Abstract
Objectives
HSV-2 biomarkers are often used in adolescent sub-Saharan HIV prevention studies, but evaluations of test performance and disclosure outcomes are rare in the published literature. Therefore, we investigated the proportion of ELISA-positive and indeterminant samples confirmed by Western blot (WB); the psychosocial response to disclosure; and whether reports of sexual behavior and HSV-2 symptoms are consistent with WB confirmatory results among adolescent orphans in Kenya.
Methods
In 2011, 837 Kenyan orphan youth in grades 7 and 8 enrolled in an HIV prevention clinical trial with HSV-2 biomarker outcomes. We used a modified algorithm for the Kalon HSV-2 ELISA to improve specificity; positive and indeterminate results were WB-tested. We developed culturally sensitive protocols for disclosing positive results and documented psychosocial responses, reports of sexual contact, and HSV-2 symptoms.
Results
28 adolescents (3.3%) were identified as HSV-2 seropositive; 6 as indeterminate. Of these, 22 positive and all indeterminants were WB-tested; 20 and 5, respectively, were confirmed positive. Most youth reported moderate brief stress after disclosure; 22% reported longer and more severe distress. Boys were more likely to be in the latter category. Self-reported virginity was highly inconsistent with WB confirmed positives.
Conclusions
The higher than manufacturer cut-off for Kalon ELISA modestly reduced the rate of false positive test results but also increased false negatives. Investigators should consider the risk-benefit ratio in deciding whether or not to disclose HSV-2 results to adolescent participants under specific field conditions.
Keywords: Herpes Simplex (Clinical), Adolescent, Africa, Serology, Sensitivity and Specificity
INTRODUCTION
Herpes Simplex Virus type 2 (HSV-2) is the primary cause of genital herpes [1], particularly in sub-Saharan Africa. Because nearly all HSV-2 infections are sexually acquired, type-specific HSV-2 antibodies in serum imply anal-genital infection.[2] Infections are life-long with intermittent clinical and subclinical viral reactivation and mucosal shedding.[1] Only 10–25% of people with HSV-2 antibodies are aware that they have genital herpes.[1] Symptoms vary widely, and infected persons may be asymptomatic. Although there is no cure, systemic antiviral drugs can partially control the signs and symptoms of herpes episodes and can be used as daily suppressive therapy.[2]
Prevalence of genital herpes in the adult general population in sub-Saharan Africa is high, ranging from 30% to 80% in women and from 10% to 50% in men, as assessed by serological detection of HSV-2 antibodies.[3–7] In Kenya, the population-based prevalence among those 15–64 years old is 42% for females and 26% for males.[8] In Nyanza Province, which has the highest HIV prevalence in the country, HSV-2 prevalence among 13–14 year olds is estimated at 9% for females and 4% for males, and among 15–19 year olds at 28% and 17% respectively.[9]
HSV-2 is an important risk factor for HIV.[1] Meta-analyses of longitudinal studies have found a three-fold increase in HIV infection with HSV-2. [10, 11] Among Kenyans, HIV prevalence is 16% among HSV-2 seropositive persons compared to 2% among seronegatives.[8]
Besides its importance as an HIV risk factor, HSV-2 has become increasingly used as a biomarker to corroborate adolescent self-report of sexual behavior, which is often inconsistent.[12–14] HSV-2 is also considered an important clue in determining the path of transmission for HIV positive youth, whether vertical or through sexual contact.[15] Because of its relatively high prevalence among sexually transmitted infections, the inclusion of HSV-2, along with HIV biomarkers, has become the scientific standard for evaluating youth prevention interventions in sub-Saharan Africa,[16–20] although some have more recently questioned its utility for this purpose.[21]
Despite the potential importance of HSV-2 as a biomarker, studies to evaluate disclosure of results to sub-Saharan adolescents are lacking. Some U.S. studies have raised concerns about disclosure after serological screening in the absence of pre-existing symptoms or diagnosis, given the low calculated positive predictive value (PPV) of existing enzyme-linked immunosorbent assay (ELISA) tests [22] and the possibility of psychosocial harm.[23] Young adolescents are likely to have a low prevalence of HSV-2 infection,[19, 16] and PPV for all diagnostic tests drops greatly when prevalence is low, resulting in a potentially high rate of false positives.[24] Nevertheless, a recent review of the extant literature concluded that HSV-2 diagnosis by type-specific serological testing did not result in long-term psychosocial harm in most persons without an identified history of genital herpes.[25] That review, however, was limited to studies with adults from developed countries. Moreover, conclusions were aimed at clinicians, who were advised to offer HSV-2 testing selectively to “appropriate” patients. Among published sub-Saharan adolescent prevention studies collecting biomarkers, most have not disclosed HSV-2 results to participants [16, 18, 20] while others have.[17, 19] No research guidelines on the disclosure of HSV-2 biomarker test results in community studies of sub-Saharan African adolescents are available in the scientific or regulatory literature.
