SUMMARY
There are six members of the tubulin superfamily in eukaryotes [1]. Alpha- and beta-tubulin form a heterodimer that polymerizes to form microtubules, and gamma-tubulin nucleates microtubules as a component of the gamma-tubulin ring complex. Alpha-, beta-, and gamma-tubulin are conserved in all eukaryotes. In contrast, delta- and epsilon-tubulin are conserved in many, but not all, eukaryotes and are associated with centrioles, although their molecular function is unclear [2–7]. Zeta-tubulin is the sixth and final member of the tubulin superfamily, and is largely uncharacterized. We find that zeta-, epsilon-, and delta-tubulin form an evolutionarily co-conserved module, the ZED module, that has been lost at several junctions in eukaryotic evolution, and that zeta- and delta-tubulin are evolutionarily interchangeable. Humans lack zeta-tubulin, but have delta-tubulin. In Xenopus multiciliated cells, zeta-tubulin is a component of the basal foot, a centriolar appendage that connects centrioles to the apical cytoskeleton, and co-localizes there with epsilon-tubulin. Depletion of zeta-tubulin results in disorganization of centriole distribution and polarity in multiciliated cells. In contrast with multiciliated cells, zeta-tubulin in cycling cells does not localize to centrioles and is associated with the TRiC/CCT cytoplasmic chaperone complex. We conclude that zeta-tubulin facilitates interactions between the centrioles and the apical cytoskeleton as a component of the basal foot in differentiated cells, and propose that the ZED tubulins are important for centriole functionalization and orientation of centrioles with respect to cellular polarity axes.
Graphical Abstract
RESULTS
The final member of the tubulin superfamily, zeta-tubulin, was identified in a recent evolutionary analysis showing that proteins annotated as zeta-tubulin, “cryptic tubulin”, and eta-tubulin form a single unique tubulin family [1, 8, 9]. Zeta-tubulins are present in many genomes, but are mostly unannotated or mis-annotated, and essentially uncharacterized. The sole functional analysis was in Paramecium, in which a zeta-tubulin mutant had defects in centriole number, rare defects in triplet microtubules, and mis-localization of gamma-tubulin [9].
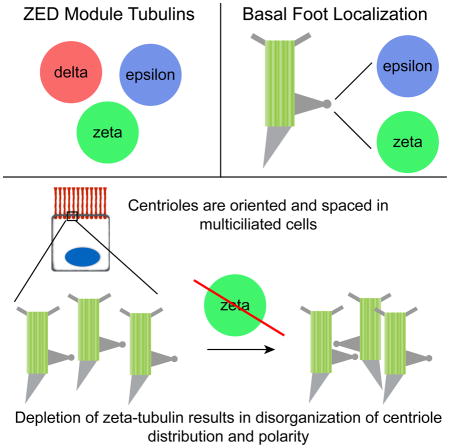
Zeta-tubulin is the last member of the tubulin superfamily to be characterized in vertebrates. We assessed the presence of zeta-, epsilon-, and delta-tubulin in genomes representing all known branches of the eukaryote tree. In contrast to the previous analysis [1], we found a unique pattern of conservation: 1) organisms that lack epsilon-tubulin always also lack delta- and zeta-tubulin, and 2) organisms that have epsilon-tubulin always also have delta- and/or zeta-tubulin. Thus, these three tubulin families form a co-conserved evolutionary module, and we will refer to these together as the “ZED module” (Table S1). The entire ZED module has been lost independently in each of the major eukaryote branches. It is absent in higher fungi, but present in the basal chytrids; it is absent in higher plants, but present in mosses; it is absent in dipteran insects, but present in hymenopterans (Figure 1A).
Figure 1. Zeta-tubulin is a conserved tubulin that associates with TRiC/CCT.
A) Presence or absence of ZED module tubulins in representative organisms. B) Unrooted phylogenetic tree, based on Clustal Omega alignment. C–E) Sucrose gradient sedimentation of zeta-tubulin from C) A6 cells, D) eggs, and E) whole oviduct. Lysates were separated on 10–40% sucrose gradients and fractions probed for zeta-tubulin, the TRiC/CCT component CCT2, and gamma-tubulin. Molecular weights in kilodaltons (left), and increasing sucrose concentration, left to right, (above) are indicated. Dividing line in E) indicates joint between two membranes, probed and developed identically. See also Table S1 and Figure S1.
