Abstract
Objective
We recently reported sex-specific percent body fat (%BF) thresholds (Males = 23%, Females =38%) above which, visceral adipose tissue (VAT) significantly increases. Using monozygotic (MZ) and dizygotic (DZ) twins, we examined the influence of genetics on regional fat distribution measured by dual-energy x-ray absorptiometry, above and below these sex-specific thresholds for VAT accumulation.
Methods
Fifty-eight twin pairs (44 MZ, 14 DZ) were recruited from the University of Washington Twin Registry. Segmented linear regression was used to assess the threshold between VAT mass and %BF by sex and by zygosity. To assess the effect of genetics on VAT accumulation, Dunnett’s T3 compared MZ and DZ pairs whether the twin pairs were both above the adiposity threshold or not.
Results
%BF thresholds for VAT accumulation were identified (%BF: M=20.6%, F=39.4%). Zygosity-specific thresholds were not significantly different (p>0.05). If at least one twin was below threshold, DZ twins still exhibited greater within-pair differences than MZ pairs in %BF (p=0.023) but not VAT (p=0.121).
Conclusions
Using a twin study approach, we observed no difference by zygosity for the threshold as which VAT accumulates. Additionally, for the first time we observed that while total BF is influenced by genetics, VAT accumulation may depend more on whether a person’s %BF is above their sex-specific adiposity threshold. These results suggest there may not be a genetic predisposition for VAT accumulation but rather it is a result of a predisposition for total fat accumulation.
Keywords: DXA, Fat Distribution, Zygosity, Ectopic Fat
1. Introduction
Although total body fat has long been recognized as a hallmark of obesity, the regional deposition of body fat has recently gained importance in our understanding of the detrimental health effects of obesity. Twin cohorts have identified the important role of genetics in total and regional fat accumulation (1-6). Additionally, overfeeding studies have been used to demonstrate that variability in changes in weight, total body fat mass as well as regional fat mass also have genetic influences (7, 8). Taken together, these studies suggest that non-proportional accumulation of fat in different regions (i.e. abdominal, hip/gluteal) is influenced to some degree by genetics.
Studies in twins have reported that monzygotic (MZ) twins have significantly lower within-twin pair variability for both total and regional fat distribution than dizygotic (DZ) twins (1, 4, 6). Heritability (h2) of total and regional fat has been identified ranging from 0.40-0.90 using twin and family cohorts (9-11). Pérusse et al. (1996) reported that visceral adipose tissue (VAT) has a stronger genetic component than abdominal subcutaneous adipose tissue (10). These studies provide evidence of a genetic predisposition toward increased VAT accumulation, which is important given the established association between VAT, dyslipidemia, insulin resistance and hypertension (12-15).
Recently, we identified sex-specific %BF thresholds (males ~23%; females ~39%) in adults, above which, the accumulation of VAT increases dramatically (14, 16). This suggests that total body fat and percent body fat (%BF) have a non-linear relationship with VAT and that the amount of VAT accumulation is strongly influenced by these thresholds. With strong heritability of total body fat, assessing the genetic components of VAT in the context of these thresholds is warranted. The purpose of this study was to identify sex-specific and zygosity-specific %BF thresholds for VAT accumulation, using dual energy x-ray absorptiometry (DXA), in adult MZ and DZ twins and to measure the between and within pair variance for total and regional fat in twin pairs that are both above threshold or have at least one twin below the %BF thresholds. We hypothesized that within pair variability for VAT would be dependent on twins being above or below threshold.
2. Material and methods
Fifty-eight same-sex twin pairs (44MZ, 14 DZ) age 19-48 yrs (mean age 28 yrs) were recruited from the University of Washington Twin Registry (Males n=27, Females n=31). Zygosity determination and registry composition are described elsewhere (17). Participants were recruited based on standard BMI criteria for normal, overweight and obese (18-24.9, >24.9-29.9, >30 kg/m2 respectively). Recruitment specifically targeted twin pairs with at least one having a BMI>30 kg/m2. However, an additional group of normal weight (BMI<25 kg/m2) and overweight (BMI>24.9-29.9 kg/m2) twin pairs were recruited to increase the range of adiposity. Pairs were excluded if they had any major medical problems (e.g. diabetes, eating disorders), history of weight loss surgery, or pregnant. The study protocol was approved by the University of Washington Institutional Review Board, and written informed consent was obtained from all participants.
