Abstract
Objective
To examine the putative adverse effects of ambient fine particulate matter (PM2.5) on brain volumes in older women.
Methods
We conducted a prospective study of 1403 community-dwelling older women without dementia enrolled in the Women's Health Initiative Memory Study (WHIMS), 1996–8. Structural brain MRI scans were performed at age of 71–89 years in 2005–6 to obtain volumetric measures of gray matter (GM) and normal-appearing white matter (WM). Given residential histories and air monitoring data, we used a spatiotemporal model to estimate cumulative PM2.5 exposure in 1999–2006. Multiple linear regression was employed to evaluate the associations between PM2.5 and brain volumes, adjusting for intracranial volumes and potential confounders.
Results
Older women with greater PM2.5 exposures had significantly smaller WM, but not GM volumes, independent of geographic region, demographics, socioeconomic status, lifestyles, and clinical characteristics including cardiovascular risk factors. For each inter-quartile increment (3.49 µg/m3) of cumulative PM2.5 exposure, the average WM volume (95% confidence interval) was 6.23 (3.72–8.74) cm3 in the total brain and 4.47 (2.27–6.67) cm3 lower in the association areas, equivalent to 1–2 years of brain aging. The adverse PM2.5 effects on smaller WM volumes were present in frontal and temporal lobes and corpus callosum (all p-values <0.01). Hippocampal volumes did not differ by PM2.5 exposure.
Interpretation
PM2.5 exposure may contribute to WM loss in older women. Future studies are needed to determine whether exposures result in myelination disturbance, disruption of axonal integrity, damages to oligodendrocytes, or other WM neuropathologies.
Introduction
Evidence is accumulating to support that exposure to ambient air pollutants, especially particulate matter (PM), contributes to increased risks and poor outcomes of various neurological disorders. Ambient fine particle (PM2.5: PM with aerodynamic diameters < 2.5µm), an ubiquitous exposure largely generated by combustion processes, has been recognized as a pervasive threat to cardiovascular health.1 An increased risk for stroke was observable even at relatively low levels of exposures.2–3 Earlier neuroepidemiological reports also linked PM to relapses in multiple sclerosis.4 More recent studies reported adverse effects of ambient air pollutants on cognitive decline and accelerated brain aging. For instance, several cross-sectional studies reported low cognitive performance among older people residing in neighborhoods with higher levels of exposure to PM2.55–7 or near major roadways.8 Findings from longitudinal studies also support the adverse effect of airborne particles on cognitive decline.9–11 Biomarkers of accelerated brain aging in autopsy studies were higher among children and young adults living in urban areas with high levels of ambient air pollutants.12 As a result, there is growing concern about potential impact of ambient air pollution on neurological health.13 Only few studies have begun to examine the pathophysiological changes resulting from air pollution exposure. One found that PM2.5 was associated with diminished cerebral blood flow,14 but two others reported inconsistent associations between PM exposure and white matter hyperintensities.15–16 Importantly, many toxicological studies have demonstrated that particle-induced neurotoxicity extends beyond cerebrovascular system.17 We examined the neurotoxic effects of PM2.5 on brain volumes of older women in the Women’s Health Initiative (WHI) Memory Study (WHIMS). We hypothesized that long-term PM2.5 exposures would be associated with smaller brain volumes.
Methods
Study Design and Population
The women in our cohort were enrolled in 1996–1998 into the WHIMS, two parallel randomized controlled clinical trials of postmenopausal hormone therapy. At WHIMS baseline, all women were community-dwelling, aged 65–80 years and free of dementia determined by cognitive function tests and clinical evaluations, as part of the WHIMS protocols at baseline and annually, described elsewhere.18–19 Briefly, WHIMS implemented a four-phase measurement protocol, which included annual screening of global cognitive function using the Modified Mini-Mental State Examination20 during each clinic visit. Participants who screened positive underwent comprehensive neuropsychological testing (including the Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] battery), assessment of behavioral symptoms, detailed clinical neurological examination /neuropsychiatric evaluation, head CAT scan and a series of laboratory tests to rule out reversible causes for dementia. A central adjudication committee (2 neurologists and 1 geriatric psychiatrist) classified the participants as normal, mild cognitive impairment (MCI), or dementia. MCI was defined as poor performance (≤10th percentile in CERAD norms) on at least one CERAD test, evidence of functional impairment (but not severe enough to interfere with activities of daily living), and absence of psychiatric or other medical disorders that could explain the cognitive impairment. Dementia was defined by DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). In 2005–2006, 1403 participated in the WHIMS Magnetic Resonance Imaging (WHIMS-MRI) study,21–22 conducted in 14 WHIMS sites. During the 7–10 years of follow-up preceding brain MRI scans, 44 participants had been classified as incident MCI and 16 as probable dementia (including 6 progressed from MCI). The WHIMS and WHIMS-MRI study was approved by the institutional review boards of all participating institutes. Written informed consent was obtained from all participants.