Within the ZED module, loss of delta- or zeta-tubulin has also occurred independently; in most cases delta-tubulin is retained (Table S1). Zeta-tubulin has been lost independently several times, including most strikingly between marsupial mammals and placental mammals (Figure 1A). In vertebrates in which zeta-tubulin is present, the gene is in a genomic region between Setd2 and Kif9. Although this locus is rearranged in some vertebrates lacking zeta-tubulin, in others (e.g. placental mammals) it remains intact but bears no remnant of zeta-tubulin coding sequence.
We characterized zeta-tubulin in Xenopus, which has all three members of the ZED module. Comparison of the tubulins from Xenopus with those from Chlamydomonas, opossum, and human confirms that the zeta-tubulins are a unique family, that zeta-tubulin is most similar to delta-tubulin, and that the zeta-tubulin family is the most divergent of the tubulin families (Figure 1B). Zeta-tubulin mRNA was detectable at low levels in most adult tissues, with much higher expression in testis (Figure S1A). We generated an antibody against the C-terminal 20 residues of X. laevis zeta-tubulin that specifically recognizes a protein of the predicted molecular weight in eggs and A6 cells (Figure S1B). In both immunoblotting and immunofluorescence, the signal can be blocked by incubation with the immunizing peptide (Figures S1C and S1D).
The other ZED module tubulins are associated with the centriole in animal cells [5, 6], and we tested whether this was also true of zeta-tubulin. Based on quantitative immunoblotting, zeta-tubulin is present in low abundance (≤ 0.002% total protein) in both egg and A6 cells. In A6 cells, zeta-tubulin antibody did not label centrioles or other microtubule-based structures (Figures S2B–S2D), and the faint cytoplasmic labeling of zeta-tubulin could be competed with immunizing peptide. Zeta-tubulin in A6 cells fractionates with the cytoplasm (Figure S2A) and sediments as a ~20 S complex (~29 S in egg extract); the zeta-tubulin peak was clearly distinct from that of the gamma-tubulin ring complex (~32 S) (Figures 1C and 1D).
To determine the nature of the zeta-tubulin complex(es), we generated an A6-derived cell line that stably expresses GFP-zeta-tubulin at approximately the same level as endogenous; this also localized to the cytoplasm (Figure S3B). GFP-zeta-tubulin was purified by GFP nanobody affinity [10] and co-purifying proteins identified by mass spectrometry. Zeta-tubulin co-purified with all eight subunits of the TRiC/CCT chaperone complex and other proteins associated with TRiC/CCT function (Figure S3A) [11–13]. Consistent with this, endogenous zeta-tubulin co-sedimented with TRiC/CCT component CCT2 in both A6 cell and egg lysates (Figures 1C and 1D). Thus, although zeta-tubulin is expressed in both of these dividing cell types, it is likely sequestered in TRiC/CCT.
We next tested whether zeta-tubulin might associate with the specialized centrioles in some differentiated cells. Zeta-tubulin expression is highest in tissues with motile cilia (Figure S1A) and is positively regulated by Rfx2, a transcription factor involved in the differentiation of cells with motile cilia [14]. Xenopus oviduct multiciliated cells have >100 motile cilia on their apical surface, each anchored by a basal body, a specialized centriole. In oviduct cell lysate, zeta-tubulin did not co-sediment with TRiC/CCT, but rather as a smaller ~12.5 S complex (Figure 1E). To assess localization of zeta-tubulin, dissociated multiciliated cells from oviduct were stained with zeta-tubulin antibody. Zeta-tubulin localized primarily to the apical domain containing the basal bodies (Figure 2A).
Figure 2. Zeta-tubulin localizes to the basal foot in multiciliated cells.
