Abstract
Background
Neurofibromatosis type 1 (NF1) is a genetic disorder that predisposes affected individuals to formation of benign neurofibromas, peripheral nerve tumors that can be associated with significant morbidity. Loss of the NF1 Ras-GAP protein causes increased Ras-GTP, and we previously found that inhibiting MEK signaling downstream of Ras can shrink established neurofibromas in a genetically engineered murine model.
Procedures
We studied effects of MEK inhibition using 1.5 mg/kg/day PD-0325901 prior to neurofibroma onset in the Nf1 flox/flox;Dhh-Cre mouse model. We also treated mice with established tumors at 0.5 and 1.5 mg/kg/day dosees of PD-0325901. We monitored tumor volumes using MRI and volumetric measurements, and measured pharmacokinetic and pharmacodynamic endpoints.
Results
Early administration significantly delayed neurofibroma development as compared to vehicle controls. When treatment was discontinued neurofibromas grew, but no rebound effect was observed and neurofibromas remained significantly smaller than controls. Low dose treatment of mice with PD-0325901 resulted in neurofibroma shrinkage equivalent to that observed at higher doses. Tumor cell proliferation decreased, although less than at higher doses with drug. Tumor blood vessels per area correlated with tumor shrinkage.
Conclusions
Neurofibroma development was not prevented by MEK inhibition, beginning at 1 month of age, but tumor size was controlled by early treatment. Moreover, treatment with PD-0325901 at very low doses may shrink neurofibromas while minimizing toxicity. These studies highlight how genetically engineered mouse models can guide clinical trial design.
Keywords: NF1, neurofibroma, nerve, MEK, therapy
Introduction
Neurofibromatosis 1 (NF1) is one of the most common autosomal dominant single-gene disorders affecting neurological function in humans, as NF1 mutations are found in about 1 of 3,500 births (1). About 50% of individuals with NF1 inherit disease through the germ line, while in others the disease is a result of spontaneous mutations (2, 3). NF1 is caused by germ line mutations in NF1, a gene that inactivates the RAS pathway (4). NF1 disease is clinically characterized by the presence of benign peripheral nerve tumors, called neurofibromas, containing Schwann cells with biallelic mutations in the NF1 gene (5), as well as other tumor and non-tumor manifestations (6, 7).
Approximately 25–30% of children with NF1 develop benign plexiform neurofibromas (PNFs) associated with large nerve trunks, which can cause significant morbidity when they compress vital structures (8). Plexiform neurofibromas can undergo malignant transfprmation into MPNST (9). Currently, there is no therapy available to shrink or to prevent growth of neurofibromas, other than surgical dissection. However, surgery is rarely curative and often impossible because neurofibromas are part of peripheral nerves, and thus many neurofibromas are inoperable. In addition, recurrence rates of resected neurofibromas are high and repeated surgery can be required. Classic chemotherapy and radiation do not slow neurofibroma growth. Clinical trials are therefore ongoing in an attempt to find effective remedies using targeted therapeutics. Recent clinical trials used volumetric MRI analysis to evaluate response and progression (10) as standard response criteria for malignant solid tumors is not applicable for the slow growing and complex shaped plexiform neurofibromas. Response is defined as a ≥20% decrease in tumor volume compared to baseline, and progression is defined as a ≥20% increase in tumor volume compared to baseline. Minor plexiform neurofibroma shrinkage in a subset of patients has been observed in clinical trials with pegintron (11) and imatinib (12) and some delay in time to progression with sirolimus (13).
Pre-clinical trials using mouse models may expedite identification of the most promising agents to test in human clinical trials. Based on this rationale, we and others have developed mouse neurofibroma models for preclinical testing (14, 15). In Nf1 mouse models, including the Nf flox/flox;DhhCre mouse model, all mice form neurofibromas which are histologically identical to human neurofibromas. Thus, neurofibromas are comprised of Schwann cells, axons, fibroblasts, perineurial cells, mast cells, macrophages and blood vessels (16–18). Mouse tumors can, like human neurofibromas, be monitored using volumetric measurement after MRI imaging (14, 19).
Because the NF1 protein encodes a Ras-GAP protein, NF1 loss results in high Ras-GTP (20, 21). Therefore, NF1 research has focused intensively on testing inhibitors in the Ras signaling pathway, including the Ras-MAPK cascade. PD-0325901 is a potent allosteric inhibitor of MEK1 and MEK2 that is currently in clinical cancer trials (22–24). Of 70 tested kinases, PD-0325901 blocked MEK1, with the related MEK5 affected at 10-fold higher concentrations; no other kinases were affected (25). PD-0325901 is specific because it is an allosteric, not a kinase active site, inhibitor. Preliminary studies reported adverse effects after prolonged treatment of patients with advanced cancers with ≥10 mg twice daily PD-0325901 (22, 23, 26); the current clinical dose is 8 mg twice daily in humans, equivalent to 1.5 mg/kg daily in mice. In the Nf flox/flox; DhhCre mouse model, PD-0325901 at 1.5, 5, and 10 mg/kg/day shrank neurofibromas in >80% of treated mice as monitored using volumetric measurements (27). At present, there are two ongoing clinical trials of MEK inhibitors to treat neurofibromas, and a third trial close to opening. (http://www.clinicaltrials.gov/NCT01362803) and NCT0296471 (PD); NCT02124772 (GSK 116540; Trametinib).