The study is unique in examining the performance of a commonly used serological HSV-2 test for Kenyan adolescents and the outcomes of a culturally sensitive protocol for disclosing results. Since the response of African youth to positive test results within the context of an HIV prevention trial was unknown, we also examined findings related to disclosure of positive HSV-2 results to Kenyan orphan adolescents. This paper addresses the following research questions:
Following disclosure, what proportion of ELISA-positive samples was confirmed by WB?
What was the psychosocial response reported by participants?
Were participants' report of sexual behavior and HSV-2 symptoms consistent with WB confirmatory tests?
Study findings provide important guidance to scientists about the ethical disclosure of HSV-2 biomarkers in adolescent African HIV prevention research.
METHODS
Sample and Setting
This study was conducted in Siaya County, in Nyanza Province, Kenya. Twenty-six primary schools were selected for a randomized controlled trial to determine whether providing orphans with school fees can prevent HIV and HIV-related risk behaviors.[19, 24] To be included, schools had to have at least 20 orphans (one or both parents deceased) in grades 7 and 8. All orphans in grades 7 and 8 at selected schools were eligible.
HSV-2 Biomarker sample collection and testing
Whole blood was obtained aseptically by venipuncture at remote study sites and transported on ice to a local laboratory where serum was prepared and stored at 4°C until weekly transport to the AMPATH Reference Laboratory in Eldoret, Kenya for ELISA testing using a type specific HSV-2 ELISA (Kalon Biological Ltd., Guildford, UK). Serum was stored at 4°C until testing. Kalon testing was performed by a single technician from October, 2011 to January, 2012. After testing, samples were frozen at −20°C until selected samples (described below) were shipped on dry ice to the Virology Laboratory at the University of Washington where confirmatory WB testing was performed in June, 2014 by a single technician without knowledge of ELISA results. [30]
Kalon testing was performed according to the manufacturer's instructions with absorbance values obtained using a microtiter platereader at 450nm wavelength blanked on air. Index values were calculated from the absorbance value of the test sample compared to a manufacturer-supplied calibrator for the kit. The manufacturer's cut-off index value defining a positive result is 1.1.[26] Numerous reports in the literature describe performance issues with HSV-2 ELISA tests in sub-Saharan African populations using manufacturers' cut-offs, particularly in low prevalence groups such as adolescents.[26–29] In an effort to improve the specificity of the test, we employed a modified algorithm for test result interpretation in the study. Samples were initially tested in single wells; samples with initial index values < 0.9 were considered negative, those with initial index values > 2.5 were considered positive, and no further ELISA testing was performed on those sera. Samples with initial index values between 0.9 and 2.5 were retested in duplicate. Final results were considered negative if retest index results were < 0.9; positive if retest results were ≥ 1.5 and indeterminant if retest results were between 0.9 and 1.5, or discordant.
