Abstract
Class D β-lactamases of Acinetobacter baumannii are enzymes of the utmost clinical importance, producing resistance to last resort carbapenem antibiotics. Although the OXA-51-like enzymes constitute the largest family of class D β-lactamases, they are poorly studied and their importance in conferring carbapenem resistance is controversial. We present the detailed microbiological, kinetic and structural characterization of the eponymous OXA-51 β-lactamase. Kinetic studies show that OXA-51 has low catalytic efficiency for carbapenems, primarily due to the low affinity of the enzyme for these substrates. Structural studies demonstrate that this low affinity results from the obstruction of the enzyme active site by the side chain of Trp222 which presents a transient steric barrier to an incoming carbapenem substrate. The Trp222Met substitution relieves this steric hindrance and elevates the affinity of the mutant enzyme for carbapenems by 10-fold, significantly increasing the levels of resistance to these antibiotics. The ability of OXA-51 to evolve into a robust carbapenemase as the result of a single amino acid substitution may, in the near future, elevate the ubiquitous enzymes of the OXA-51 family to the status of the most deleterious A. baumannii carbapenemases, with dire clinical consequences.
Over the last decade Acinetobacter baumannii has evolved from a commensal microorganism of little clinical importance into a major nosocomial pathogen. This remarkable transformation resulted from the ability of A. baumannii to rapidly spread and survive for a long time in the clinical setting and its propensity to accumulate and exploit various antibiotic resistance mechanisms.1-3 Due to its utmost clinical importance, A. baumannii was included by the Infectious Diseases Society of America (IDSA) to the list of the six clinically most important bacteria that are responsible for the majority of nosocomial infections.4 Carbapenem antibiotics such as imipenem and meropenem were the drugs of choice for treatment of A. baumannii infections. However, the emergence and rapid spread of carbapenem resistant isolates has dramatically reduced available therapeutic options. Currently, up to 40-70% of clinical A. baumannii isolates are resistant to carbapenems.5-9 As these isolates are commonly resistant to the majority of other antibiotics of various classes, mortality rates from such multi-drug-resistant pathogens often exceeds 50%. The major mechanism of resistance to carbapenem antibiotics in A. baumannii is production by bacteria of β-lactamases, enzymes capable of cleaving the four-membered ring of the drugs rendering them inactive. Among the known molecular classes of β-lactamases (A, B, C and D), class D enzymes constitute the predominant mechanism of resistance to carbapenems in Acinetobacter. Class D enzymes are also referred to as oxacillinases or OXA-type β-lactamases, as the early representatives of this class exhibited high catalytic activity for the β-lactam antibiotic oxacillin. Class D enzymes capable of conferring resistance to carbapenem antibiotics have been named CHDLs (from carbapenem-hydrolyzing class D β-lactamases) to distinguish them from class D β-lactamases that are devoid of carbapenemase activity.10 Recent studies have documented, however, that some OXA-type enzymes that behave as non-carbapenemases in various bacteria, are capable of producing clinically significant levels of resistance to carbapenem antibiotics when expressed in A. baumannii.11
Five A. baumannii CHDLs, OXA-23, -24/-40, -51, -58, -143 and -235 are responsible for the majority of the infections caused by carbapenem-resistant Acinetobacter in clinics around the world.12-15 The genes for the OXA-23, -40, -58, -143 and -235 enzymes are located on various plasmids, which could have contributed to their rapid spread in A. baumannii.16-19 Unlike all other clinically important CHDLs, the OXA-51-like β-lactamases are encoded by genes located on the chromosome of the vast majority of A. baumannii isolates and thus are native to this species.20,21 Although OXA-51 is regarded as a weak carbapenemase, it often produces resistance to carbapenems in A. baumannii when its gene acquires a strong promoter or/and changes its location from the chromosome to a plasmid, which is expected to result in the increase in the gene copy number and the amount of the enzyme produced. Mutant OXA-51 β-lactamases have also been reported in the carbapenem resistant clinical isolates of A. baumannii, but their role in enhanced resistance has not been studied.23,24 The OXA-51-like β-lactamases have thus a potential to become the most important and deleterious CHDLs of Acinetobacter, as their presence in every A. baumannii isolate and the rapid evolution of the enzyme towards more robust carbapenemase activity could render every A. baumannii isolate resistant to carbapenem antibiotics. While OXA-51-type enzymes constitute the largest family of A. baumannii CHDLs that includes more than 100 closely related enzymes, their role in carbapenem resistance of this important pathogen is currently the subject of debate. To gain insight into the kinetic and structural basis for the carbapenemase activity of OXA-51, we performed kinetic analyses and determined the crystal structure of this ubiquitous A. baumannii β-lactamase. We have further demonstrated how a single amino acid substitution in OXA-51 significantly elevates its catalytic efficiency and transforms the enzyme into a robust carbapenemase.
