Abstract
It has been suggested that the simulation of hypothetical episodes and the recollection of past episodes are supported by fundamentally the same set of brain regions. The present article specifies this core network via Activation Likelihood Estimation (ALE). Specifically, a first meta-analysis revealed joint engagement of core network regions during episodic memory and episodic simulation. These include parts of the medial surface, the hippocampus and parahippocampal cortex within the medial temporal lobes, and the lateral temporal and inferior posterior parietal cortices on the lateral surface. Both capacities also jointly recruited additional regions such as parts of the bilateral dorsolateral prefrontal cortex. All of these core regions overlapped with the default network. Moreover, it has further been suggested that episodic simulation may require a stronger engagement of some of the core network’s nodes as wells as the recruitment of additional brain regions supporting control functions. A second ALE meta-analysis indeed identified such regions that were consistently more strongly engaged during episodic simulation than episodic memory. These comprised the core-network clusters located in the left dorsolateral prefrontal cortex and posterior inferior parietal lobe and other structures distributed broadly across the default and fronto-parietal control networks. Together, the analyses determine the set of brain regions that allow us to experience past and hypothetical episodes, thus providing an important foundation for studying the regions’ specialized contributions and interactions.
Keywords: Episodic future thinking, episodic simulation, episodic memory, functional MRI, core network, meta analysis
Humans have the capacity to vividly imagine hypothetical episodes that may occur in their futures or that might have happened in their pasts (Schacter, Benoit, De Brigard, & Szpunar, 2015; Suddendorf & Corballis, 2007; Tulving, 2005). Recent years have seen a surge of interest in such episodic simulation of hypothetical events, and this surge has been fuelled by the observation that episodic simulation has much in common with episodic memory (for reviews, see Mullaly & Maguire, 2014; Schacter et al., 2012).
For example, both the capacity to remember past episodes and to imagine possible future episodes seem to emerge simultaneously during child development (Busby & Suddendorf, 2005), show a parallel decline with aging (Addis, Wong, & Schacter, 2008; Gaesser, Sacchetti, Addis, & Schacter, 2011), and are similarly impaired in amnesic patients (Hassabis, Kumaran, Vann, & Maguire, 2007a; Klein, Loftus, & Kihlstrom, 2002; Race, Keane, & Verfaellie, 2011; but see Squire et al., 2010). Recollected events and simulated episodes also exhibit comparable phenomenological properties, such as becoming less vivid and more abstract with increasing distance from the present (D’Argembeau & Van der Linden, 2004; Trope & Lieberman, 2003). Moreover, episodic specificity inductions – training that focuses on recalling specific details of past experiences – enhance both subsequent recollections of past events (e.g., Neshat-Doost et al., 2012) and imaginings of hypothetical future events (Madore, Gaesser, & Schacter, 2014).
Such similarities between episodic memory and episodic simulation have provided the basis for the constructive episodic simulation hypothesis (Schacter & Addis, 2007; for related ideas see also Suddendorf & Corballis, 1997), which posits that episodic simulation is based on an episodic memory system that provides (1) access to stored episodic details and (2) the constructive processes to recombine these details for the mental simulation of hypothetical episodes.
The constructive episodic simulation hypothesis receives further critical support from neuroimaging studies, which converge on the finding that the pattern of brain activation associated with the retrieval of autobiographical memories closely resembles the pattern associated with the simulation of future or fictitious episodes (e.g., Addis, Wong, & Schacter, 2007; Botzung, Denkova, & Manning, 2008; Hassabis, Kumaran, & Maguire, 2007b; Szpunar, Watson, & McDermott, 2007). Related research indicates that many of the same brain areas are also engaged during the simulation of novel episodes that could have happened in the past (Addis, Pan, Vu, Laiser, & Schacter, 2009; van Hoeck et al., 2013). The implicated regions comprise parts of the medial temporal lobes (MTL), the medial prefrontal cortex, the posterior cingulate, including retrospenial cortex, as well as lateral temporal and parietal regions (Hassabis & Maguire, 2007; Schacter, Addis, & Buckner, 2007; Schacter et al., 2012). This set of brain regions has thus been argued to constitute a core network that fundamentally supports both the reconstruction of past events and the construction of hypothetical events (Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Schacter et al., 2007). A recent meta-analysis provided compelling evidence that this network is consistently engaged during the simulation of future episodes (Stawarczyk & D’Argembeau, in press). However, the joint engagement of regions in the core network during various forms of episodic simulation and episodic memory is less well established. The best evidence for such joint engagement comes from an early qualitative comparison of a small-scale meta-analysis on prospection with a separate meta-analysis on autobiographical memory, which suggested that both functions share a similar brain network on a coarse spatial scale (Spreng, Mar, & Kim, 2009).
The first goal of the present meta-analysis is thus to provide a more precise and quantitative specification of the regions that are jointly engaged during episodic memory and episodic simulation. Towards this end, we conducted an activation likelihood estimation (ALE) (Eickhoff et al., 2009; Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Turkeltaub et al., 2012) that examined the concordance of brain activation patterns across neuroimaging experiments. Specifically, our analysis is characterized by two key features. First, we included only studies that had formally tested for spatial overlap between episodic memory for autobiographical events and episodic simulation (e.g., by employing a conjunction approach), thus providing stringent evidence for commonly recruited brain regions. Second, the simulated hypothetical events included possible future (e.g., Szpunar et al., 2007) and fictitious (Hassabis et al., 2007b) episodes as well as episodes that might have taken part in the past (e.g., counterfactual episodes, i.e., imaginings of alternative versions of real experienced events; e.g., de Brigard, Addis, Ford, Schacter, & Giovanello, 2013; van Hoeck et al., 2013). Examining the concordance across diverse types of hypothetical episodes allows us to specify the set of brain regions that is commonly engaged during episodic simulation irrespective of the exact nature of the imagined event.