Based on previously published evaluations of Kalon test performance in Sub-Saharan African populations using the higher index value cut-off of 1.5 to interpret results compared to the WB reference standard, [26] we assumed ELISA sensitivity of 94% and specificity of 92% for the study-specific algorithm. Positive and negative predictive values (PPV and NPV, respectively) of the Kalon test were calculated using standard formulas:
Sub-Study Procedures
University-educated male and female registered nurse researchers were trained in the study protocol as follows: nurses met with same-gender participants for disclosure at a private place (e.g., home or clinic room). If the participant was under 18 years, the nurses met first with the guardian to disclose the diagnostic findings and review a CDC adapted fact sheet on HSV-2. The participant then joined to receive the same information. The nurse answered questions, provided the fact sheet with his/her contact information for further questions, gave a referral form for treatment, and requested permission to return for follow-up in a few months. The nurse then talked with the adolescent alone about transmission, symptoms, or other questions. After disclosure, nurse researchers summarized their observations of guardian and participant behavior and questions/comments during the meeting, including participant's self-report of sexual behavior and symptoms. All participants were re-contacted several months later for a follow-up interview at a private place of participant's choice. Nurses transcribed their notes and sent them in English to investigators in the U.S. who reviewed, coded, and entered data.
Measures
Using content analysis of disclosure and follow-up interview notes, measures were coded by a senior research scientist, in consultation with the interviewers, as follows: I) participants' emotional response at follow up (1=low or no emotional distress exhibited; 2=reported distress for a few days or weeks but resolved; 3=distressed for a month or more but resolved; 4=prolonged psychosocial effects with adverse emotional, social, academic effects); II) guardian type (mother, sibling, stepmother, grandparent, aunt/uncle, spouse); III) participants' perception of guardian or family response and their relationship at follow-up (supportive or very supportive; no change in relationship, other reactions, missing); and IV) HSV-2 symptoms (0,1); and sexual activity (0,1). Other measures included baseline audio computer-assisted survey items: age; gender; ever had sexual intercourse (0,1). At follow-up, nurse researchers asked whether participants had tried to access HSV-2 treatment (most local clinics have acyclovir in stock, provided free of charge with a referral). From the laboratory analyses, we documented index values for Kalon and results of WB testing.
Data Analysis
We analyzed qualitative notes from nurse field researchers and coded responses for measures as described above. We compared the participants' interview response on sexual behavior to their baseline survey results and noted discordant results, and we compared self-reported sexual behavior and HSV-2 symptoms with WB results. PPV and negative predictive value (NPV) for the Kalon test were calculated from the estimated sensitivity (94%) and specificity (92%) of the test with the higher cut-off using standard formulas.
Human Subjects Protection
Institutional review boards at PIRE (USA) and the Moi University (Kenya) reviewed and approved study protocols, including consent procedures and disclosure procedures. Young adults aged 18 and older gave written consent, while guardians gave written permission and youth under 18 gave written assent for participation. To send samples out of the country for WB confirmatory testing, additional written consent was obtained.
RESULTS
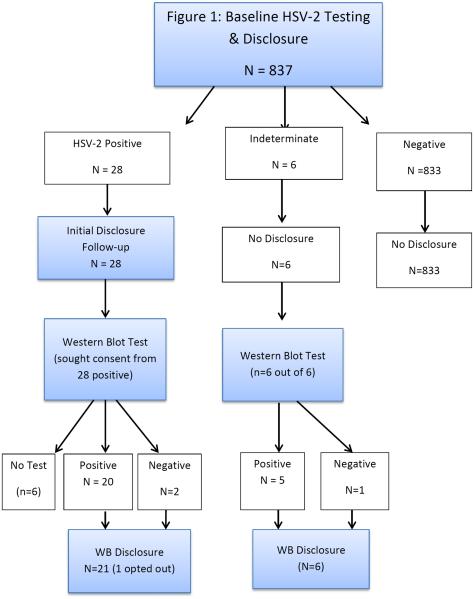
From October 2011 to January 2012, 837 youth completed baseline survey and biomarker testing. The average age of participants was 14.9 years (SD 1.5; range 11–21 years). Twenty-eight adolescents (3.3%) were identified as HSV-2 positive by Kalon ELISA at baseline, with an estimated prevalence of 4.4% for girls and 2.3% for boys. Another six were identified as indeterminate. Only participants with positive ELISA results were contacted for disclosure which was conducted from February 2012 through March 2013. Follow-up interviews were conducted within 3–9 months later.