WHIMS-MRI Scanning and Data Processing Protocols
All sites followed standardized scan acquisition and processing protocols developed by the WHIMS-MRI Quality Control Center, in compliance with the American College of Radiology MRI Quality Control Program.22 Briefly, standard T1-, T2-, proton density-weighted, and FLAIR scans were acquired using 1.5T scanners. T1-weighted volumetric MRI scans were pre-processed according to a standardized protocol: 1) alignment to the AC-PC orientation; 2) removal of extra-cranial material; and 3) segmentation of the brain into gray and white parenchyma and cerebrospinal fluid (CSF). Regional volumetric measurements of gray matter (GM), white matter (WM), and CSF were subsequently obtained via a validated, automated computer-based template warping method.23 This technique is based on a digital atlas labeled for brain lobes and individual structures in 92 anatomic regions of interest (ROIs). The volumes of GM, WM, and CSF of each labeled brain region were obtained by summing the number of respective voxels. Intracranial volume (ICV) was estimated as the total cerebral hemispheric volumes, including ventricular CSF and the CSF within the sulcal spaces. Measures of regional volumes obtained by this approach showed high test–retest stability over time.24 To segment small vessel ischemic diseases (SVID), a brain lesion segmentation algorithm25–26 was applied to multimodal images (T1, T2 and FLAIR). The computer-assisted methodology was validated against manual segmentation used by other cohorts.27 By combining the tissue segmentation and lesion segmentation algorithm, every voxel was classified as normal (not SVID-affected) or abnormal (SVID-affected), allowing calculation of normal-appearing brain volumes and SVID volumes in each region. Volumes of GM and WM reported in this article referred to normal-appearing brain tissue only. In the present study, ROI analyses focused on hippocampus and multimodal association brain areas (frontal, parietal, temporal lobes) critical to memory and complex cognitive processing. Data on SVID volumes were also acquired to explore their potential influence on the putative adverse PM2.5 exposure effect on GM or WM brain structure if any observed.
Estimation of Air Pollution Exposures
WHIMS participants’ residential addresses, prospectively collected at each clinic visit and updated at least biannually, were geocoded following a standardized protocol by a single geocoding vendor selected from 4 candidates on the basis of its accuracy. Results of reliability and validation study with high level of accuracy in geocoding of WHI addresses have been published.28 The Bayesian Maximum Entropy (BME)-based spatiotemporal modeling approach was employed to estimate the ambient concentration of PM2.5.29 We modeled the spatiotemporal interdependence of the environmental data to estimate mean trends and covariance of the air pollution fields over time and space, without making strong assumptions about the distributions of environmental processes. Our empirical data showed that BME estimates of annual exposures were highly correlated (cross-validation Spearman’s R=0.90) with the yearly PM2.5 concentrations recorded at the monitoring sites of U.S. EPA Air Quality System. This statistically-validated BME model was then applied to each geocoded residential location to generate the PM2.5 exposure yearly time-series with each estimate anchored on the calendar year. These location-specific exposure estimates were then combined with the residential histories including relocations to calculate the cumulative PM2.5 exposures. We used time-weighted averages of yearly estimates from 1999 to 2005–6 when the MRI scan was performed. We were unable to estimate the pre-1999 PM2.5 exposures because of limited monitoring data.
Measurement of Covariates
At WHIMS enrollment, participants completed structured questionnaires to provide information on: demographics (age, race/ethnicity), socioeconomic status (SES, including education, family income, employment status), lifestyle factors (smoking, alcohol consumption, physical activity), and clinical characteristics (postmenopausal hormone treatment [HT], prior depression, CVD and related risk factors). Body mass index (BMI, in kg/m2) was calculated. Hypertension was defined as antihypertensive medication or elevated blood pressure (systolic≥140 or diastolic≥90 mmHg). Treated diabetes mellitus (DM) was defined as a physician diagnosis plus oral medications or insulin therapy. History of CVD included previous coronary heart disease (myocardial infarction, coronary angioplasty, or coronary artery bypass graft), stroke, or transient ischemic attack. Good reliability and validity of both the self-reported medical histories and the physical measures have been documented.30 The Burnam screening algorithm was used to characterize the presence of prior depressive disorders.31
Statistical Analyses
We assessed relationships that cumulative PM2.5 exposures had with women’s characteristics, using chi-square tests. Using analysis of covariance (ANCOVA), ICV-adjusted brain volumes were compared among women grouped by the quartiles of cumulative PM2.5 exposures, with p-values accounting for multiple comparisons by the Benjamini–Hochberg procedure.32 Statistically significant associations were examined further with multiple linear regression to adjust for sociodemographic factors, SES, lifestyle, and clinical characteristics. To evaluate the possibility of biases resulting from self-selection among participants with better brain health at baseline, we conducted sensitivity analyses by adjusting for 3MS score at WHIMS baseline or excluding those (n=54) who had developed MCI or dementia prior to MRI scans. To evaluate the possible residual confounding by race/ethnicity, the adjusted analyses were restricted to non-Hispanic White only. To evaluate whether the results were sensitive to missing-at-random assumption in calculating the cumulative PM2.5 exposures, we also restricted the analyses to older women with the most complete exposure data. Finally, we stratified the effect estimates to assess whether PM2.5 effects differed by BMI, history of CVDDM, hypertension, or white blood cell count, using the tests of interaction. Statistical analyses were performed using the SAS System for Windows, Version 9.3 (SAS Institute, Cary, NC).
Results
Tables 1 & 2 describe the cumulative PM2.5 exposures among subgroups of WHIMS-MRI participants. Women with cumulative exposures estimated in the upper two quartiles (> 12.24 µg/m3) were more likely to be ethnic minorities (Black or Hispanic White), less physically active (with no moderate or strenuous activities ≥ 20 minutes/week), current smokers or diabetic. More detailed comparisons of other population characteristics are summarized in Supplementary Materials (Tables 1S; 2S).
Table 1.