We first determined the MICs of carbapenem antibiotics conferred by OXA-51 and compared them to the MICs produced by other CHDLs, OXA-23, -24, and -58. All of these genes were cloned into the pNT221 shuttle vector25 to express them under the same promoter in A. baumannii ATCC 17978. MICs were determined as previously described.11 In the presence of OXA-51 the MICs of imipenem, meropenem, ertapenem and doripenem increased 16-, 32-, 16-and 64-fold when compared to the background control strain. The MICs of the carbapenems produced by OXA-51 were similar to those conferred by OXA-58, but well below the levels produced by two other A. baumannii CHDLs, OXA-23 and OXA24/40 (Table 1).
Table 1. MICs of (μg/ml) carbapenem antibiotics against A. baumannii ATCC 17978 producing various CHDLs.
| Enzyme | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Antibiotic | Controla | OXA-51 | OXA-51Mb | OXA-23c | OXA-24c | OXA-58c |
| Imipenem | 0.125 | 2 | 16 | 32 | 64 | 8 |
| Meropenem | 0.25 | 8 | 64 | 64 | 128 | 4 |
| Ertapenem | 2 | 32 | 256 | 256 | >256 | 32 |
| Doripenem | 0.125 | 8 | 64 | 32 | 128 | 4 |
Parental A. baumannii ATCC 17978 strain with the plasmid pNT221 without a β-lactamase gene.
OXA-51M denotes the OXA51 Trp222Met mutant enzyme.
The MICs of OXA-23, OXA-24 and OXA-58 for carbapenems have been previously reported.11
Next we evaluated the steady-state kinetic parameters for the turnover of the carbapenems by purified OXA-51 (Table 2). The enzyme turned over these carbapenem substrates rather inefficiently (kcat values from 0.11s-1 to 0.41s-1). The kcat values for OXA-51 with meropenem, ertapenem and doripenem were overall similar to those reported for the CHDLs OXA-23 and OXA-24/40 and were 4- to 5-fold higher than those for OXA-58.11 With imipenem the kcat value for OXA-51 was identical to that for OXA-23 and was 4- to 4.5-fold below that for OXA-58 and OXA-24/40.11 The Km value of OXA-51 for imipenem was 89 μM (Table 2), well above (≥50-fold) the values determined by us for the OXA-23, OXA-24/40 and OXA-58 β-lactamases.11 For the three other carbapenems, Km values were in the 5.5 – 7.1 μM range, also well above the reported Ks values for the other three CHDLs.11
Table 2. Kinetics parameters for hydrolysis of carbapenems by OXA-51 and the OXA-51 Trp222Met mutant.
| Antibiotic | |||||
|---|---|---|---|---|---|
|
| |||||
| Enzyme | Parameter | Imipenem | Meropenem | Ertapenem | Doripenem |
| OXA-51 | kcat (s-1) | 0.41 ± 0.01 | 0.113 ± 0.001 | 0.044 ± 0.001 | 0.052 ± 0.001 |
| Km (μM) | 89 ± 7 | 7.1 ± 0.4 | 6.0 ± 0.6 | 5.5 ± 0.6 | |
| kcat/Km (s-1M-1) | (4.6 ± 0.4) × 103 | (1.6 ± 0.1) × 104 | (7.3 ± 0.8) × 103 | (9.5 ± 1.0) × 103 | |
|
| |||||
| OXA-51Ma | kcat (s-1) | 0.28 ± 0.01 | 0.089 ± 0.001 | 0.039 ± 0.001 | 0.053 ± 0.001 |
| Km (μM) | 4.7 ± 0.3 | 0.67 ± 0.08 | 0.51 ± 0.07 | 0.52 ± 0.09 | |
| kcat/Km (s-1M-1) | (6.0 ± 0.4) × 104 | (1.3 ± 0.2) × 105 | (7.7 ± 1.1) × 104 | (1.0 ± 0.2) × 105 | |
OXA-51M denotes the OXA51 Trp222Met mutant enzyme.
