Abstract
Objectives
Visual perceptual organization impairments in schizophrenia (SCZ) are well established, but their neurobiological bases are not. The current study used the previously validated Jittered Orientation Visual Integration (JOVI) task, along with fMRI, to examine the neural basis of contour integration (CI), and its impairment in SCZ. CI is an aspect of perceptual organization in which multiple distinct oriented elements are grouped into a single continuous boundary or shape.
Methods
On the JOVI, five levels of orientational jitter were added to non-contiguous closed contour elements embedded in background noise to progressively increase the difficulty in perceiving contour elements as left- or right-pointing ovals. Multi-site fMRI data were analyzed for 56 healthy control subjects and 47 people with SCZ.
Results
SCZ patients demonstrated poorer CI, and this was associated with increased activation in regions involved in global shape processing and visual attention, namely the lateral occipital complex and superior parietal lobules. There were no brain regions where controls demonstrated more activation than patients.
Conclusions
CI impairment in this sample of outpatients with SCZ was related to excessive activation in regions associated with object processing and allocation of visual-spatial attention. There was no evidence for basic impairments in contour element linking in the fMRI data. The latter may be limited to poor outcome patients, where more extensive structural and functional changes in the occipital lobe have been observed.
1.0 Introduction
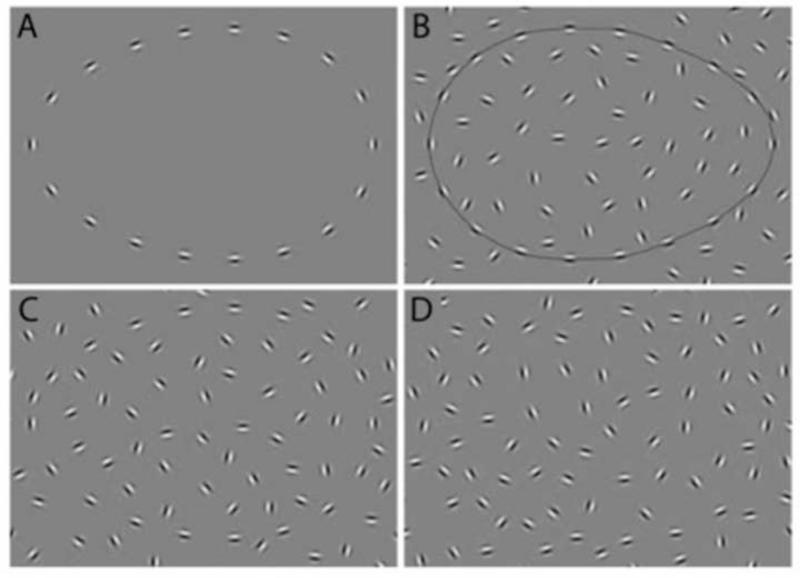
Evidence is increasing for the presence of visual perceptual and ocular impairments in schizophrenia (Silverstein & Keane, 2011b; Silverstein & Rosen, 2015). These are found in patients (Butler, Silverstein, & Dakin, 2008) and, in some cases, in unaffected offspring (Hebert et al., 2010), and, when identified in high-risk children (Schiffman et al., 2006; Schubert, Henriksson, & McNeil, 2005) and in ultra-high risk young adults (Klosterkotter, Hellmich, Steinmeyer, & Schultze-Lutter, 2001), they predict conversion to psychosis in adulthood. In addition, visual processing disturbances are related to functional impairment (Green, Hellemann, Horan, Lee, & Wynn, 2012; Rassovsky, Horan, Lee, Sergi, & Green, 2011). One well-documented visual impairment is in perceptual organization, which refers to the processes by which individual elements of sensory information are collectively structured into larger units of perceived objects and their interrelations (Palmer, 1999). Over 50 studies have now demonstrated reduced visual perceptual organization in schizophrenia across various paradigms, labs, and countries (Silverstein & Keane, 2011a; Uhlhaas & Silverstein, 2005). One of the most commonly investigated aspects of perceptual organization in the schizophrenia and basic vision literatures is contour integration (CI) (Braun, 1999; Chandna, Pennefather, Kovacs, & Norcia, 2001; Kovacs, 2000a; Levi, Yu, Kuai, & Rislove, 2007), which is a fundamental visual process that forms representations of continuous boundaries and shapes on the basis of the relative positions and orientations of multiple edge elements. CI is typically measured as the ability to detect or make a judgment about the shape, position, or presence of a closed contour made up of non-contiguous elements, embedded within a display of randomly oriented elements (see Figure 1).
Figure 1.
Examples of stimuli from the JOVI task: A) left-pointing contour from no-background catch trial; B) right-pointing contour from outline catch trial; C) left pointing contour from 0° jitter condition; D) left-pointing contour from 11° jitter condition. Note that these images emphasize the center portion of the stimulus containing the contour. Actual test stimuli included a larger background area.
The Jittered-Orientation Contour Integration task (JOVI), a measure of CI, has been recognized by the NIMH-sponsored Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative (Carter & Barch, 2007) as being reliable, valid, and recommended for use in studies of schizophrenia (Butler et al., 2012; Butler et al., 2008; Silverstein et al., 2012), including in clinical trials (Green et al., 2009). With the JOVI and other similar CI tasks, studies have shown that people with schizophrenia are less able to detect and make shape judgments about fragmented contours when compared to various healthy and psychiatric control groups (Butler et al., 2013; Feigenson, Keane, Roche, & Silverstein, 2014; Keane, Erlikhman, Kastner, Paterno, & Silverstein, 2014; Keane et al., 2012; Kozma-Weibe et al., 2006; Schallmo, Sponheim, & Olman, 2013a, 2013b; Schenkel, Spaulding, DiLillo, & Silverstein, 2005; Schenkel, Spaulding, & Silverstein, 2005; Silverstein et al., 2009; Silverstein et al., 2006; Silverstein et al., 2012; Silverstein, Kovacs, Corry, & Valone, 2000; Uhlhaas, Phillips, Schenkel, & Silverstein, 2006; Uhlhaas, Phillips, & Silverstein, 2005). Past CI studies in schizophrenia have also demonstrated that, while performance does not vary from the acute to stabilization phases of illness in briefly hospitalized (i.e., ~2 weeks) patients (Feigenson et al., 2014), it becomes worse with longer illness chronicity and a lower level of functioning (Schenkel, Spaulding, & Silverstein, 2005; Silverstein et al., 2006; Uhlhaas et al., 2005).
Despite the many demonstrations of CI impairment in schizophrenia, exploration of its brain bases has been limited to one ERP study of closure negativity (Ncl) (Butler et al., 2013) and one fMRI study (Silverstein et al., 2009). Both of these identified reduced processing, compared to healthy controls, in visual regions known to subserve CI, based on prior studies from healthy humans and monkeys (Altmann, Bulthoff, & Kourtzi, 2003; Kourtzi, Tolias, Altmann, Augath, & Logothetis, 2003; Volberg & Greenlee, 2014) [e.g., V2, V3, V4, lateral occipital complex (LOC)]. These data suggested disturbances in the coordination of feedforward (bottom-up) stimulus assembly processes with re-entrant top-down disambiguation to increase the salience of contours and to inhibit background noise – an iterative integration mechanism that has been proposed to subserve CI in healthy observers (Chen et al., 2014). In addition to disturbances in visual cortex, however, the one prior fMRI study of SCZ demonstrated reduced prefrontal cortex and parietal lobe processing in patients. These data are consistent with recent findings indicating that in addition to visual cortex, frontal-parietal connectivity and other mechanisms supporting higher-level top-down control are involved in contour integration and object processing (Castellano, Plochl, Vicente, & Pipa, 2014; Hanslmayr, Volberg, Wimber, Dalal, & Greenlee, 2013; Li, Piech, & Gilbert, 2008; Sun et al., 2012; Volberg, Wutz, & Greenlee, 2013). The CI data reviewed above are supported by data from studies of perceptual closure, where subjects view fragmented line drawings of familiar objects. These visual evoked potential and ERP studies indicate both impaired early (Foxe, Doniger, & Javitt, 2001) and later (Doniger, Foxe, Murray, Higgins, & Javitt, 2002) contributions to perceptual organization impairment. Recognition of objects in these paradigms is also associated with the generation of Ncl (Doniger et al., 2000). However, studies indicate that Ncl amplitude is attenuated in schizophrenia patients (compared to controls) and this is associated with reduced activation within occipito-temporal and parietal-occipital regions (Doniger et al., 2002; Doniger et al., 2000), as well as reduced activity in prefrontal areas (Sehatpour et al., 2010; Sehatpour, Molholm, Javitt, & Foxe, 2006). A later fMRI study of perceptual closure confirmed reduced activity within these regions during perceptual closure in schizophrenia (Sehatpour et al., 2010). Similarly, perception of illusory contours has been shown to be driven by activity within LOC (Murray et al., 2002), and schizophrenia patients are less able to perceive such stimuli compared to controls (Keane, Joseph, & Silverstein, 2014).