At the observed HSV-2 seroprevalence the calculated NPV of the ELISA was 99.8%, providing high confidence in negative test results. However, the low calculated PPV of 26.4% prompted investigator and IRB concerns that false positives could result in needless social and psychological distress. Thus, we modified our protocol and informed participants that serological results are not always accurate and could result in false positive values, and requested consent to send samples for WB confirmatory testing. All six of those with indeterminate results and 22 of those with positive results consented (five more were not found and one refused; Figure 1).
Figure 1.
Despite the low calculated PPV of the Kalon test, > 90% of positive samples and > 80% of indeterminate samples were positive by WB (Table 1). Among the samples tested by WB, 100% (9/9) with initial Kalon index values ≥ 2.5, 87.5% (14/16) with index values ≥ 1.5 and < 2.5, and 83% (5/6) with index values ≥ 0.9 and < 1.5 were confirmed positives. Using our study-specific algorithm, 2/22 positives (9%) were false positives. For samples with initial Kalon index values < 2.5, there was no association between the index value and WB result (data not shown).
Table 1.
HSV-2 Antibody Detection in Kenyan Adolescent Orphans.
| Number of samples with result indicated / Total number tested (%) Kalon ELISA Testing | ||||
|---|---|---|---|---|
|
|
||||
| Index Value Range | Initial | Product Insert Finala | Study Algorithm Finalb | Positive by Western blot |
| ≥ 2.5 | 12/837 (1.4) | 12/12 (100) Positive | 12/12 (100) Positive | 9/9 (100) |
|
| ||||
| ≥ 1.5, < 2.5 | 16/837 (2.0) | 16/16 (100) Positive | 14/16 (87.5) Positive 2/16 (12.5) Indeterminate | 13/14 (92.9) 1/2 (50.0) |
|
| ||||
| ≥ 1.1, < 1.5 | 5/837 (0.6) | 4/5 (80.0) Positive | 2/5 (40.0) Positive 2/5 (40.0) Indeterminate | 1/2 (50.0) 2/2 (100) |
|
| ||||
| 1/5 (20.0) Negative | 1/5 (20.0) Negative | ND | ||
|
| ||||
| ≥ 0.9, < 1.1 | 2/837 (0.2) | 1/2 (50.0) Positive 1/2 (50.0) Indeterminate | 2/2 (100) Indeterminate | 2/2 (100) |
|
| ||||
| < 0.9 | 802/837 (95.8) | 802/802 (100) Negative | 802/802 (100) Negative | ND |
Final Kalon results determined according to the product insert, including repeat testing when applicable; no repeat ELISA testing was performed for initial index values ≥ 2.5 or < 0.9.
Final Kalon results determined according to the algorithm described in Methods.
ND, not done.
Because we did not submit all samples for reference testing by WB, we cannot calculate valid sensitivity and specificity estimates for Kalon performance using the study-specific algorithm. However, the observed high rates of WB positives among samples submitted for confirmatory testing suggest that the sensitivity was lower than estimated (most indeterminants were true positives), and the specificity was higher than estimated (most positives were true positives). Among samples tested by WB, 26 had initial index values ≥1.1 and would have been deemed positive according to the manufacturer's suggested cut-off; three of these (11.5%) were negative by WB and would thus have been false positives.