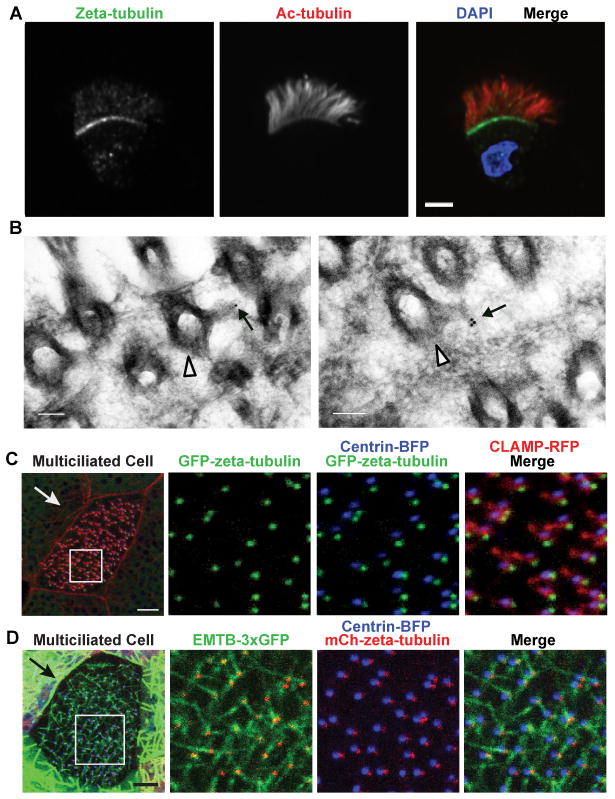
A) Dissociated Xenopus multiciliated oviduct cells stained for zeta-tubulin (green), acetylated tubulin (red), and DAPI (blue). Scale bar, 5 μm. B) Transmission EM of oviduct tissue stained with zeta-tubulin antibody and 10 nm gold-conjugated secondary antibody. Arrowhead indicates basal body, and arrow indicates labeling of basal foot. Scale bars, 100 nm. C) Confocal image of live tadpole epidermal multiciliated cell expressing GFP-zeta-tubulin (green), CLAMP-RFP (red), and centrin-BFP (blue). Arrow shows direction of ciliary beating. Scale bar, 5 μm. D) Confocal image of live tadpole epidermal multiciliated cell expressing EMTB-3XGFP (green), mCherry-zeta-tubulin (red), and centrin-BFP (blue). Arrow shows direction of ciliary beating. Scale bar, 5 μm. See also Figures S2 and S3.
In contrast to cycling cell centrioles, basal bodies in multiciliated cells have a basal foot, which links the basal body to the apical cytoskeleton, and a rootlet, which extends from the basal body into the apical cytoplasm [15–18]. Immunoelectron microscopy of oviduct multiciliated cells showed that zeta-tubulin localized specifically to the distal end of the basal foot (Figures 2B and S2E). We determined the localization of GFP-zeta-tubulin in vivo relative to centrin-BFP (basal body) and CLAMP-RFP (rootlet) in the multiciliated cells of the tadpole epidermis. In these cells the rootlet points in the opposite direction from the basal foot when viewed down the basal body axis [19]. Zeta-tubulin localized to foci in the apical cytoplasm adjacent to the centrin foci marking the basal body axis and opposite the rootlet, consistent with localization to the basal foot (Figure 2C). The basal foot interacts with cortical microtubules in multiciliated cells [16, 18]. Microtubules labeled by EMTB-3XGFP, the microtubule-binding domain of ensconsin, intersected the zeta-tubulin foci (Figure 2D). Thus, we conclude that zeta-tubulin associates with the distal end of the basal foot in multiciliated epithelial cells.
In multiciliated cells, basal bodies are polarized with respect to tissue axes such that ciliary beating generates directional fluid flow. This polarity is established and refined via the planar cell polarity pathway and fluid flow [20, 21]. Uniform spacing and directional orientation of basal bodies is mediated by the microtubule and actin cytoskeletal networks [22], which interact with the basal foot [16–18]. Disruption of the basal foot causes loss of ciliary polarity and uneven spacing of basal bodies [23]. To test whether zeta-tubulin is involved in this process, we depleted zeta-tubulin in Xenopus embryos using translation- (MO1) and splice-blocking (MO2) morpholinos. Both morpholinos reduced zeta-tubulin protein and RNA levels in injected embryos (hereafter morphants) (Figures S4A–S4C).