The single fMRI and single ERP study of CI in schizophrenia were both small (14-15 patients with schizophrenia), and consisted primarily of chronically functionally impaired patients. Therefore, it is not known whether a community-dwelling group of schizophrenia patients would demonstrate the same pattern of functional brain disturbance as observed in the two earlier studies. Specifically, there is the question of whether occipital lobe abnormalities would be found during CI in community-dwelling outpatients, given evidence that: 1) CI impairment is more severe in chronically poor outcome patients (Silverstein et al., 2006); 2) such patients are characterized by greater white and gray matter losses in the occipital lobe, which are not typically found in other people with schizophrenia (Mitelman & Buchsbaum, 2007; Onitsuka et al., 2007); and 3) CI deficits have been observed in Alzheimer's disease patients with structural changes (atrophy and gliosis of white matter) in the occipital lobe, whereas CI deficits are not found in AD patients without these tissue changes (Uhlhaas et al., 2008).
The purpose of this study, therefore, was to examine more definitively the neurobiology of CI in schizophrenia by collecting fMRI data during CI task performance in a large sample of clinically stable patients and psychiatrically healthy controls, collected across 5 sites, using a CI task that was previously validated for behavioral studies of schizophrenia. We used an updated version of the previously validated JOVI task that included several improvements over versions used in prior studies. Methodological improvements included: 1) the use of two types of catch trials (see below) to quantify and, in part, allow for statistical control for attention lapses during task performance; 2) the inclusion of new conditions at the levels of difficulty that prior behavioral work (Silverstein et al., 2012) showed best discriminated schizophrenia patients from controls; 3) the use of fewer conditions at the most extreme difficulty levels, that were associated with chance-level performance in prior studies; and 4) the use of fewer overall conditions to reduce fatigue and task duration.
2.0 Methods
2.1 Subjects
Complete details regarding recruitment and enrollment through the multi-site CNTRACS Consortium are found in Henderson et al. (Henderson et al., 2012). Briefly, participants were recruited via flyers and online advertisements across five sites: University of California – Davis, Maryland Psychiatric Research Center (MPRC) at the University of Maryland, Rutgers University, University of Minnesota – Twin Cities, and Washington University in St. Louis. All patients were outpatients, in partial hospital programs, or were attending other types of community programs. Research diagnoses (and lack thereof for controls) were confirmed via the Structured Clinical Interview for DSM-IV, patient and non-patient editions (First, Spitzer, Gibbon, & Williams, 2002a; First, Spitzer, Gibbon, & Williams, 2002b).
One hundred twenty subjects [60 psychiatrically healthy control (CON), 60 with schizophrenia (SCZ)] enrolled in the study. JOVI data were not available for 7 SCZ and 2 CON due to acquisition, stimulus delivery, or subject compliance issues. Data from 3 SCZ and 1 CON were removed due to excessive movement (i.e., greater than .37 mm mean relative movement) during the JOVI acquisitions, and from 1 SCZ who moved out of the imaging field-of-view to an excessive degree during the scan session. Data from 1 SCZ and 1 CON were removed due to technical difficulties leading to missing behavioral data in the scanner. Finally 1 SCZ was removed due to being an outlier (> 3SD) in the relationship between fMRI and behavioral data (p<0.001 in multivariate Mahalanobis distance relative to the other subjects). The final analyzed data set therefore consisted of 56 control subjects (CON) and 47 subjects with schizophrenia (SCZ).
As seen in Table 1, groups were matched on age, sex, handedness, parental socioeconomic status [SES; based on the Hollingshead index as updated using occupational prestige ratings based on the 1989 general social survey (Hollingshead & Redlich, 1958)], and estimated premorbid intelligence (WTAR). Not surprisingly, SCZ participants had fewer years of schooling, a lower SES, and a lower level of functioning than CON subjects, likely reflecting disruption caused by illness onset and chronicity. All but one patient was receiving antipsychotic medication (1 first generation only, 43 second generation only, 2 first and second generation), and all were clinically stable, with mild symptomatology (see Table 1 for demographic and clinical data). After complete description of the study, written informed consent was obtained. The study was approved by the IRB at all participating research sites.
Table 1.
Demographic and clinical data.
| Control (n=56) | Schizophrenia (n=47) | Statistics | |
|---|---|---|---|
| Gender | 41 male, 15 female | 36 male, 9 female | X2(1)=.83, p=.36 |
| Age | 34.11 (11.53) | 34.84 (11.96) | t(101)=−.31, p=.76 |
| Handedness | 46 R, 10 L | 38 R, 9 L | X2(1)=.03, p=.87 |
| SES (Mother/Father) | 14.89 (4.50) 15.06 (4.22) |
15.33 (3.36) 15.15 (4.08) |
t(99.15)=0.58, p=.58 (mother); t(92)=.10, p=.92 (father) |
| WTAR Percentile Score | 37.57 (10.67) | 35.62 (9.50) | t(101)=−.97, p=.33 |
| Years of Education | 14.75 (1.94) | 13.15 (1.82) | t(100)=−4.27, p<.001 |
| Personal SES | 35.70 (12.25) | 24.87 (6.17) | t(87.28)=−5.70, p<.001 |
| Level of Functioning (UPSA-B) | 81.91 (10.50) | 78.30 (10.91) | t(100)=−1.70, p=.09 |
Numerical values in columns 2-3 are either counts (if integers), or means (and standard deviations).
2.2 Contour Integration task
The JOVI task assesses the ability of subjects to visually group non-contiguous edge elements into a single enclosing boundary (Silverstein et al., 2012). The elements in this task are Gabor patches, which are Gaussian modified sinusoidal luminance distributions that model the receptive field properties of spatial frequency filters in primary visual cortex. Gabors are, therefore, often preferred to line elements since their integration can more directly be connected to the functional properties of cells in V1 or V2 (Field, Hayes, & Hess, 1993; Kovacs, 2000a). On all trials except no-background catch trials (see below), 15 Gabors jointly formed an oblong-shaped contour embedded in a display of 207 randomly oriented Gabor elements (see Figure 1). The ratio of the density of background elements to the density of contour elements (i.e., the signal:noise ratio) was 0.9:1.0 in all non-catch trials (see below). At this level, adjacent contour elements are farther apart than adjacent background elements, and thus subjects cannot locate the contour on the basis of density cues (i.e., integration is required for contour perception) (Kovacs, 2000b). Perceptual organization was manipulated by adding orientation jitter to the Gabor elements forming the contours, across 5 levels: +/− 0°, 7-8°, 9-10°, 11-12°, and 13-14°. This manipulation reduces the correlation between the orientations of adjacent Gabors in the contour, thereby weakening overall curvature and placing greater demands on perceptual organization processes.
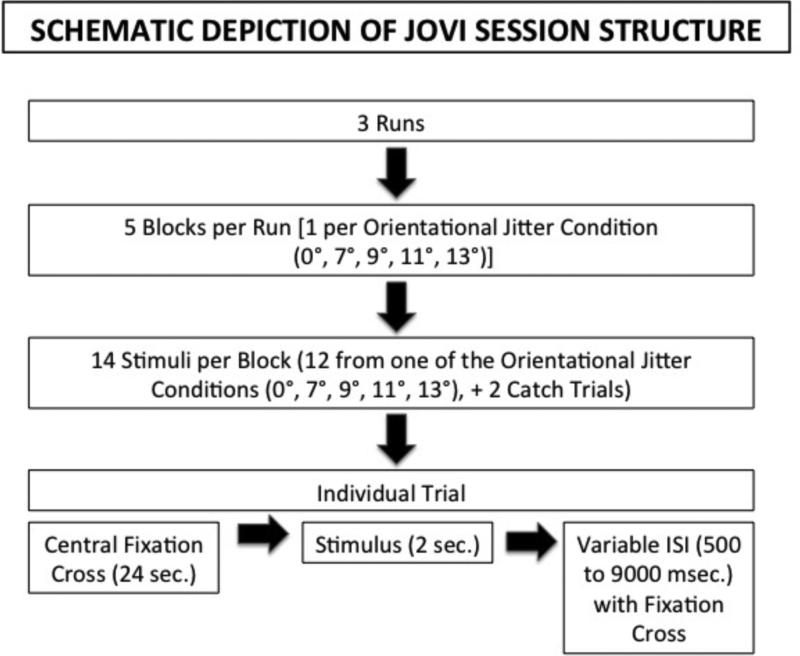
A schematic depiction of the structure of the JOVI task is presented in Figure 2. Trials were presented in three separate runs, with each run containing five blocks of trials (each 70 seconds long), interleaved with five blocks of fixation (24 seconds each). Each block contained 14 trials, 12 of one jitter level, and 2 catch trials. Each jitter level was presented once in a run, so that each jitter level was presented three times across the course of all three runs, for a total of three blocks and 36 trials at each jitter level. The order of the jitter levels was different for each run, but the same orders per run were used for each subject. On each trial, subjects determined whether the narrow end of the oblong contour was pointing left or right. Each stimulus was presented for 2 seconds followed by a variable inter-stimulus interval (500 to 9000 ms) during which responses were no longer recorded but a fixation cross appeared. Two types of catch stimuli (i.e., where no errors were expected) were administered during each block to quantify the extent to which subjects were properly attending to and understanding the task (see Figure 1). No-background catch trials had 0° jitter, and they contained no background noise elements, obviating the need for suppression of background noise. Outline catch trials also consisted of 0° jitter stimuli, and they contained the same number of noise elements as regular trials, but they also included a black line tracing out the entire contour, obviating the need for integration; All subjects completed the JOVI twice. The first time was outside of the scanner, to familiarize subjects with the task. The second session was inside of the scanner, and occurred within a week of the practice session.