Participant Reaction as Reported at Follow Up
About 28% of participants who received HSV-2 serologic test results (8/28) reported minimal stress and a positive attitude after disclosure. Another 50% said that they were worried or upset for a few days to a few weeks, but had eventually found ways to cope through support from their guardian, prayer, or hope that the result could be a false positive. Another 11% said they had been worried for a month or more, but eventually came to acceptance. The final group (11%) reported being very stressed for many months with adverse emotional, physical, social and/or academic effects. As an example, one participant was very distressed at follow up, as the family was convinced the youth was actually infected with HIV and not HSV-2, resulting in stigmatization and isolation. The nurse researcher assured the participant that this was not the case, and talked with family members to correct the misperception.
Males made up 36% of the sample, but were represented disproportionately among those with the most severe and lingering stress (66%). Females were most likely to be among those with minimal stress (80%). Only two participants reported that they had sought treatment. In one case, the participant was told the clinic was out of the medication. The second went to a health center with the referral form and was given medicine. She reported, “the ulcers disappeared four days after taking the treatment.”
Guardian Reaction
Among the guardians present at initial disclosure, seven were mothers; four brother, sister, or sister-in-law; two stepmothers; seven grandparents; and eight aunts or uncles. Guardians of participants under 18 years of age were contacted for permission to speak with the child at follow-up, but in no case did the guardian choose to be present for the follow-up visit. Most guardians were perceived as being supportive or very supportive (61%) or showing no change in the way they related to the youth (17%). As an example of perceived support, one participant said of her Aunt, “were it not for her, I don't know how I would have made it.” Another boy said of his brother, “Nowadays he is more loving and concerned about my life. Before the disclosure we could not sit together to discuss anything but nowadays we discuss most things freely and kept on encouraging me to work hard in school.”
“Other reactions” included three guardians who were convinced that HSV-2 was actually a different disease (HIV or cancer). One guardian insisted the child take a medicine (likely a traditional medicine). Another mother told the researcher, “she likes men too much,” referring to the participant daughter. Among the three youth who reported the most severe and lingering stress, two reported negative attitudes from their guardian. No other pattern was found.
Sex, symptoms and WB
Of the 22 participants with positive samples and WB, only six (27%) reported ever having a sex partner (Table 2). Of these, three reported symptoms, and of these, one had reported virginity on the survey. Of the rest (16), 10 consistently reported no sexual contact (both interview and survey) and no symptoms; of these, two were WB negative (both males) while eight were positive. Of the final six, three reported sexual intercourse by survey but not interview, and three consistently denied sex but reported HSV-2 symptoms.
Table 2.
Report of Sexual Behavior and Symptoms by gender and WB results among Kalon HSV-2 positive participants
| Gender | Age at Baseline (Oct. 2011) | Reported sexual contact in interview | Reported Sex on Survey | Reported HSV2 Symptoms | HSV2 Western blot result |
|---|---|---|---|---|---|
| F | 13 | 0 | 1 | 0 | positive |
| F | 13 | 0 | 0 | 1 | positive |
| F | 14 | 0 | 0 | 0 | positive |
| F | 14 | 0 | 0 | 0 | positive |
| F | 14 | 1 | 1 | 1 | positive |
| F | 15 | 0 | 0 | 0 | positive |
| F | 15 | 0 | 1 | 0 | positive |
| F | 15 | 0 | 0 | 1 | positive |
| F | 15 | 1 | 1 | 1 | positive |
| F | 16 | 0 | 0 | 0 | positive |
| F | 17 | 0 | 0 | 0 | positive |
| F | 17 | 1 | 1 | 0 | positive |
| F | 20 | 0 | 0 | 0 | positive |
| M | 12 | 0 | 0 | 1 | positive |
| M | 13 | 0 | 0 | 0 | negative |
| M | 14 | 1 | 1 | 0 | positive |
| M | 14 | 0 | 1 | 0 | positive |
| M | 15 | 0 | 0 | 0 | negative |
| M | 15 | 1 | 0 | 1 | positive |
| M | 15 | 0 | 0 | 0 | positive |
| M | 15 | 0 | 0 | 0 | positive |
| M | 17 | 1 | 1 | 0 | positive |
DISCUSSION
Using a higher than manufacturer cut-off (1.5 vs. 1.1), we found a relatively low prevalence of HSV-2 among orphaned 7th and 8th grade Kenya students, as compared to a 13–14 year old sample in the same region that was not limited to school-attending orphans.[9] We used a higher than manufacturer test cut-off, and with WB confirmatory testing, 91% of those who were positive by our algorithm and 83% of indeterminants were true positives. It is possible that estimates from studies using the manufacturer's cut-off may have had lower specificity, inflating prevalence. Our study, however, was not designed to formally evaluate the accuracy of the Kalon HSV-2 ELISA test; the goal was to optimize its performance for our specific study setting. Anticipating the potential trauma of informing adolescent subjects of a positive result, we opted to maximize specificity of the biomarker assay to minimize false-positives by increasing the cut-off to define a positive test. A priori, we accepted the resulting decrease in sensitivity and any attendant false negative results, recognizing that negative predictive value would be minimally affected.