Population Distribution of Long-term Exposure to Fine Particulate Matter in Relation to Sociodemographics and Lifestyle Factors in the WHIMS-MRI Study Cohort (1999–2006)
| Population Characteristics† | N** | Quartile of cumulative average of annual PM2.5 | p-value* | |||
|---|---|---|---|---|---|---|
| 5.75–10.67 ug/m3 (N=351) |
10.67–12.24 ug/m3 (N=351) |
12.24–14.16 ug/m3 (N=351) |
14.16–22.18 ug/m3 (N=350) |
|||
| U. S. Region | <0.0001 | |||||
| Northeast | 329 | 57 (17.3%) | 74 (22.5%) | 98 (29.8%) | 100 (30.4%) | |
| South | 207 | 64 (30.9%) | 59 (28.5%) | 69 (33.3%) | 15 (7.2%) | |
| Midwest | 486 | 137 (28.2%) | 126 (25.9%) | 84 (17.3%) | 139 (28.6%) | |
| West | 381 | 93 (24.4%) | 92 (24.1%) | 100 (26.2%) | 96 (25.2%) | |
| Age group at baseline | 0.24 | |||||
| 65–69 | 712 | 197 (27.7%) | 169 (23.7%) | 164 (23%) | 182 (25.6%) | |
| 70–74 | 495 | 111 (22.4%) | 132 (26.7%) | 135 (27.3%) | 117 (23.6%) | |
| ≥ 75 | 196 | 43 (21.9%) | 50 (25.5%) | 52 (26.5%) | 51 (26%) | |
| Ethnicity | <0.0001 | |||||
| Black or African-American | 64 | 7 (10.9%) | 4 (6.3%) | 27 (42.2%) | 26 (40.6%) | |
| Hispanic White | 21 | 1 (4.8%) | 2 (9.5%) | 10 (47.6%) | 8 (38.1%) | |
| White (not of Hispanic origin) | 1276 | 336 (26.3%) | 336 (26.3%) | 301 (23.6%) | 303 (23.7%) | |
| Other or Missing | 42 | 7 (16.7%) | 9 (21.4%) | 13 (31%) | 13 (31%) | |
| Smoking status | 0.0065 | |||||
| Never Smoked | 806 | 200 (24.8%) | 199 (24.7%) | 195 (24.2%) | 212 (26.3%) | |
| Past Smoker | 526 | 136 (25.9%) | 134 (25.5%) | 145 (27.6%) | 111 (21.1%) | |
| Current Smoker | 59 | 13 (22%) | 12 (20.3%) | 8 (13.6%) | 26 (44.1%) | |
| Moderate or strenuous activities ≥20 minutes | 0.07 | |||||
| No activity | 799 | 196 (24.5%) | 188 (23.5%) | 199 (24.9%) | 216 (27%) | |
| Some activity | 78 | 21 (26.9%) | 17 (21.8%) | 16 (20.5%) | 24 (30.8%) | |
| 2–4 episodes/wk | 280 | 61 (21.8%) | 78 (27.9%) | 72 (25.7%) | 69 (24.6%) | |
| ≥ 4 episodes/wk | 244 | 73 (29.9%) | 67 (27.5%) | 63 (25.8%) | 41 (16.8%) | |
presented as the number (row %) belonging to each exposure quartile, given the indicated subcategory of population characteristics
p-value: comparing the distribution of exposure quartile across subcategories of each personal characteristics
The total number of subjects summed up across each subcategory varies slightly because of missing values.
Table 2.
Population Distribution of Long-term Exposure to Fine Particulate Matter in Relation to Selected Clinical Characteristics in the WHIMS-MRI Study Cohort (1999–2006)
| Population Characteristics† | N** | Quartile of cumulative average of annual PM2.5 | p-value* | |||
|---|---|---|---|---|---|---|
| 5.75–10.67 ug/m3 (N=351) |
10.67–12.24 ug/m3 (N=351) |
12.24–14.16 ug/m3 (N=351) |
14.16–22.18 ug/m3 (N=350) |
|||
| Hormone treatment ever | 0.06 | |||||
| No | 752 | 181 (24.1%) | 201 (26.7%) | 171 (22.7%) | 199 (26.5%) | |
| Yes | 651 | 170 (26.1%) | 150 (23%) | 180 (27.6%) | 151 (23.2%) | |
| Hypertension ever | 0.23 | |||||
| No | 889 | 229 (25.8%) | 233 (26.2%) | 209 (23.5%) | 218 (24.5%) | |
| Yes | 505 | 121 (24%) | 115 (22.8%) | 140 (27.7%) | 129 (25.5%) | |
| Diabetes treated ever (pills or shots) | ||||||
| No | 1356 | 347 (25.6%) | 338 (24.9%) | 335 (24.7%) | 336 (24.8%) | |
| Yes | 46 | 4 (8.7%) | 12 (26.1%) | 16 (34.8%) | 14 (30.4%) | |
| High cholesterol requiring pills ever | 0.18 | |||||
| No | 1153 | 302 (26.2%) | 284 (24.6%) | 280 (24.3%) | 287 (24.9%) | |
| Yes | 223 | 43 (19.3%) | 62 (27.8%) | 60 (26.9%) | 58 (26%) | |
| Cardiovascular disease ever | 0.44 | |||||
| No | 1193 | 302 (25.3%) | 307 (25.7%) | 294 (24.6%) | 290 (24.3%) | |
| Yes | 193 | 45 (23.3%) | 42 (21.8%) | 51 (26.4%) | 55 (28.5%) | |
presented as the number (row %) belonging to each exposure quartile, given the indicated subcategory of population characteristics
p-value: comparing the distribution of exposure quartile across subcategories of each personal characteristics
The total number of subjects summed up across each subcategory varies slightly because of missing values.
