Abstract
Glycosaminoglycans are linear anionic polysaccharides that exhibit a number of important biological and pharmacological activities. The two most prominent members of this class of polysaccharides are heparin/heparan sulfate and the chondroitin sulfates (including dermatan sulfate). These polysaccharides, having complex structures and polydispersity, are biosynthesized in the Golgi of most animal cells. The chemical synthesis of these glycosaminoglycans is precluded by their structural complexity. Today, we depend on food animal tissues for their isolation and commercial production. Ton quantities of these glycosaminoglycans are used annually as pharmaceuticals and nutraceuticals. The variability of animal-sourced glycosaminoglycans, their inherent impurities, the limited availability of source tissues, the poor control of these source materials, and their manufacturing processes, suggest a need for new approaches for their production. Over the past decade there have been major efforts in the biotechnological production of these glycosaminoglycans. This mini-review focuses on the use of recombinant enzymes and metabolic engineering for the production of heparin and chondroitin sulfates.
Keywords: glycosaminoglycans, chemoenzymatic, metabolic engineering, recombinant enzymes, heparin, chondroitin sulfate, sulfotransferases, glycosyltransferases, PAPS
Introduction
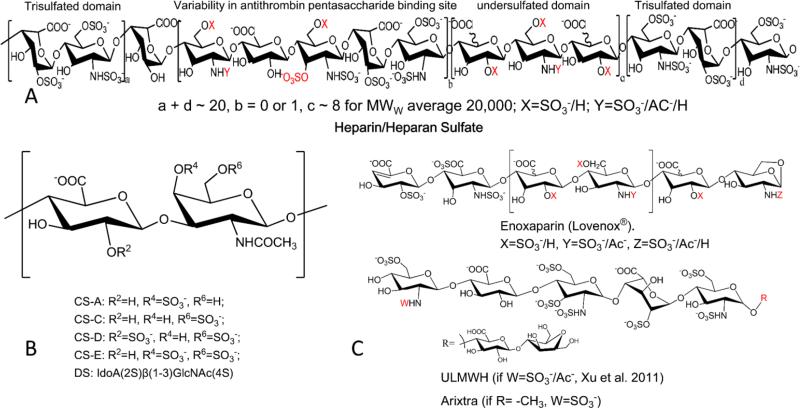
Glycosaminoglycans (GAGs) are structurally complex molecules, with varying lengths, backbone sugars, and modifications (Figure 1). GAGs are found primarily in the form of proteoglycans, composed of a core protein with varying numbers of GAG chains. The initial building blocks of a GAG are repeating disaccharide units, in the case of heparan sulfate (HS), the disaccharide unit is composed of D-glucuronic acid (GlcA) linked to N-acetyl-D-glucosamine (GlcNAc) (Figure 1A). GlcA can later be epimerized into L-iduronic acid (IdoA), and sulfo groups can be substituted at the 2-hydroxyl groups of both IdoA and GlcA, as well as the 3-hydroxyl and 6-hydroxyl groups and the 2-amino group of glucosamine residue. HS also exhibits a domain structure, with alternating NA and NS domains, composed of contiguous unsulfated N-acetyl regions and N-sulfated regions respectively, and mixed NA/NS domains (Lindahl et al. 1998). Heparin (HP) is a highly sulfated form of HS, and is often represented as a single extended NS domain. Heparin has anticoagulant properties due to the presence of 3-O-sulfation, which forms part of the antithrombin III binding site (Loganathan et al. 1990; Esko and Lindahl 2001; Linhardt 2003). Chondroitin sulfate (CS) is a similar sulfated glycosaminoglycan with the repeating disaccharide unit of GlcA linked to N-acetyl-D-galactosamine (GalNAc). The GalNAc residue can have sulfo groups substituted at the 4- and 6-hydroxyls of the GalNAc (Figure 1B). The GlcA residue in CS can also be epimerized to IdoA, present in dermatan sulfate (DS, also known as chondroitin sulfate B), and both the GlcA and IdoA residues can contain sulfo groups at their 2-positions. CS-GAGs also have domain structures, similar to HS-GAGs (Mikami and Kitagawa 2013).
Figure 1.
Structures of glycosaminoglycans and their oligosaccharides. A. Structure and common domains of heparin (b ~ 0.4, and a + d > c) or heparan sulfate (b < 0.4, and c > a + d). B. common chondroitin sulfates. C. Low molecular weight heparin (Enoxaparin) and ultra-low molecular weight heparins (ULMWH and Arixtra).
This diversity of structure poses a challenge for GAG analysis. While GAG sequencing and domain mapping methods exist, they are still relatively difficult to perform. GAG composition, however, can be readily obtained by several disaccharide analysis methods. These techniques usually involve using enzymes (heparin lyases I, II, and III, and chondroitinase ABC) to break down a GAG into its disaccharide building blocks, which are then separated by liquid chromatography, and detected by ultraviolet spectroscopy or mass spectrometry. Comparison to disaccharide standards reveals the position and number of sulfo groups in each disaccharide. Options for chromatography steps include hydrophilic liquid interaction, strong anion exchange, and ion-pairing reverse-phase chromatography. Sensitivity can be increased to picomole detection limits by labeling with 2-aminoacridone, allowing analysis of GAG mixtures in biological samples (Yang et al. 2012; Sun et al. 2015). Liquid chromatography-mass spectrometry techniques can also be used for direct characterization of low molecular weight heparin oligosaccharides (Li et al. 2012). High resolution one and two-dimensional nuclear magnetic resonance has become an equally important tool for GAG analysis, allowing determination of defined GAG structures (Zhang et al. 2011; Fu et al. 2013). Nuclear magnetic resonance spectroscopy can also provide information about structural elements such as sulfation, epimerization, and acetylation, and can be used to identify contaminants in pharmaceutical heparin (Xu et al. 2011; Xiong et al. 2013; Fu et al. 2014; Guerrini et al. 2008). Additionally, molecular weight distribution and polydispersity can be measured by size exclusion chromatography.
The structural complexity of GAGs is mirrored by the diverse functions they carry out, playing roles in signaling and development, blood coagulation, cancer and inflammation, wound healing, as well as providing unique structural properties (Bernfield et al. 1999; Vlodavsky and Friedmann 2001; Linhardt. 2003; Lauder 2009; Cress et al. 2014). CS and HS proteoglycans are present in the extracellular matrix of animals, where they work in concert with fibrous proteins to maintain cell structure. CS proteoglycans in cartilage and joints act as shock absorbers, due to their tendency as polyanions to retain water. GAGs can also bind extracellular signaling molecules such as growth factors, morphogens and other chemokines, acting as a “molecular sponge.” This allows GAGs to play twin roles in signaling, both stabilizing signal molecule gradients, and functioning as a reservoir of signal molecules, which can be released by GAG degrading enzymes (Taipale and Keski-Oja 1997). CS and HS proteoglycans are additionally found anchored at the cell surface, where they make up a tissue specific GAG coat; variations in the composition and structure of this coat can be thought of as a kind of fingerprint (Li et al., 2015). Regulation of fine structure allows for different affinities to extracellular signaling molecules, providing a level of fine tuning of GAG function (Kato et al. 1994; Shi and Zaia 2009). Endoglycosidases such as heparanase are known to be important regulators of HS function in cancer and inflammation. Breakdown of extracellular matrix HS-GAGs by heparanase removes a physical barrier for leukocytes, which can transit into organs to exert an inflammatory response to injury. This mechanism is often co-opted by metastasizing tumor cells, evidenced by the fact that HPSE expression is a prognostic marker for high metastatic potential (Sato et al. 2004). Heparanase activity also releases bound growth factors that can drive tumor cell proliferation and angiogenesis (Parish et al. 2001; Li and Vlodavsky 2009; Ramani et al. 2013). One of the most well documented and studied functions of HS-GAGs is their anticoagulant activity. HS-GAGs containing 3-O-sulfation, primarily heparin, can bind strongly to antithrombin, a serine protease inhibitor. This binding causes a conformational change that activates antithrombin, inhibiting thrombin and related serine proteases involved in the coagulation cascade (Linhardt. 2003).
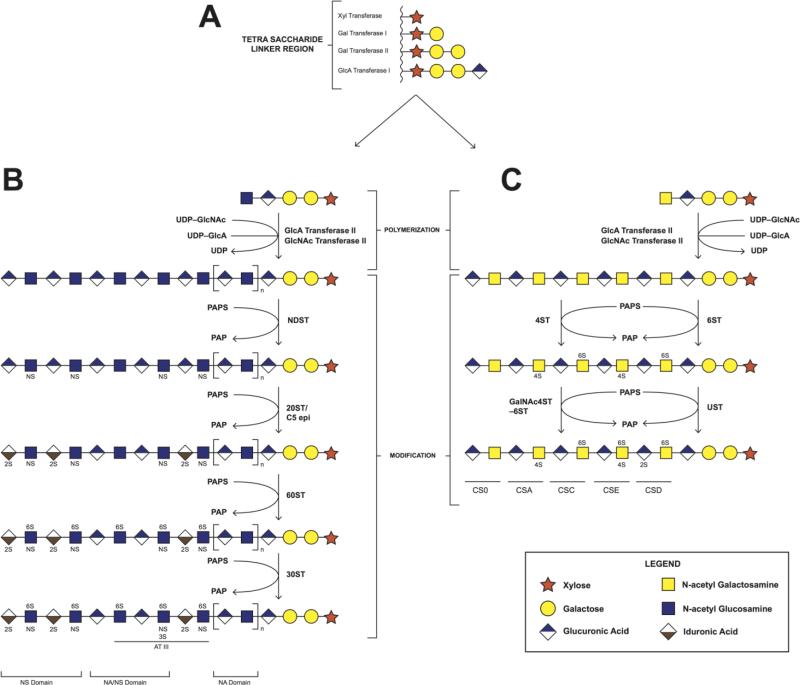
The biosynthesis of CS and HS-GAGs starts out along a similar pathway, beginning with the stepwise addition of four monosaccharides to a serine residue of a core protein (Serine-xylose-galactose-galactose-glucuronic acid), through the involvement of high-energy UDP-activated sugars (Figure 2A). The subsequent addition of either a GlcNAc or GalNAc residue, forms a pentasaccharide, determining whether an HS or CS chain is built, respectively (Mikami and Kitagawa 2013) (Figure 2B & C). The CS and HS chains are then extended by their respective glycosyltransferases, and then modified by a host of sulfotransferases and epimerases. Epimerization, of GlcA residues to IdoA, occurs in both HP and HS, while epimerization, of CS GlcA residues, forms DS. The sulfotransferases use a universal sulfo group donor, 3’-phosphoadenosine-5’-phosphosulfate (PAPS), to transfer a sulfate group to a specific hydroxyl or amino position of a sugar residue. These modifying enzymes often have tissue specific isoforms and expression patterns, giving rise to incredible heterogeneity of structure and function (Sasisekharan and Venkataraman 2000; Mikami and Kitagawa 2013).