Moreover, it has been argued that the core network is similar to the set of brain regions typically referred to as the default network (DN; Buckner & Carrol, 2007; Schacter et al., 2012). The DN comprises regions that seem to be strongly activated during internally-directed cognition (Andrews-Hanna, Smallwood, & Spreng, 2014) and that, possibly as a consequence, are often more strongly engaged during rest periods than during the performance of tasks that require an attentional focus on the external environment (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al. 2001; Shulman et al., 1997). We here gauge the spatial overlap of the regions jointly engaged by episodic memory and episodic simulation on the one hand and the DN on the other by comparing the results of our meta-analysis with a recent large-scale parcellation of the cerebral cortex that was based on resting-state functional connectivity (Yeo et al., 2011).
The second goal of this study is the identification of brain regions that are more strongly recruited during the simulation of hypothetical episodes than during the retrieval of autobiographical memories. Though the constructive episodic simulation hypothesis emphasizes the commonalities of those functions, it also posits some critical differences (Schacter & Addis, 2007; Schacter et al., 2012). For example, the simulation of a hypothetical event presumably requires the retrieval of details from multiple episodes that can then be recombined into the novel event. On the one hand, episodic simulation would thus place greater demands on nodes of the core network supporting the retrieval of such details. In line with this account, there is some evidence for a greater engagement of the hippocampus during the imagination of the future than the recollection of the past (Addis et al., 2007; Addis, Roberts, & Schacter, 2011; Okuda et al., 2003; but see also Botzung et al., 2008).
On the other hand, only episodic simulation imposes a need for novel recombination. It is also more open-ended and less constrained than the retrieval of a past episode. As such, episodic simulation would possibly require further control processes in addition to those core construction processes shared with episodic memory. Indeed, recent studies (Gerlach et al., 2014; Spreng et al. 2010) have demonstrated an increased coupling of the DN with a set of regions referred to as the frontoparietal control network (FPCN; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Vincent, Kahn, Snyder, Raichle, & Buckner et al., 2008) in situations when people presumably draw on knowledge of their past for the planning of future personal events. The episodic simulation of hypothetical events may thus require an additional engagement of parts of the FPCN in addition to a stronger recruitment of the core network.
To test these two predictions, we performed a complementary ALE meta-analysis of studies reporting greater activation during hypothetical imaginings than during the recollection of past happenings. Together the present meta-analyses thus specify core network regions that are jointly engaged during episodic memory and episodic simulations, characterize their spatial relationship to the DN, and also identify regions that are more strongly recruited during the simulation of hypothetical episodes than during the retrieval of past memories.
Methods
We conducted two ALE meta-analyses. The first was based on neuroimaging experiments reporting spatial overlap in brain activation during (i) the recollection of past episodes and (ii) the simulation of hypothetical events. Hypothetical events include specific future, fictitious, and counterfactual (i.e., alternative versions of the past) episodes that participants imagined experiencing through their own eyes (i.e., from a field perspective). The second meta-analysis examined brain regions exhibiting greater activation during the simulation of hypothetical episodes than during the retrieval of past episodes.
Study selection: inclusion and exclusion criteria
We first searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) for peer-reviewed articles that included at least one of the following key phrases in order to identify studies examining episodic simulation: episodic future thinking, prospection, episodic simulation, mental time travel, self-projection, future envisioning, or counterfactual simulation. The articles moreover had to include one of the keywords fMRI, neuroimaging, or PET. The final literature search was conducted on March 11, 2015.
From the resulting list of articles, we selected those that reported functional neuroimaging studies (i.e., fMRI or PET) of episodic simulation (leading to the exclusion of review articles, neuropsychological studies, and, e.g., Lavallee & Persinger, 2010, which reported source-localized EEG data). Moreover, the reported studies had to further include a condition that required participants to recollect autobiographical memories (leading to the exclusion of several studies that only examined episodic simulation of hypothetical events, e.g., Benoit, Gilbert, & Burgess, 2011; Benoit, Szpunar, & Schacter, 2014; D’Argembeau, Xue, Lu, Van der Linden, & Bechara, 2008; Summerfield, Hassabis, & Maguire, 2010). The included studies reported results for healthy adults or common results for healthy adults and a clinical sample (i.e., Hach, Tippett, & Addis, 2014). For the inclusion in the first meta-analysis, the articles had to report whole-brain coordinates for spatial overlap between episodic memory and episodic simulation. The overlap could have been obtained by averaging across conditions, by performing a conjunction analysis, or by identifying a latent variable coding for both conditions (leading to the exclusion of Botzung et al., 2008). For inclusion in the second meta-analysis, the studies had to report coordinates of regions exhibiting greater activation for episodic simulation than episodic memory.
Using these criteria, 12 studies were included in the first meta-analysis, and 11 in the second (Table 1). Four studies contributed to only the first meta-analysis. Of those, three had employed a data-driven approach (i.e., partial least squares) that did not explicitly test for greater activation during simulation than memory (Spreng et al., 2010; de Brigard et al., 2013; Hach et al., 2014), and the final one did not report a test for this effect (Hassabis et al., 2007). It is thus unclear whether these studies would have revealed a pattern similar to the one obtained in our second meta-analysis, or whether they would have yielded null results.
Table 1.