Figure 2.
Schematic depiction of JOVI task procedures. Note that the order of conditions was counterbalanced in each block, but that this was the same for all subjects.
The background luminance at each site was 30 cd/m2, with the exception of the Washington University site (lower bulb intensity), which had a background luminance of (14 cd/m2). Michelson contrast was 90% and the Gabor spatial frequency was 6 cycles per degree. All images were linearized, as determined by photometric measurements at each site. Each image subtended 11° of visual angle on each side. The task was implemented within E-prime (Psychology Software Tools, Pittsburgh, PA). As in prior studies (Feigenson et al., 2014; Silverstein et al., 2012), a timed-out response was scored as 0.5 correct, to not give any advantage to subjects who preferred to guess rather than time-out on a trial. Reaction time (RT) data were analyzed for correct responses only.
2.3 Functional MRI
Three of the sites (University of California - Davis, University of Minnesota, Washington University) acquired data on a 3 Tesla Siemens TIM TRIO MRI system (Siemens Medical Solutions, Inc., Erlanger, Germany) with a Siemens 12 channel phased array coil. At MPRC, images were acquired on a Phillips 3T Achieva scanner with an 8 channel phased array coil, and at Rutgers University, images were acquired on a Siemens Allegra scanner with a circularly polarized (CP) transmit/receive coil. Deformable foam pads were used at all sites to minimize head motion. An AGAR phantom was used to perform quality assurance of scanners across sites based on guidance and recommendations from the Functional Biomedical Informatics Research Network (FBIRN) initiative.
Structural T1-weighted images were acquired using an MPRAGE sequence (TR=2300ms, TE~3.0ms, FA=9°, FOV=256 × 240 mm, matrix: 256 × 240, voxel size: 1mm isotropic, 176 sagittal slices). Functional images sensitive to BOLD contrast were acquired using a gradient echo-planar imaging (EPI) sequence with the following parameters: (TR=2000ms, TE=30ms, FA=77°, FOV=220mm, matrix: 64 × 64, voxel size: 3.4375 × 3.4375 × 4-mm, 32 transverse slices). A gradient echo scan with 2 different echo times was obtained to estimate magnetic field inhomogeneity (TR=500ms, ΔTE=2.46 ms, FA=55°, FOV=220mm, matrix: 64 × 64, voxel size: 3.4375 × 3.4375 × 4-mm, 32 transverse slices). Visual stimuli were projected using a color LCD projector, and subjects responded with a custom-built 5-button fiber-optic response device.
2.4 Image processing
Pre-Processing was accomplished using the fMRI Expert Analysis Tool (FEAT) in the FMRIB Software Library (FSL version 4.1; www.fmrib.ox.ac.uk/fsl). Brain volumes were extracted from full-head functional and structural images. Functional images were motion corrected and high-pass filtered in FEAT with a cutoff of 100 s. Subjects with greater than 0.37mm of mean relative frame-to-frame movement (greater than 3 standard deviations from the mean) were excluded. Functional images were registered to each participant's MPRAGE using a rigid-body transformation (df 6) with FMRIB's Linear Image Registration Tool (FLIRT). Functional images underwent B0 unwarping using the gradient-echo fieldmap estimates for each subject to correct for magnetic field inhomogeneity. Images were smoothed with a Gaussian kernel (7mm FWHM, isotropic).
Modeling of fMRI data was performed using a general linear model (GLM) implemented in FEAT. Activity associated with each jitter block type was modeled by convolving a vector of expected neural activity with the 3 basis-function set of FMRIB's Linear Optimal Basis Sets (FLOBS). The first basis function represents a canonical hemodynamic response, while the other two effectively model delay and dispersion variability in the hemodynamic response, and are included to decrease error in first level GLM fit. However, only canonical response estimates were passed up to higher (group-level) analysis. Temporal autocorrelation due to low frequency (“1/f”) noise was accounted for by pre-whitening (FSL's FILM). The 3 runs for each subject were combined in a 2nd level fixed-effect analysis for each subject. These parameter estimates were then used in a 3rd level mixed-effect analysis to derive group-level maps using all subjects (FSL's FLAME). Because of site differences in scanner characteristics (Table 2), research site was added as a covariate in the group-level GLM design.
Table 2.
Quality assurance data.
| Measure | SCZ (n=47) | CON (n=56) | Group Effect | Site Effect | Group × Site Interaction |
|---|---|---|---|---|---|
| Absolute Motion | .419 (.0399) | .389 (.0354) | F(1,93102) = 0.32, p=.57 | F(4,93102) = 5.81 p<.001 | F(4,93102) = 0.42, p=.80 |
| Relative Motion | .127 (.0089) | .102 (.0079) | F(1,93102) = 4.32, p=.041 | F(4,93102) = 3.27, p=.015 | F(4,93102) = 0.67, p=.62 |
| tSNR | 207.6 (6.96) | 226.4 (6.18) | F(1,93102) = 4.08, p=.046 | F(4,93102) = 6.92, p<.001 | F(4,93102) = 2.03, p=.096 |
| Smoothness | 10.25 (.120) | 10.30 (.106) | F(1,93102) = 0.11, p=.75 | F(4,93102) = 6.46, p<.001 | F(4,93102) = 0.80, p=.53 |
Values for SCZ and CON are the estimated marginal means (and SE), controlling for site. Motion and smoothness measures are in mm. Absolute motion is computed relative to a fixed reference frame (middle time point of the run) and relative motion is computed from one frame to the next. Both are outputs of FSL's MCFLIRT tool for motion correction. Smoothness is the full-width half-maximum estimate of the spatial smoothness of the residuals from the first (subject) level GLM (obtained by converting the “resels” estimate from FSL's ‘smoothest’ function into units of mm). Temporal signal-to-noise (tSNR) for each run was obtained by first computing a spatial (voxel-wise) map of mean signal over time divided by the standard deviation of the residuals from the first level GLM. This tSNR map was then averaged over space using a weighted average according to the probability of gray matter at that voxel in MNI152 space. For all measures, values were averaged across the 3 runs for each subject and those subject-specific averages formed the basis for analysis of site and group effects.
2.5 Region of interest (ROI) analyses
Data analysis for this study focused on occipital, parietal, and frontal regions known to be involved in perceptual organization or its impairment. Occipital ROIs were those that have been associated with CI in past non-clinical human and monkey studies (Altmann et al., 2003; Kourtzi et al., 2003), and/or that have been associated with reduced activity during CI in schizophrenia (i.e., V2-V4, LOC) (Butler et al., 2013; Silverstein et al., 2009). Parietal ROIs included: 1) the superior parietal lobules, which are involved in the distribution of visual-spatial attention and the binding of visual features (Robertson, 2003), and which were associated with reduced CI in schizophrenia in our past study (Silverstein et al., 2009); and 2) the angular gyrus, which is involved in integrating visual information with the verbal and spatial concepts of left and right during left-right discrimination (Hirnstein, Bayer, Ellison, & Hausmann, 2011; Seghier, 2013), a process essential for accurate JOVI performance. Frontal ROIs were the middle and inferior frontal gyri (including the pars triangularis and pars operculum regions), which are involved in perceptual organization and perceptual decision making (Ploran et al., 2007; Sun et al., 2012), as was recently shown using a CI task similar to the JOVI in healthy subjects in a study of neuronal oscillations (Castellano et al., 2014).
ROI analysis was accomplished by selecting the above-mentioned regions from the Harvard-Oxford cortical atlas in FSL (thresholded at a 50% probability criterion), and intersecting these a priori regions with the map of regions showing a whole brain corrected significant linear effect of jitter level (including jitter level 0°) in a whole brain analysis including all subjects. This latter mask was created using whole-brain cluster correction (p<0.05) with a cluster-forming threshold for the individual voxels of Z>2.3, in a model that included site as an independent variable. Regions with insufficient remaining volume after intersection with the group-level map were excluded from further analysis1. We then extracted the average beta weights across voxels in each region for each subject at each jitter level in the 2nd level data, and subjected the means of subjects’ median ROI values to subsequent ANOVAs in SPSS (version 21) that included group (CON or SCZ) and site as between subject factors, jitter level as a within subject factor, and age as a covariate. Age was included as a covariate because, in adults: 1) increased age is related to reduced CI (Del Viva & Agostini, 2007; Roudaia, Bennett, & Sekuler, 2008); 2) age was related to CI in our sample overall (see Results); and 3) the SCZ and CON groups in this study did not differ in age, making it an appropriate covariate to reduce within-group variance (Miller & Chapman, 2001), but sites differed in age (see Results).
2.6 Statistical analysis
The SCZ and CON groups were compared on single variables using independent sample t-tests. In cases where the homogeneity of variance assumption was not met, the t test assuming unequal variances was used (typically producing df values that are not whole numbers). Where multiple independent variables (e.g., site, group) existed, or where repeated measures (i.e., jitter condition) were included, ANCOVAs were used (with age as covariate). In cases where the sphericity assumption was not met for repeated measures factors, Greenhouse-Geisser corrected df (often in non-whole numbers), F and p values are reported. All correlations reported are Spearman rho values, to minimize the effects of extreme values. Between-group and correlational analyses on ROI data were corrected for multiple comparisons using the false discovery rate (FDR) method (Benjamini & Hochberg, 1995) and we report “FDR adjusted p-values.”