Figure 2.
Biosynthesis of heparin/heparan sulfate and chondroitin sulfates. A. Synthesis of the tetrasaccharide linker region. B. Polymerization and modification pathway of heparin/heparan sulfates. C. Polymerization and modification pathway of chondroitin sulfates.
Because of an aging world population, the demand for glycosaminoglycan drugs such as heparin and chondroitin sulfate will continue to climb. Heparin is an invaluable drug for treatment of coagulation and thrombotic disorders, and is listed as one of the World Health Organizations essential medicines. Heparin can also be used to create anticoagulant surfaces for test tubes and in dialysis machines (Murugesan et al. 2008). Low molecular weight heparins (LMWHs), derived from heparin by depolymerization, are used subcutaneously in treating deep vein thrombosis, and are often used in cancer treatment due to the association between cancer and thromboembolic disease. The use of heparins results in additional survival benefits in cancer treatment, not attributable to their anticoagulant activities, mainly due to inhibition of heparin binding growth factors that drive tumor growth, and inhibition of heparanase and selectin mediated metastasis mechanisms (Castelli et al. 2004). CS has been shown to relieve pain and stiffness associated with osteoarthritis, one of the most common musculoskeletal conditions in the world (Schiraldi et al. 2010). In addition to the chondroprotective properties that help to prevent and relieve osteoarthritis, CS also impact many pathologies by its anti-inflammatory response, in part by inhibition of the pro-inflammatory adipokine TNF-α (Tully et al. 2006; Osterman and Lichtenstein 2007; Papoutsaki et al. 2013). In a clinical trial, CS-A and CS-E was shown to have dramatic impact on improving psoriasis and colitis (Lauder 2009). New methods to prepare them in large quantities of GAGs must be implemented to meet growing demand for these GAG-based drugs. Synthetic methods must also be improved to allow preparation of GAGs with defined sequences, improving knowledge of structure-function relationships. This review will focus on the use of recombinant enzymes and metabolic engineering for the production of GAGs.
Current state of GAG preparation
Current heparins and CSs are derived from a variety of animal tissues. Animal source materials present serious concerns for the possibility of transmission of viral and prion diseases, and the susceptibility of animal populations to infectious disease or overharvesting has potential to drastically reduce supply. Moreover, seasonal, geographical and subspecies variations may alter the product obtained from a given animal species. The process of preparing pharmaceutical grade heparin has been altered somewhat over time as the primary tissue source has changed from dog liver to beef lung and finally to porcine intestine (Linhardt. 2003). The preparation of heparin from ruminant tissues obtained at slaughterhouses present a special concern particularly following the appearance of bovine spongiform encephalopathy (BSE, “mad cow disease”) in both humans and cattle (Guerrini et al. 2008) and scrapies prion in sheep (Schonberger 1998). Thus, the use of bovine and ovine tissue products as injectable pharmaceuticals has declined and these tissues are now rarely used in heparin production.
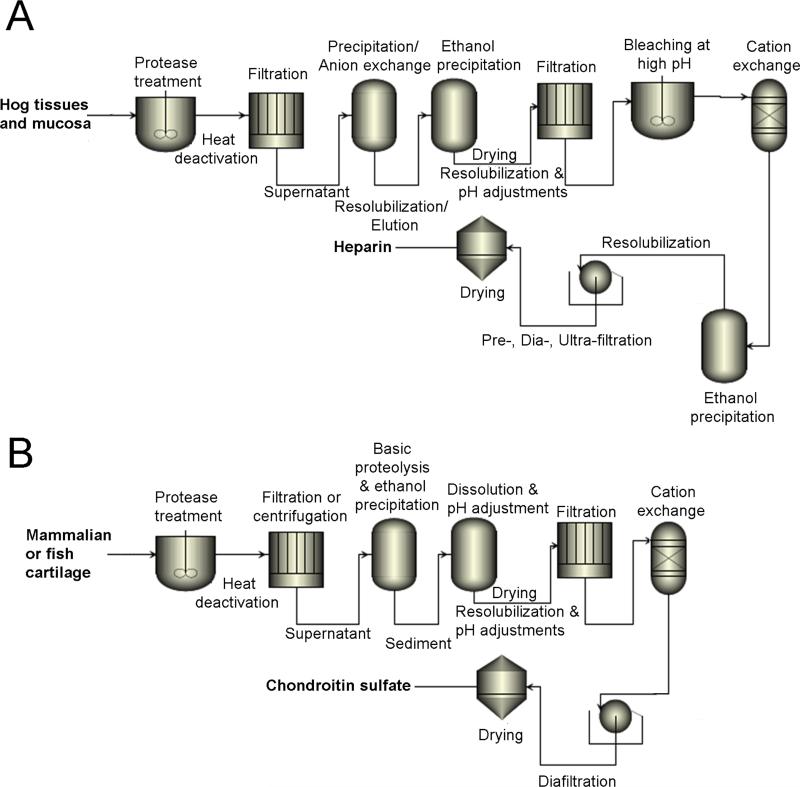
The methods used today for the commercial preparation of heparin have changed from that used early in the 20th century and involve five basic steps (Figure 3A): 1) preparation of tissue; 2) extraction of heparin from tissue; 3) recovery of raw heparin; 4) purification of heparin; and 5) recovery of purified heparin (Etal et al. 1962; Williams RE 1967; Okuyama T, Yoshida K, Sakuraik, Ogurat, Horie K, Tawada A 1975; Vidic H-J 1981; Linhardt et al. 1992; Van Gorp CL 1997; Bhaskar et al, 2012). However, to minimize the environmental impact of high-ash, high-biochemical oxygen demand hydrolyzed protein, raw heparin extraction typically takes place at the hog slaughtering facility itself (not under current good manufacturing practices (cGMP) conditions). Additional high potency heparin may be recovered by saving the waste brine solution of the hog casings operation (Vidic 1981).
Figure 3.
Extractive preparation of glycosaminoglycans. A. heparin and B. chondroitin sulfate.
There are growing concerns about porcine tissue nowadays, especially after the heparin crisis that took place in 2008. This crisis involved the introduction of an oversulfated chondroitin sulfate into heparin produced from hogs in China leading to the death of nearly 100 Americans (Liu et al. 2009). The lack of oversight and cGMP in slaughterhouses leaves the heparin supply chain open to this kind of adulteration, which can be difficult to detect. Bovine lung heparin can be distinguished from porcine intestinal heparin because it contains a different distribution of structural variants of the antithrombin pentasaccharide binding site as well as other differences in disaccharide composition (Loganathan et al. 1990; Fu et al. 2013). It is somewhat more difficult to distinguish bovine intestinal heparin or ovine intestinal heparin (Fu et al. 2013). Moreover, blends of pharmaceutical grade heparins prepared from different species might make the content of non-porcine heparin even more difficult to assess.
Currently available commercial CS is mainly extracted from trachea, nasal septa, chicken keel, shark cartilage and fish (Figure 3B). As “dietary supplements” available in the US market, the overall quality of CS is poorly regulated. Some products contain much less CS than advertised, as low as 10% in some cases (Adebowale et al. 2000). Tracheal CS may sometimes be substituted for higher priced shark-derived CS (Sakai et al. 2007; Higashi et al. 2015). In addition to problems of regulation and transmission of disease, overfishing of shark populations (Higashi et al. 2015) and porcine epidemics such as blue ear pig disease and porcine epidemic diarrhea virus (Zhou et al. 2008) highlight the precarious nature of the supply chain for these GAGs.
Chemical synthesis and enzymatic depolymerization to prepare heparin oligosaccharides
Low molecular weight heparins or fractionated heparins with a molecular weight of ~ 3–8 kDa are a group of heparin-derived anticoagulant/antithrombotic agents (Figure 1C), and their development began approximately 30 years ago (Mousa and Fareed 2001). Currently, the commercial preparation of LMWHs from unfractionated heparin includes the controlled chemical depolymerization of heparin by peroxidative cleavage, nitrous acid cleavage, and chemical β-elimination (Figure 4B). These chemically depolymerized LMWHs such as enoxaparin, Ardeparin sodium, Dalteparin sodium, Nadroparin calcium, Reviparin sodium and Certroparin sodium contain artifacts including 2,6-anhydromannitol, epoxide, 1,6-anhydroglucopyranose, and 1,6-anhydromannopyranose due to the harsh reaction conditions (Higashi et al. 2012; Keire et al. 2013). Potential side effects associated with these process artifacts still remain unknown, and what's more, the animal sourced unfractionated heparin starting material for LMWHs is still at risk.
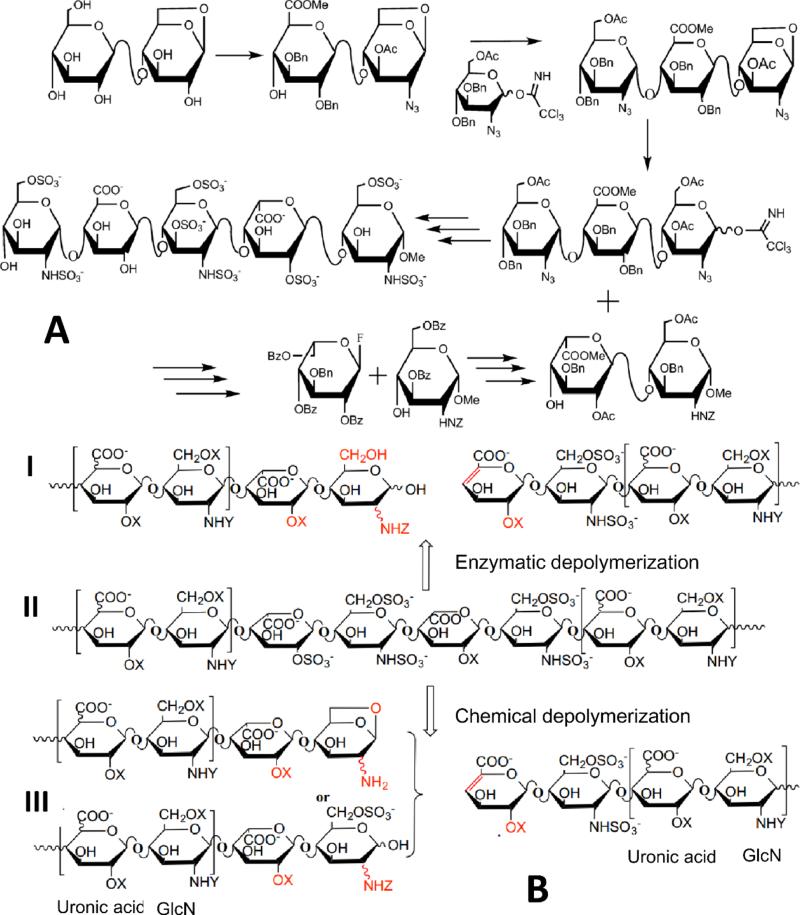
Figure 4.