2.1 Procedures
All testing was performed in the morning after the participants had fasted for a minimum of 12 hours. Height and weight were determined in participants in hospital gown and without shoes using a wall-mounted stadiometer and an electronic scale (SR555i, SR Instruments), respectively. Body mass index (BMI) was calculated as the body weight in kilograms divided by the height in meters squared. Normal-weight was defined as 18.5-24.9 kg/m2, overweight as 25.0-29.9 kg/m2, and obese as >30 kg/m2 (CDC). Physical activity was assessed by the short-form International Physical Activity Questionnaire (18). A 24-hour dietary recall was used to assess caloric intake. Fasting blood was drawn for measurement of insulin and glucose. Samples were centrifuged at 4°C and plasma was stored at −80°C. Plasma glucose was determined by hexokinase method (Roche Model P Chemistry autoanalyzer; Roche Diagnostic Inc., Indianapolis, IN) and insulin by immunoenzymatic method (Tosoh 2000; San Francisco, CA). Homeostasis model of insulin resistance (HOMA-IR) was calculated as described previously (19).
2.2 Body Composition and Visceral Adipose Quantification
Total body composition was measured using DXA, (Prodigy, General Electric Medical Systems, Madison, WI, USA) and the scans were analyzed using enCore™ software (platform version 13.6, General Electric Medical Systems, Madison, WI, USA). Estimates of abdominal visceral and subcutaneous adipose tissue were obtained using the method described previously for adults (20). The estimation of VAT using DXA was recently validated in adults (20) and has been shown to provide a reliable estimate in children (21). Android fat (abdominal) was measured using a region-of-interest automatically defined with a caudal limit placed at the top of the iliac crest and its height set to twenty percent of the distance from the top of the iliac crest to the base of the skull (22). The gynoid region (hip/gluteal) is located mid-pelvis to mid-thigh, with the upper limit set below the iliac crest a distance 1.5 times the height of the android region and the lower limit set a distance of 2 times the height of the android region (22). Subcutaneous fat and visceral fat were estimated within the android region as described previously (20). All scans were reviewed for accurate placement of the android box by the same technician.
2.3 Statistical Analysis
Averages between each twin pair were stratified by zygosity to compare demographic, physical activity, and dietary recall for the whole sample. Data are presented as means±standard deviation (SD). If data was not normally distributed it is presented as median and interquartile range (25%, 75%). Segmented linear regression “segmented package” (23, 24) was used to evaluate break-points (thresholds) in the relationship between VAT mass and %BF in both males and females. Briefly, the segmented analysis uses the linear regression (adjusted for zygosity) and estimates a new model having a change-point linear relationship with the specified variables. The break-point is determined at the point where the slope of the linear relationship changes the most based on least squares estimates above and below. This method of segmented linear regression has been detailed previously (16, 23-25). To assess zygosity-specific thresholds we combined males and females by subtracting the sex-specific %BF thresholds from the absolute body fat for each individual and segmented linear regression evaluated significant thresholds between VAT mass and difference from the adiposity thresholds. After assessment of thresholds, we assessed between- and within-twin pair differences for total body fat and VAT above and below threshold by zygosity. For this analyses, we used a subset of the total sample of twins with at least one twin with a BMI>25 kg/m2. 14 normal weight twin pairs were removed due to the fact that all DZ pairs had at least 1 twin classified as overweight/obese based on BMI. A two way -ANOVA with an interaction for zygosity and threshold compared differences for between twin averages for age, physical activity, diet and total and regional subcutaneous body fat between groups. Specific group differences were assessed using Dunnett’s T3 because of the differences in twin pair between each group. (26). Differences for between and within twin-pair variability of total fat and VAT in MZ and DZ twins above and below thresholds were assessed for significance using Dunnett’s T3 (26). Between pair differences were calculated by taken the average for each twin pair and within-pair differences were calculated by taken the difference between each pair. Significance level was set at p<0.05 for the group comparisons. All analyses were completed in R (27).
3. Results
3.1 Effect of zygosity on baseline variables
Table 1 presents a comparison of the population by zygosity. DZ twins had a significantly (0.013) higher BMI than MZ twins, which is likely a result of recruitment as the majority of normal-weight pairs were MZ twins. All other variables were similar between MZ and DZ twins. All values for the 24-hour dietary recall were verified, the extreme high and low values were given by twin pairs trying to gain and lose weight, respectively. Interestingly, the majority of the twins were categorized by the IPAQ as moderate or high activity levels (MZ=79/88; DZ=21/28).
Table 1.
