Abstract
Background
A better understanding of drivers of treatment costs may help identify effective cost containment strategies and prioritize resources. We aimed to develop a method for estimating inpatient costs for pediatric patients with acute myeloid leukemia (AML) enrolled on NCI-funded Phase III trials, compare costs between AAML0531 treatment arms (standard chemotherapy ± gemtuzumab ozogamicin (GMTZ)), and evaluate primary drivers of costs for newly diagnosed pediatric AML.
Procedure
Patients from the AAML0531 trial were matched on hospital, sex, and dates of birth and diagnosis to the Pediatric Health Information Systems (PHIS) database to obtain daily billing data. Inpatient treatment costs were calculated as adjusted charges multiplied by hospital-specific cost-to-charge ratios. Generalized linear models were used to compare costs between treatment arms and courses, and by patient characteristics.
Results
Inpatient costs did not differ by randomized treatment arm. Costs varied by course with stem cell transplant being most expensive, followed by Intensification II (cytarabine/mitoxantrone) and Induction I (cytarabine/daunorubicin/etoposide). Room/board and pharmacy were the largest contributors to inpatient treatment cost, representing 74% of the total cost. Higher AML risk group (P = 0.0003) and older age (P < 0.0001) were associated with significantly higher daily inpatient cost.
Conclusions
Costs from external data sources can be successfully integrated into NCI-funded Phase III clinical trials. Inpatient treatment costs did not differ by GMTZ exposure but varied by chemotherapy course. Variation in cost by course was driven by differences in duration of hospitalization through room/board charges as well as increased clinical and pharmacy charges in specific courses. Pediatr Blood Cancer
Keywords: acute myeloid leukemia, cost and cost analysis, pediatric, treatment cost
INTRODUCTION
Over the past several decades, cooperative oncology group clinical trials for pediatric acute myeloid leukemia (AML) have improved overall survival rates to nearly 65% through intensive chemotherapy regimens that necessitate lengthy hospitalizations. [1–6] However, none have assessed the economic cost of delivering AML chemotherapy. Comparisons of cost between treatment arms have not been performed for any pediatric cooperative group Phase III clinical trial and are infrequently assessed in adult cooperative group oncology trials.[7–9] Interpretation of clinical trial outcomes in the context of cost is of significant interest to patients, providers, policy makers, and payers. A more complete understanding of the drivers of pediatric AML treatment costs may help to identify effective cost containment strategies and prioritize resources. Thus, establishing an accurate and efficient methodology for estimating adjusted costs would substantively augment NCI-funded cooperative group Phase III trials.
Gemtuzumab ozogamicin (GMTZ), an anti-CD33 immunoconjugate, received accelerated FDA approval in 2000 for the treatment of relapsed AML based on Phase II trials that reported single agent overall response rates of approximately 30%.[10] A subsequent post-approval study in adults failed to demonstrate improvement in remission rates and found significantly higher induction mortality for GMTZ + standard chemotherapy relative to chemotherapy alone, leading to the withdrawal of GMTZ from the US market in 2010.[11] More recently, a meta-analysis of five Phase III studies concluded that GMTZ added to standard chemotherapy significantly improved event free survival (EFS) in adult patients with AML and similar results were reported for a Phase III Children’s Oncology Group (COG) trial (AAML0531).[1,12] The meta-analysis results led to a call for reconsideration of GMTZ approval in the United States.[13] Given the improved EFS for patients treated with GMTZ on the AAML0531 trial, we sought to determine the economic impact of this treatment.
The objectives of this study were to develop a methodology for estimating inpatient costs for patients enrolled on an NCI-funded cooperative oncology group Phase III clinical trial and to use the methodology to compare costs between treatment arms for AAML0531.
METHODS
Study Population
Between August 2006 and June 2010, AAML0531 enrolled children and adolescents with new-onset AML. Patients were randomized to standard chemotherapy ± GMTZ (Supplemental Figure).[1] Patients enrolled on AAML0531 who were treated at hospitals contributing to the Pediatric Health Information System (PHIS) were considered in this cost analysis.
PHIS is an administrative database containing inpatient, emergency department, ambulatory surgery, and observation data from 43 not-for-profit, tertiary care pediatric hospitals in the US, representing 85% of freestanding children’s hospitals (www.chca.com). Each patient in PHIS is assigned a unique identifier allowing records to be longitudinally linked. The methods for the merging of AAML0531 COG data with daily billing data from PHIS were described previously.[14]Briefly, a list of patients enrolled on AAML0531 generated by COG statisticians was matched on hospital, date of diagnosis, date of birth, and sex to a list of PHIS patients identified as having an ICD-9-CM code for AML (205.xx).