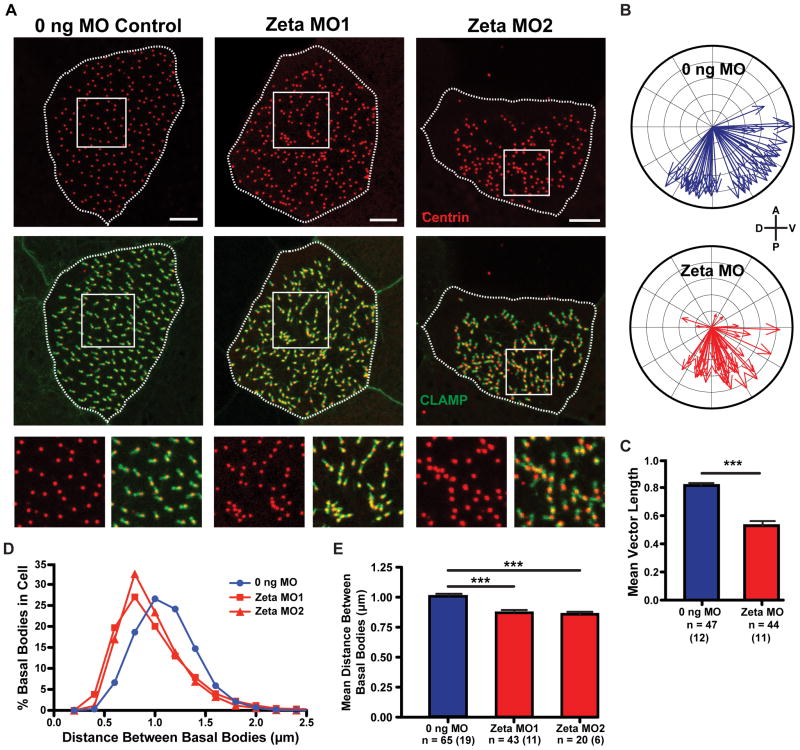
The effect of zeta-tubulin depletion was assessed by co-expressing fluorescently-tagged CLAMP and centrin and examining tadpole epidermal multiciliated cells after basal body orientation is established (stage 31–34) [20]. Basal bodies and cilia still formed in zeta-tubulin morphant cells, although the number of cilia per cell was reduced and the cilia beat with reduced metachronal fidelity (Figures S4D and S4E; Movies S1 and S2). Most strikingly, zeta-tubulin depletion caused disruption of basal body distribution and orientation (Figure 3A). Quantification of basal body orientation as assessed by angle of the rootlet with respect to the body axis revealed that the mean directionality (the average of all basal body/rootlet vectors within a cell) was unaffected (Figure 3B). However, the uniformity of basal body orientation was significantly reduced in morphants (Figure 3C). This is consistent with a previous report of the phenotype of basal foot disruption [23], which noted basal body disorientation and defective metachronal beat. The basal foot is not completely absent in zeta-tubulin morphants, as the core basal foot component ODF2 [23] was still present on basal bodies (Figure S4F). We conclude that zeta-tubulin, functioning at the basal foot, is important for intracellular basal body orientation; this phenotype might be enhanced by defective flow over the embryo.
Figure 3. Zeta-tubulin depletion disrupts basal body orientation and spacing.
A) Tadpole epidermal multiciliated cells in morphant and control embryos co-expressing CLAMP-GFP (green) and centrin-(RFP or BFP) (red). Clumps of basal bodies indicated by arrowheads and insets. Scale bars, 5 μm. B) Quantification of mean rootlet angle from experiments as in A). Each arrow represents one cell, where length indicates uniformity of rootlet angles in that cell. Mean cellular rootlet orientation was statistically similar between morphants and controls (mean vector angle −64.4° in controls and −74.8° in morphants, Watson-Williams). Number of cel ls counted is as in C). C) Vector length of plots shown in B) is significantly reduced in morphants (***p < 0.0001, Mann-Whitney). n = Number of cells counted, with total number of embryos in parentheses. D, E) Quantification of basal body clumping phenotype from experiments as in A). n = Number of cells counted, with total number of embryos in parentheses (shown in E). D) The distance between each basal body and its nearest neighbor shown as average percentage of binned nearest neighbor distances for each condition. E) Mean distance between basal bodies; this is significantly reduced in morphants (***p < 0.0001, Mann-Whitney). See also Figure S4.