Demographic, physical activity and diet by Zygosity
| mean(SD) |
||||
|---|---|---|---|---|
| MZ (n=44pairs) | DZ (n=14pairs) | p-value | Range | |
| Sex (pairs) | M=21/F=23 | M=6/F=8 | ||
| Race (pairs) | ||||
| White | 38 | 8 | ||
| African American | 2 | 3 | ||
| Other | 4 | 3 | ||
| Age (years) | 28.4 (9.0) | 27.5 (8.6) | 0.592 | 18-48 |
| Height (cm) | 172.3 (9.8) | 169.9 (8.2) | 0.198 | 153.9-193.0 |
| Weight (kg) | 86.1 (22.3) | 92.7 (19.5) | 0.141 | 46.5-143.5 |
| BMI (kg/m2) | 28.7 (5.8) | 32.0 (6.1) | 0.013 | 18.9-43.6 |
|
*IPAQ-Vigorous (METmin/week) |
480 (0,1220) | 200 (0, 1110) | 0.821 | 0-7200 |
|
*IPAQ-Moderate (METmin/week) |
1018 (120, 1440) | 440 (110, 1440) | 0.416 | 0-5040 |
|
*IPAQ-Walk (METmin/week) |
792 (396, 2772) | 1254 (272, 4158) | 0.448 | 0-4158 |
| IPAQ-Total (METmin/week) |
3535 (2650) | 3766 (3442) | 0.748 | 0-13758 |
| IPAQ Category | ||||
| 1 - low | 7 | 7 | ||
| 2 - moderate | 35 | 6 | ||
| 3 - high | 44 | 15 | ||
| Sitting (min/day) | 339 (177) | 398.9 (191.0) | 0.162 | 20-840 |
| 24 hour diet recall (kilocalories) |
2134 (990) | 2097 (685.0) | 0.828 | 676-6560 |
Data was not normally distributed and is presented as median (25th, 75th) quartiles BMI = body mass index, IPAQ = international physical activity questionnaire
3.2 Effect of gender on percent body fat threshold
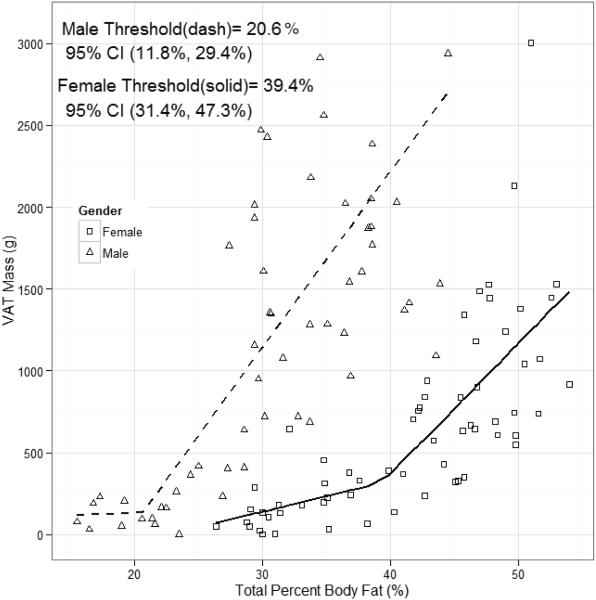
Figure 1 presents the sex-specific percent body fat thresholds within the population. In males, a threshold was observed at 20.6% body fat (95% CI: 11.8, 29.4%). The slope of VAT, with increasing %BF, below threshold was 3.5 (95% CI: −252.2, 259.2, p=0.978) and the slope above threshold was 106.8 (95% CI: 73.1, 140.6; p <0.001). The slopes above and below threshold were significantly different (p<0.001). In females, a higher %BF threshold was observed at 39.4% body fat (95% CI: 31.4, 47.3). The slope of VAT before the thresholds was 19.2 (95% CI: −31.6, 70.0; p=0.452) and the slope above threshold was 79.7 (95% CI: 45.4, 114.0; p <0.001). The slopes above and below threshold were significantly different (p<0.001). There was no gender-related difference (p>0.05) between the slopes either above or below threshold.
Figure 1.
Sex-specific percent body fat thresholds for males (open triangles) and females (open circles) for VAT mass.