Chemical synthesis, depolymerization and enzymatic depolymerization of ultra-low molecular weight heparin and low molecular weight heparin. A. Summary of a convergent multi-step chemical synthesis of Arixtra from cellobiose derivative (reagents not shown). B. Enzymatic (I) and chemical (III) depolymerization to prepare low molecular heparins from unfractionated heparin (II).
However, Arixtra® (fondaparinux sodium), a synthetic heparin pentasaccharide drug, introduced by Sanofi in 2002 (Toschi and Lettino 2007) is an example of an ultra-LMWH. This ultra-LMWH (<3 kDa) drug was based on a simplification of the elegant synthesis of the heparin antithrombin pentasaccharide binding site, first reported by Choay and coworkers in the 1980s (Petitou et al. 1986) (Figure 4A). Arixtra differs from heparin in that it is a specific anti-factor Xa agent, which lacks many of the important pharmacological properties of the polycomponent, polypharmacolgical drug heparin (Bick et al. 2005).
About 40% of all heparin used each day in US is unfractionated heparin. It is primarily used in dialysis and hospitalized patients as an intravenous drug. Approximately 55% of US heparin market is dominated by LMWHs, principally used subcutaneously for the treatment of deep vein thrombosis. The final 5% of the heparin market is comprised of the expensive synthetic ultra-LMWH, fondaparinux, which is used in select applications when a side effect, known as heparin-induced thrombocytopenia, is anticipated. The ultra-LMWH market share represents only ~ $0.5B of the total worldwide heparin market of around $4B (Bhaskar et al. 2012). Thus, the worldwide market is evenly split between the use of heparin and LMWHs with very little synthetic ultra-LMWH being used. The reasons for the low demand for fondaparinux are: 1. Expense — fondaparinux is roughly 1000-fold more expensive than heparin and 50-fold more expensive than LMWHs; 2. Poorer pharmacological profile — heparin and LMWH are polycomponent, polypharmacolgical agents that show a better overall therapeutic profile in patients than fondaparinux; 3. Safety — fondaparinux and LMWHs are not reversible with protamine posing safety concerns; and 4. Limitations — fondaparinux is ineffective in a number of applications where heparin and LMWHs are currently used. A new ultra-LMWH called semuloparin has recently been developed for the prevention of venous thromboembolism. It is a semisynthetic ultra-LMWH that is prepared by a selective and controlled depolymerization of heparin through a β-elimination reaction only at the less hindered regions using a phosphazene base (Viskov et al. 2009). Due to its bulky structure, the base cleaves the heparin chain, leaving the crowded AT-binding site intact. Studies in patients showed the antifactor Xa/antifactor IIa ratio of semuloparin to be above 30, indicating nearly pure anti-factor Xa activity (Lassen et al. 2009). Although the preparation cost is significantly lower than that of fondaparinux, it is neither homogeneous nor structurally defined and, since it is still derived from porcine intestinal heparin semuloparin, could be subject to contamination or adulteration.
Bioengineering approaches
Glycosyltransferases
The chemical syntheses of heparin or heparin-like drugs typically involve numerous steps and result in low overall yields and high costs, which limits its clinical application. Chemists are starting to turn towards enzymatic or chemoenzymatic synthesis to circumvent these problems (Gijsen et al. 1996; Karst and Linhardt 2003; Deangelis et al. 2013). Unlike most chemical reactions, these enzymatic reactions are highly chemospecific, regiospecific and stereospecific. Using recombinant technology, glycosyltransferases and heparin biosynthetic enzymes have been cloned and expressed, and are under study for the synthesis of heparin (Orellana et al. 1994; DeAngelis and White 2002). Initial efforts towards a chemoenzymatic preparation of heparin used C5-epimerase to convert the GlcA of the heparosan polysaccharide to IdoA, but relied primarily on chemical modifications for the introduction of N- and O-sulfo groups, creating unwanted sulfation sites (Naggi et al. 2001). An enzymatic synthesis of an oligosaccharide based on the structure of HS has been accomplished using the heparin/HS modification enzymes (Kuberan et al. 2003) and glycosyltransferases (Liu et al. 2010).
Enzymatic synthesis of polysaccharides and oligosaccharides of defined lengths has recently become possible due to the availability of many recombinantly expressed glycosyltransferases (Table 1). These enzymes use UDP-activated sugars produced by uridyltransferases such as GlmU, which can be used to produce UDP-GlcNAc and UDP-GalNac in vitro, building blocks for HS and CS backbones respectively. GlmU is flexible in its substrate specificity, and allows the synthesis of some unnatural UDP-sugars possessing a tag, which can be polymerized into novel glycosaminoglycans (Masuko et al. 2012). This technique has been used to incorporate labile N-trifluoroacetyl groups into HS oligosaccharides, which can later be enzymatically sulfated (Liu et al. 2010; Xu et al. 2011; Xu et al. 2014). Polymerizing enzymes can be processive, by addition of alternating UDP-sugars, or may catalyze the addition of a single sugar, as in KfiA, a UDP-GlcNAc transferase that has been used to build heparin oligosaccharides in a controlled, stepwise manner (Liu et al. 2010; Xu et al. 2011; Xu et al. 2014). Processive glycosyltransferases such as heparosan synthases 1 and 2 (PmHS1 & PmHS2) from Pasteurella multocida, and chondroitin polymerase from Escherichia coli K4, have been used to synthesize HS and CS backbones of varying molecular weight (Sugiura et al. 2002; Sismey-Ragatz et al. 2007). Additionally, site-directed mutagenesis studies have been able to isolate two single-action P. multocida PmHS2 mutants, which can be used to build oligosaccharides in a step-wise manner (Chavaroche et al. 2012).
Table 1.
Enzymes utilized in heparin and chondroitin synthesis.
| Name | Abbreviation | Organism | References |
|---|---|---|---|
| Chondroitin Polymerase/Chondroitin Synthase | K4CP | Escherichia coli K4 | Sugiura et al. 2002; Sugiura et al. 2012 |
| N-acetyl-D-glucosaminyl transferase | KfiA | Escherichia coli K5 | Chen et al. 2006; Xu et al. 2011 |
| Heparosan Synthase 1 & 2 | PmHS1, PmHS2 | Pasteurella multocida | Liu et al 2010; Sismey-Ragatz 2007; Xu et al. 2011 |
| N-acetyl-glucosamine-1-phosphate Uridyltransferase | GlmU | Escherichia coli K5 | Masuko et al. 2012 |
| Arylsulfotransferase IV | AST-IV | Rattus norvegicus | Bhaskar et al. 2014; Burkhart et al. 2000 |
| C5 Epimerase | C5 Epi | Cricetulus griseus (CHO cell) | Bhaskar et al. 2014; Liu et al. 2010; Xu et al. 2011; Zhang et al. 2015 |
| 2-O-sulfotransferase 1 | 2OST-1 | Cricetulus griseus (CHO cell) | Bhaskar et al. 2014; Zhang et al. 2015; Xu et al. 2011 |
| 6-O-sulfotransferase 1 | 6OST-1 | Mus musculus | Bhaskar et al. 2014; Liu et al. 2010; Restaino et al. 2013; Xu et al. 2011 |
| 6-O-sulfotransferase 3 | 6OST-3 | Mus musculus | Bhaskar et al. 2014; Liu et al. 2010; Xu et al. 2011; Zhang et al. 2013 |
| 3-O-sulfotransferase 1 | 3OST-1 | Mus musculus | Bhaskar et al. 2014; Liu et al. 2010; Moon et al. 2012; Xu et al. 2011 |
| 3-O-sulfotransferase 5 | 3OST-5 | Mus musculus | Liu et al. 2010 |
| 3-O-sulfotransferase 3 | 3OST-3 | Mus musculus | Moon et al. 2012 |
| N-deacetylase/N-sulfotransferase | NDST-1 | Rattus norvegicus | Liu et al 2010; Saribas et al. 2004 |
| Chondroitin 4-sulfotransferase 1 | C4ST-1 | Homo sapiens | Sugiura et al. 2012 |
| Chondroitin 6-sulfotransferase 1 | C6ST-1 | Homo sapiens | Sugiura et al. 2012 |
| N-acetyl galactosamine 4-sulfate 6-sulfotransferase | GalNAc4S-6ST | Homo sapiens | Sugiura et al. 2012 |
| uronosyl 2-sulfotransferase | UST | Homo sapiens | Sugiura et al. 2012 |
Sulfotransferases and epimerases
Many sulfotransferases involved in GAG biosynthesis have been expressed and characterized in vitro (Table 1). Unique to HS biosynthesis is the introduction of N-sulfo groups that is carried out by N-sulfotransferase/N-deacetylases (NDSTs), bifunctional enzymes with two active sites (Berninsone & Hirschberg 1998). While the bacterial recombinant expression of active N-deacetylase domain has been difficult, the bacterially expressed N-sulfotransferase domain (NST) has been used in conjunction with N-trifluoroacetyl sugars to achieve precise the introduction of N-sulfo groups sites in heparin oligosaccharides (Liu et al. 2010; Xu et al. 2011; Xu et al. 2014). The presence of N-sulfo groups are a prerequisite for the further introduction of O-sulfo groups and for C5 epimerization, thus NDST specificity controls the formation (or absence, as in heparin) of domain structures in HS (Sheng et al. 2011). C5 epimerase, which produces critical IdoA residues in HS-GAGs, is thought to act irreversibly in vivo, likely due to concurrent introduction of 2-O-sulfo groups by GAG-modifying enzyme complex of C5 epimerase and 2-O-sufotransferase (2OST). The introduction of a 2-O-sulfo group blocks the reversible activity of C5 epimerase in vitro possibly due to steric hindrance suggested from the recent crystallization of C5 epimerase in complex with a heparin oligosaccharide (Qin et al. 2015). There is only one 2OST isoform identified in humans and it can act on both IdoA and GlcA residues adjacent to an N-sulfo glucosamine (GlcNS) residue without a 6-O-sulfo group, with a preference for IdoA. A crystallization study elucidated the molecular basis of this specificity, showing favorable interactions with the N-sulfo group, and suggesting steric hindrance with the 6-sulfo groups of the adjacent residue (Liu et al. 2014). Three 6-O-sulfotransferase isoforms (6OST-1,2,3) have been identified in humans, and found to have slightly different specificities, 6OST-1 and 6OST-2 prefer to transfer a 6-O-sulfo groups to a GlcNS that is next to an GlcA residue and IdoA2S residue, respectively (Bhaskar et al. 2012). There are at least 6 different isoforms of 3-O-sulfotransferases (3OSTs) with distinct substrate specificities, two of which (3OST-1 & 3) have solved crystal structures (Moon et al. 2004; Moon et al. 2012). Comparison of the two structures reveal distinct binding modes for the two isoforms, suggesting a mechanism for recognition of fine saccharide structure. It is thought that the presence of 3-O-sulfo groups can regulate many important HS functions. This is due to the modification being critical for protein binding of at least two specific saccharide sequences, the AT-binding site and the binding of the gD envelope protein of herpes simplex virus 1 (Liu et al, 2002; Kusche-Gullberg and Kjellén 2003).