ICV-adjusted average brain volumes for women grouped by cumulative PM2.5 exposures quartiles are summarized in Table 3. Those in the lowest PM2.5 exposure quartile (<10.67 µg/m3) on average had the largest normal brain volume, and this pattern persisted in association areas of the brain. Differences in brain volumes associated with PM2.5 exposures were largely limited to the normal-appearing WM, with no statistically significant differences observed in GM. Across the quartile distribution of cumulative PM2.5 exposures, the measured total WM volumes (mean±S.D. in cm3) decreased by 3.5% (from 410.71±50.44 to 396.55±49.30), but ventricular sizes, hippocampal and basal ganglia volumes did not differ by PM2.5 exposures. A consistent pattern of smaller WM volume with greater PM2.5 exposure was found in the WM of all association areas (frontal; parietal; temporal) of the brain (Table 3S).
Table 3.
Distribution of Structural Normal Brain Volume in Relation to Cumulative Annual Exposure to Fine ParticulateMatter (1999–2006), WHIMS-MRI Study
| MRI-Measured Brain Volume (cm3)† |
Quartile of cumulative average of annual PM2.5 | |||||
|---|---|---|---|---|---|---|
| 5.75–10.67 ug/m3 (N=351) |
10.67–12.24 ug/m3 (N=351) |
12.24–14.16 ug/m3 (N=351) |
14.16–22.18 ug/m3 (N=350) |
p-value* | ||
| Total Brain Volume | 808.00±74.17 | 799.41±73.60 | 792.39±78.68 | 799.22±71.78 | <0.0001 | |
| Normal Brain Volume | 798.99±73.69 | 790.93±73.06 | 783.51±77.85 | 791.21±71.10 | <0.0001 | |
| Association Brain | 620.06±58.65 | 613.2±57.51 | 607.71±60.9 | 614.54±56.92 | 0.0002 | |
| Ventricle | 35.62±15.02 | 37.73±17.52 | 37.30±15.73 | 37.80±16.80 | 0.24 | |
| Total Gray Matter | 353.37±40.06 | 350.72±40.59 | 346.68±45.49 | 359.64±42.48 | 0.27 | |
| Association Cortices | 268.4±31.03 | 265.68±32.31 | 261.96±37.03 | 273.3±34.57 | 0.32 | |
| Total White Matter | 410.71±50.44 | 405.28±54.38 | 402.03±56.37 | 396.55±49.3 | <0.0001 | |
| Association Brain White Matter | 351.65±44.07 | 347.52±46.81 | 345.75±48.9 | 341.25±42.73 | <0.0001 | |
| Corpus Callosum | 9.21±1.28 | 9.20±1.30 | 9.14±1.28 | 9.08±1.37 | 0.06 | |
| Hippocampus | 5.68±1.01 | 5.77±1.04 | 5.77±1.14 | 5.72±1.03 | 0.80 | |
| Basal Ganglia | 34.9±3.41 | 34.93±3.51 | 34.8±3.58 | 35.02±3.26 | 0.91 | |
Mean ± S.D. of brain volume in each quartile of exposure
p-values from ICV-adjusted ANCOVA testing the linear trend or difference in the indicated brain (with log-transformation if needed) and accounting for multiple comparisons
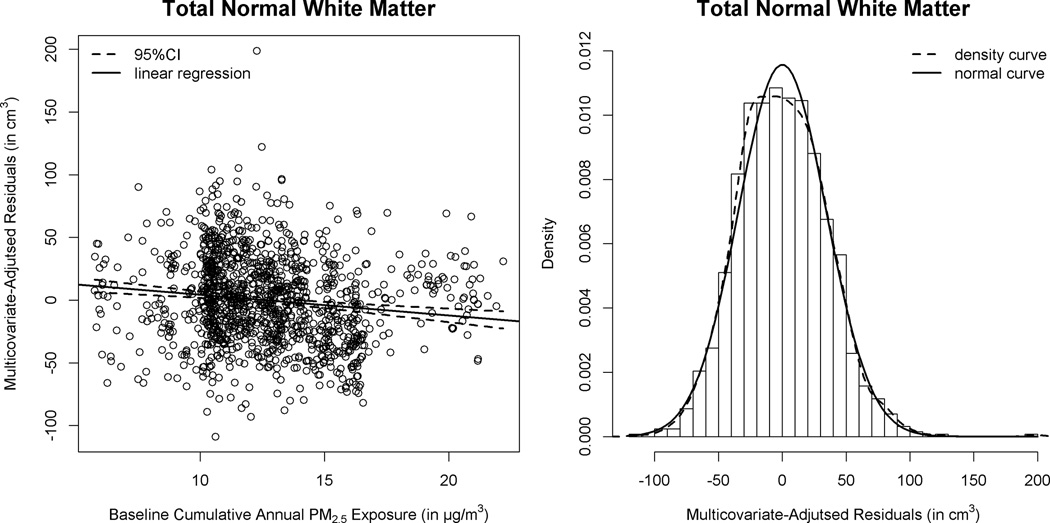
Results of multiple linear regression models, with additional covariate adjustment, are summarized in Table 4. The volume of corpus callosum was included as post-hoc analyses in the adjusted models. In order to estimate the PM2.5 effect on each hierarchically-organized WM volume while accounting for possible confounders, we added covariates stepwise (Models A–C in Table 4). The inverse association between PM2.5 exposure and WM volumes was present in the total brain, association areas, and corpus callosum. The estimated effect sizes were not materially diminished with multicovariate adjustment. For each inter-quartile (3.49 µg/m3) increment of cumulative PM2.5 exposure in the full models (Model C: including ICV, geographic region, sociodemographics, SES, lifestyles, HT use, depressive symptoms, BMI, CVD and associated risk factors), the adjusted mean of WM volume (in cm3) was lowered by 6.23±1.28 in total brain and by 4.47±1.12 in all association areas. In our adjusted analyses, these effect estimates were comparable to differences in WM volume between WHIMS women who were 1–2 years apart in age. Except for parietal lobe, multicovariate-adjusted difference in WM volume across the interquartile range of (3.49µg/m3) PM2.5 exposure all reached the pre-determined level of significance (p<0.01) for the comparison in frontal lobe (lowered by 2.04±0.59), temporal lobe (lowered by 1.70±0.33), and corpus callosum (lowered by 0.12±0.04). The distributions of modeled residuals (Figure 1) before and after including the cumulative PM2.5 exposure support our use of multiple linear region models in these adjusted analyses.