3.3 Zygosity specific thresholds
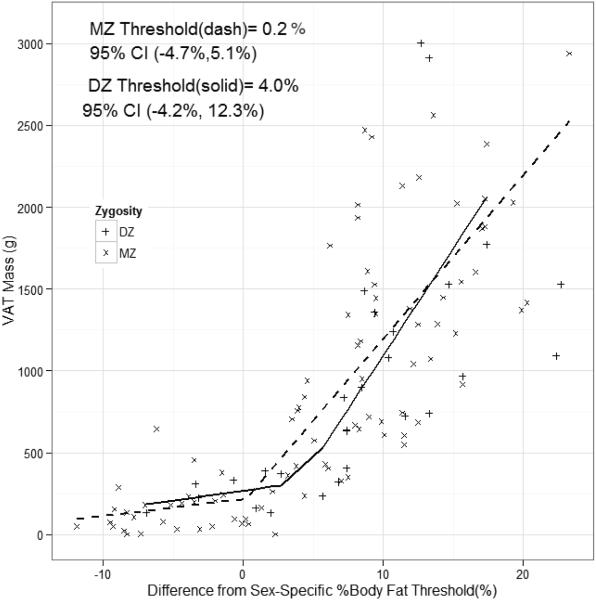
Figure 2 presents the zygosity-specific thresholds for the population. Because of the relatively small sample size, especially for DZ twins, males and females were combined for the analysis by subtracting the sex-specific body fat thresholds identified in Figure 1 from the absolute percent body fat of each individual. Zygosity-specific thresholds were identified in the relationship between VAT mass and difference from %BF thresholds. In MZ twins, a threshold was identified at −0.6%, and in DZ twins a threshold was identified at 4.0%. These zygosity-specific thresholds were not significantly different (p>0.05). The slope of VAT for MZ twins below thresholds was 14.0 (95%CI: −50.4, 78.3; p=0.667) and the slope above threshold was 86.8 (95% CI: 65.0, 108.7; p<0.001). In DZ twin pairs, the slope of VAT mass below threshold was 11.7 (95% CI: −120.9, 144.3; p=0.856) and the slope above threshold was 130.6 (95% CI: 47.3, 213.8; p<0.001).
Figure 2.
Zygosity-specific thresholds for VAT mass based on the difference from the sex-specific adiposity thresholds, MZ (open triangles), DZ (open circles).
3.4 Zygosity differences on between-twin variability above and below threshold
Based on the sex-specific thresholds identified in Figure 1, twin pairs were classified as “above threshold” if both twins were above the %BF threshold or “below threshold” if one or both twins were below the %BF threshold. Of note, DZ twins had a greater proportion (5/6) of discordant twins (i.e., 1 twin above threshold and 1 twin below threshold) compared to MZ twins (1/5). However, the proportion difference biases towards significant differences in comparing within twin variability for %BF and VAT. Table 2 presents a comparison of the threshold groups by zygosity for the 44 twin pairs classified as overweight/obese by BMI. All values are the between twin averages for each pair. MZ twins had a greater percentage with both twins above threshold (84% vs 57%) compared to DZ twins. Age and physical activity levels were similar across the groups. Interestingly DZ twins below threshold had similar total and regional subcutaneous (i.e. android, gynoid) fat mass as MZ and DZ twins above threshold for all measurements. MZ twins below threshold had significantly lower body fat across all measurements compared to MZ and DZ twins above threshold, but were similar to DZ twins below threshold.
Table 2.
Descriptive characteristics, physical activity and body composition of each group Values are the mean of the between twin averages
| mean(sd) |
||||
|---|---|---|---|---|
| Both twins Above threshold MZ pairs (n=26pairs) |
Both twins Above threshold DZ pairs (n=8pairs) |
1 or both twins Below threshold MZ pairs (n=5pairs) |
1 or both twins Below threshold DZ pairs (n=6pairs) |
|
| Sex | F=13/M=13 | F=3/M=5 | F=4/M=1 | F=5/M=1 |
| Age (years) | 30.9A (9.8) | 28.8A (8.1) | 29.3A (9.2) | 25.8A (6.1) |
| IPAQ Total (METmin/wk) |
3429A (2729) | 4054A (4171) | 2593A (2122) | 3382A (2251) |
| 24-hr diet recall (kilocalories) |
2079A (749) | 2245A (817) | 2021A (1854) | 1901A (410) |
| Sitting (min/day) | 381A (191) | 462A (210) | 247A (174) | 320A (134) |
| BMI (kg/m2) | 32.4A (3.5) | 32.9A (4.4) | 26.9B (2.2) | 30.8AB (1.9) |
| Percent Fat (%) | 40.6A (7.6) | 39.4A (8.1) | 30.0B (7.6) | 38.6AB (8.8) |
| Total Fat (kg) | 38.5A (9.9) | 37.7A (14.3) | 21.6B (7.7) | 33.7AB (11.6) |
| Total Lean (kg) | 56.5A (13.8) | 55.6A (10.5) | 49.8A (6.7) | 52.1A (10.3) |
| Android Fat (kg) | 3.7A (1.2) | 3.5A (1.7) | 1.5B (0.9) | 2.7AB(1.3) |
| Gynoid Fat (kg) | 6.3A (1.8) | 6.4A (2.6) | 3.8B (1.3) | 6.1A* (2.0) |
If groups do not share a letter within the same row they are significantly different (p<0.05)
trend towards significance (p=0.06)
IPAQ – international physical activity questionnaire,
3.5 Threshold effect on genetic variability of VAT and Insulin Resistance