Patients who were enrolled on AAML0531 but subsequently determined not to meet inclusion criteria, enrolled at one of three PHIS centers that did not submit cost-to-charge ratios, or who were not admitted to a PHIS hospital during the on-protocol period were excluded.
Course Definitions
The start of each treatment course was defined as the first day on which chemotherapy was administered and continued until either the start of the subsequent course or the AAML0531 off-protocol date.
Covariates
Patient information including age at enrollment (<1 year, 1 to <5 years, 5 to <10 years, 10 to <15 years and ≥15 years), sex, race (white or non-white), insurance status (private, government, or other), AML risk classification (high, intermediate, or low), and minimal residual disease percentage were obtained from the COG AAML0531 database.
PHIS Adjusted Inpatient Costs
Volumes of services, wage- and price-adjusted charges for each unit of service, and department-specific ratio of cost-to-charge (RCC) for each hospital were obtained from PHIS. Adjusted inpatient treatment costs were calculated by multiplying the adjusted charge by the relevant RCC then further adjusted to 2011 US dollars using the consumer price index. Costs for each day of hospitalization were summarized into the following categories: room and board, pharmacy, laboratory, clinical (e.g., respiratory, rehabilitative services, etc.), supply, and imaging. Commercial GMTZ supplies were utilized on AAML0531, thus hospitals billed for the drug and these costs are included in pharmacy costs (mean daily cost for GMTZ: $2,609 ± 1,677). Adjusted overall cost and total costs for each category were calculated for each patient as the sum of the daily costs during the entire on-protocol period. Similar calculations were performed separately for each course. Mean cost per inpatient day was calculated for each patient as the total cost for the given period (entire on-protocol period or specific course) divided by the number inpatient days in the given period. The current analyses include direct medical costs, with the exception of provider, surgery and procedure fees, accrued during inpatient admissions to PHIS hospitals. Indirect costs, outpatient costs, and costs accrued at non-PHIS hospitals were not captured.
Statistical Analyses
Distributions (frequencies and percent) of patient characteristics were calculated by treatment for the current study population as well as the full AAML0531 patient population for those included and not included in cost analyses. Distributions were compared using χ2 tests. Overall survival (OS) and EFS with 95% confidence intervals (CIs) were estimated using the Kaplan–Meier method and were compared using the log-rank statistic. Cox proportional hazards regression was used to estimate hazard ratios (HRs) with 95%CIs. Course duration and number of inpatient days were summarized as median (range) and compared by treatment using Wilcoxon rank sum tests.
PHIS adjusted inpatient treatment costs (for the entire on-protocol period and by course), were summarized in box-and-whisker plots. Due to the skewed nature of the cost data, generalized linear models with a log link and gamma distribution were used to calculate the crude and adjusted cost ratios (CR) with corresponding 95%CIs, for comparisons of total inpatient cost per patient by treatment arm, between courses, and by remission status (Induction II only), and for comparisons of daily cost per patient between courses, and by treatment arm and patient characteristics. Robust variance estimates were obtained using generalized estimating equation methods with an exchangeable correlation matrix to account for clustering by hospital. Each covariate described above was added independently to the crude model, and the change in the cost ratio was assessed. A change >10% was considered evidence of meaningful confounding. Based on the similarity of estimates between crude and adjusted models, there was no evidence of confounding; thus, the results of crude models are presented. All statistical analyses were performed using SAS (version 9.2, SAS Institute, Inc., Cary, NC). A two-sided P-value <0.05 was used as the threshold for statistical significance.
RESULTS
Thirty-nine percent (n = 416) of patients on AAML0531 (n = 1,070) were enrolled at PHIS institutions, of which 96% (n = 378) were matched and had PHIS data available.[14] Twelve patients identified in PHIS were excluded from the cost analyses because they did not meet AAML0531 inclusion criteria (n = 2), were enrolled at a PHIS hospital that did not submit cost-to-charge ratios (n = 5), were discharged from a PHIS hospital on the date of AAML0531 enrollment (n = 2), or were only admitted to a PHIS hospital after the last date of on-protocol follow-up (n = 3). Therefore, the current study population included 366 patients with daily billing data available for at least one course: 47% were randomized to standard chemotherapy and 53% were randomized to standard chemotherapy + GMTZ.
There were no significant differences in sex, age, race, insurance status, or risk classification distributions between treatment groups for the subset of AAML0531 patients included in this cost analysis (Supplemental Table I). The covariate distributions and three-year OS and EFS among AAML0531 patients included in this analysis were comparable to those among patients who were not included (Supplemental Table II). The observed hazard ratio comparing EFS between treatment arms among the subpopulation included in the current cost analyses (HR = 0.85, 95%CI: 0.63, 1.14) was consistent with that observed for the full AAML0531 study population (HR = 0.83, 95%CI: 0.70, 0.99).[1] These results suggest that our study subpopulation is representative of the full AAML0531 population.