The amount of soluble β-lactamase produced by the bacterial cell has a significant impact on the produced level of antibiotic resistance. To evaluate the relative amount of OXA-51 produced by A. baumannii ATCC 17978 versus the amount of the three other CHDLs (OXA-23, OXA-24/40 and OXA-58) produced by the same host strain, the hexa-His tag was introduced to the C-termini of these enzymes. The amount of produced β-lactamases were subsequently evaluated as described by us recently.11 This analysis demonstrated that while A. baumannii ATCC 17978 produced similar amounts of each of the CHDLs, the amount of soluble β-lactamases differed. Production of soluble OXA-51 was the highest (100%), followed by OXA-58, OXA-24/40 and OXA-23 (80%, 60% and 30% of the OXA-51 amount, respectively; Figure 1). These data combined with our kinetic measurements demonstrate that it is not the amount of enzyme produced but rather its lower relative affinity for carbapenems is responsible for lower levels of resistance to these antibiotics conferred by OXA-51 when compared to the three other CHDLs.
Figure 1.
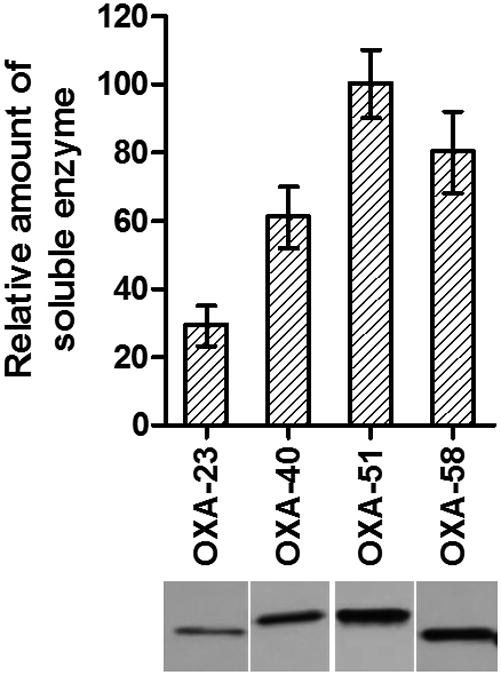
Expression levels of OXA carbapenemases. (Bottom) Immunoblot of the soluble OXA-23, -40, -51 and -58 produced by A. baumannii ATCC 17978. (Top) Relative amounts of enzymes produced are normalized to that of OXA-51.
The OXA-51 carbapenemase was purified to homogeneity and crystallized prior to diffraction data collection at SSRL beamline BL12-2. The final refined OXA-51 model comprises 243 amino acids (residues Ser38 to Leu280), folded into two structural domains (Figure 2A). Some representative electron density is shown in Figure S1. The overall structure of OXA-51 resembles that of other class D β-lactamases whose structures have been determined, including OXA-1,26,27 OXA-2 (unpublished, PDB code 1K38), OXA-10,28,29 OXA-13,30 OXA-23,25,31 OXA-24,32,33 OXA-45 (unpublished, PDB code 4GN2), OXA-46,34 OXA-48,35 OXA-58,36 and OXA-146.31 Superposition of OXA-51 onto these enzymes gives rmsds for all matching Cα atoms ranging from 0.6 Å for OXA-23 and OXA-24 up to 1.8 Å for OXA-45 (Table S1). Not surprisingly, OXA-51 shows high structural agreement with the four other OXA enzymes from A. baumannii (OXA-23, -24, -58 and -143), consistent with a significant degree of sequence identity (54% to 67%) between these five enzymes. The largest structural deviations among the OXA enzymes occur in the loops connecting strands β1 and β2 (L1), helices α6 and α7 (L2), strands β8 and β9 (L3) and in the final turn between strand β10 and helix α10 (L4). The two latter loops are near the opening of the active site (Figure 2A).