We found that youth were generally resilient in the disclosure of results. Most reported that they felt stressed and upset initially but, with support from their guardians, they soon accepted. Most orphaned participants described their guardians as supportive, even though few had a living parent to come with them for disclosure. In a few cases, however, participants reported feeling very stressed over prolonged periods of time.
We also found that the great majority of youth who were HSV-2 seropositive denied any sexual contact when interviewed. Since HSV-2 is almost always transmitted by direct sexual contact with an infected person [2, 31] this suggested that either the participant's self-report (corroborated or not by ACASI survey and symptoms) or the Kalon result was false. When examining WB results, we found that the two false positives samples corresponded to consistent verbal and survey reports denying sexual contact or symptoms. Eight others, however, likewise consistently denied sexual behavior or symptoms, suggesting inaccurate reporting.
A limitation of the present study is the small sample size of participants with both WB and qualitative (disclosure) data. Findings can only be generalized to adolescent orphans attending Grades 7 and 8 in western Kenya, almost all of whom were members of the Luo tribe. Since over 80% of indeterminants in our study were found to be WB positive, adjustments to our protocol may be needed in order to maximize both sensitivity and specificity of the ELISA test. A standardized measure for distress was not used. For future studies, we recommend using such a measure at follow-up.
In summary, the Kalon ELISA used with a higher than manufacturer recommended cut-off modestly reduced the rate of false positive test results but also increased false negatives. Investigators should carefully consider the relative benefits of increased confidence in positive test results versus the potential risk of missed positives in making such adjustments. They should also consider the risk-benefit ratio in deciding whether or not to disclose HSV-2 results to adolescent participants under specific field conditions. Including a parent/guardian or trusted adult at disclosure, and following up with the youth a month later, may help to minimize psychosocial harm, especially for boys, who may be more vulnerable than girls.
Key messages.
The study is unique in examining the specificity of a common serological HSV-2 test along with adolescent outcomes of a culturally sensitive disclosure protocol.
Using a modified algorithm for interpreting results, 91% of tested ELISA positive results were WB confirmed; 83% of ELISA indeterminants were WB positive.
Boys in this study were more susceptible than girls to prolonged distress after HSV-2 disclosure.
Self-reported virginity was highly inconsistent with WB-confirmed detection of HSV-2 antibodies.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH092215 (Hyunsan Cho, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful for the contributions of Janet Itindi who participated in the initial disclosure field work; Anne Cent and Rhoda Ashley Morrow at the University of Washington Virology Laboratory for WB testing; AMPATH Reference Laboratory for storing, analyzing, and preparing samples for WB; AMPATH's Fidelis Mambo and Linda Lumbasi who provided consultation and monitored quality control for data processing and analysis of the HSV-2 samples; Luke Opondo (DASCO), Bonita Iritani, Arlene Sena-Soberano, and Judith Pokorni who labored with us to establish ethical protocols under conditions of uncertainty; Susannah Allison who generously provided ongoing support and guidance; and all of the Kenyan youth and guardians who courageously participated in this research.