Table 3 presents the between- and within-twin pair measures for total fat mass, %BF and VAT and a surrogate measure of insulin resistance (HOMA-IR) calculated for pairs above and below the thresholds and stratified by zygosity. The between twin variability was computed by taking the average value for each twin pair. Within-pair differences were computed by taking the difference in values within each twin pair. There were no significant differences between MZ and DZ pairs in the between pair measures in pairs above the threshold, reflecting a similar range of body composition and insulin resistance between the groups. However, MZ twins had significantly lower within-twin variability for total fat, %BF and VAT measurements but no difference for HOMA-IR. Similar to twins above threshold, there were no significant differences in between twin variability for MZ and DZ twins below threshold. Likewise, within-twin variability for total fat mass and %BF were significantly lower (p=0.009 and p=0.023 respectively) in MZ twins compared to DZ twins below threshold. However, when comparing within-twin variability for VAT and HOMA-IR, no differences were observed between MZ and DZ twins below threshold. As expected, between twin average HOMA-IR was significantly higher in MZ twins above threshold compared to below (2.7±1.3 vs 1.1±0.6, p<0.034). This same observation was not observed in DZ twins above and below threshold (2.4±1.2 vs 2.1±1.5, p=0.785). Similarly within-twin variability was not different above or below threshold for MZ and DZ twins below threshold (p>0.05 for both).
Table 3.
Comparison of genetic effect in MZ and DZ twin pairs on between and within pair differences of total and regional fat measurements.
|
Both twins within the pair are above threshold [mean difference between and within pairs (sd)]
| ||||
| MZ (n=26 pairs) | DZ (n=8 pairs) | Δ (95% CI) | p-value | |
|
*Between twin pair Total Fat (kg) |
39.2 (8.3) | 37.7 (11.4) | 1.5 (−12.5, 15.6) | 0.970 |
|
*Between twin pair Percent Fat (%) |
41.0 (7.3) | 39.4 (7.2) | 1.6 (−5.1, 8.2) | 0.606 |
|
*Between twin pair VAT (g) |
1320 (626) | 1192 (686) | 128 (−498, 754) | 0.648 |
|
*Between twin pair VAT% (%) |
3.5 (1.8) | 3.1 (1.8) | 0.4 (−1.3, 2.0) | 0.678 |
|
*Between twin pair HOMA-IR |
2.7 (1.3) | 2.4 (1.2) | 0.3 (−1.1, 1.8) | 0.926 |
|
† Within twin pair Total Fat (kg) |
6.1 (6.1) | 15.3 (9.7) | −9.2 (−16.4, −2.1) | 0.007 |
|
† Within twin pair Percent Fat (%) |
3.1 (2.9) | 6.9 (4.3) | −3.8 (−7.6, −0.1) | 0.041 |
|
† Within twin pair VAT (g) |
352 (350) | 820 (504) | −468 (−912, −24) | 0.036 |
|
† Within twin pair VAT% (%) |
0.46 (0.46) | 1.35 (0.46) | −0.89 (−1.32, −0.46) | <0.001 |
|
† Within twin pair HOMA-IR |
1.1 (0.9) | 1.3 (1.3) | −0.2 (−1.9,1.4) | 0.964 |
|
| ||||
| At least 1 twin is below threshold [Mean difference between and within pairs (sd)] | ||||
|
| ||||
| MZ (n=5 pairs) | DZ (n=6 pairs) | Δ (95% CI) | p-value | |
|
*Between twin pair Total Fat (kg) |
21.6 (7.0) | 33.7 (5.7) | −12.0 (−28.6, 4.6) | 0.148 |
|
*Between twin pair Percent Fat (%) |
29.6 (7.6) | 38.6 (7.2) | −9.0 (−20.2, 2.2) | 0.061 |
|
*Between twin pair VAT (g) |
407 (269) | 534 (284) | −127 (−596, 342) | 0.487 |
|
*Between twin pair VAT% (%) |
1.7 (0.9) | 1.4 (0.6) | 0.3 (−1.2, 1.7) | 0.659 |
|
*Between twin pair HOMA-IR |
1.1 (0.6) | 2.1 (1.5) | −1.0 (−3.6, 0.9) | 0.370 |
|
† Within twin pair Total Fat (kg) |
5.2 (6.6) | 19.5 (2.6) | −14.3 (−26.7, −2.8) | 0.009 |
|
† Within twin pair Percent Fat (%) |
4.4 (3.1) | 10.2 (2.5) | −5.8 (−11.3, −0.2) | 0.023 |
|
† Within twin pair VAT (g) |
189 (177) | 549 (450) | −360 (−908, 187) | 0.121 |
|
† Within twin pair VAT% (%) |
0.43 (0.76) | 0.80 (0.78) | −0.37 (−1.8, 1.1) | 0.471 |
|
† Within twin pair HOMA-IR |
0.7 (0.9) | 0.9 (0.8) | −0.2 (−1.1,0.7) | 0.708 |
VAT = visceral adipose tissue, VAT% = percent of fat stored in the visceral region
Between twin pair variability was computed by taking the average value for each twin pair (T1 + T2/2), then calculating group means and variance from the pair averages for MZ and DZ groups.