Zeta-tubulin depletion also caused striking defects in basal body distribution. In control embryos, basal bodies are evenly distributed in the apical domain of multiciliated cells, but in morphants basal bodies were frequently clumped (Figure 3A, arrowheads), displaying a significant reduction in the mean distance between each basal body and its nearest neighbor (Figures 3D and 3E). This phenotype was similar to that caused by actin depolymerization [22].
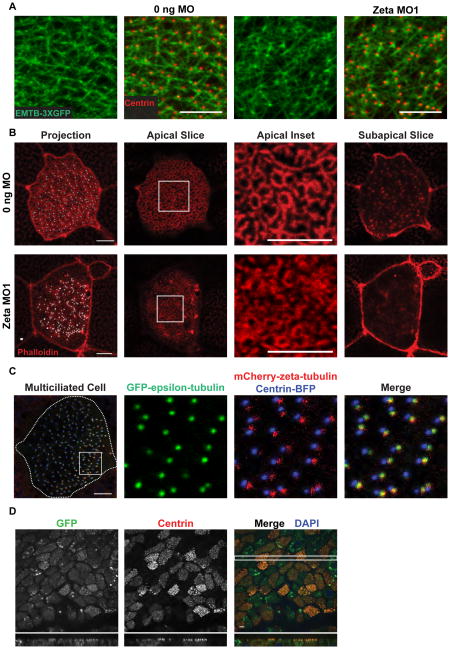
The basal body orientation and distribution defects suggested that the interaction of basal bodies with the apical actin and microtubule networks might be defective in zeta-tubulin morphants. Microtubules in control embryos, visualized by expression of EMTB-3XGFP, formed a dense apical meshwork linking adjacent basal bodies (Figure 4A, see also Figure 2D). Microtubules in morphant embryos still appeared to link basal bodies, although the distribution of these basal bodies was defective (Figure 4A). Actin filaments in control embryos, visualized by phalloidin staining, formed an apical mesh surrounding each basal body and sub-apical foci linking adjacent basal bodies, as described [22]. Both the apical and subapical actin networks were disrupted in morphants (Figure 4B). Morphant cells had fewer sub-apical foci, and the annular aspect of the apical actin network was less defined (Figures 4B and S4G). Phalloidin labeling intensity was also reduced in zeta-tubulin morphant cells compared to neighboring uninjected cells in mosaic embryos [24], but not in those injected with a mismatch morpholino based on MO2 (MM-MO2) (Figures S4H and S4I). Thus, depletion of zeta-tubulin disrupts the actin cytoskeleton associated with basal bodies.
Figure 4. Zeta-tubulin function and ZED tubulin localization.
A) Microtubule organization in tadpole epidermal multiciliated cells visualized by co-expressing EMTB-3XGFP (green) and centrin-RFP (red). Scale bars, 5 μm. B) Tadpole epidermal multiciliated cells expressing centrin-BFP (white) fixed and stained with Alexa Fluor 568 phalloidin (red). Apical actin (Apical Inset) and subapical foci (Subapical Slice, ~1.2 μm below the Apical section) are shown. Scale bars, 5 μm. C) Tadpole epidermal multiciliated cell expressing GFP-epsilon-tubulin (green), mCherry-zeta-tubulin (red), and centrin-BFP (blue). Scale bar, 5 μm. D) Mouse tracheal epithelial cell cultures infected with GFP-zeta-tubulin-expressing lentivirus and stained for GFP (green), centrin (red), and DAPI (blue). X–Z projection (below) shows a slice through the area marked by the white rectangle. Scale bar, 5 μm. See also Figures S3 and S4.