Patients with NF1 and plexiform neurofibroma may require prolonged dosing to decrease tumor size and prevent tumor growth. We therefore sought to define a minimum dose required to retain efficacy in preclinical models, in an attempt to inform clinical trials and ultimately help decrease the risk of potential toxicities in clinical testing. Furthermore, we tested whether we could prevent neurofibromas, if we administered MEK inhibitor to mice before tumors formed, and whether tumors would rebound after treatment. Here we report that we can significantly de-escalate drug concentrations and retain efficacy, and that tumors are not prevented by treatment beginning at 1 month of age, but are significantly smaller in size compared to vehicle controls. We also observed that tumors did not show accelerated growth after drug withdrawal.
Material and Methods
Mouse husbandry
We housed mice in temperature- and humidity-controlled facilities on a 12-hr dark–light cycle with free access to food and water as described. The animal care and use committee of Cincinnati Children’s Hospital Medical Center approved all animal use. The mice were in a mixed C57/129/FVBN strain background and were interbred to obtain the required genotype. Mouse genotyping was carried out as described (18).
PD-0325901 Dosing
We obtained PD-0325901 from Pfizer (New York City, NY). Mice were administered vehicle control (0.5% [w/v] methylcellulose solution with 0.2% [v/v] polysorbate 80 [Tween 80] or PD-0325901 in the same vehicle by oral gavage for 60–90 days, at doses noted in the text.
MRI and Neurofibroma Volumetric Measurement
MRI in anesthetized Nf1 flox/flox; DhhCre mice using a 7T Bruker Biospec (Ettlingen,Germany) and neurofibroma volumetric measurement were conducted as previously described (14, 27).
Plasma collection
We anesthetized tumor bearing mice with iso-fluorane and collected blood by cardiac puncture, prior to mouse sacrifice and harvest of neurofibromas. Blood samples were transferred to anti-clotting EDTA tubes on ice, and centrifuged within 30 minutes of collection at 4°C for 15 minutes at 14,000g. Plasma was transferred to 1.5ml Eppendorf tubes on ice and stored at −80°C until analysis.
Pharmakokinetic analysis
PD-0325901 was quantified in mouse plasma using midazolam (MDZ) as the internal standard and HPLC-MS/MS (Thermo TSQ Quantum Ultra). PD-0325901 and MDZ were removed from plasma by protein precipitation with acetone. PD-0325901 and MDZ were separated by a gradient mobile phase (acetonitrile: 5mM ammonium acetate) with a C-18 column (Inertsil® ODS-3; 4.6 × 150 mm, 5 μm). The Q1/Q3’s (in positive mode) for PD-0325901 and MDZ were 483/249 and 326/291, respectively. The lower limit of quantification was 1 ng/mL using 20 μL of mouse plasma. Pharmacokinetic parameters for PD-0325901 including area under the curve (AUC) and the elimination rate constant, kel, were estimated (from average data) using non-compartmental methods with add-ins on Excel®.
Pharmacadynamic analysis
Tumors were harvested from mice at several different time points after final dose and snap frozen immediately in liquid nitrogen and stored subsequently at −80°C until further use. The day prior to analysis tumors were defrosted on wet ice, lysed in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Halt™ Protease & Phosphatase Single-Use Inhibitor Cocktail 100X, Thermo Scientific, Florence, KY) using the Qiagen Tissue Ruptor. Protein concentration was estimated using Coomassie® Plus Protein Assay Reagent (Bio-Rad, Berkeley, Ca). Proteins (1μg/μl) were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis on 7.5% tris-glycine gel (Bio-Rad, Berkeley, Ca) and transferred to polyvinylidene diflouride membrane by electrophoresis. Membranes were blocked with 5% nonfat milk in 0.1% TBST to minimize nonspecific binding. Antibodies recognizing pERK at a dilution of 1:5000 (# 4370S, Cell Signaling, Danvers, MA) and ERK at a dilution of 1:5000 (#4695S, Cell Signaling, Danvers, MA) were probed ON at 4°C, followed the next by incubation with appropriate secondary HRP conjugated antibodies for 1 hour at RT. Antibody binding to the membrane was visualized using the Immobilon™ Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA).