Within twin pair variability was computed by taking the difference in values within each twin pair (T1-T2), then calculating group means and variance from the pair differences for MZ and DZ groups.
4. Discussion
The purpose of this study was to examine the effect of the %BF thresholds on VAT accumulation in a group of MZ and DZ twins. The sex-specific %BF thresholds identified in this current study, males = 20.6% and females = 39.4% body fat, are consistent with our previously reported thresholds (14, 16) and did not differ based on zygosity. These thresholds had a strong influence on VAT variability between twin pairs. If both twins were not above threshold, then VAT was not significantly more variable among the DZ twin pairs even though DZ twins were more variable in their %BF than MZ twins. Taken together, the results of this study suggest within-twin pair variability for VAT and VAT% is influenced by the %BF thresholds for VAT accumulation and not solely by genetics; if both twins were not above threshold VAT was similar between and within twin pairs regardless of zygosity. Additionally we observed higher average HOMA-IR in twin pairs above threshold suggesting that insulin resistance tracks with these changes in VAT accumulation. This study provides further support for a threshold capacity in preferential subcutaneous deposition of excess fat and suggests that VAT accumulation is, at least in part, driven by total body adiposity itself rather than any genetic predisposition.
Significant variation exists in changes in weight, total fat mass and regional fat mass after long term overfeeding in identical twins, suggesting that genetic factors are involved in total and regional fat accumulation (8). Since these classic studies, others have provided evidence to support a role for genetic influences on total and regional fat distribution. These studies have estimated the range of genetic influence for total body fat (h2) from 0.40-0.90 and 0.40-0.80 for trunk, abdominal and visceral fat accumulation depending on the methods used for measuring fat mass (i.e. skinfolds, DXA, computed tomography) (1-3, 9-11). Taken together, previous data suggest strong evidence for genetic influence on total and regional fat. This study expands upon previous work, by measuring differences in VAT accumulation between MZ and DZ twins with respect to recently reported VAT accumulation thresholds (14,16). Additionally similar to our recent report (14), insulin resistance was significantly increased in twin pairs above threshold. Our results suggest that the threshold also influences changes in HOMA-IR. MZ twin pairs above threshold had a significantly higher average HOMA-IR. While this same observation was not observed in DZ pairs, most of the DZ pairs below threshold were discordant for threshold. In those twins discordant for threshold, the twin above threshold had higher HOMA-IR than their twin pair below threshold but it was not significant (3.1±2.6 vs 1.8±1.0 n=5 pairs).
To illuminate the influence of genetic factors on VAT accumulation, we examined between- and within-twin pair variability of total and regional fat in MZ and DZ twin with respect to sex-specific %BF thresholds for increased VAT. The results of this study support the strong influences of genetics on total body fat. However, increased levels of VAT and within-twin variability in VAT and VAT% were dependent on being above %BF thresholds, suggesting total body fat or %BF may have a greater influence on VAT differences than genetic components. Regardless of threshold, within-twin variability was always higher in DZ twins for measures of total body fat (%BF, BMI, total fat mass) compared to MZ twins. Additionally, in DZ twins below threshold between- and within-twin variability for regional measurements of subcutaneous fat mass (i.e. gynoid, trunk) were higher than MZ twin pairs below thresholds but similar to MZ and DZ twins above threshold. These results suggest preferential accumulation of fat in subcutaneous depots in individuals below threshold and that the differences in VAT variability between MZ and DZ twins is strongly influenced by within-twin %BF differences only when both twins are above threshold.
One of the challenges in assessing the role of genetics in regional and VAT distribution is controlling for the influence of total fat mass, while some studies have adjusted for total fat mass (10, 14), the multicollinearity between total and regional fat makes the interpretation difficult since you cannot have increases in VAT without increases in total fat mass. In the present study, we controlled for the relationship in total fat mass by also measuring VAT%; VAT% takes into account the differences in total fat mass by expressing VAT as a percentage of total fat mass. We observed similar differences above and below thresholds for total VAT and VAT%.