REFERENCES
- 1.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–37. doi: 10.1016/S0140-6736(07)61908-4. Epub 2007/12/25. [DOI] [PubMed] [Google Scholar]
- 2.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2010;59(RR-12):1–110. Epub 2010/12/17. [PubMed] [Google Scholar]
- 3.Ghebremichael M, Larsen U, Paintsil E. Association of age at first sex with HIV-1, HSV-2, and other sexual transmitted infections among women in northern Tanzania. Sex Transm Dis. 2009;36(9):570–6. doi: 10.1097/OLQ.0b013e3181a866b8. Epub 2009/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjetland EF, Gwanzura L, Ndhlovu PD, et al. Herpes simplex virus type 2 prevalence of epidemic proportions in rural Zimbabwean women: association with other sexually transmitted infections. Archives of gynecology and obstetrics. 2005;272(1):67–73. doi: 10.1007/s00404-004-0689-8. Epub 2005/01/14. [DOI] [PubMed] [Google Scholar]
- 5.Munjoma MW, Kurewa EN, Mapingure MP, et al. The prevalence, incidence and risk factors of herpes simplex virus type 2 infection among pregnant Zimbabwean women followed up nine months after childbirth. BMC Womens Health. 2010;10:2. doi: 10.1186/1472-6874-10-2. Epub 2010/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobian AA, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;199(7):945–9. doi: 10.1086/597074. Epub 2009/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. Epub 2004/04/30. [PubMed] [Google Scholar]
- 8.Mugo N, Dadabhai SS, Bunnell R, et al. Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis. 2011;38(11):1059–66. doi: 10.1097/OLQ.0b013e31822e60b6. Epub 2011/10/14. [DOI] [PubMed] [Google Scholar]
- 9.Amornkul PN, Vandenhoudt H, Nasokho P, et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in Rural Western Kenya. Plos One. 2009;4(7):e6470. doi: 10.1371/journal.pone.0006470. Epub 2009/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. Epub 2005/12/06. [DOI] [PubMed] [Google Scholar]
- 11.Glynn JR, Biraro S, Weiss HA. Herpes simplex virus type 2: a key role in HIV incidence. AIDS. 2009;23(12):1595–8. doi: 10.1097/QAD.0b013e32832e15e8. [DOI] [PubMed] [Google Scholar]
- 12.Palen LA, Smith EA, Caldwell LL, et al. Inconsistent reports of sexual intercourse among South African high school students. J Adolesc Health. 2008;42(3):221–7. doi: 10.1016/j.jadohealth.2007.08.024. Epub 2008/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowan FM, Langhaug LF, Mashungupa GP, et al. School based HIV prevention in Zimbabwe: feasibility and acceptability of evaluation trials using biological outcomes. AIDS. 2002;16(12):1673–8. doi: 10.1097/00002030-200208160-00013. [DOI] [PubMed] [Google Scholar]
- 14.Plummer ML, Ross DA, Wight D, et al. “A bit more truthful”: the validity of adolescent sexual behaviour data collected in rural northern Tanzania using five methods. Sex Transm Infect. 2004;80(Suppl 2):ii49–56. doi: 10.1136/sti.2004.011924. Epub 2004/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51(7):844–51. doi: 10.1086/656361. Epub 2010/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowan FM, Pascoe SJ, Langhaug LF, et al. The Regai Dzive Shiri Project: a cluster randomised controlled trial to determine the effectiveness of a multi-component community-based HIV prevention intervention for rural youth in Zimbabwe--study design and baseline results. Trop Med Int Health. 2008;13(10):1235–44. doi: 10.1111/j.1365-3156.2008.02137.x. Epub 2008/09/10. [DOI] [PubMed] [Google Scholar]