Contrasts included in the respective meta-analyses
| Studies reporting joint activation for episodic memory and episodic simulation | |||||
|---|---|---|---|---|---|
| Study | episodes | control task | analysis | n | foci |
| Abraham et al. (2008) | future & past | semantic future & past | MU: CO | 20 | 25 |
| Addis et al. (2007) | future & past (construction) | semantic retrieval & mental imagery (const.) | MU: CO | 14 | 5 |
| future & past (elaboration) | semantic retrieval & mental imagery (elab.) | MU: CO | 14 | 23 | |
| Addis et al. (2009) | future & possible past & past | semantic retrieval & mental imagery | PLS: LV | 18 | 21 |
| Addis et al. (2011) | future & past | routine future & past events | MU: CO | 23 | 7 |
| de Brigard et al. (2013) | counterfactual & past | semantic retrieval & mental imagery | PLS: LV | 17 | 5 |
| Hach et al. (2014) | future & past | semantic retrieval & mental imagery | PLS: LV | 33* | 14 |
| Hassabis et al. (2007) | fictitious & recall of fictitious & past | mental imagery & recall of individual objects | MU: CO | 21 | 18 |
| Spreng et al. (2010) | future & past | mentalizing | PLS: LV | 16 | 17 |
| Szpunar et al. (2007) | future & past | mentalizing | MU: AV | 21 | 15 |
| van Hoeck et al. (2013) | future & counterfactual & past | semantic retrieval & mental imagery | MU: CO | 14 | 22 |
| Viard et al. (2011) | future & past | reading & letter identification | MU: CO | 12** | 29 |
| Weiler et al. (2010) | future & past (construction) | mentalizing (construction) | MU: CO | 17 | 50 |
| future & past (elaboration) | mentalizing (elaboration) | MU: CO | 17 | 49 | |
|
| |||||
| Studies reporting greater activation for episodic simulation than episodic memory | |||||
| Study | contrast | analysis | n | foci | |
|
| |||||
| Abraham et al. (2008) | future > past | MU | 20 | 9 | |
| Addis et al. (2007) | future > past (construction) | MU | 14 | 18 | |
| future > past (elaboration) | MU | 14 | 1 | ||
| Addis et al. (2009) | future & imagined past > past | PLS | 18 | 10 | |
| Addis et al. (2011) | future > past | MU | 23 | 11 | |
| Gilmore et al. (2014) | future > past | MU | 21 | 11 | |
| Kirwan et al. (2014) | fictitious > past | MU | 14 | 8 | |
| Szpunar et al. (2007) | future > past | MU | 21 | 8 | |
| Szpunar et al. (2009) | future > past | MU | 27 | 10 | |
| van Hoeck et al. (2013) | counterfactual > past | MU | 14 | 17 | |
| future > past | MU | 14 | 12 | ||
| Viard et al. (2011) | future > past | MU | 12 | 9 | |
| Weiler et al. (2010) | future > past (construction) | MU | 17 | 6 | |
| future > past (elaboration) | MU | 17 | 6 | ||
16 controls and 17 depressed patients;
older subjects (mean age ± SD = 67.2 ± 5.2 years); AV: averaged; CO: conjunction; LV: latent variable; MU: mass-univariate; PLS: partial-least squares
For a study that reported results for nested contrasts, we chose to include the contrast with the more complex control task (i.e., specific versus general episodes rather than all episodes versus a semantic and imagery control task; Addis et al., 2011). For studies reporting results for multiple time points of the same contrast (Addis, Pan, Vu, Laiser, & Schacter, 2009; de Brigard et al., 2013), we selected the one that covered the time window of 4 to 6 s following simulation onset, thus adjusting for the hemodynamic lag. In the one case where an analogous effect was reported with two analyses, we report the a-priori contrast testing for commonalities between episodic memory and simulation rather than the complementary data-driven result (i.e., the non-rotated rather than the mean-centered partial-least square analysis; Addis et al., 2009). This procedure led to the inclusion of 14 contrasts for each of the meta-analyses. They are listed, with additional details, in Table 1. Together, the contrasts yielded 300 and 136 foci for the respective meta-analyses (Fig. 1), of which 4 and 1, respectively, were excluded from the analyses because they were located outside the employed brain mask. Coordinates reported in Talairach space were converted to the Montreal Neurological Institute stereotactic space using the algorithm developed by Lancaster et al. (2007) and implemented in GingerALE 2.3 (http://brainmap.org).
Table 2.
Regions consistently engaged during both episodic simulation and episodic memory, i.e., the core network
| Region | ~BA | Volume (mm3) | Peak ALE value | MNI peak | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| dlPFC | 8, 9 | 960 | 0.015 | 24 | 46 | 48 |
| 0.015 | 26 | 50 | 38 | |||
| ACC, rmPFC, vmPFC | 10, 11, 24, 32 | 3408 | 0.022 | −4 | 42 | 4 |
| 0.016 | −4 | 50 | −10 | |||
| 0.010 | 2 | 34 | −4 | |||
| 0.010 | 4 | 34 | 2 | |||
| dmPFC | 6, 9 | 856 | 0.016 | −6 | 38 | 38 |
| 0.013 | −4 | 36 | 32 | |||
| oIFG / insula | 47, 13 | 576 | 0.015 | −34 | 26 | −8 |
| pdlPFC | 6, 8 | 608 | 0.014 | −38 | 18 | 52 |
| LTC | 21 | 2264 | 0.025 | 60 | −6 | −10 |
| 0.014 | 58 | −4 | −24 | |||
| LTC | 21 | 1112 | 0.018 | −60 | −4 | −16 |
| PHC, HC | 28, 35 | 1280 | 0.019 | −20 | −18 | −24 |
| PHC, HC | 28, 35, 36 | 3080 | 0.021 | 22 | −20 | −16 |
| 0.016 | 30 | −28 | −20 | |||
| PCC | 24, 31 | 688 | 0.012 | 0 | −20 | 38 |
| 0.011 | −6 | −10 | 50 | |||
| LTC | 21 | 768 | 0.014 | −58 | −22 | −6 |
| PCC | 31 | 808 | 0.022 | −2 | −38 | 42 |
| PHC, HC | 30, 36, 37 | 2624 | 0.027 | −20 | −38 | −12 |
| PCC incl. Resp, Prec, PHC | 23, 29, 30, 31 | 6728 | 0.023 | 10 | −52 | 10 |
| 0.019 | −8 | −48 | 8 | |||
| 0.019 | −6 | −58 | 24 | |||
| 0.016 | 14 | −46 | 2 | |||
| 0.014 | −14 | −54 | 30 | |||
| pIPL, pSTL | 39 | 1720 | 0.018 | 54 | −62 | 26 |
| 0.015 | 46 | −60 | 26 | |||
| 0.010 | 48 | −70 | 28 | |||
| pIPL, pSTL | 39 | 1792 | 0.018 | −48 | −64 | 30 |
| 0.016 | −44 | −56 | 28 | |||
| dlOC | 19 | 736 | 0.016 | −34 | −78 | 38 |
Thresholded at p < 0.05, cluster-level corrected; aCC: anterior cingulate cortex; aI: anterior insula; BA: Brodmann area; dlOC: dorsolateral occipital cortex; dlPFC: dorsolateral prefrontal cortex; dmPFC: dorsomedial prefrontal cortex; dpCC: dorsal posterior cingulate cortex; HC: hippocampus; LTC: lateral temporal cortex; oIFC: orbital inferior frontal gyrus; PHC: parahippocampal cortex; pCC: posterior cingulate cortex; pdlPFC: posterior dorsolateral prefrontal cortex; pIPL: posterior inferior parietal lobe; pSTL: posterior superior temporal lobe; Resp: retrosplenial cortex; rmPFC: rostral medial prefrontal cortex; vmPFC: ventromedial prefrontal cortex
Figure 1.