Overall and Course-Specific Costs
The total adjusted costs per patient overall and for each course were comparable for the GMTZ and standard arms (Fig. 1). Likewise, neither the course duration nor the number of inpatient days differed by treatment (Table I). However, there were substantial differences in cost between specific chemotherapy courses (Figures 1 and 2).
Fig. 1.
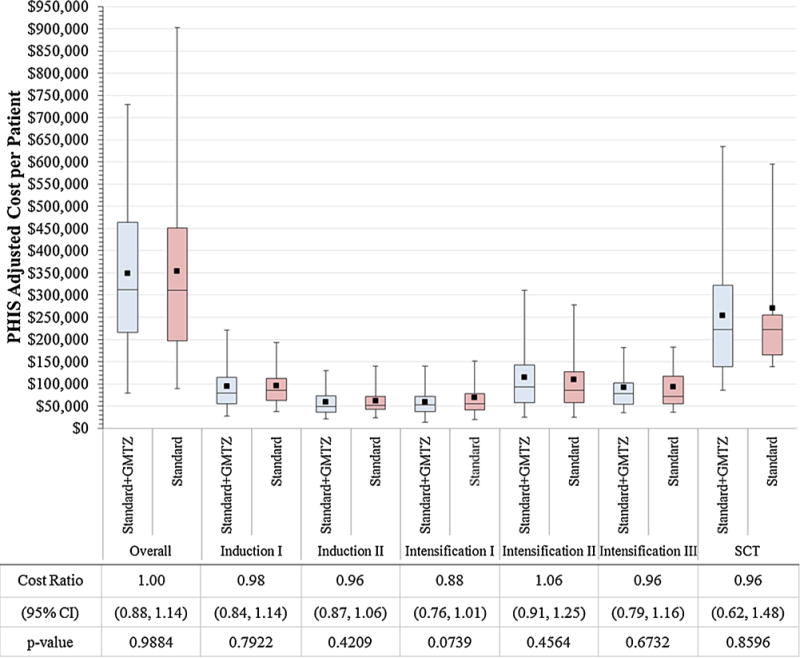
Comparisons of overall and course-specific PHIS adjusted costs per patient by treatment arm. The horizontal lines that form the top and bottom of each box are the 75th and 25th percentiles, respectively. The horizontal line that intersects the box is the median. The whiskers present the 5th and 95th percentiles. The solid squares represent the mean values. Cost Ratios compare cost by treatment regimen (standard + GMTZ vs. standard). Abbreviations: GMTZ = gemtuzumab ozogamicin; SCT = stem cell transplant; CI = confidence interval.
TABLE I.
Course Duration and Total Number of Inpatient Days
| Course | Treatment | n | Course Duration, days
|
Inpatient Days
|
||||
|---|---|---|---|---|---|---|---|---|
| Median | Range | P-valuea | Median | Range | P-valuea | |||
| Induction I | Standard + GMTZ | 187 | 36 | (1–61) | 0.9885 | 30 | (1–54) | 0.2352 |
| Standard | 169 | 36 | (1–58) | 29 | (1–52) | |||
| Induction II | Standard + GMTZ | 176 | 34 | (24–108) | 0.3375 | 25 | (9–72) | 0.4987 |
| Standard | 151 | 34 | (7–69) | 25 | (6–46) | |||
| Intensification I | Standard + GMTZ | 163 | 37 | (21–81) | 0.9068 | 25 | (1–53) | 0.7595 |
| Standard | 136 | 36 | (1–99) | 25 | (1–99) | |||
| Intensification II | Standard + GMTZ | 115 | 53 | (8–175) | 0.0565 | 35 | (7–110) | 0.1518 |
| Standard | 99 | 48 | (6–121) | 33 | (6–97) | |||
| Intensification III | Standard + GMTZ | 90 | 44 | (27–125) | 0.1591 | 34 | (11–89) | 0.1853 |
| Standard | 87 | 41 | (9–89) | 33 | (9–72) | |||
| Stem Cell Transplant | Standard + GMTZ | 31 | 108 | (29–126) | 0.4862 | 43 | (29–124) | 0.4051 |
| Standard | 24 | 106 | (34–168) | 43 | (29–158) | |||
GMTZ, gemtuzumab ozogamicin.
P-values for comparison between treatment regimens (standard + GMTZ versus standard).
Fig. 2.