In this study, %BF thresholds for VAT accumulation were comparable regardless of zygosity. The consistency in the identified thresholds supports a sex-specific physiological limit to subcutaneous fat storage such that regardless of genetics, reaching this threshold results in a proportional shift in fat accumulation to the visceral region. It is important to note that genetics, in regards to sex, still plays an important role in VAT accumulation. The sex-specific thresholds demonstrate the genetic differences between males and females that result in a greater subcutaneous storage capacity for females. Sex differences in adipocyte number, size increases (hypertrophy vs hyperplasia) and regional storage depots have been identified previously which all have strong genetic influences (7, 28-30). We acknowledge that above threshold there is still variability in VAT and VAT% that could be genetically influenced. However, we hypothesize that these differences are based on the genetic ability to store fat subcutaneously (i.e. number of adipocytes, hyperplasia vs hypertrophy), which is supported by the sex-specific %BF thresholds and similar zygosity-specific thresholds identified in this study. While total body fat seems to be strongly influenced by genetics, we observed that VAT is dependent on surpassing sex-specific adiposity thresholds. As such, twin congruence in VAT accumulation may reflect genetic predispositions for increased total adiposity more so than a strong genetic component as previously reported.
The strengths of this study are the use of both MZ and DZ twin pairs with a wide range of adiposities and the use of sophisticated methods for measuring total and regional body composition. One limitation to this study was the sample size difference between MZ and DZ pairs and the imbalance in normal weight participants between MZ and DZ groups. Additionally our sample size below threshold may have limited our ability to detect a difference between zygosity. However, as stated previously, this comparison was biased towards significant differences with a greater proportion of DZ twin pairs discordant for the threshold. Another limitation is that this study was cross-sectional and had limited racial diversity. Thus causality cannot be determined and generalization is limited.
In conclusion, we identified sex-specific %BF thresholds in a genetically informative sample of twins that are consistent with previously reported thresholds and are not significantly influenced by zygosity. Additionally, the within-twin pair variability for VAT and VAT% is influenced by the %BF thresholds; if both twins are not above thresholds there is not a significant difference in VAT or VAT% even with dramatic differences in total body fat. The findings, coupled with previous reports observing robust genetic influence on total BF accumulation, imply that previous observations may have overestimated the heritable influences on VAT accumulation. Further research is needed to assess the mechanism behind a subcutaneous threshold resulting in a shift in fat accumulation, however because of the strong relationship between total BF and VAT above threshold we suggest the use of VAT% to account for the differences in total BF between and within twin pairs. The higher VAT% in twin pairs above threshold suggests a proportional shift in the amount of fat stored in VAT. We conclude that while total BF is influenced by genetics, VAT accumulation also depends on whether a person’s %BF is above his or her sex-specific adiposity threshold.
Acknowledgements
Research from this grant was supported by RO1DK089036(ES),, NORC P30DK035816, UL1TR000423, KL2TR000421, and TL1TR000422, NIH/NIDDK 1R01DK098203-01 (LC).
Abbreviations
- VAT
visceral adipose tissue
- DXA
dual energy X-ray absorptiometry
- BMI
body mass index
- VAT%
percent of fat, relative to total fat, stored in the visceral region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The Authors have no conflicts to report
Author Contributions
TAB drafted the manuscript, TAB, LC, SJM and EAS contributed to the experimental design, discussion of the result and critical revision of the manuscript. DRD contributed to the data collection and critical revision of the manuscript. MW, DY, HC MRBD and VT contribute to the conduct of the experiment.