Because we used a variable, and often large, inter-stimulus interval, the fMRI data could be analyzed at the block or the event-level. For the purposes of this paper, the block level results only are described. However, the event-related results did not differ in any significant way from the results reported below.
3.0 Results
3.1 Clinical and demographic data
Mean scores on all 24 Brief Psychiatric Rating Scale (BPRS) items ranged from 1.13 to 2.88 [i.e., within the normal range (1-2), to approaching mild levels (3)], confirming that, on the whole, the patient group was relatively asymptomatic. Demographic, academic achievement, and level of functioning data are shown in Table 1.
3.2 Behavioral data
3.2.1 Accuracy
For the sample as a whole, age was significantly and inversely correlated with accuracy in all jitter conditions (all r values > |−.36|, all p values < .001), but not with catch trial performance. In addition, although the groups did not differ in age (see Table 1), there was a significant main effect of site regarding age [F(4, 102=7.79, p<.001; subjects at UC - Davis were younger than those at all other sites by between 9-16 years]. Therefore, age was used as a covariate in all analyses reported below (behavioral and imaging).
Catch trial performance did not provide evidence that the groups differed in their attentiveness to the task or their frequency of random responding. See Supplemental Results for description of catch trial data.
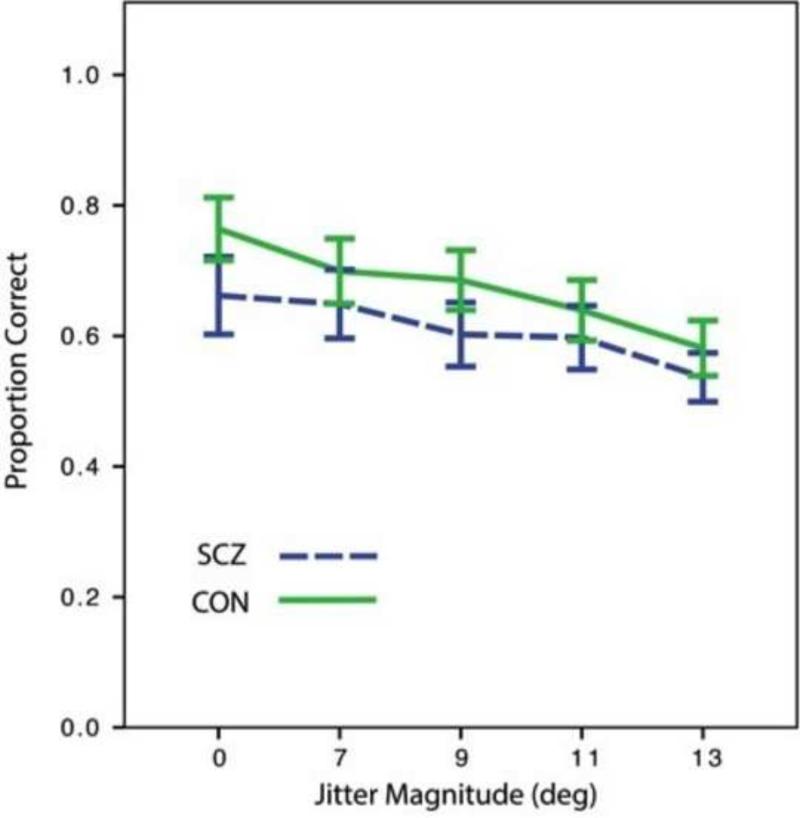
Means and standard error for accuracy data, on non-catch trials, are presented in Figure 3. There was a main effect of site [F(4, 93)=4.95, p=.001; UC-Davis subjects were more accurate than those at all other sites for all jitter conditions, presumably due to also being the youngest (see Supplemental Results for further analysis of site effects), but the group x site effect was not significant [F(4, 93)=1.11, p=.36]. There were the hypothesized effects of orientational jitter [F(3.23, 300.2)=4.48, p=.003], and group [F(1, 93)=9.40, p=.003], but the group x jitter interaction was not significant [F(3.23, 300.2)=2.52, p=.054]. After covarying for number of catch trial errors, there were still significant main effects of orientational jitter [F(3.54, 322.33)=5.73, p<.001], and group [F(1, 91)=4.49, p=.037], but the group x jitter interaction was still not significant [F(3.54, 322.33)=1.58, p=.18]. These results replicate prior studies(Kozma-Weibe et al., 2006; Silverstein et al., 2009; Silverstein et al., 2012), in indicating that the performance decrement in SCZ patients is consistent across difficulty levels. There were no between-group differences in reaction time (see Supplemental Results).
Figure 3.
Accuracy data, by group and orientational jitter level. Error bars = 1 SE.
3.3 Imaging data
3.3.1 Quality assurance (QA)
Table 2 presents data on QA indices, which were calculated with and without age as a factor in the statistical analyses. With age not in the model, site effects were observed on all variables and a significant difference between groups was observed for relative motion and temporal signal:noise ratio (tSNR). However, the group x site interaction effect was not significant for any QA variable. When age was included as a main effect (with no interactions with age) in the model, the effect of age itself was significant for smoothness (p=0.0007) and at a trend level for relative motion (p=0.065), but not significant for absolute motion (p=0.84) and tSNR (p=0.19). However, adding age as a factor in the analysis did not materially impact the p values of the site, group, or group x site effects.
3.3.2 ROI based analyses
ROI analyses revealed regions where SCZ showed greater activity than CON, or where there were no group differences, but did not reveal any regions with greater activity in CON than SCZ (see Table 3). There were no group differences in early visual cortex (V1-V4). However, the SCZ group demonstrated increased activation in the LOC, an area critical for shape and object processing, an effect that survived FDR correction for the number of ROIs. Patients also demonstrated increased activation in the superior parietal lobules, a region known to be involved in binding of visual features and in distribution of visual-spatial attention. This effect also survived FDR correction. As shown in Table 3, there were no significant group x jitter effects in the ROI data. Moreover, there was a significant site effect for only one ROI (left SPL). Post-hoc pairwise comparisons on the left SPL data indicated that the Baltimore site had higher levels of BOLD signal in that region compared to all the other sites (all Bonferroni p values <.005) except Rutgers. There were no other pairwise site differences. There were no significant Group x Site or Group x Site x Jitter Effects in any ROI.
Table 3.
Summary of ROI volumes, and group, jitter, and site effects in ROI analyses.
| ROI | ROI Volume (mm3) | Jitter Effect | Group Effect | Site Effect | Group × Jitter Effect |
|---|---|---|---|---|---|
| Occipital Lobe | |||||
| Left V2 | 3296 | .80 | .46 | .07 | .26 |
| Right V2 | 5368 | .65 | .53 | .17 | .22 |
| Left V3 | 1248 | .68 | .57 | .87 | .48 |
| Right V3 | 1680 | .65 | .48 | .54 | .42 |
| Left V4 | 1608 | .56 | .46 | .16 | .23 |
| Right V4 | 1472 | .65 | .32 | .34 | .16 |
| Left Sup. LOC | 10,240 | .28 | .01* | .08 | .11 |
| Right Sup. LOC | 12,104 | .49 | .03* | .06 | .11 |
| Parietal Lobe | |||||
| Left SPL | 1640 | .09 | .01* | .01* | .11 |
| Right SPL | 2776 | .02* | .03* | .06 | .11 |
| Frontal Lobe | |||||
| Left IFG Operculum | 832 | .49 | .17 | .06 | .18 |
| Right IFG Operculum | 1240 | .02* | .57 | .28 | .11 |
| Left MFG | 2696 | .95 | .21 | .53 | .18 |
| Right MFG | 4376 | .65 | .19 | .06 | .22 |
Table values in columns 3-6 are FDR adjusted p values, corrected for each set of analyses (columns). In all cases of statistically significant findings, the SCZ group demonstrated greater activation than the CON group.
p-value <.05 after FDR adjustment
3.3.3 Correlations between fMRI indices and task performance
We examined correlations between: 1) the accuracy difference between the 13° (most difficult to perceive contours) and 0° (easiest to perceive contours) jitter conditions; and 2) activation differences in each ROI between these two conditions across individuals. Here, after FDR adjustment for multiple comparisons, significant correlations were observed for the patient group only [note, none of the correlation values for controls were significant even before the FDR correction]. Notably, decreased accuracy with increased jitter was consistently related to increased activity in visual cortex regions earlier than the LOC (i.e., right V2, bilateral V3 and V4). Further, all of the correlations that were significant in the SCZ group were significantly stronger in the SCZ than in the CON group, based on the Fisher r-to-z transformation, as shown in Table 4.
Table 4.