In addition to the HS sulfotransferases, there are several recombinant CS sulfotransferases with demonstrated in vitro activity, including chondroitin-4-sulfotransferase 1 (C4ST-1), chondroitin-6-sulfotransferase 1 (C6ST-1), N-acetylgalactosamine-4-sulfate 6-sulfotransferase (GalNAc4S-6ST), and uronosyl 2-sulfotransferase (UA2ST) (Sugiura et al. 2012) (Table 1). Less is known about the CS sulfotransferases and the two CS C5 epimerases (Silbert and Sugumaran 2002; Pacheco et al. 2009a; Pacheco et al. 2009b; Thelin et al. 2013), but it is likely that the activities, specificity and biosynthetic control parallels that of the HS biosynthetic enzymes. Moreover, specific CS structures seem to play prominent roles in nervous tissues and in brain development and function (Higashi et al. 2015).
Finally, while not directly involved in GAG biosynthesis, arylsulfotransferase-IV (AST-IV), a mammalian liver detoxification enzyme involved in transferring sulfo groups to the hydroxyl groups of phenols, has been indispensable for chemoenzymatic synthesis of sulfated GAGs. While normally catalyzing the transfer of a sulfo group from PAPS to a phenol, at high concentrations of p-nitrophenyl sulfate, AST-IV can be used to catalyze the reverse reaction transferring a sulfate group from p-nitrophenyl sulfate to PAP, thus, forming PAPS, the universal sulfate donor for sulfotransferases. This reverse reaction can be used as a cofactor regeneration system when coupled to HS or CS sulfotransferase reactions and overcomes strong product inhibition of these sulfotransferases by PAP (Burkart et al. 2000). This cofactor regeneration also produces p-nitrophenol, a yellow colored product which can be easily monitored at a 400 nm wavelength, forming the basis of a commonly used sulfotransferase assay (Burkart and Wong 1999; Sterner et al, 2014). Collectively, this cofactor regeneration system and colorimetric assay represents a valuable enzymatic toolbox for GAG synthesis.
Further structural elucidation of the GAG biosynthetic enzymes and enzymes for cofactor recycling may lead to new, engineered forms with novel specificities, further expanding the range of tools available. While protein engineering offers opportunities to improve the stability and activity of these recombinant enzyme catalysts, the lack of crystal structures for many of these enzymes posses a barrier to progress. Further efforts to scale-up the production of these enzymes in fed-batch fermenters are underway, and have been demonstrated for 4 out of 5 of the HS sulfotransferases including 2OST-1, C5 epimerase, 6OST-1 and 6OST-3 (Restaino et al. 2013a; Zhang et al. 2015a; Zhang et al. 2015b). This opens the way for the industrial scale production of GAGs.
Chemoenzymatic synthesis/depolymerization of heparin oligosaccharides
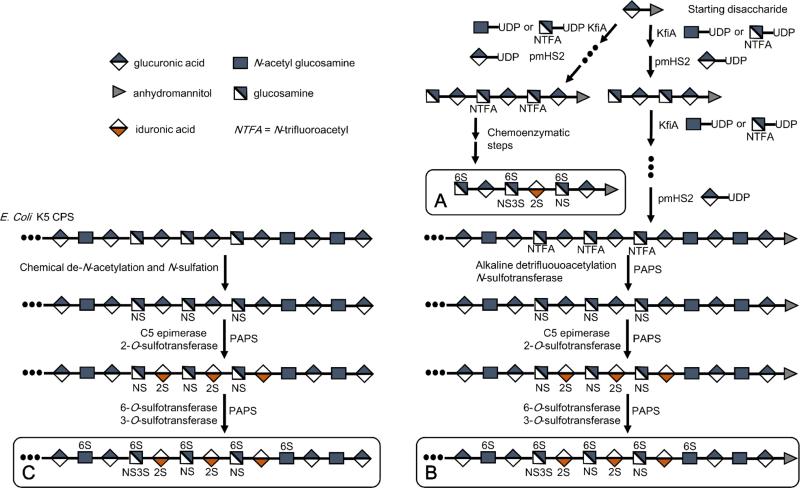
Efforts to produce high-value oligosaccharide targets using this enzymatic toolbox are underway. Two fondaparinux-like ultra-LMWHs (Figures 1 & 5) that showed excellent in vivo and in vitro anticoagulant activity have been chemoenzymatically synthesized using heparin biosynthetic enzymes (Xu et al. 2011). By using a chemoenzymatic approach, it is notable that these homogeneous heptasaccharides were synthesized through an approach biomimetic to heparin biosynthesis and within 12 steps at multi-milligram scale and in approximately 40% overall yield. Both heparin constructs were synthesized initially on a heparosan-derived disaccharide acceptor containing a ring-contracted anhydromannitol residue. Using the N-acetyl glucosaminyltransferase (KfiA) and the heparosan synthase (pmHS2) (Sismey-Ragatz et al. 2007), the acceptor was elongated stepwise from a disaccharide to a heptasaccharide, using the unnatural GlcN-trifluoroacetyl donor, which was later deprotected and N-sulfonated. KfiA transferred GlcN-trifluoroacetyl smoothly, demonstrating that uridine diphosphate sugar is a compatible unnatural substrate for KfiA. Compared with chemical glycosylation, enzymatic glycosylation proceeded with over 80% yield and in a stereospecific manner, giving the correct stereochemistry at each anomeric center.
Figure 5.
Chemoenzymatic synthesis of A. ultra-low molecular weight heparins; B. low molecular weight heparins; and C. bioengineered heparin.
The selective epimerization and sulfation of heparin oligosaccharide backbones are done using C5-epimerase and O-sulfotransferases, which converts GlcA into its C5-epimer IdoA and transfers sulfo groups to desired positions, respectively. The selectivities of these modification enzymes provide excellent control over products but require careful reaction scheme design and careful selection of the appropriate isoforms to obtain the desired target structures. The most effective schemes are those that follow the reaction order found in natural heparin synthesis. Investigation of ideal reaction order and enzymatic activity, based on the heparin biosynthesis pathway, has shown that C5-epimerase only converts GlcA residues between two GlcNS residues (Liu et al. 2010) to IdoA and works best collaborating with 2OST, which locks the normally reversible epimerization into the IdoA conformation upon introduction of the 2-O-sulfo group. This specificity requires the NST pre-treatment before C5-epimerase and 2OST (Sheng et al. 2012).
Recently, one-pot enzymatic synthesis has been explored for the preparation of certain heparin oligosaccharide targets (Chen et al. 2013). Chemoenzymatic strategies appear to be the next step in the development of efficient syntheses of heparin oligosaccharides having up to 20 saccharide units.
Sugiura and coworkers have chemoenzymatically synthesized various CS species with defined lengths and defined sulfate compositions, using bacterial chondroitin polymerase and recombinant CS sulfotransferases, including chondroitin-4-sulfotransferase-1 (C4ST-1), chondroitin-6-sulfotransferase-1 (C6ST-1), N-acetylgalactosamine 4-sulfate 6-sulfotransferase (GalNAc4S-6ST), and uronosyl 2-sulfotransferase (UA2ST). Chemoenzymatic synthesis enables the generation of CS chains of the desired lengths, compositions, and distinct structures, and the resulting library will be a useful tool for studies of CS functions (Sugiura et al. 2012).
Another approach to oligosaccharide synthesis, used in preparing LMWHs involves the controlled enzymatic depolymerization of heparin using recombinant heparinases. In contrast to chemical depolymerization, enzymatic depolymerization using recombinant heparin lyases was proven to be a relatively artifact-free method (Fu et al. 2014b). Enzymatic depolymerization of heparin in scalable and potentially provides more access to LMWHs with specific in vivo biological and pharmacological activities. LMWHs, such as tinzaparin, prepared through controlled heparinase treatment have already been successfully commercialized.
Bioengineered heparin
A concerted effort is currently underway to chemoenzymatically synthesize a full length bioengineered heparin, based on the overexpression of the E. coli K5 capsular polysaccharide (CPS) heparosan, and subsequent modification with recombinant HP biosynthetic enzymes (Zhang et al. 2008). Such a bioengineered heparin might one day be approved as a generic heparin and also used in the preparation of LMWHs, increasing the supply and eliminating the risks that come with drugs derived from animal tissues (Liu et al. 2009; Wang et al. 2011; Bhaskar et al. 2012). Small amounts of bioengineered heparin have been prepared from this E. coli heparosan in several laboratories (Kuberan et al. 2003; Lindahl et al. 2005; Kane et al. 2006; Zhang et al. 2008). Over the past 5 years, research has focused on developing a scalable process capable of producing sufficient quantities of a bioengineered heparin for pre-clinical and clinical evaluation. Even greater challenges are anticipated to meet global demand (over 100 tons/y) if a bioengineered, generic version of heparin, chemically and biologically equivalent to current USP heparin, is to be introduced in the future (Liu et al. 2009; Wang et al. 2011; Linhardt and Liu, 2012).
Unlike the chemoenzymatic synthesis of LMWHs and ULMWH oligosaccharides, the process for preparing bioengineered heparin begins with an E. coli fermentation to prepare the CPS, heparosan, followed by its chemical (or enzymatic) de-N-acetylation and N-sulfonation. Treatment of N-sulfo, N-acetyl heparosan with recombinant O-sulfotransferases and C5-epimerase in the presence of a PAPS cofactor recycling system results in a bioengineered heparin that closely resembles the chemical and biological properties of heparin. Key elements for the commercialization include: process control, scale-up, and a reduction in the costs of CPS, recombinantly expressed biosynthetic enzymes, and PAPS cofactor (Burkart et al. 2000; Zhou et al. 2011) (Wang et al. 2013).