Table 4.
Multiple Linear Regression of White Matter Volumes (†) with Fine Particulate Matter Exposures, WHIMS-MRI Study
| Adjusted Analyses |
Total White Matter |
Association Brain White Matter |
Frontal White Matter |
Parietal White Matter |
Temporal White Matter |
Corpus Callosum |
|---|---|---|---|---|---|---|
|
aModel A (N=1403) |
−5.52±1.22 (p<0.001) |
−3.90±1.08 (p<0.001) |
−1.74±0.56 (p=0.004) |
−0.56±0.33 (p=0.09) |
−1.60±0.32 (p<0.001) |
−0.11±0.04 (p=0.005) |
|
bModel B (N=1310) |
−5.79±1.25 (p<0.001 |
−4.13±1.1 (p<0.001) |
−1.83±0.57 (p=0.003) |
−0.68±0.34 (p=0.04) |
−1.62±0.33 (p<0.001) |
−0.11±0.04 (p=0.006) |
|
cModel C (N=1272) |
−6.23±1.28 (p<0.001) |
−4.47±1.12 (p<0.001) |
−2.04±0.59 (p=0.001) |
−0.73±0.34 (p=0.03) |
−1.70±0.33 (p<0.001) |
−0.12±0.04 (p=0.005) |
Expressed as the linear regression coefficients (±standard errors) per inter-quartile (3.49µg/m3) increase in the continuous variable of cumulative annual PM2.5 exposure (1999–2006); all p-values adjusted for multiple comparisons
Model A: adjusted for and the intracranial volume, geographic region, age and race/ethnicity
Model B: adjusted for Model A covariates + SES (education, income, and employment status), lifestyle factors (smoking; alcohol use; physical activities), HRT use, depressive symptoms, and BMI
Model C: adjusted for Model B covariates + conventional CVD risk factors (hypertension; diabetes mellitus; hypercholesterolemia) and CVD histories
Figure 1. Distributions of Multiple Linear Regression Modeled Residuals of Normal-Appearing White Matter Total Volumes (†).
†Regressing the brain volumes on fine particulate matter exposure adjusted for multiple covariates (Model C of Table 4): before (left) and after (right) including cumulative PM2.5 exposures.
The adverse effects of long-term PM2.5 exposures on WM atrophy remained significant in the sensitivity analyses (Table 4S). Further adjusting for baseline 3MS scores or excluding subjects who had developed MCI or dementia before the MRI scans resulted in very little changes to these effect estimates. In the analyses restricted to non-Hispanic White, there was a consistent pattern of PM2.5–smaller WM associations across the brain regions examined, with effect estimates comparable to those found in the primary analyses including minorities. Despite modest reduction in effect sizes among women with more than 60% complete data on the yearly exposure estimates, the presumed PM2.5–associated neurotoxic effects on WM atrophy remained statistically significant (p<0.01) for the total brain, association areas, and corpus callosum.
In the additional analyses exploring whether SVID could affect the observed associations, we found the adverse PM2.5 effects on smaller WM volume was still present across these same brain regions (Model E of Table 4S). The resulting effect sizes were not materially altered with covariate adjustment for SVID volume in WM, although there was a statistically significant (p<0.0001) and negative correlation between SVID in WM and the normal-appearing WM volume in all the association brain regions. Furthermore, the observed associations were fairly consistent across subgroups defined by BMI, CVD history, DM, hypertension, and white blood cell count (Table 5S). Almost all the tests of interactions did not reach statistical significance in the examined WM regions (except for parietal WM), although greater adverse PM2.5 effects with much smaller WM volumes were found among women who were obese (BMI> 30 kg/m2) or had prior CVD. For instance, the association of frontal WM with PM2.5 was strongest (–3.04±1.00) in obese women, less (–2.49±0.94) in overweight group, and apparently null (–0.34±1.06) in those with BMI<25 kg/m2. Women with CVD compared to those with no history, had stronger associations between PM2.5 exposures and smaller volumes of total brain WM (–8.35±3.42 vs. –5.90±1.37) and frontal WM (–3.40±1.57 vs. –1.83±0.63).
Discussions
In this large-scale study of residential exposure to fine particles and human brain structure, we found that older women had smaller brain volumes especially in the normal-appearing WM if they resided in places with higher levels of long-term exposure to PM2.5 over 6–7 years preceding the brain MRI scans. The observed associations were not explained by demographic factors, socioeconomic status, lifestyle, and clinical characteristics we explored. These novel epidemiologic findings support the emerging concept that late-life exposure to ambient particulate air pollutants has a deleterious effect on brain aging.