Spearman correlations between fMRI and behavioral (accuracy) indices of the difference between the 13° and 0° jitter conditions, for SCZ and CON groups.
| ROI | SCZ rs (N=47) | Adjusted p value | CON rs (N=56) | Adjusted p value | Adjusted p value of SCZ-CON rs Difference |
|---|---|---|---|---|---|
| Occipital Lobe | |||||
| Left V2 | −.31 | .07 | .07 | .99 | |
| Right V2 | −.42 | .01* | .00 | .99 | .02* |
| Left V3 | −.36 | .04* | −.02 | .99 | .04* |
| Right V3 | −.48 | .007** | −.01 | .99 | .02* |
| Left V4 | −.51 | .007** | −.11 | .99 | .02* |
| Right V4 | −.44 | .009** | −.03 | .99 | .02* |
| Left Sup. LOC | −.19 | .25 | −.09 | .99 | |
| Right Sup. LOC | −.21 | .24 | −.18 | .99 | |
| Parietal Lobe | |||||
| Left SPL | −.24 | .20 | −.17 | .99 | |
| Right SPL | −.22 | .24 | −.07 | .99 | |
| Frontal Lobe | |||||
| Left IFG Operculum | −.20 | .25 | −.16 | .99 | |
| Right IFG Operculum | −.18 | .26 | −.22 | .99 | |
| Left MFG | −.15 | .34 | −.11 | .99 | |
| Right MFG | .08 | .61 | −.13 | .99 |
The right column represents p values for z tests of the between-group difference in these correlations in cases where one of the groups demonstrated a significant correlation. FDR adjusted p-values are reported.
<.05 after FDR adjustment
<.01 after FDR adjustment
4.0 Discussion
There are two primary findings from this study. First, we replicated prior findings of reduced CI in schizophrenia, and unlike most prior studies which involved only inpatients or day hospital patients [except (Silverstein et al., 2012)], this was observed in a relatively asymptomatic population on stable doses of medication. This is consistent with recent data demonstrating that CI ability is stable across the acute and stabilization phases of illness in SCZ (Feigenson et al., 2014). Second, there was a clear pattern of brain activity that differentiated the SCZ and CON groups. Most notably, the SCZ group demonstrated increased levels of activity during task performance in the LOC and superior parietal lobules. These data suggest reduced efficiency during processing of global form in schizophrenia compared to healthy individuals. Further evidence in support of this conclusion is discussed below.
While we replicated some prior findings, there were three important sets of results that differed from those of prior studies and that, therefore, require explanation. These are: 1) the lack of between-group differences in occipital regions V2-V4; 2) evidence for relative hyper-activation in higher visual regions in the SCZ group, and the lack of any findings of increased ROI activation in the CON group; and 3) the consistent pattern of relationships between visual cortex activation and CI condition difficulty in the SCZ group in contrast to the lack of any such relationships for the CON group. Each of these issues is considered, in turn, below.
4.1 Accounting for between-group similarities in V2-V4
In contrast to this study where there were no between-group differences in activation in V2-V4, in the sole prior fMRI study of CI in schizophrenia (Silverstein et al., 2009), the CON group was characterized by greater activity in these regions, even when matched to patients on accuracy level. Several factors may account for the differences in results between the two studies, however. One is that the earlier fMRI study included three orientational jitter conditions that were much more difficult than those used in this study, and that are typically associated with chance-level performance, namely 15°, 19°, and 23°. This means that exposure to well-formed, perceivable, contours occurred approximately 60% less in our 2009 study than it did in this study (which focused on conditions engendering above chance performance). The fact that in the current study patients and controls had more exposure to well-formed perceivable contours could account for more efficient processing in early visual regions. Evidence consistent with this view comes from (non-clinical) human and monkey studies, where repeated exposure to simple stimuli such as Gabor-defined contours embedded within noise leads to decreases in activation as circuits become more efficient at processing the increasingly familiar and expected stimuli (Altmann et al., 2003; Grill-Spector & Malach, 2001; Kourtzi et al., 2003). Evidence that consistent exposure to well formed contours within a single testing session aids CI comes from our 2012 JOVI validation paper (Silverstein et al., 2012; Silverstein & Keane, 2009), where inclusion of a 0° jitter condition in one experiment led to 10%-15% higher levels of accuracy in the 7° and 11° jitter conditions, for both controls and SCZ patients, compared to a second experiment (with different patients), where a 7° jitter condition was the ‘baseline’ condition. While this did not eliminate the between-group difference between patients and controls, it did demonstrate that for patients (and controls), more consistent exposure to perceivable contours during a CI task facilitates CI.
Another factor that may have contributed to perceptual learning effects in the current imaging study is that this study used a pre-scan behavioral practice session with the JOVI, while the prior study did not. Thus, all subjects in the current study had significantly more exposure to the stimuli prior to fMRI data collection, and this level of exposure is often sufficient to produce perceptual learning (Hussain, Sekuler, & Bennett, 2009). Moreover, subjects in the current study had several nights to consolidate memory traces of the stimuli, which has been shown to facilitate perceptual learning (Baeck, Rentmeesters, Holtackers, & Op de Beeck, 2014; Censor, Karni, & Sagi, 2006), including in the visual cortex (Bang, Khalilzadeh, Hamalainen, Watanabe, & Sasaki, 2014), and on measures of CI (Gervan & Kovacs, 2010). An argument against the hypothesis that perceptual learning can account for some of our findings is that JOVI performance in the scanner was lower than in prior studies using the task. However, as discussed below, and in Supplemental Results, performance in the subsequent scanning session may have been interfered with by factors related to being in the scanner, or being in it for an extended period of time. For example, attention lapses for both patients and controls were more frequent during the scanner session compared to the practice session.
4.2 Accounting for greater brain activation in the SCZ group
It is known, from microelectrode recordings in awake monkeys, that top-down modulation, leading to perceptual enhancement of task-relevant stimuli, is necessary for contour integration within distractor noise (Li et al., 2008). Therefore, we hypothesize that the increased activation, relative to controls, that we observed in higher level visual processing regions (LOC and SPL) in the SCZ group reflects greater difficulty consolidating output from earlier visual regions into a coherent shape representation (in LOC), and subsequently, greater demands on visual attention processes (subserved by SPL). In this scenario, while there were low-level perceptual learning effects in both groups due to extended exposure to well-formed contours over two testing sessions (as described in the paragraph above), these effects did not extend into higher visual regions to increase efficiency in global shape discriminations for patients. Note also that this scenario assumes that perceptual learning effects (resulting in reduced activation for controls) did not occur in the prior fMRI and ERP studies of CI in schizophrenia. As discussed in the section above, our prior imaging study of CI in schizophrenia (Silverstein et al., 2009) did not have a pre-scan behavioral session, and, as noted earlier, there was greater exposure to well-formed contours (i.e., a greater proportion of conditions with perceivable contours) in the current study. A similar situation exists regarding the ERP study of CI (Butler et al., 2013), which only included 2 conditions, one with visible contours, and one where contours were not visible (i.e., 27 −28° jitter). Within that single session (compared to 2 sessions in this study), with only 80 trials (compared to over 350 across both sessions in this study), and with contours visible (for the average participant) on only 50% of the trials (compared to in 80% of the conditions in this study), we consider it unlikely that perceptual learning would have occurred.
What is not accounted for by this interpretation is why the SCZ group did not show greater activity in frontal regions associated with perceptual decision making (e.g., MFG and IFG). It should be noted that the lack of greater activation for the CON versus SCZ group is unlikely to be an artifact, as greater activation in CON than SCZ was seen during working and relational memory paradigms that were completed during the same scanning session (Ragland et al., in Press; Sheffield et al., 2015). However, a fuller exploration of the effects of higher level visual processing on JOVI performance was precluded by the lack of sufficient data for analysis of activity in bilateral IFG pars triangularis and parietal angular gyrus, as described in Footnote 1.
4.3 Accounting for relationships between task difficulty and visual cortex activation occurring only in the SCZ group
The significant relationships between increased task difficulty and greater activation in occipital regions for patients, but not for controls, suggests that perceptual learning and processing efficiency had reached asymptotic levels for controls but not for patients. This hypothesis is supported by data suggesting that the level of brain activation in regions V1 and V2 no longer predicts performance on perception tasks after behavioral evidence of perceptual learning has occurred (Ghose, Yang, & Maunsell, 2002; Yotsumoto, Watanabe, & Sasaki, 2008). It is also consistent with prior data on reduced within-session perceptual learning during perceptual organization tasks in schizophrenia (Place & Gilmore, 1980; Silverstein, Bakshi, Nuernberger, Carpinello, & Wilkniss, 2005; Silverstein, 1998, 2009; Silverstein et al., 1996), especially when top-down input is required for efficient task performance [see (Silverstein, 2009) for review]. For example, a prior 4-day perceptual learning study by our group (Silverstein et al., 2006) demonstrated that while the contour integration performance of controls saturated by day two, the performance of schizophrenia patients was continuing to improve on the 4th day of exposure, even though it began to improve on Day 2. This suggests that, in the current study, even with the earlier practice session, patients would not have reached their maximum levels of performance during the scan session.