Figure 2.
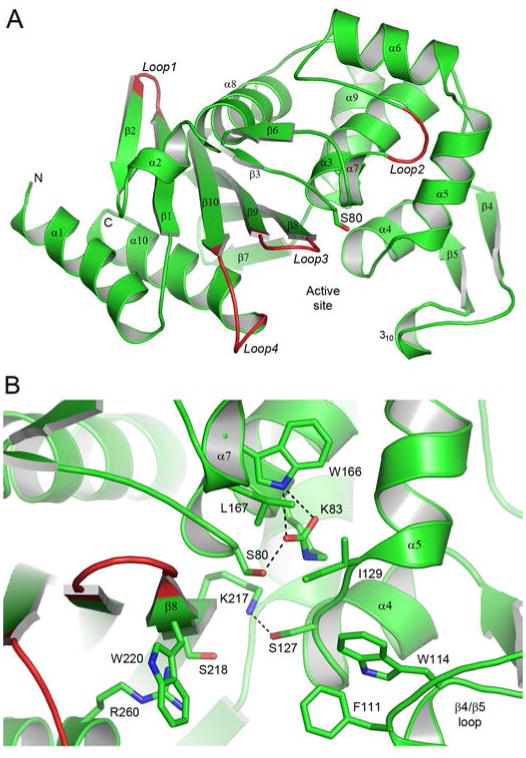
The OXA-51 structure. (A) Ribbon representation of the A. baumannii OXA-51 β-lactamase showing the secondary structure labelling used. The location of the active site is indicated by the side chain of the catalytic serine, Ser80. Four loops (loops 1 - 4) which show the largest structural variation between OXA-51 and the other OXA enzymes, whose structures are known, are also indicated. (B) Close-up view of the OXA-51 active site showing some of the conserved and partially-conserved residues which are important for substrate binding and/or the enzyme mechanism.
The active site is located in a cleft at the interface of the two structural domains (Figure 2A), which is exposed to the external solvent and filled with a number of water molecules. A number of universally conserved residues line the active site including the catalytic Ser80 and the neighboring residues Lys83, Ser127, Trp166, Lys217 and Arg260 (Figure 2B). Ser127 from the loop between helices α4 and α5 forms a hydrogen bond with the side chain of Lys217, and this is the only direct connection between the two sides of the cleft. The Trp166 residue on helix α7 helps to anchor the carboxylated Lys83 via a hydrogen bonding interactions (Figure 2B). The Arg260 residue has been shown to be responsible for anchoring the carboxylate moiety, a common feature on the ring immediately adjacent to the β-lactam ring of penicillins, cephalosporins and carbapenems, in known OXA enzyme complex structures.25,26,30,33 There are also several partially conserved amino acids which have been suggested to play a role in either substrate specificity or/and the reaction mechanism. These include two aromatic residues at the end of the long β4/β5 loop, Phe111 and Trp114 in OXA-51 (motif 2, Figure S2), small hydrophobic residues on the α4/α5 loop (Ile129, motif 3) and helix α7 on the Ω-loop (Leu167, motif 5) and a hydroxyl (Ser218) and an aromatic residue(Trp220) on strand β8 (motif 7) (Figure 2B). Table S2 indicates the most common amino acids at these positions in 156 OXA enzymes. The two aromatic residues (Phe111 and Trp114 in OXA-51) on the β4/β5 loop appear to make hydrophobic interactions with substrates in some OXA enzyme-substrate complexes.25,33 Another role for the phenylalanine residue in some OXA enzymes seems to be the formation of a hydrophobic “bridge” or “tunnel” across the mouth of the active site. In these enzymes, the phenylalanine interacts with a partially-conserved methionine residue from motif 7 at the C-terminal end of strand β8 (Figure 3A). This hydrophobic interaction was first observed in the structure of OXA-2433 and subsequently in OXA-23,25 and it was suggested that the carbapenemase activity of these enzymes might be dependent upon the formation of this bridge, whereby it can direct the orientation of the carbapenem substrate and make it more susceptible to deacylation. The recent structure of the carbapenemase OXA-58 shows that this hydrophobic bridge does not exist in this enzyme despite the presence of the same residues (Phe114 and Met225, OXA-58 numbering) which would normally form the hydrophobic interaction. In OXA-51 and almost all of the known OXA-51-like variants, the residue at the equivalent position to this methionine is a tryptophan (Trp222) (Figure 3A).