Recombinant heparin biosynthetic enzymes, C5 epimerase, 2OST, 6OST and 3OST, are currently being expressed fused to maltose binding protein or (His)6 tagged at their N-termini (Table 1). This affords a handle that allows for the convenient purification of these enzymes and their immobilization onto beaded supports. Immobilization both stabilizes these enzymes and allows for their easy recovery and reuse, which simplifies product purification. A recent investigation showed that the enzymes maintain greater than 80% of activity after immobilization (Xiong et al. 2013). These recombinant enzymes have been immobilized on amino-linked agarose gel beads at a loading of 20 mg/ml of gel with enhanced thermo stability (Clarke et al. 2000).
The control of number and weight average molecular weight of the final bioengineered heparin is another challenge for making a product that closely resembles porcine intestinal heparin. The heparosan CPS from E. coli K5 has a higher average molecular weight (75 KD) than heparin (~ 15 KD) (Zhang et al. 2008). Moreover, as sulfo groups are transferred to heparosan the molecular weight of a given chain increases by 1.60-fold to 1.75-fold. The average molecular weight of the CPS can be conveniently decreased in the base-catalyzed de-N-acetylation to between 8 KD to 10 KD, affording a precursor polysaccharide that will afford a bioengineered heparin of the same average molecular weight as porcine intestinal heparin. Process control, time, temperature, base and heparosan concentration can be optimized based on the starting CPS to afford an intermediate with desired molecular weight properties and N-acetyl content (Wang et al. 2011). It might also be possible to control the molecular weight of heparosan through manipulation of culture conditions, chain termination and genetic manipulation (Griffiths et al. 1999). Infection with phage carrying heparosan lyase has been examined as a means to control molecular weight (Clarke et al. 2000). Molecular weight can be rapidly estimated by polyacrylamide gel electrophoresis and analyzed by size-exclusion chromatography and comparison made to a USP heparin standard. The chemical N-sulfonation step also needs to be controlled to ensure all the amino groups in the glucosamine residues are either substituted with an N-sulfo or N-acetyl group (Fu et al. 2014a).
Recently, a successful one-pot chemoenzymatic synthesis of complex full-length heparin/HS polysaccharides has been achieved beginning from N-sulfoheparosan in our laboratory. This approach of modulating enzymatic activity through use of an optimized enzyme/substrate ratio is suited for high throughput screening studies aimed at better understanding of heparin's structural heterogeneity and its impact on structure activity relationship. Diversity in 3OST family (7 different isoforms) is primarily responsible for wide array of biological functions attributed to heparin/HS glycosaminoglycans. One-pot chemoenzymatic synthesis of heparin/HS chains provides a way to decipher the substrate specificity of various 3OST isoforms with easy availability of polysaccharide/oligosaccharide substrates (Bhaskar et al. 2015).
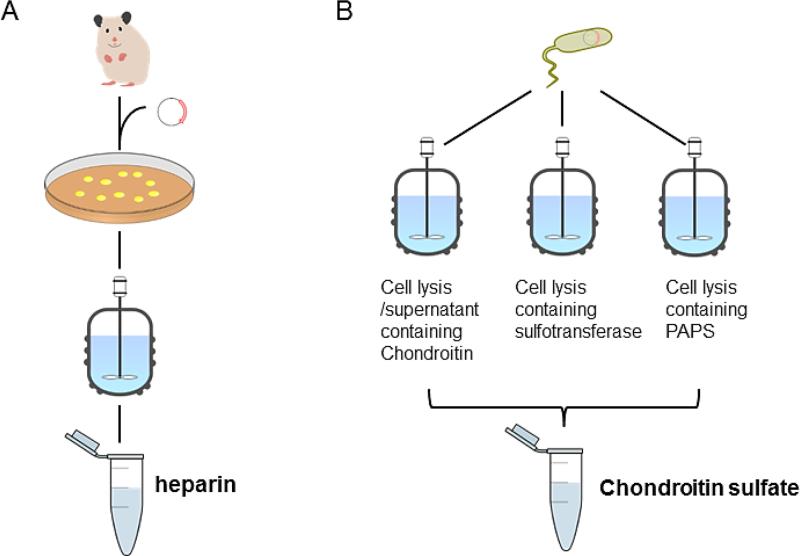
Metabolic engineering of CHO cells
More and more effort has been focused on producing GAG through metabolic engineering (Figure 6A). Chinese hamster ovary (CHO) cells have been widely used in producing therapeutic proteins, due to glycosylation that more closely resembles native human proteins, and relative safety from biological contamination and industrial scalability (Datta et al. 2013b). On transfecting CHO cells with human gene encoding NDST-2 (NDST2) and the gene encoding 3OST-1 (Hs3st1), a substantial increase of AT-binding and anticoagulant activity was observed in CHO cell-produced HS (Baik et al. 2012). However, the disaccharide composition of this GAG was not consistent with that of the pharmaceutical heparin, as it showed an exceptionally high composition of GlcA-GlcNS disaccharide units (Baik et al. 2012). One hypothesis supporting this observation is that NDST is involved in the termination of HS/heparin chains (Esko and Selleck 2002). The expression level of NDST2 and its effect on the introduction of N-sulfo groups may overwhelm the functional expression of Hs3st (Baik et al. 2012). Refinement of the CHO cell system by designing Golgi-targeted Hs3st1 enhanced the expression of 3OST-1 (Datta et al. 2013a). The improvement of anticoagulant activity was further confirmed the structural signature of the AT-binding site by tetrasaccharide analysis (Datta et al. 2013a). Moreover, the overexpression of Hs3st1 in the Golgi compartment may also result in the up-regulation of other sulfotransferases natively expressed in CHO cell Golgi. Engineering CHO cell HS into a more heparin-like structure will clearly require better control of heparin/HS polymerization, as well as up-regulation of the genes controlling the introduction of critical 2-O-sulfo and 6-O-sulfo groups (Datta et al. 2013a).
Figure 6.
Alternate strategies for metabolic engineering of glycosaminoglycans. A. CHO cell engineering to produce heparin, based on manipulation of existing pathway for HS biosynthesis. B. An approach for the E. coli based production of chondroitin sulfate using microbial biotransformation. Three E. coli strains produce components for CS synthesis, including chondroitin, sulfotransferases, and the sulfate donor PAPS, which are then combined to produce CS.
Metabolic engineering of E. coli strains producing chondroitin and heparosan
Capsular polysaccharides produced by pathogenic E. coli strains such as K4 and K5 are an important source of precursors for chemoenzymatic synthesis of CS and heparin polysaccharides. Several studies have been performed to improve the production and yield of K4 CPS comprised of fructosylated chondroitin (Manzoni et al. 1996; Zoppetti et al. 2004; Cimini et al. 2010; Restaino et al. 2011). The growth of E. coli K4 has been optimized by altering medium composition, including the use of glucose, glycerol and soya peptone. Direct feeding monosaccharides precursors including GlcA, GalNAc, and fructose results in an increased yield of CPS (Restaino et al. 2013b). High cell density cultivation, accomplished through microfiltration fermentation to prevent acetate accumulation increased the amount of K4 CPS (Restaino et al. 2011).
In addition to fermentation optimization, increasing attention has been focused on genetic modification and the preparation of recombinant strains to produce CPS (Zanfardino et al. 2010; Cimini et al. 2010; Doherty et al. 2011). More information on the precise function of biosynthetic genes should be useful for improving CPS production. Non-pathgenetic production of non-fructosylated chondroitin can be achieved by integrating group I, II, and III genes related to the transportation and biosynthesis of E. coli K4 capsular polysaccharide into the chromosomes of E. coli K-12 and Xanthomonas campestris (pv. campestris) (Doherty et al. 2011). Homologous overexpression of kfoC in K4 reportedly increased CPS productivity by 100%, although the compatibility of the plasmid in wild type E. coli K4 strain may cause gene expression stability issues during scale-up (Cimini et al. 2010). A recent publication suggests that utilizing engineered IS2 transposable elements from K4 fused with kfoC will result in a more stable overexpression system upon integration into the genome (He et al. 2015). A 2.5-fold increase is reported in large-scale fermenter (He et al. 2015). Moreover, a single mutation on chondroitin polymerase kfoC (R313Q) potentially enhances the affinity of the polymerase to the UDP-GalNAc increasing K4 CPS productivity by 80% (Zanfardino et al. 2010). Recently, our group investigated optimizing expression levels for biosynthetic genes of K4 CPS in E. coli BL21 Star™ (DE3). By using ePathbrick platform vectors designed for tunable gene copy number and promoter strength in a single plasmid, a maximum production of 2.4 g/L was observed in DO-STAT fed batch fermenter (Cimini et al. 2013). A similar strategy has also been applied to the production of heparosan. Introduction of the four heparosan biosynthetic genes (KfiA-D) from E. coli K5 into E. coli BL21 resulted in a final yield of 1.88 g/L in a DO-STAT fed batch fermenter (Zhang et al. 2012).
Transcription factors may also play a major role in regulating CPS biosynthesis. Homologous overexpression of rfaH in E. coli K4 resulted in a total CPS yield of 5.3 g/L in a fed-batch experiment, representing the highest reported level of bacterial chondroitin production. The rfaH gene is a transcriptional activator that carries out an anti-termination process during CPS expression. It binds to the operon polarity suppressor element located just upstream of many CPS gene starts and controls promoter distal gene expression by preventing the termination of transcripts and promoting transcription over a long distance (Cimini et al. 2013). Overexpression of the transcriptional regulator slyA enhances the E. coli K4 CPS production by 1.85-fold higher than the wild type strain (Wu et al. 2013). As a global transcriptional regulator, slyA may up-regulate region II gene cluster expression for E. coli K4 CPS synthesis while down-regulating the genes involved in glycolysis and citrate cycle pathway (Wu et al. 2013).
Conclusions and future prospects
The small-scale chemoenzymatic synthesis of bioengineered heparin has been demonstrated and work is proceeding on scaling-up and commercializing its production. It should not be long before similar studies are successful in the chemoenzymatic synthesis of various chondroitin sulfates. Enzyme engineering should be useful to improve the folding of the recombinant GAG biosynthetic enzymes increasing their production levels in E. coli. Similarly enzyme engineering or alternative expression systems should be useful in increasing the activity and stability of these recombinant catalysts.