- 17.Baird SJ, Garfein RS, McIntosh CT, et al. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012;379(9823):1320–9. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 18.Jewkes R, Nduna M, Levin J, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ. 2008;337:a506. doi: 10.1136/bmj.a506. Epub 2008/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H, Luseno W, Halpern C, et al. Discordance of HIV and HSV-2 biomarkers and self-reported sexual behaviour among orphan adolescents in Western Kenya. Sex Transm Infect. 2014 doi: 10.1136/sextrans-2014-051720. Epub 2014/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallfors DD, Cho H, Rusakaniko S, et al. The Impact of School Subsidies on HIV-Related Outcomes Among Adolescent Female Orphans. J Adolesc Health. 2015;56(1):79–84. doi: 10.1016/j.jadohealth.2014.09.004. Epub 2014/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastien S, Mason-Jones AJ, De Koker P, et al. Herpes simplex virus type 2 infection as a biomarker for sexual debut among young people in sub-Saharan Africa: a literature review. Int J STD AIDS. 2012;23(11):761–6. doi: 10.1258/ijsa.2012.011433. Epub 2012/11/17. [DOI] [PubMed] [Google Scholar]
- 22.Mark H, Nanda JP, Joffe A, et al. Serologic screening for herpes simplex virus among university students: a pilot study. Journal of American college health : J of ACH. 2008;57(3):291–6. doi: 10.3200/JACH.57.3.291-296. Epub 2008/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melville J, Sniffen S, Crosby R, et al. Psychosocial impact of serological diagnosis of herpes simplex virus type 2: a qualitative assessment. Sex Transm Infect. 2003;79(4):280–5. doi: 10.1136/sti.79.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luseno WK, Hallfors DD, Cho H, et al. Use of HIV and HSV-2 biomarkers in sub-saharan adolescent prevention research: a comparison of two approaches. J Prim Prev. 2014;35(3):181–91. doi: 10.1007/s10935-014-0343-6. Epub 2014/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross K, Johnston C, Wald A. Herpes simplex virus type 2 serological testing and psychosocial harm: a systematic review. Sex Transm Infect. 2011;87(7):594–600. doi: 10.1136/sextrans-2011-050099. [DOI] [PubMed] [Google Scholar]
- 26.Biraro S, Mayaud P, Morrow RA, et al. Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Dis. 2011;38(2):140–7. doi: 10.1097/OLQ.0b013e3181f0bafb. Epub 2010/08/14. [DOI] [PubMed] [Google Scholar]
- 27.Ng'ayo MO, Friedrich D, Holmes KK, et al. Performance of HSV-2 type specific serological tests in men in Kenya. J Virol Methods. 2010;163(2):276–81. doi: 10.1016/j.jviromet.2009.10.009. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JS, Bailey RC, Westreich DJ, et al. Herpes simplex virus type 2 antibody detection performance in Kisumu, Kenya, using the Herpeselect ELISA, Kalon ELISA, Western blot and inhibition testing. Sex Transm Infect. 2009;85(2):92–6. doi: 10.1136/sti.2008.031815. Epub 2008/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamiel J, Tobian A, Laeyendecker O, et al. Improved performance of enzyme-linked immunosorbent assays and the effect of human immunodeficiency virus coinfection on the serologic detection of herpes simplex virus type 2 in Rakai, Uganda. Clinical and Vaccine Immunology. 2008;15(5):888. doi: 10.1128/CVI.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashley RL, Militoni J, Lee F, et al. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26(4):662–7. doi: 10.1128/jcm.26.4.662-667.1988. Epub 1988/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (US) Genital Herpes - CDC Fact Sheet; detailed version. Centers for Disease Control and Prevention; Atlanta, Georgia: 2013. [updated 2013 Feb 13; cited 2014 Nov 25]. Available from: http://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm. [Google Scholar]