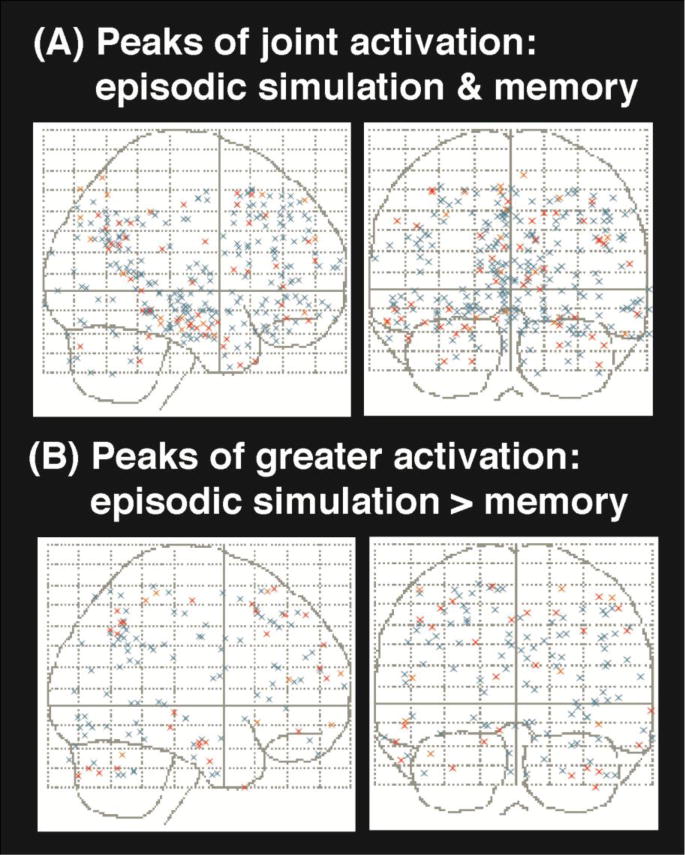
Peak coordinates of all contrasts reporting (A) joint activation for episodic simulation and episodic memory and (B) greater activation for episodic simulation than episodic memory. Blue markers identify studies examining simulations of future episodes, orange of fictitious events, and red include counterfactual episodes.
Activation likelihood estimation (ALE)
We performed the meta-analyses using activation likelihood estimation (ALE) as implemented in GingerALE 2.3 (Eickhoff et al., 2009; Eickhoff et al., 2012; Turkeltaub, Eden, Jones, & Zeffiro, 2002; Turkeltaub et al., 2012). ALE tests for above-chance clustering of peak foci from the included experiments in a random-effects approach by comparing the union of the smoothed foci across studies with the null hypothesis that the foci are spatially independent from each other (Eickhoff et al., 2009).
First, for each experiment, a Model Activation (MA) map is generated based on all reported foci. Each focus gets smoothed by a full-width half-maximum (FWHM) Gaussian kernel, with a smaller kernel-width for studies with larger samples as determined empirically by Eickhoff et al. (2009). Each voxel of the MA map then gets assigned the maximum probability associated with any of the foci (thus reducing the impact of studies reporting multiple foci in close proximity; Turkeltaub et al., 2012). (Also note that foci were entered into the meta-analysis arranged by study rather than contrast, as recommended by Turkeltaub et al. (2012). This approach prevents studies reporting more than one contrast from influencing the analysis more than other studies.) Second, the ALE map is calculated as the voxelwise union of all MA maps. It thus summarizes the patterns of foci across the included studies. Third, the ALE map is thresholded to show clusters where the convergence between foci exceeds what would be expected under the null hypothesis (i.e., spatial independence of the foci across studies). To correct for multiple comparisons, we used cluster-level inference with a threshold of p < 0.05, based on a null distribution determined by 1000 permutations of the data and a cluster-forming threshold of p < 0.005 (Eickhoff et al., 2012).
To assess the overlaps of the thresholded maps with the DN and FPCN, the results of the meta-analyses were rendered on the inflated PALS template using Caret v5.65 (van Essen, 2005; http://brainvis.wustl.edu/wiki/index.php/Caret:About), with borders of the DN and FPCN derived from a large-scale analysis of resting-state functional connectivity (Yeo et al., 2011).
Finally, we performed a conjunction analysis (Nichols, Brett, Andersson, Wager, & Poline, 2005) to identify brain regions that were associated (i) with activation during both episodic memory and simulation, as well as (ii) with greater activation during episodic simulation than memory. Specifically, using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), we combined the thresholded maps of the two ALE analyses. This approach thus identifies voxels that were part of significant clusters in both meta-analyses.
Results
We first report regions exhibiting consistent activation during both episodic memory of autobiographical events and episodic simulation before describing those areas that are consistently more strongly engaged during episodic simulation than episodic memory.