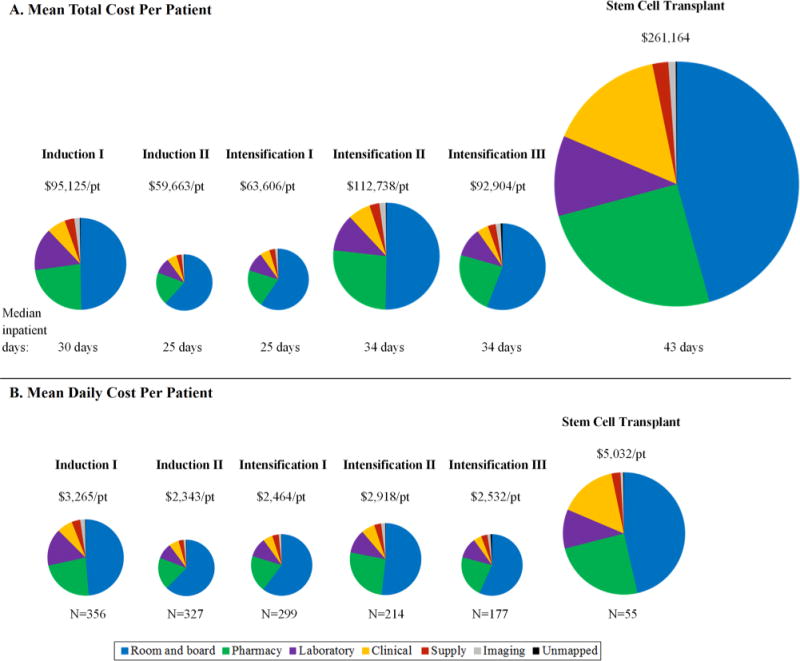
Distribution of component costs by treatment course. The treatment course-specific pie graphs are sized proportional to their mean total cost per patient (Panel A) and mean daily cost per patient (Panel B).
Stem cell transplant (SCT) was associated with the highest inpatient cost across all courses (Figures 1 and 2). Mean total cost per patient for SCT was significantly higher than for Intensification II (CR = 2.36, 95%CI: 1.96, 2.84) and Intensification III (CR = 2.84, 2.41, 3.35), due to the longer duration of hospitalization (43 vs. 34 days, P < 0.001) and corresponding increase in room and board costs as well as higher daily pharmacy and clinical costs (Fig. 2).
The mean costs per patient were significantly higher for Intensification II (CR = 1.51, 95%CI: 1.41, 1.62) and Intensification III (CR = 1.39, 95%CI: 1.25, 1.54) compared to Intensification I (Fig. 2). This difference was attributed to the difference in the number of inpatient days for each course (25 vs. 34 days, P < 0.0001) resulting in an increase in total room and board costs.
Inpatient cost for Induction I was significantly higher than for Induction II (CR = 1.60, 95%CI: 1.50, 1.70). While the higher overall cost associated with Induction I was due in part to greater room and board costs associated with a longer median duration of hospitalization (30 vs. 25 days, P < 0.0001), assessments of costs per inpatient day that account for differences in duration of hospitalization (Fig. 2, Panel B) still showed significantly greater laboratory (CR = 2.44, 95%CI: 2.10, 2.83), pharmacy (CR = 1.74, 95%CI: 1.58, 1.93), and clinical (CR = 1.57, 95%CI: 1.36, 1.81) expenditures for Induction I versus Induction II.
Component Costs
Distributions of component costs did not differ by treatment (Table II). Overall, room and board was the principle component accounting for approximately 53% of the total cost per patient of on-protocol treatment. Despite the significant variability in total cost per patient by course, the principle drivers of cost were similar across courses (Fig. 2).
TABLE II.
Component Costs Per Patient for the Entire On-Protocol Period
| Department | Overall Cohorta
|
Standard + GMTZa
|
Standarda
|
Cost Ratiob
|
P-valuec |
|---|---|---|---|---|---|
| (n = 366) | (n = 194) | (n = 172) | (95% CI) | ||
| Room & board | 187,232 (102,715) [1,673–590,803] |
186,648 (98,640) [1,673–508,144] |
188,799 (107,858) [2,041–590,803] |
1.00 (0.91, 1.13) | 0.8081 |
| Pharmacy | 80,313 (72,860) [0–656,110) |
78,463 (60,070) [0–357,428] |
82,700 (58,174) [0–656,110] |
0.95 (0.78, 1.17) | 0.6379 |
| Lab | 41,259 (27,599) [2,342–160,719] |
42,792 (28,024) [2,342–157,656] |
39,879 (27,244) [3,539–160,719] |
1.04 (0.91, 1.21) | 0.5020 |
| Clinical | 24,482 (52,871) [0–490,002] |
24,669 (52,263) [0–490,002] |
22,993 (49,770) [0–435,391] |
1.07 (0.77, 1.48) | 0.7003 |
| Supply | 10,498 (21,393) [0–216,156] |
8,762 (16,348) [0–111,747) |
12,613 (26,052) [0–216,156] |
0.84 (0.63, 1.11) | 0.2102 |
| Imaging | 6,064 (5,754) [0–44,242] |
6,132 (5,692) [0–44,241] |
6,075 (5,900) [0–33,462] |
1.00 (0.86, 1.16) | 0.9844 |
GMTZ, gemtuzumab ozogamicin.