Given the co-conservation of zeta- and epsilon-tubulin, we tested whether epsilon-tubulin might also localize to the basal foot in multiciliated cells. Xenopus embryos were injected with mRNAs for mCherry-zeta and GFP-epsilon-tubulin. In tadpole epidermal multiciliated cells, the foci of epsilon- and zeta-tubulin co-localized adjacent to the centrin foci marking basal bodies, consistent with a model in which these ZED tubulins act together (Figure 4C).
Multiciliated cells are a conserved feature of all vertebrates [25], yet some vertebrates, including human, lack zeta-tubulin but retain delta-tubulin. Given this relationship, and that delta- and zeta-tubulin are more similar to each other than other tubulins, we considered whether zeta-tubulin might be recognized even in contexts where it is naturally absent. To test this, Xenopus GFP-zeta-tubulin was expressed in cultured multiciliated cells derived from mice, which lack zeta-tubulin. Remarkably, Xenopus GFP-zeta-tubulin localized to basal bodies in these cells (Figures 4D and S3C), presumably reflecting conservation of ZED tubulin interactions.
DISCUSSION
We have identified the co-conserved ZED tubulin module and characterized zeta-tubulin, the final member of the tubulin superfamily and the last studied in vertebrates. Zeta-tubulin is expressed in cycling and differentiated cells, is restricted to TRiC/CCT in cycling cells, but is a functionally important component of the basal foot in multiciliated cells. As for other basal foot proteins, depletion of zeta-tubulin causes defects in the orientation and distribution of basal bodies and disorganization of associated cytoskeletal elements. The basal foot of basal bodies and the sub-distal appendages of centrioles are structurally and functionally analogous; they have common components (e.g. ODF2 [23, 26]) and they link to the cytoskeletal network. Epsilon-tubulin ([7] and here) and gamma-tubulin [27, 28] also localize to both structures. The localization of delta-tubulin is less clear [5, 6], but the apparent evolutionary interchangeability of delta- and zeta-tubulin may reflect a functional overlap between these two tubulins. We propose that the ZED tubulin module is an essential component of such appendages, which orient centrioles with respect to cellular polarity axes. Organisms without the ZED module either lack centrioles or have centrioles that lack such appendages (e.g. C. elegans and D. melanogaster). Most single-celled ciliated organisms have the ZED tubulin module (Table S1) and have basal body appendages that are functionally analogous to those in vertebrates [29, 30]. Disruption of ZED tubulins in these organisms results in significant cytoskeletal defects, consistent with the ZED tubulins having a conserved role in basal body-cytoskeleton interactions [31, 32]. For example, disruption of epsilon-tubulin in Tetrahymena resulted in defective orientation and spacing of basal bodies [33], similar to depletion of zeta-tubulin in Xenopus.
We did not observe a strong defect in centriole number associated with zeta-tubulin depletion, in contrast to previous reports for the ZED tubulins [7, 9, 31–33]. The evolutionary conservation of the ZED module clearly indicates that these tubulins cannot be absolutely required for centriole formation, since organisms such as Drosophila have centrioles but lack all ZED tubulins [34]. It is possible that in some contexts, ZED appendages are required for centriole stability rather than duplication per se, or that vertebrate zeta-tubulin has become specialized for multiciliated cells that do not have this requirement. Also, we note that a basal body attached to a motile cilium is subjected to forces not experienced by those without, and that the importance of the ZED tubulins to centriole orientation and/or stability might differ by context. Lastly, we have not determined a molecular mechanism for the function of the ZED tubulins at centriole appendages. Since the characterized tubulin superfamily members all interact with each other in microtubule structures, it is tempting to speculate that the ZED tubulins similarly function by interaction with each other and/or other tubulins at the intersection of appendage and cytoskeleton. Deletion of ZED tubulin genes from vertebrates, and further characterization of the ZED tubulin proteins, will be needed to resolve these questions.
Experimental Procedures
See supplemental experimental procedures. Methods are adequately described in the figure legends.
Supplementary Material
Highlights.