Brain regions jointly activated by episodic memory and episodic simulation
The first meta-analysis identified 17 clusters that were consistently engaged during both episodic memory and episodic simulation (Table 2; Fig. 2a). The clusters comprised, in both hemispheres, all nodes that had been suggested to be part of the core network (Schacter et al., 2007; Hassabis and Maguire, 2007). Within the MTL, these were the parahippocampal cortex (PHC) and hippocampus (HC); on the medial surface, the anterior cingulate cortex (aCC) and adjacent rostral and ventral medial prefrontal cortex (vmPFC), the dorsomedial prefrontal cortex (dmPFC) as well as the posterior cingulate cortex (pCC), including retrosplenial cortex (Resp); and on the lateral surface parts of the temporal cortex (LTC), as well as clusters encompassing posterior inferior parietal (pIPL) and superior temporal lobes (pSTL). Clusters that had not typically been linked to the core network, and that survived thresholding, were situated in the right dorsolateral prefrontal cortex (dlPFC), in the left posterior dorsolateral prefrontal cortex (pdlPFC), in a region comprising parts of the left anterior insula and of the orbital inferior frontal gyrus, and in left dorsolateral occipital cortex.
Figure 2.
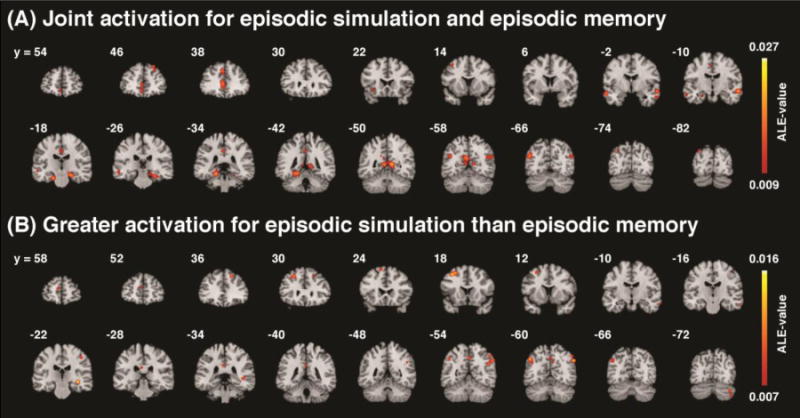
(A) Results of the ALE meta-analysis identifying the core network, i.e., regions showing consistent engagement during both, various forms of episodic simulation and episodic memory, and (B) results of the complementary analysis revealing regions showing consistently greater activation during simulation than during memory retrieval. Thresholded at p < 0.05, cluster-level corrected.
Moreover, inspection of the whole brain rendering indicated that all clusters were predominantly located within the DN as demarcated by Yeo et al. (2011) (Fig. 3a). Only the right dlPFC cluster similarly overlapped with both the DN and the FPCN.
Figure 3.
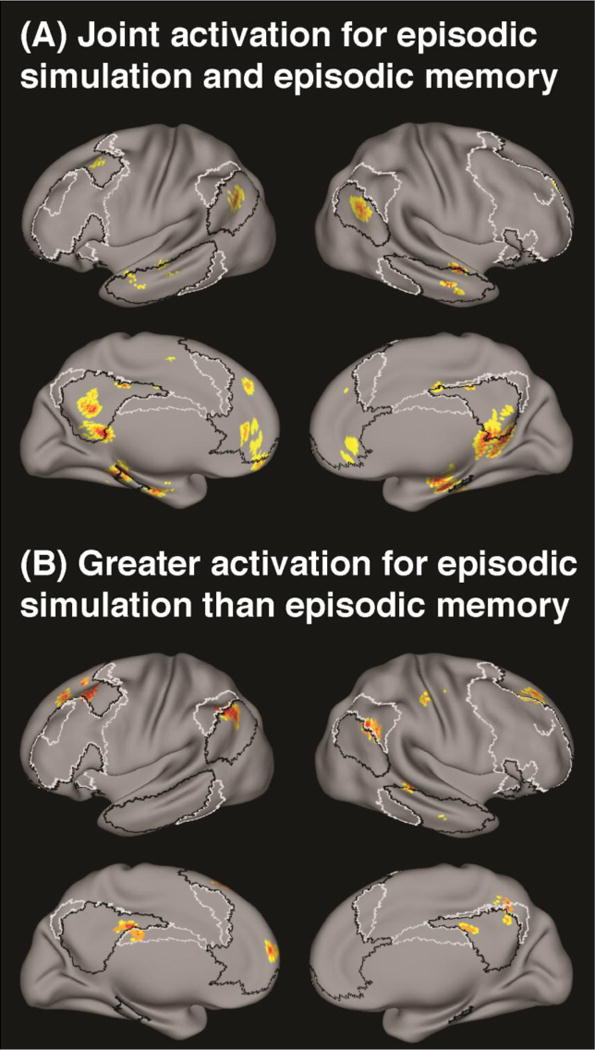
Results of the meta-analyses revealing (A) common activation during episodic simulation and episodic memory and (B) greater activation during simulation than memory. Thresholded at p < 0.05, cluster-level corrected. Black dashed lines indicate the borders of the default network, and white dashed lines of the fronto-parietal control network as demarcated by Yeo et al. (2011).
Brain regions more strongly activated by episodic simulation than episodic memory
The second meta-analysis revealed 14 clusters that were consistently more strongly engaged during the simulation of hypothetical events than during the retrieval of past memories. These comprised bilateral clusters in the dlPFC and pIPL, clusters in dmPFC, pCC, and precuneus (Prec), clusters in right MTL (including the hippocampus), LTC, postcentral gyrus (PCG) and cerebellum, and in left pdlPFC, (Fig. 2b). Inspection of the whole-brain rendering indicated that all clusters but the ones in PCG and cerebellum overlapped with the DN; five of those also covered parts of the FPCN (i.e., right dlPFC, pCC, Prec, bilateral pIPL) (Fig. 3b).