Presented as mean (standard deviation) [range];
Cost ratio for comparison between arms (standard + GMTZ versus standard);
P-values for comparison between arms (standard + GMTZ versus standard).
Predictors of Cost Per Inpatient Day
While daily costs per patient did not differ by treatment group, sex, race, or insurance status, older age and higher risk classification were each independently associated with higher daily cost (Table III).
TABLE III.
Comparisons of Mean Adjusted Inpatient Cost Per Day Per Patient by Patient Characteristics
| Characteristic | Cost Per Day Mean (sd) | Crude Cost Ratio (95%CI) | P-valuea |
|---|---|---|---|
| Treatment Regimen | |||
| Standard + Gemtuzumab | 2,983 (1,501) | 0.95 (0.88, 1.03) | 0.2038 |
| Standard | 3,175 (1,485) | 1 (reference) | |
| AML Risk Classificationb | |||
| High | 3,563 (1,730) | 1.30 (1.13, 1.51) | 0.0004 |
| Intermediate | 3,068 (1,536) | 1.11 (1.02, 1.20) | 0.0125 |
| Low | 2,677 (940) | 1 (reference) | |
| Sex | |||
| Female | 2,953 (1,507) | 0.94 (0.87, 1.01) | 0.1043 |
| Male | 3,199 (1,486) | 1 (reference) | |
| Agec, years | |||
| <1 | 2,604 (1,346) | 1 (reference) | |
| 1 to <5 | 2,757 (1,505) | 1.04 (0.88, 1.24) | 0.6280 |
| 5 to <10 | 2,970 (1,076) | 1.09 (0.91, 1.31) | 0.3436 |
| 10 to <15 | 3,427 (1,837) | 1.29 (1.09, 1.53) | 0.0029 |
| 15+ | 3,369 (1,238) | 1.27 (1.08, 1.50) | 0.0036 |
| Race | |||
| Non-white | 2,898 (1,320) | 0.94 (0.83, 1.07) | 0.3315 |
| White | 3,139 (1,515) | 1 (reference) | |
| Insurance | |||
| Public | 2,972 (1,350) | 0.99 (0.89, 1.09) | 0.7725 |
| Other | 2,861 (1,372) | 0.95 (0.71, 1.26) | 0.7040 |
| Private | 3,179 (1,603) | 1 (reference) |
sd, standard deviation; CI, confidence interval.
P-values for comparison between arms (standard + GMTZ versus standard);
Linear trend with increasing AML risk classification, P = 0.0003;
Linear trend with increasing age, P < 0.0001.
DISCUSSION
By merging clinical trial and billing data, we are able to estimate adjusted inpatient costs of AML chemotherapy on AAML0531, a NCI-funded phase III pediatric cooperative group clinical trial. AAML0531 evaluated standard intensive chemotherapy ± GMTZ for de novo AML and found similar OS, but significantly improved EFS through a reduced risk for relapse with GMTZ compared to standard therapy alone.[1] The current analyses demonstrate that overall and course-specific inpatient treatment costs as well as the distribution of component costs were comparable between treatment arms. Thus, the addition of GMTZ to standard pediatric AML therapy improved EFS without an observed increase in inpatient treatment cost.
These results have important implications for clinical practice. In light of recent calls for the reintroduction of GMTZ in the US and its continued use in Europe and Japan, our findings provide reassurance that a decision to make GMTZ available to pediatric patients with AML will not increase health care expenditures. [12,13,15] Additionally, these data demonstrate that a biologically targeted therapy can be added to intensive chemotherapy without increasing inpatient supportive care costs. Thus, payers and national health systems may make decisions regarding provision of adjuvant biologic therapies based on the cost of the biologic itself rather than costs of associated supportive care.