Zeta-, epsilon-, and delta-tubulin form an evolutionarily co-conserved module
Zeta-tubulin localizes to the basal foot in multiciliated cells
Zeta-tubulin participates in cytoskeletal organization in multiciliated cells
Epsilon-tubulin co-localizes with zeta-tubulin to basal feet
Acknowledgments
We gratefully acknowledge members of the Stearns and Wallingford labs for helpful advice. We acknowledge Dan Van de Mark, Yin Loon Lee, and Christian Hoerner for assistance with the MTEC experiment. We thank Dan Gestaut and Judith Frydman for consultation on TRiC/CCT and gift of the CCT2 antibody. We would also like to thank Christine Reid for donation of Xenopus oviducts. Immunoelectron microscopy was performed with assistance from John Perrino in the Stanford Cell Sciences Imaging Facility; this work was supported, in part, by ARRA Award Number 1S10RR026780-01 from the National Center for Research Resources (NCRR). Mass spectrometry was done with the Stanford Mass Spectrometry Core. This work was supported by the Gabilan Stanford Graduate Fellowship (SGF) to E.T.; the Cancer Biology Program Training Grant (T32 CA09302) to E.T.; NIH grant R01GM052022 to T.S.; Ruth L. Kirschstein National Research Service Award for Postdoctoral Fellows (NIGMS) F32GM103146 to A.A.W.; and NIH grant R01HL117164 and HHMI Early Investigators grant to J.B.W.
Footnotes
Author Contributions:
E.T., A.A.W., T.S., and J.B.W designed the experiments, E.T. and A.A.W. performed the experiments. E.T., A.A.W., T.S., and J.B.W. wrote the paper. T.K. analyzed the RNA-Seq data. J.S. produced the algorithm used to analyze basal body spacing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Findeisen P, Mühlhausen S, Dempewolf S, Hertzog J, Zietlow A, Carlomagno T, Kollmar M. Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family. Genome Biol Evol. 2014;6:2274–2288. doi: 10.1093/gbe/evu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato A, Nagata Y, Todokoro K. δ-Tubulin is a component of intercellular bridges and both the early and mature perinuclear rings during spermatogenesis. Dev Biol. 2004;269:196–205. doi: 10.1016/j.ydbio.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Dutcher SK, Morrissette NS, Preble AM, Rackley C, Stanga J. Epsilon-tubulin is an essential component of the centriole. Mol Biol Cell. 2002;13:3859–3869. doi: 10.1091/mbc.E02-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutcher SK, Trabuco EC. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes delta-tubulin, a new member of the tubulin superfamily. Mol Biol Cell. 1998;9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smrzka OW, Delgehyr N, Bornens M. Tissue-specific expression and subcellular localisation of mammalian delta-tubulin. Curr Biol. 2000;10:413–416. doi: 10.1016/s0960-9822(00)00418-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang P, Stearns T. Delta-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat Cell Biol. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- 7.Chang P, Giddings TH, Winey M, Stearns T. Epsilon-Tubulin is required for centriole duplication and microtubule organization. Nat Cell Biol. 2003;5:71–76. doi: 10.1038/ncb900. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan S, Attwood T, Navarro M, Scott V, McKean P, Gull K. New tubulins in protozoal parasites. Current Biology. 2000;10:R258–R259. doi: 10.1016/s0960-9822(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz F, Krzywicka A, Klotz C, Keller AM, Cohen J, Koll F, Balavoine G, Beisson J. The SM19 gene, required for duplication of basal bodies in Paramecium, encodes a novel tubulin, eta-tubulin. Current Biology. 2000;10:1451–1454. doi: 10.1016/s0960-9822(00)00804-6. [DOI] [PubMed] [Google Scholar]
- 10.Rothbauer U, Zolghadr K, Muyldermans S, Schepers A, Cardoso MC, Leonhardt H. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics. 2008;7:282–289. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Melki R, Vainberg IE, Chow RL, Cowan NJ. Chaperonin-mediated folding of vertebrate actin-related protein and gamma-tubulin. J Cell Biol. 1993;122:1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 13.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Chung MI, Kwon T, Tu F, Brooks ER, Gupta R, Meyer M, Baker JC, Marcotte EM, Wallingford JB. Coordinated genomic control of ciliogenesis and cell movement by RFX2. Elife. 2014;3:e01439. doi: 10.7554/eLife.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi N, Hirokawa N. Cytoskeletal architecture and immunocytochemical localization of fodrin in the terminal web of the ciliated epithelial cell. Cell Motil Cytoskeleton. 1988;11:167–177. doi: 10.1002/cm.970110304. [DOI] [PubMed] [Google Scholar]