We had also predicted that some of the regions identified by this second ALE analysis would overlap with the jointly engaged regions specified by the preceding analysis. We tested for such spatial convergence by performing a conjunction analysis of the thresholded maps of the two meta-analyses (Nichols et al., 2005). This analysis reveals only voxels that were significant in both meta-analyses, i.e., (i) jointly activated by episodic simulation and episodic memory as well as (ii) more strongly recruited during episodic simulation. Only voxels in the left pIPL and pdlPFC fulfilled these criteria (Fig. 4).
Figure 4.
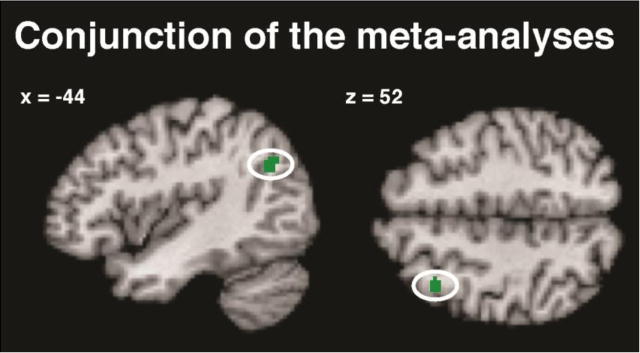
Conjunction analysis identifying regions that were significant in both meta-analyses, i.e., part of the core network and exhibiting greater activation for episodic simulation than episodic memory.
Discussion
In the current study we set out to specify the neural networks supporting our ability to simulate hypothetical episodes. We therefore performed two meta-analyses. The first examined the concordance of brain regions jointly activated during both episodic simulation and episodic memory for autobiographical events. It thus specified the nodes of the hypothesized core network supporting both functions (Hassabis & Maguire, 2007; Schacter et al., 2007).
We only included data from studies that had performed a formal analysis testing for spatial overlap of episodic simulation and memory, and which thus provided stringent evidence for joint activation. Observing functional overlap within the same set of subjects suggests that these functions recruit overlapping neuronal populations. By contrast, observing such overlap between studies is more ambiguous. For example, subtle between-subject differences in functional neuroanatomy could result in both over- and underestimations of common activation, and study differences in the spatial signal-to-noise pattern may artificially reduce the observed overlap.
At the same time, we included studies examining various forms of hypothetical events that differed in their temporal orientation (i.e., future, past, atemporal) and accordingly may vary in features such as plausibility and affective content (for general discussion, see Schacter et al., 2012). The identified network is thus likely to support core-construction processes that generalize across such features. However, a majority of the included studies concentrated on future simulations, reflecting the focus of the extant literature. With further maturation of this field, it would be desirable to corroborate the recruitment of the network separably for the simulation of each event type. Indeed, the notion of a core network does not suggest that any episodic simulation will similarly recruit each of the network’s nodes. For example, the functional profile of the hippocampus seems to differ between future and counterfactual simulations, with the former eliciting greater activation for implausible (Weiler et al., 2010) and the latter for plausible events (de Brigard et al., 2013).
Finally, a challenge for neuroimaging investigations of episodic simulation is the use of appropriate control conditions, and the included studies varied considerably in their choice (from simple visual imagery to complex mentalizing; see Table 1). By collapsing across those experiments, the current description of the core network is less influenced by the exact features of any single control task.
The brain regions identified by the first analysis were largely identical to the hypothesized core network (Hassabis & Maguire, 2007; Schacter et al., 2007). These were parts of the medial temporal lobes, including the HC and PHC, the ACC and adjacent vmPFC, dmPFC, pCC with Resp, parts of the lateral temporal cortex and areas within the pIPL and pSTL. In addition, the analysis revealed consistent engagement of parts of the dlPFC in both hemispheres, left anterior insula and dorsolateral occipital cortex. The results are thereby broadly consistent with the earlier qualitative assessment by Spreng et al. (2009), and the analysis restricted to episodic future simulation by Stawarczyk & D’Argembeau (in press). The current study extrapolates this pattern to the simulation of various kinds of hypothetical episodes, and critically reveals the overlap with regions supporting episodic recollection. It thus provides a quantitative demonstration of the core network for episodic memory and episodic simulation.
It has further been suggested that the core network is similar to the DN (Buckner, Andrews-Hanna, & Schacter, 2008; Schacter et al., 2007; Spreng et al., 2008). Consistent with this proposal, we observed that all constituent parts of the identified network overlapped extensively with areas of the DN as demarcated by a recent large-scale parcellation of the cerebral cortex (Yeo et al., 2011). The regions were broadly distributed across all of the proposed fractions of the DN, i.e., its medial temporal lobe subsystem (PHC, HC, Resp, pIPL, and vmPFC), the dorsomedial prefrontal subsystem (dmPFC, dlPFC, LTC), and the intertwining hubs (i.e., pCC and rostral mPFC) (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Yeo et al., 2011), consistent with the suggestion that autobiographical and future thoughts require the contributions of each DN subsystem (Andrews-Hanna, Saxe, & Yarkoni, 2014). However, we note that the DN is broader than the core network, and the former includes parts of the cerebral cortex that are absent from the latter (e.g., parts of the superior frontal gyri).
The constructive episodic simulation hypothesis proposes that the identified network supports processes involved in the retrieval of episodic details and in the construction of past and hypothetical episodes (Schacter & Addis, 2007). However, this idea does not imply that the regions uniquely support mnemonic processes. In fact, a largely overlapping set of regions has also been associated with the seemingly disparate faculties of mentalizing and spatial navigation (Buckner & Carrol, 2007; Spreng et al., 2008; 2010). Some of the identified brain areas may thus contribute to even broader cognitive functions. Buckner and Carroll (2007; see also Buckner et al., 2008), for example, suggest that episodic memory, future simulation, spatial navigation, and mentalizing share the requirement to project oneself into alternative situations that are decoupled from the current environment (i.e., self-projection). Alternatively, it has been argued that at least the first three of those capacities share the requirement to build spatial models of the respective situation (i.e., scene construction) (Hassabis & Maguire, 2007; Mullally & Maguire, 2014). Though the current data cannot differentiate between these accounts, they all suggest that the core network, comprised of regions jointly engaged by episodic memory and simulation, would also support various forms of rich spontaneous thoughts such as those arising during periods of mindwandering (cf., Andrews-Hanna, Smallwood, & Spreng, 2014; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; Smallwood et al., 2013; Stawarczyk & D’Argembeau, in press).
