18p::mom-5 extrachromosomal transgenic array that was created by co-injecting a mix of mCherry markers (S1 Table). These segregated some RFP(-) hermaphrodites that lacked the gly-18p::mom-5 array, which served as the evIs129 control. Error bars indicate standard error of the sample proportion. ***P <0.00001; ns = not significant (P≥0.01).
(EPS)
Upper panels: DIC and fluorescence micrographs of hermaphrodites carrying an integrated mom-5p::gfp single copy insertion (evIs462). Anterior is left and dorsal is up. Arrows mark the DTC. Sequential stages of DTC migration are shown starting at the L1 stage to the L3 to L4 stage (end of phase 1). Except for L1 and L2 only a single gonad arm is shown. For evIs462 no GFP is visible in the DTCs (gain and exposure times are identical to those of images presented in Fig 9). Lower panels: DIC and fluorescence micrographs of hermaphrodites carrying an integrated unc-5p::unc-5gfp transgene array (evIs98C). DTC GFP expression (arrows) is first visible just before or during the first DTC turn.
(EPS)
The average pixel intensity of mom-5p::gfp expression in the DTC is represented in several stages throughout DTC migration. The corresponding one way ANOVA column statistics data are presented in S7 Table.
(EPS)
(DOCX)
1DTC migration patterns were analyzed by DIC and fluorescence optics of anterior and posterior DTCs in L4 larvae or adults. ***P<0.00001; nsP≥0.01 n = number of gonad arms scored (anterior and posterior combined). SE = standard error of the proportion. 2The penetrance of defects in the evIs129 transgenic line was highly temperature sensitive and variable. Care was taken to analyze the respective control for each experiment grown under the same conditions. 3Grown on empty vector RNAi feeding bacteria.
(DOCX)
1DTC migration patterns were analyzed for anterior and posterior DTCs by DIC and fluorescent optics in L4 larvae or adults. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. 2 mom-5(gk812) and mom-5(zu193) are maternal effect embryonic lethal mutations. Balanced heterozygotes were injected with unc-5(RNAi) and the mutant homozygous progeny were analyzed for DTC migration defects; gk812 were identified as non-gfp, zu193 identified as Unc (S1 Table). 3 unc-5(RNAi) was introduced by ds RNA injection into the different strains, the D/V migration defects in the unc-5(RNAi) set of experiments reflect the efficacy of the RNAi treatment, which due to the method of delivery can be variable from strain to strain. 4 mom5(RNAi) was administered by feeding to bypass the associated embryonic lethality. This also allowed comparison of the RNAi induced defect across all strains, all grown on the same RNAi feeding bacteria under the same growth conditions.
(DOCX)
1DTC migration patterns for anterior and posterior DTCs were analyzed by DIC and fluorescence optics in L4 larvae or adults. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. The A/P polarity reversal phenotype is highly temperature sensitive. Care was taken to analyze a respective control grown under the same incubation conditions for each set of experiments.
(DOCX)
1DTC migration patterns of anterior and posterior DTCs were analyzed by DIC and florescence optics in L4 larvae or adults. ***P<0.00001; *P<0.01; nsP≥0.01. n = number of gonad arms scored. SE = standard error of the proportion. 2D/V guidance defects result from impairing unc-5 function and reflect the efficacy of the unc-5(RNAi) in the population. 2 ced-10(n3417) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were injected with unc-5(RNAi) and the ced-10(n3417) homozygous progeny (identified as non-dpy) were analyzed for DTC migration defects. 3 ced-10(t1875) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were injected with unc-5(RNAi) and the ced-10(t1875) homozygous progeny identified as GFP(-) were analyzed for DTC migration defects.
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC and florescence optics in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. ***P<0.00001; **P<0.001; *P<0.01; nsP≥0.01. 2It should be noted that we refrained from comparing the penetrance of D/V migration failures when unc-5(RNAi) was used. unc-5(RNAi) was delivered by injection; the efficacy of the RNAi treatment can vary between the injected strains. The percent D/V defects in the unc-5(RNAi) set of experiments reflect the efficacy of the RNAi. 3 mig-2(gm103) evIs129/+ were used. Only the emb-9p::gfp positive animals were included in the analysis; these may have been either heterozygous or homozygous for evIs129.
(DOCX)
One way ANOVA column statistics.
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. nsP≥0.01. 2 mom-5(gk812) is a maternal effect embryonic lethal mutation. Balanced heterozygotes were fed with mom-5(RNAi) and the mom-5 mutant homozygous progeny were analyzed for DTC migration defects; the balancer chromosome is marked with gfp hence gk812 homozygotes were identified as non-gfp (S1 Table).
(DOCX)
1DTC migration patterns of anterior and posterior DTC were analyzed by DIC in L4 larvae or adults. n = number of gonad arms scored. SE = standard error of the proportion. **P<0.001; *P<0.01; nsP≥0.01 2RNAi was introduced by feeding; controls were grown on empty vector feeding bacteria. 3The penetrance of defects in the evIs129 transgenic line was highly temperature sensitive and variable. Care was taken to analyze the respective control for each experiment grown under the same conditions.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.