Although inpatient treatment costs for AML did not differ by study arm, there was significant variability in cost by course. Mean adjusted inpatient cost per patient of Induction I was 60% higher than Induction II, a difference likely due to higher patient acuity at diagnosis with subsequent longer hospitalizations and greater pharmacy and laboratory resource utilization. There were also large differences in the inpatient cost of the post-induction courses with SCT having the highest cost followed by Intensification II (cytarabine/mitoxantrone). Mean inpatient costs for SCT were up to 3-fold greater than other post-induction chemotherapy, and averaged over $260,000 per patient, an estimate that is consistent with other reports.[16,17]
Despite the significant variability in costs across courses, the distributions of the component costs were similar. Room and board during long hospital stays was the primary cost driver in the treatment of AML, representing 45–62% of course-specific costs. Bed charges have been consistently identified as the largest contributor to direct medical costs for treatment of cancer in children and adults.[16–22] Pediatric patients with AML traditionally remain hospitalized during treatment courses until ANC recovery to reduce infectious complications. However, studies suggest that outpatient management is safe and feasible for low-risk adult and pediatric patients with AML.[23–26] Furthermore, studies assessing the outpatient management of febrile neutropenia in adult and pediatric cancer populations and home chemotherapy treatment for pediatric ALL demonstrate reduced costs and improved quality of life for patients and their families.[27–31] In this context, our results suggest that judicious use of outpatient management may have distinct advantages, including cost savings. Work is ongoing to determine the relative risks and potential costs savings of outpatient AML management.
Consistent with prior cost prediction analyses, we observed older age and higher risk classification to be associated with higher daily cost.[16,17] These findings are compatible with the increased treatment-related mortality in older patients and use of SCT in high risk patients, and thus provide further validation to the cost data. The absence of differences in inpatient cost by race, gender, and insurance status is heartening particularly given the previously reported racial disparities in pediatric AML outcomes and extensively described impact of race, gender, and insurance status on outcomes in adults with malignancy.[32–37]
The merger of PHIS cost data with an NCI-funded randomized trial establishes an enhanced data set that leverages the specific strengths of the individual sources. First, COG clinical trial data, particularly diagnostics, risk classification, and remission status, are unsurpassed in quality. Second, inpatient costs from PHIS are very reliable as these data are used routinely by member hospitals for billing, fiscal planning, and practice optimization.[38] Finally, the merging process and estimation of patient level costs is more efficient and potentially more broadly applicable than methods previously used by others, such as applying single institution cost data to multicenter trials or abstraction of costs from case report forms.[39,40]
Despite these strengths, several limitations should be recognized. Although patients with PHIS inpatient cost data were a representative sample of the AAML0531 trial, the lack of cost data on all study subjects reduces the precision of cost estimates. Non-PHIS institutions, specifically non-freestanding pediatric hospitals and non-US sites, may have different cost structures than PHIS institutions. While pediatric AML therapy is administered in the inpatient setting and treatment complications typically require hospitalization, outpatient costs are potentially important and are unmeasured in this analysis. Work is ongoing to merge COG data with other administrative databases. These data sources will expand the number of patients with cost data and allow for assessment of treatment costs in both the inpatient and outpatient settings. Finally, these analyses are limited to direct hospital costs and do not include physician fees, procedure costs, or other costs carried by patients and families. Further methodological work is needed to develop an efficient process for the acquisition of such data.
This study demonstrates the feasibility of merging NCI-funded cooperative oncology group clinical trial data with inpatient costs from a secondary source and allowed for the comparison of the costs across AAML0531 trial treatment arms. The addition of GMTZ to standard chemotherapy did not impose additional supportive care or inpatient cost, while improving EFS. The methodology employed in this study can be adapted to other NCI trials. Additionally, these results can inform clinicians and hospital administrators on the components of inpatient costs. Specifically for children with AML, these data indicate that inpatient costs are driven by room/board and pharmacy charges and that these costs are highest in SCT. Our findings also suggest that AML therapy costs may be decreased with selective use of outpatient management.
Supplementary Material
Acknowledgments
National Institutes of Health; Grant number: NIH R01 CA165277 (PI: Aplenc).
Abbreviations
- ALL
acute lymphblastic leukemia
- AML
acute myeloid leukemia
- ANC
absolute neutrophil count
- CI
confidence intervals
- COG
Children’s Oncology Group
- CR
cost ratios
- EFS
event free survival
- GMTZ
gemtuzumab ozogamicin
- HR
hazard ratios
- NCI
National Cancer Institute
- OS
overall survival
- PHIS
Pediatric Health Information Systems
- RCC
ratio of cost-to-charge
- SCT
stem cell transplant
- US
United States
Footnotes
Additional supporting information may be found in the online version of this article.
Conflicts of Interest Statement: T. A. A. reported serving as a consultant to Hologic and Swiss Precision Diagnostic. B. F. reported research funding from Merck and Pfizer unrelated to this project. R. K. reported serving as a member of a CVS Caremark advisory board regarding subject matters outside of this study. The remaining authors declare no conflicts of interest.