What is more difficult to account for is why the correlations between left and right LOC activity and task performance were not significant for patients when patients demonstrated significantly more activation in these regions than controls. The correlations for patients were in the predicted direction (−.19 and −.21), but were small in both cases. This may simply be due to the large number of patients performing at a low accuracy level in the scanner session (i.e., to floor effects), a statistical confound which may have suppressed the values of all correlations in this study (Poldrack, 2007). An additional clue to understand the lack of correlations with LOC activity may lie in the significant correlations between ROI activation and task performance (for patients only) in earlier occipital regions (L and R V2, V3, and V4). This suggests that these earlier regions are more involved in assembling, extracting (from noise), and/or (although less likely) classifying (L vs R) contours in the JOVI stimuli, compared to the LOC, which would be consistent with the findings of our earlier imaging study (Silverstein, 2009), where no between-group differences in LOC were observed. However, there is clearly a discrepancy, in this study, between which brain regions’ activity correlated with task performance, and which regions were more activated in patients than controls. Clarifying this apparent paradox is an important task for future studies of CI in schizophrenia. Issues that may be relevant in clarifying this discrepancy are that the total amount of activity in a given region is less important than connectivity between regions (which was not captured in our ROI data) and the possibility that subregions within each ROI may be more or less responsible for task-relevant processes, with these potential differences being obscured in analyses that use overall ROI activation values. For example, the LOC is generally considered to be comprised of two distinct regions, the lateral occipital cortex and the posterior fusiform gyrus, and there is evidence the latter is more involved in global shape perception than the former (Kourtzi et al., 2003).
4.4 Patient factors that may have contributed to novel findings
The earlier fMRI and ERP studies of CI in schizophrenia recruited inpatients or mostly partial hospital patients, whereas the current study included mostly outpatients, with no inpatients. It is possible that patients who are chronically lower functioning have more impaired CI, and several behavioral studies of perceptual organization in schizophrenia (Schenkel, Spaulding, DiLillo, et al., 2005; Silverstein et al., 2006; Silverstein & Keane, 2011a; Silverstein et al., 1996; Silverstein, Osborn, & Palumbo, 1998; Uhlhaas, Phillips, Mitchell, & Silverstein, 2006; Uhlhaas, Phillips, Schenkel, et al., 2006) including some of CI in particular (Schenkel, Spaulding, DiLillo, et al., 2005; Silverstein et al., 2006; Uhlhaas, Phillips, Schenkel, et al., 2006) suggest this. The fact that, in the present study, there were no RT differences between patients and controls on the non-catch trials, and that there were no group differences in the number of timed-out trials – both of which are not typical in studies of schizophrenia – also suggests that our subjects may have been more intact cognitively than patient samples in prior studies of CI. All of this raises the possibility that in the more severely ill patient groups in prior studies, abnormalities in earlier visual regions may have contributed to reduced CI by adding internal noise (due to impaired processing as early as V2 (Silverstein et al., 2009)) to higher visual processing stages, consistent with the known losses of occipital gray and white matter in poor outcome patients (Mitelman & Buchsbaum, 2007; Onitsuka et al., 2007). In contrast, better functioning outpatients with SCZ are less impaired at contour representation formation (Keane, Joseph, et al., 2014) (which relies primarily on occipital lobe activity (Castellano et al., 2014)), but they nevertheless appear to be impaired at higher levels of shape perception and discrimination (Keane, Joseph, et al., 2014) that rely on frontal-parietal connectivity (Castellano et al., 2014).
4.5 Limitations
Several limitations of the study must be noted. One is that, due to the multi-site design, there were differences in subject age and stimulus presentation parameters (e.g., in-scanner luminance levels) across sites, and these contributed to variance in the data. While we attempted to account for site differences in the statistical model, and while the effects on the data were understandable conceptually (i.e., in terms of known effects of age and reduced luminance on contour integration), site effects may nevertheless have increased between-site and within-group variance, and so may have reduced our ability to detect between-group effects.
A second issue is that, as noted above, task performance was lower in this study than in our prior studies, including our prior imaging study. Because many subjects, especially patients, did not perform at a significantly greater than chance level in multiple conditions, the extent to which brain activity in those conditions reflects perception vs. confounds from other mental processes associated with an inability to perform the task is unknown. However, to the extent that an active effort at contour perception was superseded by other forms of mental activity, this would have added additional error variance to the data set. Although we do not know for certain why performance was lower in this study than in prior studies, we believe it is due to a combination of completing the JOVI within a long battery of other tests (in both the practice and scanning sessions), and effects of the scanner environment itself. Support for this hypothesis comes from several aspects of the data: 1) accuracy during the practice session was lower than accuracy in prior behavioral studies using the JOVI, despite increasing the proportion of conditions with visible contours for this study; 2) performance during the practice session was significantly better than performance during the scan session (see Supplemental Results); and 3) there were more attention lapse errors (i.e., errors on catch trials) for both controls and patients, during the scanner session compared to the practice session (see Supplemental Results). While we cannot be certain why performance on non-catch trials was worse in the scanner than during the practice session (note – for outline type catch trials, poorer performance in the scanner was likely due to a lower luminance level in one of the scanners, see Supplemental Results), it may be due to a combination of luminance issues, fatigue due to engaging in multiple tests and to lying still for several minutes for the structural scan, and taking the test in a cold, dark, noisy, and unfamiliar environment while lying relatively rigid for a long period of time. Note that while all tests were taken during the practice session as well (and test order was counterbalanced in both the practice and scan), only one break was allowed during the scan session, which may have increased demands on attention beyond that which characterized the practice session (which was also briefer because it did not include a structural scan). One strategy for avoiding these effects is simply to not include the JOVI in long scanner sessions. Another strategy (if this cannot be avoided) is to use a staircase procedure instead of the method of constant stimuli. This would ensure that all subjects are performing at a pre-specified level, regardless of internal or external conditions.
A third limitation relates to implementing visual tasks like the JOVI in multiple scanner environments. With psychophysical measures of visual processing, it is optimal to ensure several conditions in the testing environment, including linearization of display device gamma level, equivalence of luminance across display devices, and equivalence of stimulus parameters such as contrast and visual angles subtended by stimuli. In most respects we achieved these goals, but in some cases it was not possible (e.g., differences in scanner environment luminance levels across sites). Even though we addressed this issue as much as possible by creating unique stimulus sets for each site (to offset in-scanner luminance differences), perfect matching on stimulus brightness could not be achieved. While implementation of any psychophysical task across sites can involve issues (e.g., differences in amounts of scanner acoustic noise can affect performance on any cognitive task; differences in type of response device used can affect stimulus-response mapping difficulty, etc.), because vision is studied far less in schizophrenia than other domains of cognition (Silverstein & Keane, 2011b) (e.g., memory, executive function), sites that do not specialize in studying vision may be relatively less proficient at tasks such as luminance measurement and control. This limitation may have introduced further uncertainty into luminance measurements that could have affected the degree to which the stimuli were matched on brightness in the scanner. These issues highlight the trade-offs inherent in multi-site studies: the potential for larger samples, faster data collection, and direct assessment of generalizability must be weighed against the potential for a higher level of variance (and increased sources of variance) in the data compared to single-site studies, which can reduce the ability to detect within-task and between-group effects and their interactions.
A potential limitation to any study where people with schizophrenia perform more poorly than controls is that the findings can reflect generalized cognitive and performance deficits (Chapman & Chapman, 1978) to unknown degrees, rather than the specific process of interest (Knight & Silverstein, 2001; Silverstein, 2008), in this case CI. However, a strong circumstantial argument can be made for interpreting the group differences in this study in terms of CI. The first is that the between group difference in accuracy was present even at the easiest jitter level, and the magnitude of the between group difference did not get larger as the task became more difficult due to increased orientational jitter, as might be expected if a generalized deficit was the main factor driving performance (Knight & Silverstein, 2001). Second, patients differed from controls only on one of the catch trial types, and all statistical effects involving group in the catch trial data were eliminated when data from the Washington University site (where the group difference was due to more difficult viewing conditions) were excluded (see Supplemental Results), suggesting that patients were not generally having more trouble paying attention to, or understanding or maintaining representations of the task instructions. Related to this, most of the effects in the accuracy data for the sample as a whole remained even after covarying for catch trial performance. Third, in our earlier fMRI study of CI (Silverstein et al., 2009), the brain activation differences between groups remained even after the groups were matched on accuracy level.
4.6 Summary
In conclusion, this first multi-site fMRI study of CI replicated past findings of reduced CI in schizophrenia, and generated novel insights into the cortical mechanisms involved in performance impairment, and the conditions under which specific patterns of abnormal brain activity are expressed. The data suggest that - in clinically stable schizophrenia patients with low levels of symptoms who are exposed to the CI task stimuli under conditions that are likely to lead to deactivation of early visual regions subsequent to repeated exposure to well-formed contours in healthy subjects - abnormal CI performance is associated with a reduced ability to increase efficiency of shape processing after repeated stimulus exposure, leading to increased demands on visual attention. In the fMRI data, this was expressed as normal levels of activity (relative to controls) in visual regions as anterior as V4, but increased activity in the LOC and SPL. Differences between our findings and those of a past fMRI study of CI in schizophrenia appear to be due to differences in task design and patient samples. However, confirmation of our findings and interpretations could be achieved by a direct investigation of behavioral and brain activity differences in CI in schizophrenia as a function of factors such as task difficulty, level of prior and within-session exposure to clearly perceivable Gabor-defined contours, stimulus brightness, and patient age, illness chronicity and extent of structural changes in the occipital lobe. This is especially important because some of the interpretations of our unexpected findings are clearly post-hoc, although they are all based on multiple sources of evidence.
The results of this study extend prior findings from CI studies, as well as findings regarding perceptual closure using stimuli such as drawings of common objects (reviewed in the Introduction), in people with schizophrenia. Moreover, they suggest that the JOVI is an efficient assay of the cognitive and neural processes associated with (normal) stimulus assembly, and with impairments such as object fragmentation and excessive amounts of attention being needed to integrate and appreciate the behavioral significance of real-world stimuli, as noted in prior research (Silverstein & Keane, 2011a; Uhlhaas & Silverstein, 2005) and clinical reports (Carr & Wale, 1986; McGhie, 1961; Uhlhaas & Mishara, 2007).