In the future the metabolic engineering of GAGs may also be possible. While some initial progress has been made in the metabolic engineering of CHO cells, the use of CRISPR might allow the controlled up-regulation and down-regulation of Golgi enzymes to control expression levels required for GAG targets with different fine structures. Metabolic engineering of prokaryotes (without a Golgi), such as E. coli, posses even greater challenges (Bhan et al. 2013; Xu et al. 2013; Cress et al. 2015; Jones et al. 2015). One intermediate approach can be envisioned using currently available technology to couple metabolic engineering with biotransformation. For example, the production of chondroitin CPS from glucose using an engineered E. coli is currently possible (He et al. 2015). Moreover, PAPS can be produced from ATP and inorganic sulfate using three E. coli expressed recombinant enzymes, adenosine 5’-triphosphate (ATP) sulfurylase, adenosine 5’-phosphosulfate (APS) kinase, and pyrophosphatase (Zhao et al. 2011) (Figure 6B). Finally, recombinant C4ST-1 can be expressed in E. coli. A relatively straightforward process to prepare chondroitin-4-sulfate can be envisioned by combining chondroitin produced in a first E. coli fermentation, PAPS is produced in a second E. coli fermentation, and recombinant C4ST-1 is produced in a third E. coli fermentation (Figure 6). Certainly, it is also possible, but considerably more challenging, to metabolically engineer a single E. coli to biosynthesize CS and shed it into the culture media.
Acknowledgements
The authors are grateful for support from the National Institutes of Health (HL094463, GM102137, HL62244, HL096972) and the National Science Foundation (MCB-1448657).
Footnotes
Competing Interests
The authors declare no competing interests.
References
- Adebowale AO, Cox DS, Liang Z, Eddington ND. Analysis of Glucosamine and Chondroitin Sulfate Content in Marketed Products and the Caco-2 Permeability of Chondroitin Sulfate Raw Materials. 2000;3:37–44. [Google Scholar]
- Baik JY, Gasimli L, Yang B, Datta P, Zhang F, Glass C a., Esko JD, Linhardt RJ, Sharfstein ST. Metabolic engineering of Chinese hamster ovary cells: Towards a bioengineered heparin. Metab Eng. 2012;14:81–90. doi: 10.1016/j.ymben.2012.01.008. doi: 10.1016/j.ymben.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan N, Xu P, Koffas M a G. Pathway and protein engineering approaches to produce novel and commodity small molecules. Curr Opin Biotechnol. 2013;24:1137–1143. doi: 10.1016/j.copbio.2013.02.019. doi: 10.1016/j.copbio.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Bhaskar U, Li G, Fu L, Onishi A, Suflita M, Dordick J, Linhardt RJ. Combinatorial one-pot chemoenzymatic synthesis of heparin. 2015;122:399–407. doi: 10.1016/j.carbpol.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar U, Sterner E, Hickey AM, Onishi A, Zhang F, Dordick JS, Linhardt RJ. Engineering of routes to heparin and related polysaccharides. Appl Microbiol Biotechnol. 2012;93:1–16. doi: 10.1007/s00253-011-3641-4. doi: 10.1007/s00253-011-3641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick RL, Frenkel EP, Walenga J, Fareed J, Hoppensteadt DA. Unfractionated heparin, low molecular weight heparins, and pentasaccharide: basic mechanism of actions, pharmacology, and clinical use. Hematol Oncol Clin North Am. 2005;19:1–51. v. doi: 10.1016/j.hoc.2004.09.003. doi: 10.1016/j.hoc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Burkart M, Izumi M, Chapman E, Lin C, Wong C. Regeneration of PAPS for the enzymatic synthesis of sulfated oligosaccharides. J Org Chem. 2000;65:5565–5574. doi: 10.1021/jo000266o. [DOI] [PubMed] [Google Scholar]
- Burkart M, Wong C. A continuous assay for the spectrophotometric analysis of sulfotransferases using aryl sulfotransferase IV. Anal Biochem. 1999;274:131–137. doi: 10.1006/abio.1999.4264. doi: 10.1006/abio.1999.4264. [DOI] [PubMed] [Google Scholar]
- Castelli R, Porro F, Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc Med. 2004;9:205–213. doi: 10.1191/1358863x04vm566ra. doi: 10.1191/1358863x04vm566ra. [DOI] [PubMed] [Google Scholar]
- Chavaroche A a E, Van Den Broek L a M, Boeriu C, Eggink G. Synthesis of heparosan oligosaccharides by Pasteurella multocida PmHS2 single-action transferases. Appl Microbiol Biotechnol. 2012;95:1199–1210. doi: 10.1007/s00253-011-3813-2. doi: 10.1007/s00253-011-3813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li Y, Yu H, Sugiarto G, Thon V, Hwang J, Ding L, Hie L, Chen X. Tailored design and synthesis of heparan sulfate oligosaccharide analogues using sequential one-pot multienzyme systems. Angew Chem Int Ed Engl. 2013;52:11852–6. doi: 10.1002/anie.201305667. doi: 10.1002/anie.201305667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, De Rosa M, Carlino E, Ruggiero A, Schiraldi C. Homologous overexpression of rfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide. Microb Cell Fact. 2013;12:46. doi: 10.1186/1475-2859-12-46. doi: 10.1186/1475-2859-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Restaino OF, Catapano A, De Rosa M, Schiraldi C. Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl Microbiol Biotechnol. 2010;85:1779–87. doi: 10.1007/s00253-009-2261-8. doi: 10.1007/s00253-009-2261-8. [DOI] [PubMed] [Google Scholar]
- Clarke BR, Esumeh F, IS R. Cloning, expression, and purification of the K5 capsular polysaccharide lyase (KflA) from coliphage K5A: evidence for two distinct K5 lyase enzymes. J Bacteriol. 2000;182:3761–3766. doi: 10.1128/jb.182.13.3761-3766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress BF, Englaender J a., He W, Kasper D, Linhardt RJ, Koffas M a G. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev. 2014;38:660–697. doi: 10.1111/1574-6976.12056. doi: 10.1111/1574-6976.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress BF, Toparlak ÖD, Guleria S, Lebovich M, Stieglitz JT, Englaender J a., Jones JA, Linhardt RJ, Koffas M a. G. CRISPathBrick: Modular Combinatorial Assembly of Type II-A CRISPR Arrays for dCas9-Mediated Multiplex Transcriptional Repression in E. coli. ACS Synth Biol. 2015 doi: 10.1021/acssynbio.5b00012. 150330104005005. doi: 10.1021/acssynbio.5b00012. [DOI] [PubMed] [Google Scholar]
- Datta P, Li G, Yang B, Zhao X, Baik JY, Gemmill TR, Sharfstein ST, Linhardt RJ. Bioengineered chinese hamster ovary cells with golgi-targeted 3-O-sulfotransferase-1 biosynthesize heparan sulfate with an antithrombin-binding site. J Biol Chem. 2013a;288:37308–37318. doi: 10.1074/jbc.M113.519033. doi: 10.1074/jbc.M113.519033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P, Linhardt RJ, Sharfstein ST. An ’omics approach towards CHO cell engineering. Biotechnol Bioeng. 2013b;110:1255–1271. doi: 10.1002/bit.24841. doi: 10.1002/bit.24841. [DOI] [PubMed] [Google Scholar]
- Keire David A., Buhse Lucinda F., al-Hakim A, Keire DA, Buhse LF, Al-Hakim A, Keire David A., Buhse Lucinda F., al-Hakim A. Characterization of currently marketed heparin products: composition analysis by 2D-NMR. Anal Methods. 2013;5:2984–2994. [Google Scholar]
- Deangelis PL, Liu J, Linhardt RJ. Chemoenzymatic synthesis of glycosaminoglycans: Re-creating, re-modeling and re-designing nature's longest or most complex carbohydrate chains. Glycobiology. 2013;23:764–777. doi: 10.1093/glycob/cwt016. doi: 10.1093/glycob/cwt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis PL, White CL. Identification and molecular cloning of a heparosan synthase from Pasteurella multocida Type D. J Biol Chem. 2002;277:7209–7213. doi: 10.1074/jbc.M112130200. doi: 10.1074/jbc.M112130200. [DOI] [PubMed] [Google Scholar]
- Doherty DH, Weaver crag ACA, Miyamoto K, Minamisawa T. Compositions and methods for bacterial production of chondroitin. 2011 [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–73. doi: 10.1172/JCI13530. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Etal MMM, Evans TD, Mozen MMET. Process for purifying heparin. 1962 US Pat. #3058884.