Tissue sections, histology and immunohistochemistry
Formalin-fixed neurofibromas were paraffin embedded. Paraffin blocks were sectioned at 5μm thickness; sections were baked at 60°C for 1 h and air dried. Paraffin sections were deparaffinized, re-hydrated through a graded series of alcohol to double distilled water and transferred to 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Slides were boiled for 10 minutes in citrate buffer, cooled at room temperature for 30 minutes, and then rinsed in water twice and PBS 3 times. Sections were quenched with 3% hydrogen peroxide to block endogenous peroxidases for 10 minutes, rinsed in PBS, and blocked in 10% normal goat serum with 0.3% Triton X-100. Sections were incubated overnight in primary antibody diluted in blocking medium; rabbit pERK (#4370, 1:200, Cell Signaling, Boston, MA), rabbit Ki67 (1:10,000; Leica Biosystems, Buffalo Grove, IL), rabbit anti-Iba1 (Wako Chemicals, Richmond, VA, 1:2,000) and rat MECA 32 (1:10; Developmental Studies Hybridoma Bank, Iowa City, IA). Sections were then incubated in appropriate biotinylated secondary antibodies; goat anti-rabbit or goat anti-rat (1:200; Vector). Some sections were counterstained with Harris hematoxylin. Mast cells were counted at 40X magnification in 4 high power fields per section, 3 sections per animal in at least 3 animals per condition. Iba1 positive cells were counted as a percentage of total numbers of cells in 3 sections per animal at 40X in a minimum of 3 animals per condition. Numbers of MECA+ blood vessels were counted in 5 × 20X fields per neurofibroma (n = 5) for each condition. Statistical comparisons between control and neurofibroma samples were conducted using Student’s t test or One Way Anova. All microscopic images were acquired with Openlab software suites on a Zeiss Axiovert 200.
Electron Microscopy
Mice were perfused (intracardially) with Karnovsky’s fixation solution (3% paraformaldehyde and 3% glutaraldehyde in 0.1M phosphate buffer, pH 7.4 to 7.6). Saphenous nerves were dissected, embedded, and evaluated as described (28).
Statistical Analysis
Neurofibroma shrinkages from the last pre-treatment value to the post treatment value were compared using Fisher’s exact test. Neurofibroma growth was compared by analyzing log transformed tumor volume data with a random effects model using the SAS mixed procedure (14). In the prevention model differences in axonal ensheathing by Schwann cells, cell proliferation, number of macrophages, number of mast cells and blood vessel quantification were calculated using an unpaired, two-tailed, Student’s t test. In the low dose treatment paradigm statistical differences between groups were calculated using an Ordinary one way ANOVA.
Results
Prevention treatment with PD-0325901 significantly reduces neurofibroma size
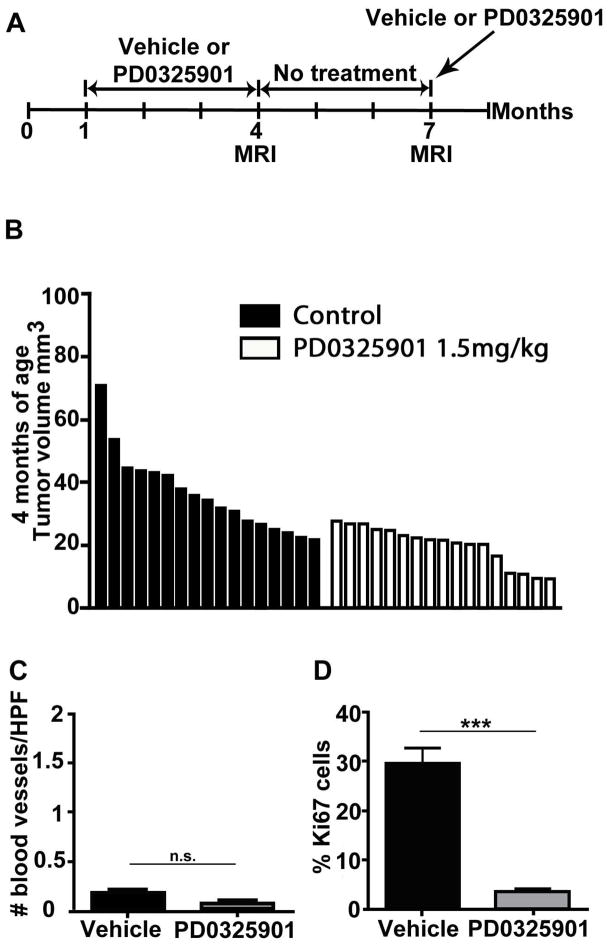
Nf1 flox/flox; Dhh-Cre mice (n=23) were treated for 90 days with either vehicle (n=6) or PD-0325901 1.5 mg/kg (n=17). All mice in the PD-0325901 treatment group survived without significant weight loss. Treatment began when mice were 1 month of age, when tumors are not yet present, and neurofibroma volumes were measured at 4 months of age, at the end of the treatment period by which time neurofibromas are detectable. To increase the control group for statistical power, neurofibroma volumes of untreated age-matched controls were included (n=11) (Fig. 1A). Neurofibroma volumes for the PD-0325901 treated group were significantly smaller compared to the control group (p=0.0003) (Fig. 1B).