Figure 3.
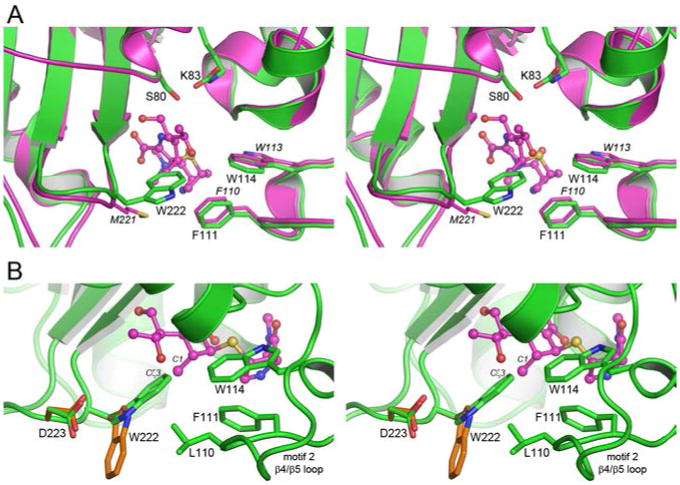
The Trp222 side chain in OXA-51 would occlude the active site. (A) Stereoview of the active site of OXA-51 (green) superimposed on OXA-23 (magenta). The location of meropenem in the OXA-23 structure is shown as magenta ball-and-stick. The position of Ser80 in OXA-51 shows the location of the site of acylation. (B) Stereoview of the outer end of the OXA-51 active site, showing the location of Trp222 and the residues from the β4/β5 (Leu110, Phe111 and Trp114) loop which make hydrophobic contacts with Trp222. Meropenem from OXA-23 is shown as magenta ball-and-stick. The critical clash between the indole ring of Trp222 and the C1 methyl atom of the substrates is indicated. The alternate rotamer configuration of Trp222 from energy minimization calculations is shown as orange sticks, and the small rotation of the Asp223 side chain is also indicated.
In OXA-51 the side chain of Trp222 projects across the opening of the active site (Figure 3B) and is anchored in this conformation by several aromatic and hydrophobic interactions with the side chains of Leu110, Phe111, Trp114 with the edge-to-face interaction with Phe111, in particular, being moderately strong.37 Moreover, the Leu110/Trp222 pairing, albeit on opposite sides of the active site cleft, is a characteristic sequence motif for the OXA-51-like family of class D enzymes. This leucine residue precedes the partially conserved phenylalanine of motif 2 (Phe111 in OXA-51) and is universally conserved in all of the OXA-51-like enzymes, whereas in the 156 non-OXA-51 enzymes, the residue at this position is highly variable.
Superposition of the apo OXA-51 structure with the published structures of the OXA-1-doripenem,26 OXA-13-meropenem,30 OXA-23-meropenem25 and OXA-24-doripenem33 acyl enzyme complexes show that the presence of Trp222 in OXA-51 would severely hinder the binding of carbapenem substrates (Figure 3B). The indole ring of Trp222 is directed towards the pyrolline ring of the carbapenem, such that the indole Cζ3 atom and the C1 methyl group of meropenem and doripenem almost overlap. There are also additional potential clashes between Ile129 and Leu167 of the enzyme and the α-hydroxyethyl group of the carbapenem, but those can be readily alleviated by small rotations of the isoleucine and leucine side chains. Occlusion of the active site of OXA-51 by the Trp222 side chain is further exacerbated by the extensive hydrophobic interactions it makes with the β4/β5 loop, which may present an additional energy barrier to efficient carbapenem binding. According to our kinetic data, OXA-51 is an active enzyme with modest carbapenemase activity, an indication that the enzyme is capable of the binding and hydrolysis of carbapenem antibiotics. The only way carbapenems can productively bind to the active site of OXA-51 is by displacing the side chain of Trp222 from the position it occupies in the current apo-OXA-51 structure.