Supplementary Material
Acknowledgments
We thank the staff at each of the CNTRACs sites for their hard work, and our participants for their time, energy and cooperation. This research was supported by NIH grants: 5R01MH084840-03 (DMB), 5R01MH084826-03 (CSC), 5R01MH084828-03 (SMS), 5R01MH084821-03 (JMG), 5R01MH084861-03 (AWM), 5R01MH059352 (APY).
Financial Disclosures
Dr. Barch has received grants from the NIMH, NIA, NARSAD, Allon, Novartis, and the McDonnell Center for Systems Neuroscience. Dr. Carter has received research grants from the NIMH, NIDA, the Robert Wood Johnson Foundation and from Glaxo Smith Kline and has been an external consultant for Roche, Servier, Lilly, Merck and Pfizer. Dr. Gold has received grants from NIMH, receives royalty payments from the BACS, and has consulted with Pfizer, Merck, Astra Zenaca, Solvay, and Glaxo Smith Kline. Dr. Harms has no financial disclosures. Dr. Keane has received grants from NIMH. Dr. MacDonald has received research grants from the NIH and NARSAD. Dr. Ragland has received research grants from the NIH and NARSAD. Dr. Silverstein has received research grants from NIMH, NARSAD, The Stanley Medical Research Institute, The New England Research Institutes, the van Ameringen Foundation, The Jacob and Valeria Langaloth Foundation, The New York State Office of Mental Health, the New Jersey Division of Mental Health and Addiction Services, Janssen, Pfizer, and AstraZeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The left and right inferior frontal gyrus pars triangularis and the left angular gyrus all had less than the equivalent of 2 voxels at the original fMRI resolution that were active in the group level map. Therefore these regions, as well as their bilateral counterpart (i.e., right angular gyrus) were excluded from further analyses.
References
- Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Curr Biol. 2003;13(4):342–349. doi: 10.1016/s0960-9822(03)00052-6. doi: S0960982203000526 [pii] [DOI] [PubMed] [Google Scholar]
- Baeck A, Rentmeesters N, Holtackers S, Op de Beeck HP. The effect of sleep in perceptual learning with complex objects. Vision Res. 2014;99:180–185. doi: 10.1016/j.visres.2013.10.003. doi: 10.1016/j.visres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Bang JW, Khalilzadeh O, Hamalainen M, Watanabe T, Sasaki Y. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vision Res. 2014;99:162–171. doi: 10.1016/j.visres.2013.12.014. doi: 10.1016/j.visres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- Braun J. On the detection of salient contours. Spatial Vision. 1999;12(2):211–225. doi: 10.1163/156856899x00120. [DOI] [PubMed] [Google Scholar]
- Butler PD, Abeles IY, Silverstein SM, Dias EC, Weiskopf NG, Calderone DJ, Sehatpour P. An event-related potential examination of contour integration deficits in schizophrenia. Front Psychol. 2013;4:132. doi: 10.3389/fpsyg.2013.00132. doi: 10.3389/fpsyg.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr Bull. 2012;38(1):81–91. doi: 10.1093/schbul/sbr106. doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64(1):40–47. doi: 10.1016/j.biopsych.2008.03.023. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr V, Wale J. Schizophrenia: an information processing model. Aust N Z J Psychiatry. 1986;20(2):136–155. doi: 10.3109/00048678609161327. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. doi: sbm081 [pii] 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M, Plochl M, Vicente R, Pipa G. Neuronal oscillations form parietal/frontal networks during contour integration. Front Integr Neurosci. 2014;8:64. doi: 10.3389/fnint.2014.00064. doi: 10.3389/fnint.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46(23):4071–4074. doi: 10.1016/j.visres.2006.07.022. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Chandna A, Pennefather PM, Kovacs I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 2001;42(3):875–878. [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14(1-4):303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Chen M, Yan Y, Gong X, Gilbert CD, Liang H, Li W. Incremental integration of global contours through interplay between visual cortical areas. Neuron. 2014;82(3):682–694. doi: 10.1016/j.neuron.2014.03.023. doi: 10.1016/j.neuron.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Del Viva MM, Agostini R. Visual spatial integration in the elderly. Invest Ophthalmol Vis Sci. 2007;48(6):2940–2946. doi: 10.1167/iovs.06-0729. doi: 48/6/2940 [pii] 10.1167/iovs.06-0729. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59(11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. doi: yoa10242 [pii] [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Snodgrass JG, Schroeder CE, Javitt DC. Activation timecourse of ventral visual stream object-recognition areas: high density electrical mapping of perceptual closure processes. J Cogn Neurosci. 2000;12(4):615–621. doi: 10.1162/089892900562372. [DOI] [PubMed] [Google Scholar]
- Feigenson KA, Keane BP, Roche MW, Silverstein SM. Contour integration impairment in schizophrenia and first episode psychosis: state or trait? Schizophr Res. 2014;159(2-3):515–520. doi: 10.1016/j.schres.2014.09.028. doi: 10.1016/j.schres.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field”. Vision Res. 1993;33(2):173–193. doi: 10.1016/0042-6989(93)90156-q. doi: 0042-6989(93)90156-Q [pii] [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP) New York State Psychiatric Institute; New York, NY: 2002a. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York State Psychiatric Institute; New York, NY: 2002b. [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(17):3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Gervan P, Kovacs I. Two phases of offline learning in contour integration. J Vis. 2010;10(6):24. doi: 10.1167/10.6.24. doi: 10.1167/10.6.24. [DOI] [PubMed] [Google Scholar]
- Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol. 2002;87(4):1867–1888. doi: 10.1152/jn.00690.2001. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35(1):163–181. doi: 10.1093/schbul/sbn156. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From Perception to Functional Outcome in Schizophrenia: Modeling the Role of Ability and Motivation. Arch Gen Psychiatry. 2012:1–9. doi: 10.1001/archgenpsychiatry.2012.652. doi: 10.1001/archgenpsychiatry.2012.652 1362793 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107(1-3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Dalal SS, Greenlee MW. Prestimulus oscillatory phase at 7 Hz gates cortical information flow and visual perception. Curr Biol. 2013;23(22):2273–2278. doi: 10.1016/j.cub.2013.09.020. doi: 10.1016/j.cub.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Hebert M, Gagne AM, Paradis ME, Jomphe V, Roy MA, Merette C, Maziade M. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2010;67(3):270–274. doi: 10.1016/j.biopsych.2009.08.016. doi: 10.1016/j.biopsych.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Henderson D, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, MacDonald AW. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2012;38(1):104–113. doi: 10.1093/schbul/sbr172. doi: 10.1093/schbul/sbr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnstein M, Bayer U, Ellison A, Hausmann M. TMS over the left angular gyrus impairs the ability to discriminate left from right. Neuropsychologia. 2011;49(1):29–33. doi: 10.1016/j.neuropsychologia.2010.10.028. doi: 10.1016/j.neuropsychologia.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Hollingshead AD, Redlich FC. Social Class And Mental Illness. Wiley; New York, NY: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Sekuler AB, Bennett PJ. How much practice is needed to produce perceptual learning? Vision Research. 2009;49:2624–2634. doi: 10.1016/j.visres.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Keane BP, Erlikhman G, Kastner S, Paterno D, Silverstein SM. Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency? Neuropsychologia. 2014;65:221–233. doi: 10.1016/j.neuropsychologia.2014.10.031. doi: 10.1016/j.neuropsychologia.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Joseph J, Silverstein SM. Late, not early, stages of Kanizsa shape perception are compromised in schizophrenia. Neuropsychologia. 2014;56:302–311. doi: 10.1016/j.neuropsychologia.2014.02.001. doi: 10.1016/j.neuropsychologia.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Silverstein SM, Barch DM, Carter CS, Gold JM, Kovacs I, Strauss ME. The spatial range of contour integration deficits in schizophrenia. Exp Brain Res. 2012;220(3-4):251–259. doi: 10.1007/s00221-012-3134-4. doi: 10.1007/s00221-012-3134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Knight RA, Silverstein SM. A process-oriented approach for averting confounds resulting from general performance deficiencies in schizophrenia. J Abnorm Psychol. 2001;110(1):15–30. doi: 10.1037//0021-843x.110.1.15. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: monkey and human FMRI studies. Neuron. 2003;37(2):333–346. doi: 10.1016/s0896-6273(02)01174-1. doi: S0896627302011741 [pii] [DOI] [PubMed] [Google Scholar]
- Kovacs I. Human development of perceptual organization. Vision Res. 2000a;40(10-12):1301–1310. doi: 10.1016/s0042-6989(00)00055-9. doi: S0042-6989(00)00055-9 [pii] [DOI] [PubMed] [Google Scholar]
- Kovacs I, Polat U, Pennefather PM, Chandna A, Norcia AM. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vision Res. 2000b;40(13):1775–1783. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Kozma-Weibe P, Silverstein SM, Feher A, Kovacs I, Uhlhaas P, Wilkniss S. Development of a World-Wide-Web based contour integration test: Reliability and validity. Computers in Human Behavior. 2006;22:971–980. [Google Scholar]