- Fu L, Li G, Yang B, Onishi A, Li L, Sun P, Zhang F, Linhardt R. Structural Characterization of Pharmaceutical Heparins Prepared from Different Animal Tissues. J Pharm Sci. 2013;102:1447–1457. doi: 10.1002/jps.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li L, Cai C, Li G, Zhang F, Linhardt RJ. Heparin stability by determining unsubstituted amino groups using hydrophilic interaction chromatography mass spectrometry. Anal Biochem. 2014a;461:46–48. doi: 10.1016/j.ab.2014.05.028. doi: 10.1016/j.ab.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Zhang F, Li G, Onishi A, Bhaskar U, Sun P, Linhardt RJ. Structure and Activity of a New Low-Molecular-Weight Heparin Produced by Enzymatic Ultrafiltration. J Pharm Sci. 2014b;100:1375–1383. doi: 10.1002/jps.23939. DOI 10. doi: 10.1002/jps.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsen HJM, Qiao L, Fitz W, Wong C-H. Recent advances in the chemoenzymatic synthesis of carbohydrates and carbohydrate mimetics. Chem Rev. 1996;96:443–474. doi: 10.1021/cr950031q. doi: 10.1021/cr950031q. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Barrett B, Cook N, Roberts IS. Biosynthesis of the Escherichia coli K5 capsular polysaccharide. Biochem Soc Trans. 1999;27:507–512. doi: 10.1042/bst0270507. [DOI] [PubMed] [Google Scholar]
- Guerrini M, Beccati D, Shriver Z. Oversulfated chondroitin sulfate is a major contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Fu L, Li G, Andrew Jones J, Linhardt RJ, Koffas M. Production of chondroitin in metabolically engineered E. coli. Metab Eng. 2015;27:92–100. doi: 10.1016/j.ymben.2014.11.003. doi: 10.1016/j.ymben.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Higashi K, Hosoyama S, Ohno A, Masuko S, Yang B, Sterner E, Wang Z, Linhardt R, Toida T. Photochemical preparation of a novel molecular weight heparin. Carbohyd Polym. 2012;87:1737–1743. doi: 10.1016/j.carbpol.2011.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Takeuchi Y, Mukuno A, Tomitori H, Miya M, Linhardt RJ, Toida T. Composition of Glycosaminoglycans in Elasmobranchs including Several Deep-Sea Sharks: Identification of Chondroitin/Dermatan Sulfate from the Dried Fins of Isurus oxyrinchus and Prionace glauca. PLoS One. 2015;10:e0120860. doi: 10.1371/journal.pone.0120860. doi: 10.1371/journal.pone.0120860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Toparlak ÖD, Koffas MA. Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol. 2015;33:52–59. doi: 10.1016/j.copbio.2014.11.013. doi: 10.1016/j.copbio.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Cimini D, Rosa M De, Viggiani A, Restaino OF, Carlino E, Schiraldi C. Improved fructosylated chondroitin production by kfoC overexpression in E. coli K4. J Biotechnol. 2010;150:324–31. doi: 10.1016/j.jbiotec.2010.09.954. doi: 10.1016/j.jbiotec.2010.09.954. [DOI] [PubMed] [Google Scholar]
- Karst NA, Linhardt RJ. Recent Chemical and Enzymatic Approaches to the Synthesis of Glycosaminoglycan Oligosaccharides | BenthamScience. Curr Med Chem. 2003;10:1993–2031. doi: 10.2174/0929867033456891. [DOI] [PubMed] [Google Scholar]
- Kato M, Wang H, Bernfield M, Gallagher JT, Turnbull JE. Cell surface syndecan-1 on distinct cell types differs in fine structure and ligand binding of its heparan sulfate chains. J Biol Chem. 1994;269:18881–18890. [PubMed] [Google Scholar]
- Kuberan B, Lech MZ, Beeler DL, Wu ZL, Rosenberg RD. Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nat Biotechnol. 2003;21:1343–1346. doi: 10.1038/nbt885. doi: 10.1038/nbt885. [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Kjellén L. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 2003;13:605–611. doi: 10.1016/j.sbi.2003.08.002. doi: 10.1016/j.sbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Dahl OE, Mismetti P, Destrée D, Turpie a GG. AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replacement surgery--TREK: a dose-ranging study. J Thromb Haemost. 2009;7:566–572. doi: 10.1111/j.1538-7836.2009.03301.x. doi: 10.1111/j.1538-7836.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- Lauder RM. Chondroitin sulphate: a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med. 2009;17:56–62. doi: 10.1016/j.ctim.2008.08.004. doi: 10.1016/j.ctim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Li G, Li L, Tian F, Zhang L, Xue C, Linhardt RJ. Glycosaminoglycanomics of Cultured Cells using a Rapid and Sensitive LC-MS/MS Approach. ACS Chem Biol. 2015 doi: 10.1021/acschembio.5b00011. 150213154901005. doi: 10.1021/acschembio.5b00011. [DOI] [PubMed] [Google Scholar]
- Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102:823–828. doi: 10.1160/TH09-02-0091. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U, Kusche-Gullberg M, Kjellen L, Lindahl U, Kusche-Gullberg M, Kjellén L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, Ampofo SA, Fareed J, Hoppensteadt D, Folkman J, Mulliken JB. Isolation and characterization of human heparin. Biochemistry. 1992;31:12441–12445. doi: 10.1021/bi00164a020. doi: 10.1021/bi00164a020. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, Liu J. Synthetic heparin. Curr Opin Pharmacol. 2012;12:217–219. doi: 10.1016/j.coph.2011.12.002. doi: 10.1016/j.coph.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt RJ. Heparin: Strucuture and Activity. J Med Chem. 2003;46:2521–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- Liu C, Sheng J, Krahn JM, Perera L, Xu Y, Hsieh PH, Dou W, Liu J, Pedersen LC. Molecular mechanism of substrate specificity for heparan sulfate 2-O-sulfotransferase. J Biol Chem. 2014;289:13407–13418. doi: 10.1074/jbc.M113.530535. doi: 10.1074/jbc.M113.530535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Xu Y, Chen M, Weïwer M, Zhou X, Bridges AS, DeAngelis PL, Zhang Q, Linhardt RJ, Liu J. Chemoenzymatic design of heparan sulfate oligosaccharides. J Biol Chem. 2010;285:34240–34249. doi: 10.1074/jbc.M110.159152. doi: 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan D, Wang HM, Mallis LM, RJ L. Structural variation in the antithrombin III binding site region and its occurrence in heparin from different sources. Biochemistry. 1990;29:4362–4368. doi: 10.1021/bi00470a015. [DOI] [PubMed] [Google Scholar]
- Manzoni M, Bergomi S, Molinari F, Cavazzoni V, Di S, Studi D, Milano DI. production and purification of an extracellularly produced K4 polysaccharide from Escherichia coli. Biotechnol Lett. 1996;4:383–386. doi: 10.1007/BF00143456. [Google Scholar]
- Masuko S, Bera S, Green DE, Weïwer M, Liu J, Deangelis PL, Linhardt RJ. Chemoenzymatic synthesis of uridine diphosphate-GlcNAc and uridine diphosphate-GalNAc analogs for the preparation of unnatural glycosaminoglycans. J Org Chem. 2012;77:1449–1456. doi: 10.1021/jo202322k. doi: 10.1021/jo202322k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield Merton, Götte Martin, PWP, Reizes Ofer, Fitzgerald Marilyn L., JL, Zako M, Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions Of Cell Surface Heparan Sulfate Proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta - Gen Subj. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Moon a. F, Xu Y, Woody SM, Krahn JM, Linhardt RJ, Liu J, Pedersen LC. Dissecting the substrate recognition of 3-O-sulfotransferase for the biosynthesis of anticoagulant heparin. Proc Natl Acad Sci. 2012;109:5265–5270. doi: 10.1073/pnas.1117923109. doi: 10.1073/pnas.1117923109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AF, Edavettal SC, Krahn JM, Munoz EM, Negishi M, Linhardt RJ, Liu J, Pedersen LC. Structural analysis of the sulfotransferase (3-O-sulfotransferase isoform 3) involved in the biosynthesis of an entry receptor for herpes simplex virus 1. J Biol Chem. 2004;279:45185–45193. doi: 10.1074/jbc.M405013200. doi: 10.1074/jbc.M405013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Fareed J. Overview: from heparin to low molecular weight heparin: beyond anticoagulation. Curr Opin Investig Drugs. 2001;2:1077–80. [PubMed] [Google Scholar]
- Murugesan S, Xie J, Linhardt RJ. Immobilization of heparin: approaches and applications. Curr Top Med Chem. 2008;8:80–100. doi: 10.2174/156802608783378891. doi: 10.2174/156802608783378891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggi A, Torri G, Casu B, Oreste P, Zoppetti G, Li JP, Lindahl U. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin Thromb Hemost. 2001;27:437–43. doi: 10.1055/s-2001-17954. doi: 10.1055/s-2001-17954. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Yoshida K, Sakuraik Ogurat, Horie K, Tawada AHT. Method of separating and recovering mucopolysaccharides from connective tissues of animals. 1975 US Pat. #3862003.
- Orellana A, Hirschberg CB, Wei Z, Swiedler SJ, Ishihara M. Molecular cloning and expression of a glycosaminoglycan N-acetylglucosaminyl N-deacetylase/N-sulfotransferase from a heparin-producing cell line. J Biol Chem. 1994;269:2270–2276. [PubMed] [Google Scholar]
- Osterman MT, Lichtenstein GR. Current and future anti-TNF therapy for inflammatory bowel disease. Curr Treat Options Gastroenterol. 2007;10:195–207. doi: 10.1007/s11938-007-0013-3. doi: 10.1007/s11938-007-0013-3. [DOI] [PubMed] [Google Scholar]
- Pacheco B, Maccarana M, Goodlett DR, Malmström A, Malmström L. Identification of the active site of DS-epimerase 1 and requirement of N-Glycosylation for enzyme function. J Biol Chem. 2009a;284:1741–1747. doi: 10.1074/jbc.M805479200. doi: 10.1074/jbc.M805479200. [DOI] [PubMed] [Google Scholar]
- Pacheco B, Malmström A, Maccarana M. Two dermatan sulfate epimerases form iduronic acid domains in dermatan sulfate. J Biol Chem. 2009b;284:9788–9795. doi: 10.1074/jbc.M809339200. doi: 10.1074/jbc.M809339200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsaki M, Osório F, Morais P, Torres T, Magina S, Chimenti S, Costanzo A. Infliximab in psoriasis and psoriatic arthritis. BioDrugs. 2013;27(Suppl 1):13–23. doi: 10.1007/BF03325638. doi: 10.1007/BF03325638. [DOI] [PubMed] [Google Scholar]
- Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Petitou M, Duchaussoy P, Lederman I, Choay J, Sinaÿ P, Jacquinet J-C, Torri G. Synthesis of heparin fragments. A chemical synthesis of the pentasaccharide O-(2-Deoxy-2-Sulfamido-6-O-Sulfo-alpha-D-Glucopyranosyl)-(1-4)-O-(beta-D-Glucopyranosyluronic Acid)-(1-4)-O-(2-O-Sulfo-alpha-L-Idopyranosyluronic acid)-(1-4)-2-Deoxy-2-Sulfamido-6-. Carbohydr Res. 1986;147:221–236. doi: 10.1016/s0008-6215(00)90633-5. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ke J, Gu X, Fang J, Wang W, Cong Q, Li J, Tan J, Brunzelle JS, Zhang C, Jiang Y, Melcher K, Li J, Xu HE, Ding K. Structural and functional study of D-glucuronyl C5-epimerase. J Biol Chem. 2015;290:jbc.M114.602201. doi: 10.1074/jbc.M114.602201. doi: 10.1074/jbc.M114.602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Purushothaman A, Stewart M, Thompson C, Vlodavsky I, Au J, Sanderson RD. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS. 2013;280:2294–2306. doi: 10.1111/febs.12168. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restaino OF, Bhaskar U, Paul P, Li L, De Rosa M, Dordick JS, Linhardt RJ. High cell density cultivation of a recombinant E. coli strain expressing a key enzyme in bioengineered heparin production. Appl Microbiol Biotechnol. 2013a;97:3893–3900. doi: 10.1007/s00253-012-4682-z. doi: 10.1007/s00253-012-4682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restaino OF, Cimini D, De Rosa M, Catapano A, Schiraldi C, Rosa M De, Catapano A, Schiraldi C, De Rosa M, Catapano A, Schiraldi C. High cell density cultivation of Escherichia coli K4 in a microfiltration bioreactor : a step towards improvement of chondroitin precursor production. Microb Cell Fact. 2011;10:1–11. doi: 10.1186/1475-2859-10-10. doi: 10.1186/1475-2859-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restaino OF, di Lauro I, Cimini D, Carlino E, De Rosa M, Schiraldi C. Monosaccharide precursors for boosting chondroitin-like capsular polysaccharide production. Appl Microbiol Biotechnol. 2013b;97:1699–709. doi: 10.1007/s00253-012-4343-2. doi: 10.1007/s00253-012-4343-2. [DOI] [PubMed] [Google Scholar]
- Sakai S, Otake E, Toida T, Goda Y. Identification of the origin of chondroitin sulfate in “health foods”. Chem Pharm Bull (Tokyo) 2007;55:299–303. doi: 10.1248/cpb.55.299. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R, Venkataraman G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr Opin Chem Biol. 2000;4:626–631. doi: 10.1016/s1367-5931(00)00145-9. doi: 10.1016/S1367-5931(00)00145-9. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schiraldi C, Cimini D, De Rosa M. Production of chondroitin sulfate and chondroitin. Appl Microbiol Biotechnol. 2010;87:1209–20. doi: 10.1007/s00253-010-2677-1. doi: 10.1007/s00253-010-2677-1. [DOI] [PubMed] [Google Scholar]
- Schonberger L. New variant Creutzfeldt-Jakob disease and bovine spongiform encephalopathy. Infect Dis Clin North Am. 1998;12:111–121. doi: 10.1016/s0891-5520(05)70412-8. [DOI] [PubMed] [Google Scholar]
- Sheng J, Liu R, Xu Y, Liu J. The dominating role of N-deacetylase/N-sulfotransferase 1 in forming domain structures in heparan sulfate. J Biol Chem. 2011;286:19768–19776. doi: 10.1074/jbc.M111.224311. doi: 10.1074/jbc.M111.224311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Xu Y, Dulaney SB, Huang X, Liu J. Uncovering biphasic catalytic mode of C5-epimerase in heparan sulfate biosynthesis. J Biol Chem. 2012;287:20996–21002. doi: 10.1074/jbc.M112.359885. doi: 10.1074/jbc.M112.359885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zaia J. Organ-specific heparan sulfate structural phenotypes. J Biol Chem. 2009;284:11806–11814. doi: 10.1074/jbc.M809637200. doi: 10.1074/jbc.M809637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert JE, Sugumaran G. Biosynthesis of Chondroitin / Dermatan Sulfate. IUBMB Life. 2002;54:177–186. doi: 10.1080/15216540214923. doi: 10.1080/15216540290114450. [DOI] [PubMed] [Google Scholar]
- Sismey-Ragatz AE, Green DE, Otto NJ, Rejzek M, Field R a., DeAngelis PL. Chemoenzymatic synthesis with distinct Pasteurella heparosan synthases: Monodisperse polymers and unnatural structures. J Biol Chem. 2007;282:28321–28327. doi: 10.1074/jbc.M701599200. doi: 10.1074/jbc.M701599200. [DOI] [PubMed] [Google Scholar]
- Sterner E, Li L, Paul P, Beaudet JM, Liu J, Linhardt RJ, Dordick JS. Assays for determining heparan sulfate and heparin O-sulfotransferase activity and specificity. Anal Bioanal Chem. 2014;406:525–536. doi: 10.1007/s00216-013-7470-4. doi: 10.1007/s00216-013-7470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N, Shioiri T, Chiba M, Sato T, Narimatsu H, Kimata K, Watanabe H. Construction of a chondroitin sulfate library with defined structures and analysis of molecular interactions. J Biol Chem. 2012;287:43390–43400. doi: 10.1074/jbc.M112.412676. doi: 10.1074/jbc.M112.412676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N, Tawada A, Sugimoto K, Watanabe H. Molecular cloning and characterization of chondroitin polymerase from Escherichia coli strain K4. J Biol Chem. 2002;277:21567–21575. doi: 10.1074/jbc.M201719200. doi: 10.1074/jbc.M201719200. [DOI] [PubMed] [Google Scholar]
- Sun X, Li L, Overdier KH, Ammons LA, Douglas IS, Burlew CC, Zhang F, Schmidt EP, Chi L, Linhardt RJ. Analysis of Total Human Urinary Glycosaminoglycan Disaccharides by Liquid Chromatography–Tandem Mass Spectrometry. Anal Chem. 2015;87:6220–6227. doi: 10.1021/acs.analchem.5b00913. doi: 10.1021/acs.analchem.5b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J Off Publ Fed Am Soc Exp Biol. 1997;11:51–9. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Thelin M a., Bartolini B, Axelsson J, Gustafsson R, Tykesson E, Pera E, Oldberg Å , MacCarana M, Malmstrom A. Biological functions of iduronic acid in chondroitin/dermatan sulfate. FEBS J. 2013;280:2431–2446. doi: 10.1111/febs.12214. doi: 10.1111/febs.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi V, Lettino M. Fondaparinux: pharmacology and clinical experience in cardiovascular medicine. Mini Rev Med Chem. 2007;7:383–7. doi: 10.2174/138955707780363819. [DOI] [PubMed] [Google Scholar]
- Tully SE, Rawat M, Hsieh-Wilson LC. Discovery of a TNF-alpha antagonist using chondroitin sulfate microarrays. J Am Chem Soc. 2006;128:7740–1. doi: 10.1021/ja061906t. doi: 10.1021/ja061906t. [DOI] [PubMed] [Google Scholar]
- Van Gorp CL SR Protein hydrolysate derived from mucosa tissue. 1997 US Pat. #5607840.
- Vidic H-J. Process for the preparation of heparin. 1981 US patent # 4,283,530.
- Viskov C, Just M, Laux V, Mourier P, Lorenz M. Description of the chemical and pharmacological characteristics of a new hemisynthetic ultra-low-molecular-weight heparin, AVE5026. J Thromb Haemost. 2009;7:1143–1151. doi: 10.1111/j.1538-7836.2009.03447.x. doi: 10.1111/j.1538-7836.2009.03447.x. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y. Heparan sulfate proteoglycans Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Investig. 2001;108:341–347. doi: 10.1172/JCI13662. doi: 10.1172/JCI200113662.Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Englaender JA, Xu P, Mehta KK, Suwan J, Dordick JS, Zhang F, Yuan Q, Linhardt RJ, Koffas M. Expression of low endotoxin 3-o-sulfotransferase in Bacillus subtilis and Bacillus megaterium. Appl Biochem Biotechnol. 2013;171:954–962. doi: 10.1007/s12010-013-0415-8. doi: 10.1007/s12010-013-0415-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang B, Zhang Z, Mellisa L, Takieddin M, Mousa S, Liu J, Dordick JS, Linhardt RJ. Control of the heparosan N-deacetylation leads to an improved bioengineered heparin. Appl Microbiol Biotechnol. 2011;91:91–99. doi: 10.1007/s00253-011-3231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RE. Process for the recovery of heparin. 1967 US Pat. #3337409.
- Wu Q, Yang A, Zou W, Duan Z, Liu J, Chen J, Liu L. Transcriptional engineering of Escherichia coli K4 for fructosylated chondroitin production. Biotechnol Prog. 2013;29:1140–9. doi: 10.1002/btpr.1777. doi: 10.1002/btpr.1777. [DOI] [PubMed] [Google Scholar]
- Xiong J, Bhaskar U, Li G, Fu L, Li L, Zhang F, Dordick JS, Linhardt RJ. Immobilized enzymes to convert N-sulfo, N-acetyl heparosan to a critical intermediate in the production of bioengineered heparin. J Biotechnol. 2013;167:241–247. doi: 10.1016/j.jbiotec.2013.06.018. doi: 10.1016/j.jbiotec.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Bhan N, Koffas MAG. Engineering plant metabolism into microbes: From systems biology to synthetic biology. Curr Opin Biotechnol. 2013;24:291–299. doi: 10.1016/j.copbio.2012.08.010. doi: 10.1016/j.copbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cai C, Chandarajoti K, Hsieh P-H, Li L, Pham TQ, Sparkenbaugh EM, Sheng J, Key NS, Pawlinski R, Harris EN, Linhardt RJ, Liu J. Homogenous low -molecular-weight heparins with reversible anticoagulant activity. Nat Chem Biol. 2014;10:248–250.–50. doi: 10.1038/nchembio.1459. doi: 10.1038/nchembio.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Masuko S, Takieddin M, Xu H, Liu R, Jing J, Mousa S a., Linhardt RJ, Liu J, Xu Y, Masuko S, Takieddin M, Xu H, Liu R, Jing J, Mousa S, Linhardt RJ, Liu J. Chemoenzymatic synthesis of structurally homogeneous ultra-low molecular weight heparins. Science (80-) 2011;334:498–501. doi: 10.1126/science.1207478. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J Chromatogr A. 2012;1225:91–98. doi: 10.1016/j.chroma.2011.12.063. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanfardino A, Restaino OF, Notomista E, Cimini D, Schiraldi C, De Rosa M, De Felice M, Varcamonti M. Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin. Microb Cell Fact. 2010;9:34. doi: 10.1186/1475-2859-9-34. doi: 10.1186/1475-2859-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu L, Teng L, Chen J, Liu J, Li J, Du G, Chen J. Metabolic engineering of Escherichia coli BL21 for biosynthesis of heparosan, a bioengineered heparin precursor. Metab Eng. 2012;14:521–527. doi: 10.1016/j.ymben.2012.06.005. doi: 10.1016/j.ymben.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Suflita M, Fiaschetti CM, Li G, Li L, Zhang F, Dordick JS, Linhardt RJ. High cell density cultivation of a recombinant Escherichia coli strain expressing a 6-O-sulfotransferase for the production of bioengineered heparin. J Appl Microbiol. 2015a;118:92–98. doi: 10.1111/jam.12684. doi: 10.1111/jam.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Suflita M, Li G, Zhong W, Li L, Dordick JS, Linhardt RJ, Zhang F. High Cell Density Cultivation of Recombinant Escherichia coli Strains Expressing 2-O-Sulfotransferase and C5-Epimerase for the Production of Bioengineered Heparin. Appl Biochem Biotechnol. 2015b;175:2986–2995. doi: 10.1007/s12010-014-1466-1. doi: 10.1007/s12010-014-1466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, McCallum SA, Xie J, Nieto L, Corzana F, Jimenez-Barbero J, Chen M, Liu J, Linhardt RJ. Solution Structures of Chemoenzymatically Synthesized Heparin and Its Precursors. J Am Chem Soc. 2008;130:12998–13007. doi: 10.1021/ja8026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chandarajoti K, Pham TQ, Liu R, Liu J. Expression of heparan sulfate sulfotransferases in Kluyveromyces lactis and preparation of 3′-phosphoadenosine-5′-phosphosulfate. Glycobiology. 2011;21:771–780. doi: 10.1093/glycob/cwr001. doi: 10.1093/glycob/cwr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC, Yuan XF. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis. 2008;55:152–164. doi: 10.1111/j.1865-1682.2008.01020.x. doi: 10.1111/j.1865-1682.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- Zoppetti G, Oreste P, Application F, Data P. process for the preparation of chondroitin sulfates from k4 polysaccharide and obtained products. 2004 US Patent 6777398 B2.