To the best of our knowledge, only one recent study (N=943) has explored the neurotoxic effects of PM exposure on brain volumes.16 Using data from the Framingham Offspring Study participants (median age: 68 years; 52% female) in New England and New York State, Wilker et al. found smaller total cerebral brain volumes were associated with increased residential exposure to PM2.5. The reported exposure-effect, equivalent to one year of brain aging in their study, became statistically non-significant after adjusting for vascular risk factors (e.g., CVD, DM, and hypertension) that might be associated with long-term PM2.5 exposure and potentially confound the un-adjusted association. Their analyses did not separate the neurotoxic effects on GM vs. WM volumes. The investigators did not account for changes in residential locations and used one year of data to represent long-term exposure. In the present study, longitudinal data on residential locations allowed us to estimate the cumulative PM2.5 exposures over 6–7 years before MRI scans. We discovered that the adverse effect on brain structure in older women was primarily driven by smaller WM volumes associated with cumulative PM2.5 exposures. These effects were present in the WM underlying frontal, parietal, and temporal association regions as well as the corpus callosum. The observed neurotoxic effects of long-term PM2.5 exposure on WM volume were robust to statistical adjustments for potential confounders including CVD and vascular risk factors (Models B&C of Table 4). However, there were no differences in GM or hippocampal volumes by PM2.5 exposures. Interestingly, Wilker et al. also reported no association between PM2.5 exposure and hippocampal volume.16
Our study findings imply that WM architecture may represent a novel target of particle-induced neurotoxicity, but the neuroanatomical correlates and metabolic abnormalities in the decreased normal-appearing WM are unknown and should be investigated further. For instance, myelin-related or axonal biomarkers choline and N-acetylaspartate could be assessed by proton magnetic resonance spectroscopy.33 Diffusion tensor images can show loss of fractional anisotropy in normal-appearing white matter, but such neuroimaging data were not included in the WHIMS MRI protocols. Changes in axial or radial diffusivity in diffusion tensor image may shed light on the relative predominance of myelin versus axonal injuries.34 Lipid-rich myelin membranes are known to be particularly susceptible to oxidative stress, which could be assessed by measuring levels of isoprostanoids in the cerebrospinal fluid.35
The underlying mechanism and possible mediators for the pervasive adverse PM2.5 effects on WM volumes demonstrated by our study warrant comment. In our exploratory analyses, the association between PM2.5 and smaller WM could not be explained by SVID. Since SVID includes WM hyperintensities, we observed an expected negative correlation between WM and SVID volumes; however, adjustments for SVID did not significantly change our findings. This suggests that the mechanisms underlying increased WM hyperintensities associated with hypertension and the mechanisms underlying loss of normal-appearing WM related to PM2.5 are likely distinct and independent. Similarly in the Framingham Offspring Study,16 adjustment for covert brain infarcts did not alter the association between PM2.5 and cerebral brain volume. CVD risk factors and systemic inflammation are known to affect WM integrity,36 independent of WM hyperintensities.37–38 Our analyses did not reveal statistically significant modification of adverse effects on WM volumes by cardiovascular risk factors (Table 5S), but these findings did provide some support for possible mechanistic interactions between PM2.5 neurotoxicity and traditional vascular pathways. Stronger associations in older women with prior CVD suggested possible interactions of CVD-related neurovascular damages with the underlying neuropathology of smaller WM associated with PM2.5 exposure. Since the putative adverse effects on WM were still present in older women without DM or hypertension, it is more likely that CVD may moderate, but is unlikely to mediate, the relationship between PM2.5 and WM loss. We did not assess associations between inflammatory markers (IL10, TNF alpha, etc) in this study. Future epidemiologic studies are needed to investigate the possible role of low-grade systemic inflammation resulting from PM2.5 exposure39 to the WM atrophy. In experimental animals, exposures to airborne particles can induce oxidative stress and cause wide-spread neuroinflammation in multiple brain regions.40 Female mice exposed to concentrated particles had extensive astrocyte activation in corpus callosum.41 PM-induced WM damages, as reflected by decreased myelin percentage and reduced size of corpus callosum, was recently demonstrated in mice models with early-life exposure to concentrated ambient ultrafine particles.42 Neurotoxicological studies using animal models with inhalation exposures may help elucidate the underlying neuropathology and mechanistic pathways linking particle exposure with WM neurotoxicity.