Turning to our second meta-analysis, we also identified a set of brain regions that are more strongly engaged during episodic simulation than episodic memory. We had suggested that these would comprise parts of the core network (e.g., Addis et al., 2007), as well as additional brain regions supporting cognitive control processes required for the recombination of disparate details into novel episodes (including those constituting the FPCN; see Gerlach et al., 2014; Spreng et al. 2010). To test the first part of this prediction, we conducted a conjunction analysis of the two meta-analyses. This analysis revealed that, of the regions encompassing the core network, only the left pIPL and posterior dlPFC were more active during episodic simulation. Thus, episodic simulation was associated with a stronger engagement of a few nodes of the specified core network. Further targeted research is required to determine the exact contribution of these regions. As expected, we also observed a greater engagement of the MTL, including the HC, though the cluster did not overlap with the one identified in the first meta-analysis.
Regarding the second part of our prediction, we first note that all of the identified clusters but the PCG and cerebellum overlapped with parts of the DN. Importantly, five of those clusters fell, partly predominantly, also within the borders of the FPCN (Power et al., 2011; Yeo et al., 2011). These clusters comprised the Prec, anterior parts of the pCC, somewhat more dorsal parts of the bilateral pIPL, and right dlPFC. The FPCN is particularly engaged in situations requiring the flexible adaptation to novel demands, and seems to implement cognitive control by influencing processing in other parts of the brain (Cole et al., 2013; Dosenbach et al., 2007; Duncan, 2013). Consistent with this account, the control network has been shown to exhibit increased functional connectivity with the DN (and, thus, putatively with regions of the core network) in situations requiring the flexible planning for future events (Spreng et al., 2010). Accordingly, the fronto-parietal regions identified in the current meta-analysis may interact with the core network to support novel recombination in service of the simulation of hypothetical episodes.
The concordance of brain activation across studies, of course, does not imply that all nodes of the network support the same processes. A fundamental goal for future research is the functional deconstruction of the network, and indeed some progress has already been made in our understanding of the specific contributions of individual regions. For example, the Resp and PHC may be particularly important for the specification of the episode’s spatiotemporal context (Benoit et al., 2014; Gilmore, Nelson, & McDermott, 2014; Szpunar, St. Jacques, Robbins, Wig & Schacter, 2014). The mPFC, by comparison, may process the anticipated affective quality of the simulated event (Benoit et al., 2011; Benoit et al., 2014; D’Argembeau et al., 2008), and also contribute to episodic simulations by integrating knowledge about the episode’s elements (Benoit et al., 2014). However, the mPFC might exhibit a further functional differentiation on a fine spatial scale (cf., Benoit, Gilbert, Frith, & Burgess, 2012; Gilbert, Henson, & Simon, 2010), similar to a possible functional fractionation within the HC (Addis & Schacter, 2012). For example, different parts of the HC may support the retrieval of episodic details, the binding of those details into novel episodes, and the encoding of those episodes into long-term memory (Addis & Schacter, 2012; Gaesser, Spreng, McLelland, Addis, & Schacter, 2013; Martin, Schacter, Corballis, & Addis, 2011; see also Viard, Desgranges, Eustache, & Piolino, 2012).
The precise specification of the core network as well as of the auxiliary set of regions that further support episodic simulation provides an important foundation for examining the interactions between those regions and for determining their specialized contributions. Such future investigations will not only enhance our understanding of those processes that allow us to imagine a plethora of possible episodes and thus inform future-oriented behavior (Benoit et al., 2011; Boyer, 2008; Schacter et al., 2015), but may also shed light on how we employ these constituent processes during rich spontaneous thought (cf., Andrews-Hanna et al., 2014; Fox et al., 2015; Smallwood et al., 2013).
Table 3.
Regions showing consistently greater activation during episodic simulation than during episodic memory
| Region | ~BA | Volume (mm3) | Peak ALE value | MNI peak | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| dmPFC, rmPFC | 9, 10 | 432 | 0.0102 | −6 | 54 | 18 |
| dlPFC | 8 | 696 | 0.0109 | 20 | 36 | 46 |
| 0.0089 | 24 | 26 | 46 | |||
| dlPFC | 8 | 712 | 0.0115 | −24 | 30 | 46 |
| pdmPFC | 6 | 400 | 0.0106 | −12 | 24 | 62 |
| pdlPFC | 6, 8 | 1504 | 0.0149 | −36 | 18 | 50 |
| 0.0126 | −30 | 16 | 54 | |||
| LTC | 21 | 336 | 0.0100 | 66 | −12 | −20 |
| MTL, HC | 568 | 0.0157 | 36 | −22 | −6 | |
| pCG | 3 | 360 | 0.0097 | 44 | −22 | 50 |
| pCC | 23, 31 | 672 | 0.0101 | −4 | −34 | 32 |
| 0.0099 | −6 | −30 | 26 | |||
| 0.0095 | −2 | −40 | 32 | |||
| LTC | 41 | 376 | 0.0105 | 42 | −34 | 0 |
| Prec | 680 | 0.0131 | 0 | −56 | 44 | |
| 0.0087 | 6 | −58 | 36 | |||
| pIPL | 40 | 2048 | 0.0149 | 54 | −58 | 38 |
| 0.0126 | 50 | −56 | 50 | |||
| 0.0106 | 46 | −52 | 32 | |||
| pIPL | 40 | 1536 | 0.0148 | −48 | −58 | 42 |
| Cerb | 520 | 0.0102 | 38 | −72 | −32 | |
| 0.0089 | 42 | −72 | −44 | |||
Thresholded at p < 0.05, cluster-level corrected; Cerb: cerebellum; dlPFC: dorsolateral prefrontal cortex; dmPFC: dorsomedial prefrontal cortex; LTC: lateral temporal cortex; pCG: postcentral gyrus; pCC: posterior cingulate cortex; pIPL: posterior inferior parietal lobe; pdlPFC: posterior dorsolateral prefrontal cortex; pdmPFC: posterior dorsomedial prefrontal cortex; pIPL: posterior inferior parietal lobe; Prec: precuneus; rmPFC: rostral medial prefrontal cortex
Highlights.