References
- 1.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, Hirsch BA, Kahwash SB, Heerema-McKenney A, Winter L, Glick K, Davies SM, Byron P, Smith FO, Aplenc R. Gemtuzumab Ozogamicin in children and adolescents with de novo acute myeloid leukemia (AML) improves event-free survival by reducing relapse risk - Results from the randomized Phase III Children’s Oncology Group trial, AAML0531l. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, Degar B, Airewele G, Raimondi SC, Onciu M, Coustan-Smith E, Downing JR, Leung W, Pui CH, Campana D. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, Jónsson ÓG, Lausen B, Palle J, Zeller B, Nordic Society of Paediatric Haematology and Oncology (NOPHO) Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: Results from NOPHO-AML 2004. Blood. 2012;120:978–984. doi: 10.1182/blood-2012-03-416701. [DOI] [PubMed] [Google Scholar]
- 4.Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J, Sander A, Schrauder A, von Stackelberg A, Starý J, Reinhardt D. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: Results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 5.Kaspers GJL, Creutzig U. Pediatric acute myeloid leukemia: International progress and future directions. Leukemia. 2005;19:2025–2029. doi: 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 6.Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K, United Kingdom Childhood Leukaemia Working Group and the Dutch Childhood Oncology Group Results of a randomized trial in children with acute myeloid leukaemia: Medical research council AML12 trial. Br J Hematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 7.Russell HV, Panchal J, Vonville H, Franzini L, Swint JM. Economic evaluation of pediatric cancer treatment: A systematic literature review. Pediatrics. 2013;131:e273. doi: 10.1542/peds.2012-0912. [DOI] [PubMed] [Google Scholar]
- 8.Kasteng F, Sobocki P, Svedman C, Lundkvist J. Economic evaluations of leukemia: A review of the literature. Int J Technol Assess Health Care. 2007;23:43–53. doi: 10.1017/S0266462307051562. [DOI] [PubMed] [Google Scholar]
- 9.Barr RD, Feeny D, Furlong W. Economic evaluation of treatments for cancer in childhood. Eur J Cancer. 2004;40:1335–1344. doi: 10.1016/j.ejca.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 11.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR. A phase 3 study of gemtuzumab ozogamicin during induction and post consolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, Estey EH, Dombret H, Chevret S, Ifrah N, Cahn JY, Récher C, Chilton L, Moorman AV, Burnett AK. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukemia: A meta-analysis of individual patient data from randomized controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharfan-Dabaja MA. A new dawn for gemtuzumab ozogamicin? Lancet Oncol. 2014;15:913–914. doi: 10.1016/S1470-2045(14)70289-X. [DOI] [PubMed] [Google Scholar]
- 14.Aplenc R, Fisher BT, Huang YS, Li Y, Alonzo TA, Gerbing RB, Hall M, Bertoch D, Keren R, Seif AE, Sung L, Adamson PC, Gamis A. Merging of the National Cancer Institute -funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: A report from the Children’s Oncology Group. Pharmacoepidemiol Drug Saf. 2012;21:37–43. doi: 10.1002/pds.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravandi F, Estey EH, Appelbaum FR, Lo-Coco F, Schiffer CA, Larson RA, Burnett AK, Kantarjian HM. Gemtuzumab ozogamicin: Time to resurrect? J Clin Oncol. 2012;30:3921–3923. doi: 10.1200/JCO.2012.43.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YF, Lairson DR, Chan W, Du XL, Leung KS, Kennedy-Nasser AA, Martinez CA, Gottschalk SM, Bollard CM, Heslop HE, Brenner MK, Krance RA. The costs and cost-effectiveness of allogeneic peripheral blood stem cell transplantation versus bone marrow transplantation in patients with acute leukemia. Biol Blood Marrow Transplant. 2010;54:1272–1281. doi: 10.1016/j.bbmt.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenman MB, Vik T, Hui SL, Breitfeld PP. Hospital resource utilization in childhood cancer. J Pediatr Hematol Oncol. 2005;27:295–300. doi: 10.1097/01.mph.0000168724.19025.a4. [DOI] [PubMed] [Google Scholar]
- 18.Leunis A, Blommestein HM, Huijgens PC, Blijlevens NM, Jongen-Lavrencic M, Uly-de Groot CA. The costs of initial treatment for patients with acute myeloid leukemia in the Netherlands. Leukemia Res. 2013;37:245–250. doi: 10.1016/j.leukres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Fedele P, Avery S, Patil S, Spencer A, Haas M, Wei A. Health economic impact of high dose versus standard dose cytarabine induction chemotherapy for acute myeloid leukemia. Internal Med. 2014 doi: 10.1111/imj.12478. [DOI] [PubMed] [Google Scholar]