- Levi DM, Yu C, Kuai SG, Rislove E. Global contour processing in amblyopia. Vision Res. 2007;47(4):512–524. doi: 10.1016/j.visres.2006.10.014. doi: S0042-6989(06)00494-9 [pii] 10.1016/j.visres.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Learning to link visual contours. Neuron. 2008;57(3):442–451. doi: 10.1016/j.neuron.2007.12.011. doi: 10.1016/j.neuron.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. British Journal of Medical Psychology. 1961;34:103–115. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345–357. doi: 10.1080/09540260701486563. doi: 780491577 [pii] 10.1080/09540260701486563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J Neurosci. 2002;22(12):5055–5073. doi: 10.1523/JNEUROSCI.22-12-05055.2002. doi: 22/12/5055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitsuka T, McCarley RW, Kuroki N, Dickey CC, Kubicki M, Demeo SS, Shenton ME. Occipital lobe gray matter volume in male patients with chronic schizophrenia: A quantitative MRI study. Schizophr Res. 2007;92(1-3):197–206. doi: 10.1016/j.schres.2007.01.027. doi: 10.1016/j.schres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SE. Vision science: photons to phenomenology. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Place EJ, Gilmore GC. Perceptual organization in schizophrenia. J Abnorm Psychol. 1980;89(3):409–418. doi: 10.1037//0021-843x.89.3.409. [DOI] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27(44):11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Harms MP, Barch DM, Gold JM, Layher E, Carter CS. Functional and neuroanatomical specificity of episodic memory dysfunction in schizophrenia: An fMRI study of the relational and item-specific encoding task. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2015.0276. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41(3):487–497. doi: 10.1017/S0033291710001054. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC. Binding, spatial attention and perceptual awareness. Nat Rev Neurosci. 2003;4(2):93–102. doi: 10.1038/nrn1030. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudaia E, Bennett PJ, Sekuler AB. The effect of aging on contour integration. Vision Res. 2008;48(28):2767–2774. doi: 10.1016/j.visres.2008.07.026. doi: S0042-6989(08)00367-2 [pii] 10.1016/j.visres.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Schallmo MP, Sponheim SR, Olman CA. Abnormal contextual modulation of visual contour detection in patients with schizophrenia. PLoS One. 2013a;8(6):e68090. doi: 10.1371/journal.pone.0068090. doi: 10.1371/journal.pone.0068090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallmo MP, Sponheim SR, Olman CA. Correction: Abnormal Contextual Modulation of Visual Contour Detection in Patients with Schizophrenia. PLoS One. 2013b;8(10) doi: 10.1371/journal.pone.0068090. doi: 10.1371/annotation/f082ec4d-419c-43ce-ae50-e05107539bf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2005;76(2-3):273–286. doi: 10.1016/j.schres.2005.03.003. doi: S0920-9964(05)00089-7 [pii] 10.1016/j.schres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Spaulding WD, Silverstein SM. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res. 2005;39(5):499–508. doi: 10.1016/j.jpsychires.2005.01.001. doi: S0022-3956(05)00005-1 [pii] 10.1016/j.jpsychires.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Maeda JA, Hayashi K, Michelsen N, Sorensen HJ, Ekstrom M, Mednick SA. Premorbid childhood ocular alignment abnormalities and adult schizophrenia-spectrum disorder. Schizophr Res. 2006;81(2-3):253–260. doi: 10.1016/j.schres.2005.08.008. doi: S0920-9964(05)00373-7 [pii] 10.1016/j.schres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Schubert EW, Henriksson KM, McNeil TF. A prospective study of offspring of women with psychosis: visual dysfunction in early childhood predicts schizophrenia-spectrum disorders in adulthood. Acta Psychiatr Scand. 2005;112(5):385–393. doi: 10.1111/j.1600-0447.2005.00584.x. doi: ACP584 [pii] 10.1111/j.1600-0447.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamis of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. Neuroimage. 2006;29(2):605–618. doi: 10.1016/j.neuroimage.2005.07.049. doi: S1053-8119(05)00563-X [pii] 10.1016/j.neuroimage.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald Iii AW, Barch DM. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM. Measuring specific, rather than generalized, cognitive deficits and maximizing between-group effect size in studies of cognition and cognitive change. Schizophr Bull. 2008;34(4):645–655. doi: 10.1093/schbul/sbn032. doi: sbn032 [pii] 10.1093/schbul/sbn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Bakshi S, Nuernberger S, Carpinello K, Wilkniss S. Effects of stimulus structure and target-distracter similarity on the development of visual memory representations in schizophrenia. Cogn Neuropsychiatry. 2005;10(3):215–229. doi: 10.1080/13546800444000029. doi: 10.1080/13546800444000029. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Bakshi S, Chapman RM, Nowlis G. Perceptual organization of configural and nonconfigural visual patterns in schizophrenia: Effects of repeated exposure. Cognitive Neuropsychiatry. 1998;3:209–223. [Google Scholar]
- Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009;8(2):175–202. doi: 10.1142/s0219635209002113. doi: S0219635209002113 [pii] [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Hatashita-Wong M, Schenkel LS, Wilkniss S, Kovacs I, Feher A, Savitz A. Reduced top-down influences in contour detection in schizophrenia. Cogn Neuropsychiatry. 2006;11(2):112–132. doi: 10.1080/13546800444000209. doi: U577866551327J47 [pii] 10.1080/13546800444000209. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 2011a;37(4):690–699. doi: 10.1093/schbul/sbr052. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, Barch DM, Carter CS, Gold JM, Kovacs I, Strauss ME. Optimization and validation of a visual integration test for schizophrenia research. Schizophr Bull. 2012;38(1):125–134. doi: 10.1093/schbul/sbr141. doi: 10.1093/schbul/sbr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Perceptual organization in schizophrenia: plasticity and state-related change. Learning and Perception. 2009;1:229–261. [Google Scholar]
- Silverstein SM, Knight RA, Schwarzkopf SB, West LL, Osborn LM, Kamin D. Stimulus configuration and context effects in perceptual organization in schizophrenia. J Abnorm Psychol. 1996;105(3):410–420. doi: 10.1037//0021-843x.105.3.410. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Kovacs I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000;43(1):11–20. doi: 10.1016/s0920-9964(99)00180-2. doi: S0920-9964(99)00180-2 [pii] [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Osborn LM, Palumbo DR. Rey-Osterrieth Complex Figure Test performance in acute, chronic, and remitted schizophrenia patients. J Clin Psychol. 1998;54(7):985–994. doi: 10.1002/(sici)1097-4679(199811)54:7<985::aid-jclp12>3.0.co;2-g. doi: 10.1002/(SICI)1097-4679(199811)54:7<985::AID-JCLP12>3.0.CO;2-G [pii] [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Perceptual organization in schizophrenia: plasticity and state-related change. Learning and Perception. 2009;1:229–261. [Google Scholar]
- Silverstein SM, Keane BP. Vision Science and Schizophrenia Research: Toward a Re-view of the Disorder Editors' Introduction to Special Section. Schizophr Bull. 2011b;37(4):681–689. doi: 10.1093/schbul/sbr053. doi: sbr053 [pii] 10.1093/schbul/sbr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophrenia Research: Cognition. 2015;2(2):46–55. doi: 10.1016/j.scog.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Grutzner C, Bolte S, Wibral M, Tozman T, Schlitt S, Uhlhaas PJ. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci. 2012;32(28):9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophr Bull. 2007;33(1):142–156. doi: 10.1093/schbul/sbl047. doi: sbl047 [pii] 10.1093/schbul/sbl047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pantel J, Lanfermann H, Prvulovic D, Haenschel C, Maurer K, Linden DE. Visual perceptual organization deficits in Alzheimer's dementia. Dement Geriatr Cogn Disord. 2008;25(5):465–475. doi: 10.1159/000125671. doi: 000125671 [pii] 10.1159/000125671. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006;145(2-3):105–117. doi: 10.1016/j.psychres.2005.10.016. doi: S0165-1781(05)00417-8 [pii] 10.1016/j.psychres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Schenkel LS, Silverstein SM. Theory of mind and perceptual context-processing in schizophrenia. Cogn Neuropsychiatry. 2006;11(4):416–436. doi: 10.1080/13546800444000272. doi: 769903309 [pii] 10.1080/13546800444000272. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Silverstein SM. The course and clinical correlates of dysfunctions in visual perceptual organization in schizophrenia during the remission of psychotic symptoms. Schizophr Res. 2005;75(2-3):183–192. doi: 10.1016/j.schres.2004.11.005. doi: S0920-9964(04)00420-7 [pii] 10.1016/j.schres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol Bull. 2005;131(4):618–632. doi: 10.1037/0033-2909.131.4.618. doi: 2005-08334-007 [pii] 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- Volberg G, Greenlee MW. Brain networks supporting perceptual grouping and contour selection. Front Psychol. 2014;5:264. doi: 10.3389/fpsyg.2014.00264. doi: 10.3389/fpsyg.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg G, Wutz A, Greenlee MW. Top-down control in contour grouping. PLoS One. 2013;8(1):e54085. doi: 10.1371/journal.pone.0054085. doi: 10.1371/journal.pone.0054085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57(6):827–833. doi: 10.1016/j.neuron.2008.02.034. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.