A total of seven rotamers of the Trp222 side chain, with probabilities of greater than 1%, are plausible based on the statistical analysis of side chain rotamer preferences in proteins.38 Analysis of these rotamers with the graphics program COOT39 indicates that five of the rotamer variants are sterically unfavored (Figure S3). The only sterically viable rotamer configuration is a 180° rotation about the Cβ-Cγ bond of the tryptophan side chain, pointing the indole ring in the opposite direction to that observed in the OXA-51 structure (Figure 3B). In this alternate configuration, although the indole ring would still be stabilized by the extensive hydrophobic interactions with the side chains of Leu110 and Phe111, it would potentially clash with the side chain of Asp233. To confirm whether this alternate rotamer configuration is accessible in the context of the OXA-51 structure, optimization of the five residue β8/β9 loop (with sequence WGWDV), centered on Trp222, with a meropenem fixed in the OXA-51 active site based upon a superposition of the OXA-23-meropenem structure25 was performed. The minimization resulted in a side chain configuration for Trp222 closely resembling the predicted viable rotamer variant, with the plane of the indole rings system within 10° of the indole of the rotamer variant. Moreover the neighboring Asp223 side chain also showed a small concerted movement in order to avoid the potential clash with the newly positioned Trp222 indole ring. Such transitioning from the observed tryptophan rotamer to the alternate configuration, would require an energy barrier to be overcome, which may explain the higher Km for OXA-51 with the carbapenem substrates (Table 2) when compared to other class D carbapenemases.11 It should be noted that there is no evidence of this alternate conformation in the apo-OXA-51 electron density (Figure S1).
An alignment of the 117 reported OXA-51-like sequences shows that the residue at position 222 is almost invariably tryptophan; only four enzymes, OXA-79 (glycine) and OXAs-172, -173 and -200 (all leucine), have other amino acid residues at this position. While these mutant enzymes have not been isolated and characterized, it has been reported that clinical isolates carrying these OXA-51 variants have increased resistance to carbapenems.40
To evaluate whether the bulky Trp222 in OXA-51 is responsible for the lower apparent affinity of the enzyme for carbapenem antibiotics when compared to the OXA-23, -24 and -58 carbapenemases and, ultimately, attenuates its carbapenemase activity, we have substituted it with methionine, a residue that is found in this position in other clinically important CHDLs and their variants. When expressed from the same plasmid and promoter in the A. baumannii ATCC 17978 recipient, this mutant enzyme produced an 8-fold increase in MICs of all four carbapenems tested when compared to the parental enzyme (Table 1). The resulting MIC values of imipenem, meropenem, ertapenem and doripenem were similar to those against A. baumannii ATCC 17978 producing the OXA-23 and OXA-24/40 CHDLs and are well above the MICs against the same strain producing the OXA-58 carbapenemase (Table 1). The Trp222Met substitution had little effect on the kcat values of the enzyme but resulted in a significant increase in its affinity for carbapenem antibiotics as judged by lower Km values. Overall, the catalytic efficiency of the Trp222Met mutant enzyme against increased 10-fold when compared to the parental OXA-51 β-lactamase. Of importance, several mutant derivatives of OXA-51 have recently been reported in carbapenem-resistant clinical A. baumannii isolates. DNA sequencing of the gene for OXA-51 has identified several amino acid substitutions, including mutations of Trp222.40 While the contribution of the mutant OXA-51 enzymes to the decreased susceptibility of the clinical isolates has not been evaluated, our present study demonstrates that Trp222 indeed is crucial for the ability of the OXA-51-type β-lactamase to produce high, clinically important levels of resistance to carbapenem antibiotics
Conclusions
The OXA-51-like enzymes comprise the largest family of A. baumannii β-lactamases. Our studies of OXA-51 have demonstrated that the bulky Trp222 residue transiently occludes the active site and is responsible for the weak carbapenemase activity of the enzyme. We have demonstrated that substitution of this tryptophan with a less bulky methionine converts this enzyme into a robust CHDL that produces high levels of resistance to these last resort antibiotics. As the gene for OXA-51 is ubiquitous in A. baumannii, its evolutionary plasticity may, in short time, transform the OXA-51-like family of enzymes into carbapenemases of major clinical importance
Experimental Procedures
Supplementary Material
Acknowledgments
Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource (SSRL), a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. We would like to thank SSRL Summer Intern C. Milianta for help with the structure determination and refinement.