- 16.Chailley B, Nicolas G, Lainé MC. Organization of actin microfilaments in the apical border of oviduct ciliated cells. Biol Cell. 1989;67:81–90. doi: 10.1111/j.1768-322x.1989.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 17.Reed W, Avolio J, Satir P. The cytoskeleton of the apical border of the lateral cells of freshwater mussel gill: structural integration of microtubule and actin filament-based organelles. J Cell Sci. 1984;68:1–33. doi: 10.1242/jcs.68.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Sandoz D, Chailley B, Boisvieux-Ulrich E, Lemullois M, Lainé MC, Bautista-Harris G. Organization and functions of cytoskeleton in metazoan ciliated cells. Biol Cell. 1988;63:183–193. doi: 10.1016/0248-4900(88)90057-3. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Am J Anat. 1968;122:19–55. doi: 10.1002/aja.1001220103. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 21.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J Cell Biol. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunimoto K, Yamazaki Y, Nishida T, Shinohara K, Ishikawa H, Hasegawa T, Okanoue T, Hamada H, Noda T, Tamura A, et al. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200. doi: 10.1016/j.cell.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 24.Kieserman EK, Lee C, Gray RS, Park TJ, Wallingford JB. High-Magnification In Vivo Imaging of Xenopus Embryos for Cell and Developmental Biology. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5427. pdb.prot5427. [DOI] [PubMed] [Google Scholar]
- 25.Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol. 2014;24:R973–82. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa Y, Yamane Y, Okanoue T, Tsukita S, Tsukita S. Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol Biol Cell. 2001;12:1687–1697. doi: 10.1091/mbc.12.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moudjou M, Bordes N, Paintrand M, Bornens M. Gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109(Pt 4):875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 28.Clare DK, Magescas J, Piolot T, Dumoux M, Vesque C, Pichard E, Dang T, Duvauchelle B, Poirier F, Delacour D. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat Commun. 2014;5:4888. doi: 10.1038/ncomms5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galati DF, Bonney S, Kronenberg Z, Clarissa C, Yandell M, Elde NC, Jerka-Dziadosz M, Giddings TH, Frankel J, Pearson CG. DisAp-dependent striated fiber elongation is required to organize ciliary arrays. J Cell Biol. 2014;207:705–715. doi: 10.1083/jcb.201409123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubusson-Fleury A, Bricheux G, Damaj R, Lemullois M, Coffe G, Donnadieu F, Koll F, Viguès B, Bouchard P. Epiplasmins and epiplasm in paramecium: the building of a submembraneous cytoskeleton. Protist. 2013;164:451–469. doi: 10.1016/j.protis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 31.O’Toole ET, Giddings TH, McIntosh JR, Dutcher SK. Three-dimensional organization of basal bodies from wild-type and delta-tubulin deletion strains of Chlamydomonas reinhardtii. Mol Biol Cell. 2003;14:2999–3012. doi: 10.1091/mbc.E02-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preble AM, Giddings TH, Dutcher SK. Extragenic bypass suppressors of mutations in the essential gene BLD2 promote assembly of basal bodies with abnormal microtubules in Chlamydomonas reinhardtii. Genetics. 2001;157:163–181. doi: 10.1093/genetics/157.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross I, Clarissa C, Giddings TH, Winey M. ε-tubulin is essential in Tetrahymena thermophila for the assembly and stability of basal bodies. J Cell Sci. 2013;126:3441–3451. doi: 10.1242/jcs.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González C, Tavosanis G, Mollinari C. Centrosomes and microtubule organisation during Drosophila development. J Cell Sci. 1998;111(Pt 18):2697–2706. doi: 10.1242/jcs.111.18.2697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.