The neurobehavioral consequences of WM injuries are difficult to predict, since WM tracts interconnect widely-distributed cognitive networks. Impaired executive function and slowing of cognitive processing speed are often associated with WM hyperintensities,43 likely due to their predilection to spread centrifugally from the periventricular regions with early engagement of longitudinal fasciculi connecting anterior frontal with posterior parietal-occipital lobes.44 On the other hand, the observed WM loss associated with PM2.5 appears to be anatomically diffuse and could potentially impact all cognitive domains. Global memory loss associated with PM2.5 exposure was seen in two longitudinal studies.10–11 Using data from the Nurses' Health Study Cognitive Cohort, Weuve et al. reported memory function declined in older women (70 to 81 years) living in locations with higher PM2.5 exposures. Tonne et al. found the adverse PM2.5 effect on memory decline among older men and women (N=2687; aged 66± 6 years) enrolled in the Whitehall II cohort and residing in greater London.11 However, prior findings from PM epidemiologic studies examining the associations of executive function (including working memory and attention) and episodic memory were either inconsistent nor non-conclusive. Weuve et al. suggested that PM exposure would accelerate the decline of executive functions in older women.10 However, results from the longitudinal study on older men (of the Normative Aging Study)9 and three cross-sectional analyses6, 8, 45 including men and women did not support the hypothesized neurotoxic PM effects on executive function. Two studies (one cross-sectional7 and one longitudinal11) showed the association between PM2.5 exposure and low performance of episodic memory, but two other longitudinal studies9–10 with instruments assessing episodic memory had not yet reported such findings. We found no published data on PM2.5 and information processing speed. These identified data inconsistency and knowledge gaps speak to the need for comprehensive analyses with longitudinal data on subdomain cognitive declines in air pollution-neuroepidemiology. Future studies, especially those planned with integrated analyses of brain imaging and cognitive function data, need to put these heterogeneous results in the context of growing knowledge about brain morphology in relation to cognitive aging across life span.46
Results of our analyses revealed no statistically significant association between GM volume and PM2.5 exposure, consistent with the current toxicological literature with only limited evidence for neuronal death in animals with inhaled exposures to concentrated fine particles.17 However, interpretation of this observation needs to consider the selected study population, exposure characteristics, and analytic approaches. The WHIMS-MRI population might remain appropriate for studying late-life (e.g., aged >65) exposure effects on accelerated WM loss in advanced ages, because WM volumes of nondemented individuals are generally preserved until after age of 50–60 years.47 On the other hand, the age-related loss in GM starts in young adulthood,47 and its putative association with PM exposures would be better captured had we been able to examine the population before the age of 60–65. Because our exposure estimation relied exclusively on EPA’s ambient monitoring data, the observed associations reflected the adverse effects of PM2.5 exposures from regional sources. Therefore, we could not exclude the possibility that smaller GM might be found in the elderly exposed to other PM with different profiles of neurotoxicity (e.g., the ultrafine particles from vehicular exhausts).48 Also, we only conducted the ROI-based analyses, which aggregate the volumetric measures within pre-defined neuroanatomic regions but discard the local variation. Future research with finer grained analyses (e.g., using voxel-based techniques) may be needed to uncover local targets with small-area variations in GM if any associated with long-term PM2.5 exposure.
We recognize several limitations to our study. First, our analyses only included one-time assessment of brain volume, raising the possibility of reverse causation. Because our analyses had already adjusted for SES, lifestyle and various health factors, it was unclear what the unmeasured factors were that might have made older women with smaller WM volumes live and stay in places with higher PM2.5 exposures. Longitudinal studies with repeated brain MRI scans will be needed to address this limitation. Second, because we only studied older women, the reported findings may not be generalized to older men or younger women. However, it has been shown that sex has negligible effects on the age-related change in brain volume in healthy population.49 Third, our study only focused on PM2.5 as a regional pollutant, with no information on emission sources, particle constituents, or interactions with other pollutant mixtures. Although researches have begun to investigate these complexities of PM exposures in the context of cardiopulmonary endpoints, such data sources are both costly and also limited for nationwide cohorts. Fourth, our analyses did not include genetic determinants of brain structure. Although ApoE allele frequencies vary by geographic region,50 there is no clear link between WM volume and ApoE polymorphism which does determine the hippocampal volume. Since our analyses did not reveal significant associations with hippocampal volumes, it was unlikely that the observed adverse effects of PM2.5 on WM volumes were due to confounding by ApoE. Fifth, the employed BME models only allowed us to estimate late-life exposure to PM2.5 after 1999. Since air pollution levels have been declining over the past 20 years, previous chronic exposure especially during the mid- or earlier life might have different and likely greater adverse effects than what observed over 6–7 years before the brain MRI scans. There were several notable strengths in our study. Our study was likely the largest neuroimaging study conducted in community-dwelling elderly to examine the association between long-term PM2.5 exposures and in vivo endophenotype of GM and WM in different regions of aging brain. The WHIMS-MRI population was a well-characterized, geographically-diverse cohort, providing an optimal environmental context with sufficient exposure gradients to study the neurotoxic effects of ambient air pollutants. The comprehensive and high-quality WHI covariate data enabled the rigorous adjustment for multiple potential confounders in studying air pollution and brain aging.
Supplementary Material
ACKNOWLEDGEMENT
The authors are grateful for the dedicated efforts of all investigators and staff at the WHI and WHIMS clinical centers as well as the WHI & WHIMS clinical coordinating center listed in: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
The authors appreciate Dr. Deborah Cory-Slechta (University of Rochester) for her help to access and interpret the latest reports in inhalation neurotoxicology.
Funding/Support and Role of Funder/Sponsor
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The WHIMS was funded by Wyeth Pharmaceuticals, Inc, St. Davids, PA, and Wake Forest University. Chen was supported in part by the NIH R01AG033078 and by the Rosenblith Award from the Health Effects Institute (HEI). Research described in this article was conducted under contract to the HEI, an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers. All these funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Several authors (JC, XW, GAW, MLS, RC, JJM, HCC, MAE) have received grants or funding support to study related topics from the National Institutes of Health, and/or the Health Effects Institute
Dr. Wellenius has received consulting fees from Environmental Health and Engineering, Inc., for work unrelated to this manuscript.