- 20.Bloom BS, Knorr RS, Evans AE. The epidemiology of disease expenses. JAMA. 1985;253:2393–2397. [PubMed] [Google Scholar]
- 21.Berman E, Little C, Teschendorf B, Jones M, Heller G. Financial analysis of patients with newly diagnosed acute myelogenous leukemia on protocol therapy or standard therapy. Cancer. 2002;95:1064–1070. doi: 10.1002/cncr.10805. [DOI] [PubMed] [Google Scholar]
- 22.Majhail NS, Mothukuri JM, Macmillan ML, Verneris MR, Orchard PJ, Wagner JE, Weisdorf DJ. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller T1, Nielsen OJ, Welinder P, Dünweber A, Hjerming M, Moser C, Kjeldsen L. Safe and feasible outpatient treatment following induction and consolidation chemotherapy for patients with acute leukemia. Eur J Hematol. 2010;84:316–322. doi: 10.1111/j.1600-0609.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- 24.Walter RB, Lee SJ, Gardner KM, Chai X, Shannon-Dorcy K, Appelbaum FR, Estey EH. Outpatient management following intensive induction chemotherapy for myelodyplastic syndromes and acute myeloid leukemia: A pilot study. Hematologica. 2011;96:914–917. doi: 10.3324/haematol.2011.040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue S, Khan I, Mushtaq R, Carson D, Saah E, Onwuzurike N. Postinduction supportive care of pediatric acute myelocytic leukemia: Should patients be kept in the hospital? Leukemia Research and Treatment. 2014 doi: 10.1155/2014/592379. Article ID 592379:10.1155/2014/592379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba H, Gaur AH, Cao X, Flynn PM, Pounds SB, Avutu V, Marszal LN, Howard SC, Pui CH, Ribeiro RC, Hayden RT, Rubnitz JE. Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia. Cancer. 2014;120:1985–1992. doi: 10.1002/cncr.28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Cumsille MA, O’Ryan ML, Payá E, Salgado C, Silva P, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. J Clin Oncol. 2004;22:3784–3789. doi: 10.1200/JCO.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 28.Teuffel O, Amir E, Alibhai SM, Beyene J, Sung L. Cost-effectiveness of outpatient management for febrile neutropenia in children with cancer. Pediatrics. 2011;127:e279–e286. doi: 10.1542/peds.2010-0734. [DOI] [PubMed] [Google Scholar]
- 29.Hendricks AM, Loggers ET, Talcott JA. Costs of home versus inpatient treatment for fever and neutropenia: analysis of a multicenter randomized trial. J Clin Oncol. 2011;29:3984–3989. doi: 10.1200/JCO.2011.35.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Close P, Burkey E, Kazak A, Danz P, Lange B. A prospective, controlled evaluation of home chemotherapy for children with cancer. Pediatrics. 1995;95:896–900. [PubMed] [Google Scholar]
- 31.Stevens B, Croxford R, McKeever P, Yamada J, Booth M, Daub S, Gafni A, Gammon J, Greenberg M. Hospital and home chemotherapy for children with leukemia: A randomized cross-over study. Pediatr Blood Cancer. 2006;47:285–292. doi: 10.1002/pbc.20598. [DOI] [PubMed] [Google Scholar]
- 32.Aplenc R, Alonzo TA, Gerbing RB, Smith FO, Meshinchi S, Ross JA, Perentesis J, Woods WG, Lange BJ, Davies SM. Ethnicity and survival in childhood acute myeloid leukemia: A report from the Children’s Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 34.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2009;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung KW, Park S, Shin A, Oh CM, Kong HJ, Jun JK, Won YJ. Do female cancer patients display better survival rates compared to males? Analysis of the Korean National Registry data 2005–2009. PLoS ONE. 2012;7:e52457. doi: 10.1371/journal.pone.0052457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2:403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, Siegel R, Stewart A, Jemal A. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 38.Kittle K, Currier K, Dyk L, Newman K. Using a pediatric database to drive quality improvement. Semin Pediatr Surg. 2002;11:60–63. doi: 10.1053/spsu.2002.29367. [DOI] [PubMed] [Google Scholar]
- 39.Bennett CL, Stinson TJ, Tallman MS, Stadtmauer EA, Marsh RW, Friedenberg W, Lazarus HM, Kaminer L, Golub RM, Rowe JM. Economic analysis of a randomized placebo-controlled phase III study of granulocyte macrophage colony stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia. Ann Oncol. 1999;10:177–182. doi: 10.1023/a:1008318930947. [DOI] [PubMed] [Google Scholar]
- 40.Bennett CL, Stinson TJ, Lane D, Amylon M, Land VJ, Laver JH. Cost analysis of filgrastim for the prevention of neutropenia in pediatric T-Cell leukemia and advanced lymphoblastic lymphoma: A case for prospective economic analysis in cooperative group trials. Med Pediatr Oncol. 2000;34:92–96. doi: 10.1002/(sici)1096-911x(200002)34:2<92::aid-mpo3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