Footnotes
Supporting Information: Tables of crystallographic data and crystallographic refinement statistics. Figures of the final electron density in the active site, a structure-based sequence alignment of the OXA enzymes whose structures are known, and the alternate rotamer conformations for Trp222. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Numbers: The atomic coordinates and the structure factors for OXA-51 were deposited to the Protein Data Bank41 with PDB code 4ZDX.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roca I, Espinal P, Vila-Farres X, Vila J. The Acinetobacter baumannii oxymoron: Commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol. 2012;3:1–30. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Davies TA, Queenan AM, Morrow BJ, Shang W, Amsler K, He W, Lynch AS, Pillar C, Flamm RK. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the USA in 2007-09. J Antimicrob Chemother. 2011;66:2298–2307. doi: 10.1093/jac/dkr290. [DOI] [PubMed] [Google Scholar]
- 6.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 7.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31:528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 8.Rhomberg PR, Jones RN. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: A 10-year experience in the United States (1999-2008) Diagn Microbiol Infect Dis. 2009;65:414–426. doi: 10.1016/j.diagmicrobio.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, Ramirez Wong FM, Barkat A, Pino OR, Duenas L, Mitrev Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Ben Jaballah N, Raka L, Gikas A, Ahmed A, Thu le TA, Guzman Siritt ME. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95–104. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 11.Antunes NT, Lamoureaux TL, Toth M, S NK, Frase H, Vakulenko SB. Class D β-lactamases: Are they all carbapenemases? Antimicrob Agents Chemother. 2014;58:2119–2125. doi: 10.1128/AAC.02522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Queenan AM, Bush K. Carbapenemases: The versatile β-lactamases. Clin Microbiol Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao WH, H ZQ. Acinetobacter: A potential reservoir and dispenser for β-lactamases. Crit Rev Microbiol. 2012;38:30–51. doi: 10.3109/1040841X.2011.621064. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PG, Perez-Llarena FJ, Zander E, Fernandez A, Bou G, Seifert H. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bou G, Oliver A, Martinez-Beltran J. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob Agents Chemother. 2000;44:1556–1561. doi: 10.1128/aac.44.6.1556-1561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:5035–5038. doi: 10.1128/AAC.00856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, Nordmann P. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49:202–208. doi: 10.1128/AAC.49.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaife W, Young HK, Paton RH, Amyes SG. Transferable imipenem-resistance in Acinetobacter species from a clinical source. J Antimicrob Chemother. 1995;36:585–586. doi: 10.1093/jac/36.3.585. [DOI] [PubMed] [Google Scholar]
- 20.Brown S, Young HK, Amyes SGB. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin Microbiol Infec. 2005;11:15–23. doi: 10.1111/j.1469-0691.2004.01016.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans BA, Amyes SG. OXA β-lactamases. Clin Microbiol Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YT, Turton JF, Chen TL, Wu RC, Chang WC, Fung CP, Chen CP, Cho WL, Huang LY, Siu LK. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J Chemother. 2009;21:514–520. doi: 10.1179/joc.2009.21.5.514. [DOI] [PubMed] [Google Scholar]
- 23.Chen TL, Lee YT, Kuo SC, Hsueh PR, Chang FY, Siu LK, Ko WC, Fung CP. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob Agents Chemother. 2010;54:4575–4581. doi: 10.1128/AAC.00764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zander E, Chmielarczyk A, Heczko P, Seifert H, Higgins PG. Conversion of OXA-66 into OXA-82 in clinical Acinetobacter baumannii isolates and association with altered carbapenem susceptibility. J Antimicrob Chemother. 2013;68:308–311. doi: 10.1093/jac/dks382. [DOI] [PubMed] [Google Scholar]