We report two meta-analyses on episodic simulation and episodic memory.
Both capacities jointly recruited a core network of brain regions.
These regions largely overlapped with the default network.
Simulation more strongly engaged some of the network’s nodes plus further regions.
Some of the additional regions fell into the fronto-parietal control network.
Acknowledgments
We thank Grace Xiao for assistance in extracting coordinate data. This work was supported by National Institute of Mental Health Grant R01MH60941 (to D.L.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Schubotz RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Research. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;18:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, Schacter DL. Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia. 2011;49:3656–3669. doi: 10.1016/j.neuropsychologia.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The hippocampus and imagining the future: Where do we stand? Frontiers in Human Neuroscience. 2012;5:173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage. 2014;91:324–35. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. The Journal of Neuroscience. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Frith CD, Burgess PW. Rostral prefrontal cortex and the focus of attention in prospective memory. Cerebral Cortex. 2012;22:1876–1886. doi: 10.1093/cercor/bhr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proceedings of the National Academy of Sciences USA. 2014;111:16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends in Cognitive Sciences. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carrol DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. The Year in Cognitive Neuroscience, Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Busby J, Suddendorf T. Recalling yesterday and predicting tomorrow. Cognitive Development. 2005;20:362–372. [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multitask connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: influence of valence and temporal distance. Consciousness and Cognition. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brigard F, Addis DR, Ford JH, Schacter DL, Giovanello KS. Remembering what could have happened: Neural correlates of episodic counterfactual thinking. Neuropsychologia. 2013;51:2401–2414. doi: 10.1016/j.neuropsychologia.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, Schacter DL. Characterizing age-related changes in remembering the past and imagining the future. Psychology & Aging. 2011;26:80–84. doi: 10.1037/a0021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: Default network couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social, Cognitive, and Affective Neuroscience. 2014;9:1942–1951. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Henson RNA, Simons JS. The scale of functional specialization within human prefrontal cortex. Journal of Neuroscience. 2010;30:1233–1237. doi: 10.1523/JNEUROSCI.3220-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, McDermott KB. The Contextual Association Network Activates More for Remembered than for Imagined Events. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu223. [DOI] [PubMed] [Google Scholar]
- Hach S, Tippett LJ, Addis DR. Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia. 2014;65:41–55. doi: 10.1016/j.neuropsychologia.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences USA. 2007a;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience. 2007b;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallee CF, Persinger MA. A LORETA study of mental time travel: similar and distinct electrophysiological correlates of re-experiencing past events and pre-experiencing future events. Consciousness and Cognition. 2010;19:1037–1044. doi: 10.1016/j.concog.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Madore KP, Gaesser B, Schacter DL. Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40:609–622. doi: 10.1037/a0034885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proceedings of the National Academy of Sciences USA. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. Memory, Imagination, and Predicting the Future: A Common Brain Mechanism? Neuroscientist. 2013;20:220–234. doi: 10.1177/1073858413495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshat-Doost HT, Dalgleish T, Yule W, Kalantari M, Ahmadi SJ, Dyregrov A, Jobson L. Enhancing autobiographical memory specificity through cognitive training: An intervention for depression translated from basic science. Clinical Psychological Science. 2012;20:1–9. [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. The Journal of Neuroscience. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B) 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: Intersections between memory and decisions. Neurobiology of Learning and Memory. 2015;117:14–21. doi: 10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, et al. Common blood flow changes across visual tasks: II.: decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Tipper C, Brown K, Baird B, Engen H, Michaels J, Grafton S, Schooler JW. Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage. 2013;69:120–125. doi: 10.1016/j.neuroimage.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, D’Argembeau A. Neural correlates of personal goal processing during episodic future thinking and mind-wandering: an ALE meta-analysis. Human Brain Mapping. doi: 10.1002/hbm.22818. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genetic, Social, and General Psychology Monographs. 1997;123:133–167. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: what is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. discussion 313–351. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia. 2010;48:1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, St Jacques PL, Robbins CA, Wig GS, Schacter DL. Repetition-related reductions in neural activity reveal component processes of mental simulation. Social, Cognitive, and Affective Neuroscience. 2014;9:712–722. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SG, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proceedings of the National Academy of Sciences USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobe Y, Liberman N. Temporal Construal. Psychological Review. 2003;110:403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and autonoesis: uniquely human? In: Terrace HS, Metcalfe J, editors. The Missing Link in Cognition: Origins of Self-reflective Consciousness. New York: Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Hoeck N, Ma N, Ampe L, Baetens K, Vandekerckhove M, Van Overwalle F. Counterfactual thinking: an fMRI study on changing the past for a better future. Social Cognitive Affective Neuroscience. 2013;8:556–564. doi: 10.1093/scan/nss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Chetelat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: an fMRI study. Brain and Cognition. 2011;75:1–9. doi: 10.1016/j.bandc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Viard A, Desgranges B, Eustache F, Piolino P. Factors affecting medial temporal lobe engagement for past and future episodic events: an ALE meta-analysis of neuroimaging studies. Brain and Cognition. 2012;80:111–125. doi: 10.1016/j.bandc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: differences in brain activation patterns for episodic memory and episodic future thinking. Behavioral Brain Research. 2010;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