Disclosure Summary
The authors have nothing to report
References
- 1.Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, Beck-Nielsen H, Astrup A, Vaag AA. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obesity research. 2005;13:2139–2145. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–543. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 3.Selby J, Newman B, Quesenberry C, Jr, Fabsitz R, Carmelli D, Meaney F, Slemenda C. Genetic and behavioral influences on body fat distribution. International journal of obesity. 1990;14:593–602. [PubMed] [Google Scholar]
- 4.Rose KM, Newman B, Mayer-Davis EJ, Selby JV. Genetic and Behavioral Determinants of Waist-Hip Ratio and Waist Circumference in Women Twins. Obesity research. 1998;6:383–392. doi: 10.1002/j.1550-8528.1998.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 5.Korkeila M, Kaprio J, Rissanen A, Koskenvuo M. Effects of gender and age on the heritability of body mass index. International journal of obesity. 1991;15:647–654. [PubMed] [Google Scholar]
- 6.Schousboe K, Visscher P, Erbas B, Kyvik KO, Hopper J, Henriksen JE, Heitmann B, Sørensen T. Twin study of genetic and environmental influences on adult body size, shape, and composition. International journal of obesity. 2004;28:39–48. doi: 10.1038/sj.ijo.0802524. [DOI] [PubMed] [Google Scholar]
- 7.Johannsen DL, Tchoukalova Y, Tam CS, Covington JD, Xie W, Schwarz J-M, Bajpeyi S, Ravussin E. Effect of 8 Weeks of Overfeeding on Ectopic Fat Deposition and Insulin Sensitivity: Testing the “Adipose Tissue Expandability” Hypothesis. Diabetes Care. 2014;37:2789–2797. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard C, Tremblay A, Després J-P, Nadeau A, Lupien PJ, Thériault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. New England Journal of Medicine. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 9.Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ. Heritability of body composition measured by DXA in the diabetes heart study. Obesity research. 2005;13:312–319. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- 10.Pérusse L, Despres J, Lemieux S, Rice T, Rao D, Bouchard C. Familial aggregation of abdominal visceral fat level: results from the Quebec family study. Metabolism. 1996;45:378–382. doi: 10.1016/s0026-0495(96)90294-2. [DOI] [PubMed] [Google Scholar]
- 11.Choh AC, Demerath EW, Lee M, Williams KD, Towne B, Siervogel RM, Cole SA, Czerwinski SA. Genetic analysis of self-reported physical activity and adiposity: the Southwest Ohio Family Study. Public health nutrition. 2009;12:1052–1060. doi: 10.1017/S1368980008003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments - Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 13.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch TA, Steinberger J, Sinaiko AR, Moran A, Jacobs DR, Kelly AS, Dengel DR. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity. 2015;23(2):375–382. doi: 10.1002/oby.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch TA, Burruss TP, Weir NL, Fielding KA, Engel BE, Weston TD, Dengel DR. Abdominal Body Composition Differences in NFL Football Players. The Journal of Strength & Conditioning Research. 2014;28:3313–3319. doi: 10.1519/JSC.0000000000000650. 3310.1519/JSC.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 17.Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, Watson N, Goldberg J, Buchwald D. University of Washington Twin Registry: Poised for the next generation of twin research. Twin Research and Human Genetics. 2013;16:455. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med sci sports Exerc. 2003;195:3508–1381. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. HOMEOSTASIS MODEL ASSESSMENT - INSULIN RESISTANCE AND BETA-CELL FUNCTION FROM FASTING PLASMA-GLUCOSE AND INSULIN CONCENTRATIONS IN MAN. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-Energy X-Ray Absorptiometry for Quantification of Visceral Fat. Obesity. 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatric Obesity. 2015;10:172–179. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stults-Kolehmainen M, Stanforth P, Bartholomew J, Lu T, Abolt C, Sinha R. DXA estimates of fat in abdominal, trunk and hip regions varies by ethnicity in men. Nutrition & diabetes. 2013;3:e64. doi: 10.1038/nutd.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muggeo V. segmented: An {R} package to Fit Regression Models with Broken-Line Relationships. R News. 2008;8:20–25. [Google Scholar]
- 24.Muggeo VMR. Estimating regression models with unknown break-points. Statistics in Medicine. 2003;22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 25.Bosch TA, Steinberger J, Sinaiko AR, Moran A, Jacobs D, JR, Kelly AS, Dengel DR. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity. 2014 doi: 10.1002/oby.20961. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Multiple, M.K.L.D.D.-T.-K.P., Sample, C.T.A.f.U.V.a.U., and http://CRAN.R-project.org/package=DTK, S.R.p.v.
- 27.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. URL http://www.R-project.org/ [Google Scholar]
- 28.McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KN. Downregulation of adipose tissue fatty acid trafficking in obesity a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alligier M, Gabert L, Meugnier E, Lambert-Porcheron S, Chanseaume E, Pilleul F, Debard C, Sauvinet V, Morio B, Vidal-Puig A. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2013;98:802–810. doi: 10.1210/jc.2012-3289. [DOI] [PubMed] [Google Scholar]
- 30.Auguet T, Guiu-Jurado E, Berlanga A, Terra X, Martinez S, Porras JA, Ceausu A, Sabench F, Hernandez M, Aguilar C. Downregulation of lipogenesis and fatty acid oxidation in the subcutaneous adipose tissue of morbidly obese women. Obesity. 2014;22:2032–2038. doi: 10.1002/oby.20809. [DOI] [PubMed] [Google Scholar]