Abbreviations
- 3MS
Modified Mini-Mental State
- BME
Bayesian Maximum Entropy
- BMI
body mass index
- CSF
cerebrospinal fluid
- CVD
cardiovascular disease
- DM
diabetes mellitus
- GM
gray matter
- HT
hormone treatment
- ICV
intracranial volumes
- PM2.5
fine particulate matter
- ROI
region of interest
- SES
socioeconomic status
- WHI
Women’s Health Initiative
- WHIMS
Women’s Health Initiative Memory Study
- WHIMS-MRI
WHIMS Magnetic Resonance Imaging
- WM
white matter
Footnotes
Author Contributions
Conceived and designed the work: Chen, Serre, McArdle, Chui, Espeland
Acquisition of data: Chen, Serre, Manson, Espeland
Analysis the data: Chen, Wang, Serre
Interpretation of the data: Chen, Wang, Wellenius, Serre, Driscoll, Casanova, McArdle, Manson, Chui, Espeland
Drafting of the manuscript: Chen
Critical revision of the manuscript for important intellectual content: Chen, Wang, Wellenius, Serre, Driscoll, Casanova, McArdle, Manson, Chui, Espeland
Administrative, technical, or material support: Espeland
Study supervision: Chen, Espeland
Conflicts of Interest and Financial Disclosures
No other competing interests were declared.
References
- 1.Kaiser J. Epidemiology. Mounting evidence indicts fine-particle pollution. Science. 2005;307:1858–1861. doi: 10.1126/science.307.5717.1858a. [DOI] [PubMed] [Google Scholar]
- 2.Lisabeth LD, Escobar JD, Dvonch JT, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellenius GA, Burger MR, Coull BA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonen M, Laaksonen M, Laippala P, et al. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. 2003;22:95–99. doi: 10.1159/000067108. [DOI] [PubMed] [Google Scholar]
- 5.Ailshire JA, Clarke P. Fine Particulate Matter Air Pollution and Cognitive Function Among U.S. Older Adults. J Gerontol B Psychol Sci Soc Sci. 2014 doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ailshire JA, Crimmins EM. Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. Am J Epidemiol. 2014 doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellenius GA, Boyle LD, Coull BA, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60:2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power MC, Weisskopf MG, Alexeeff SE, et al. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weuve J, Puett RC, Schwartz J, et al. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonne C, Elbaz A, Beevers S, et al. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology. 2014;25:674–681. doi: 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 13.Dekosky ST, Gandy S. Environmental exposures and the risk for Alzheimer disease: can we identify the smoking guns? JAMA Neurol. 2014;71:273–275. doi: 10.1001/jamaneurol.2013.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellenius GA, Boyle LD, Wilker EH, et al. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke. 2013;44:1532–1536. doi: 10.1161/STROKEAHA.111.000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semmens EOB. Ann Arbor: University of Washington; 2012. Effects of Traffic-Related Air Pollution on Cognitive Function, Dementia Risk and Brain MRI Findings in the Cardiovascular Health Study [Ph.D.] [Google Scholar]
- 16.Wilker EH, Preis SR, Beiser AS, et al. Long-Term Exposure to Fine Particulate Matter, Residential Proximity to Major Roads and Measures of Brain Structure. Stroke. 2015 doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 19.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 21.Jaramillo SA, Felton D, Andrews L, et al. Enrollment in a brain magnetic resonance study: results from the Women's Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI) Acad Radiol. 2007;14:603–612. doi: 10.1016/j.acra.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 24.Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharaki EI, Kanterakis S, Bryan RN, et al. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
- 27.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitsel EA, Rose KM, Wood JL, et al. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160:1023–1029. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- 29.Christakos G, Bogaert P, Serre ML. Temporal GIS: advanced functions for field-based applications. Berlin; New York: Springer; 2001. [Google Scholar]
- 30.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 31.Goveas JS, Espeland MA, Woods NF, et al. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women's Health Initiative Memory Study. J Am Geriatr Soc. 2011;59:57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 33.Kantarci K, Lowe V, Przybelski SA, et al. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Back SA, Kroenke CD, Sherman LS, et al. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montine TJ, Peskind ER, Quinn JF, et al. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer's disease as identified by biomarkers. Neuromolecular Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Groot M, Ikram MA, Akoudad S, et al. Tract-specific white matter degeneration in aging: The Rotterdam Study. Alzheimers Dement. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Bettcher BM, Yaffe K, Boudreau RM, et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging. 2015;36:948–954. doi: 10.1016/j.neurobiolaging.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verstynen TD, Weinstein A, Erickson KI, et al. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. Neuroimage. 2013;79:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajat A, Allison M, Diez-Roux AV, et al. Long-term Exposure to Air Pollution and Markers of Inflammation, Coagulation, and Endothelial Activation: A Repeat-measures Analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen JL, Liu X, Weston D, et al. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140:160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen JL, Morris-Schaffer K, Sobolewski M, et al. Corpus callosum damage induced by early life exposure to ultrafine particulate matter. The Toxicologist, Supplement to Toxicological Sciences. 2015;144(1):378. [Google Scholar]
- 43.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranft U, Schikowski T, Sugiri D, et al. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Sandu AL, Staff RT, McNeil CJ, et al. Structural brain complexity and cognitive decline in late life--a longitudinal study in the Aberdeen 1936 Birth Cohort. Neuroimage. 2014;100:558–563. doi: 10.1016/j.neuroimage.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 47.Ge Y, Grossman RI, Babb JS, et al. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 48.Davis DA, Akopian G, Walsh JP, et al. Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NO pathway in vitro. J Neurochem. 2013;127:509–519. doi: 10.1111/jnc.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fjell AM, Westlye LT, Amlien I, et al. Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer's disease. J Neurosci. 2009;29:8774–8783. doi: 10.1523/JNEUROSCI.0115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward A, Crean S, Mercaldi CJ, et al. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.