- 25.Smith CA, Antunes NT, Stewart NK, Toth M, Kumarasiri M, Chang M, Mobashery S, Vakulenko SB. Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem Biol. 2013;20:1107–1115. doi: 10.1016/j.chembiol.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider KD, Karpen ME, Bonomo RA, Leonard DA, Powers RA. The 1.4 Å crystal structure of the class D β-lactamase OXA-1 complexed with doripenem. Biochemistry. 2009;48:11840–11847. doi: 10.1021/bi901690r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun T, Nukaga M, Mayama K, Braswell EH, Knox JR. Comparison of β-lactamases of classes A and D: 1.5-Å crystallographic structure of the class D OXA-1 oxacillinase. Protein Sci. 2003;12:82–91. doi: 10.1110/ps.0224303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golemi D, Maveyraud L, Vakulenko S, Tranier S, Ishiwata A, Kotra LP, Samama JP, Mobashery S. The first structural and mechanistic insights for class D β-lactamases: Evidence for a novel catalytic process for turnover of β-lactam antibiotics. J Am Chem Soc. 2000;122:6132–6133. [Google Scholar]
- 29.Paetzel M, Danel F, de Castro L, Mosimann SC, Page MGP, Strynadka NCJ. Crystal structure of the class D β-lactamase OXA-10. Nature Struct Biol. 2000;7:918–924. doi: 10.1038/79688. [DOI] [PubMed] [Google Scholar]
- 30.Pernot L, Frénois F, Rybkine T, L'Hermite G, Petrella S, Delettré J, Jarlier V, Collatz E, Sougakoff W. Crystal structures of the class D beta-lactamase OXA-13 in the native form and in complex with meropenem. J Mol Biol. 2001;310:859–874. doi: 10.1006/jmbi.2001.4805. [DOI] [PubMed] [Google Scholar]
- 31.Kaitany KC, Klinger NV, June CM, Ramey ME, Bonomo RA, Powers RA, Leonard DA. Structures of the class D carbapenemases OXA-23 and OXA-146: Mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins and aztreonam. Antimicrob Agents Chemother. 2014;58:4848–4855. doi: 10.1128/AAC.00762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santillana E, Beceiro A, Bou G, Romero A. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc Natl Acad Sci. 2007;104:5354–5359. doi: 10.1073/pnas.0607557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider KD, Ortega CJ, Renck NA, Bonomo RA, Powers RA, Leonard DA. Structures of the class D carbapenase OXA-24 from Acinetobacter baumanii in complex with doripenem. J Mol Biol. 2011;406:583–594. doi: 10.1016/j.jmb.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docquier JD, Benvenuti M, Calderone V, Giuliani F, Kapetis D, De Luca F, Rossolini GM, Mangani S. Crystal structure of the narrow-spectrum OXA-46 class D beta-lactamase: relationship between active-site lysine carbamylation and inhibition by polycarboxylates. Antimicrob Agents Chemother. 2010;54:2167–2174. doi: 10.1128/AAC.01517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Docquier JD, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, Tafi A, Nordmann P, Botta M, Rossolini GM, Mangani S. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol. 2009;16:540–547. doi: 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Smith CA, Antunes NT, Toth M, Vakulenko SB. Crystal Structure of Carbapenemase OXA-58 from Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58:2135–2143. doi: 10.1128/AAC.01983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter CA, Singh J, Thornton JM. Pi-pi interactions: The geometry and energetics of phenylalanine-phenylalanine interactions in proteins. J Mol Biol. 1991;218:837–846. doi: 10.1016/0022-2836(91)90271-7. [DOI] [PubMed] [Google Scholar]
- 38.Dunbrack RL, Cohen FE. Bayesian statistical analysis of protein sidechain rotamer preferences. Prot Sci. 1997;6:1661–1681. doi: 10.1002/pro.5560060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 40.Zander E, Nemec A, Seifert H, Higgins PG. Association between β-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J Clin Microbiol. 2012;50:1900–1904. doi: 10.1128/JCM.06462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