Fig. 1. Early exposure to MEK inhibitor delays neurofibroma growth.
(A) Treatment paradigm. Mice were treated from 1 to 4 months of age with either vehicle or PD-0325901 1.5 mg/kg, left untreated from 4 to 7 months of age and for a brief period (12 days) some mice were re-exposed to PD-0325901 or vehicle for pharmacodynamics (PD) assessment. (B) Waterfall plot showing neurofibroma volume in mice at 4 months of age. Volumes of neurofibromas in mice treated with PD-0325901 are on average significantly smaller than controls. Each bar represents the volume of neurofibroma in an individual mouse. The y-axis shows the neurofibroma volume in mm3. (C, D) Immunohistochemistry at 4 months of age; Blood vessel recruitment was not affected by PD-0325901 when compared to vehicle treated neurofibroma (C). A significant decrease in cell proliferation was found after 90 days of treatment with PD-0325901 (D).
We stained neurofibroma sections to evaluate underlying processes involved in neurofibroma growth and development. Firstly, we quantified effects of PD-0325901 on blood vessels per area, by staining with anti-MECA 32 (an endothelial cell marker). Very few blood vessels had invaded neurofibromas at the early 4 month time point, and there was no significant change in the number of blood vessels when mice were treated with PD-0325901 (Fig. 1C). Treatment with PD-0325901 highly significantly decreased neurofibroma cell proliferation, assessed by Ki-67 staining after 90 days of drug exposure (p=0.0002) (Fig. 2G). In contrast, no significant differences were found in the number of Iba1 positive macrophages or numbers of metachromatic mast cells per high power field (HPF) in PD-0325901 treated neurofibromas (not shown).
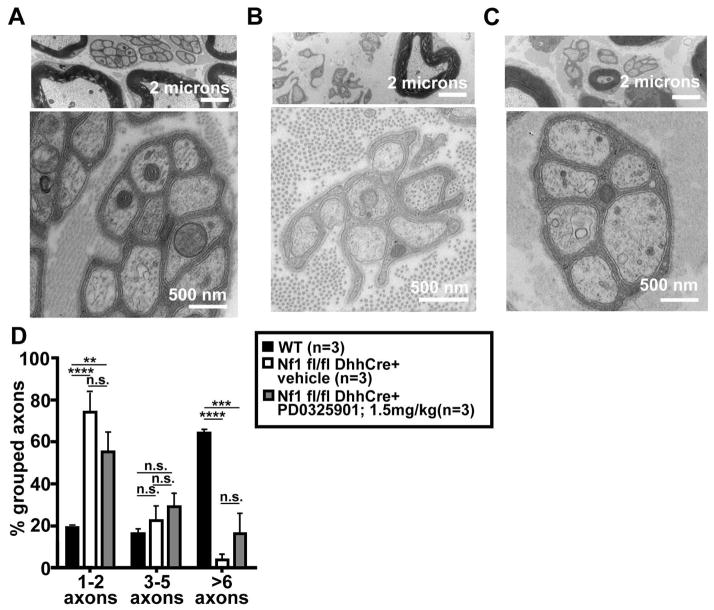
Fig. 2. Representative electron micrograph of saphenous nerves.
Untreated wild type (A), vehicle treated Nf1 flox/flox; Dhh-Cre (B) and PD-0325901 treated Nf1 flox/flox; Dhh-Cre (C) mice. The higher magnification in (A) indicates a well-organized un-myelinated Schwann cell ensheathing multiple small axons, cut in cross section. The low magnification micrographs in (B) and (C) show unorganized non-myelinated Schwann cells in Nf1 flox/flox; Dhh-Cre mice treated with vehicle (B) or PD-0325901 (C). At higher magnification (C, bottom) one can appreciate a rare bundle with improved Remak bundle organization. Percentage of grouped axons in wild type control, Nf1 flox/flox; Dhh-Cre vehicle treated control mice and Nf1 flox/flox; Dhh-Cre PD-0325901 treated mice at 4 months of age (D). A high percentage (6 or more) of grouped axons per Schwann cell signifies normal Remak bundle organization.
Neurofibromas are characterized by dissociation between Schwann cells and axons in nerve Remak bundles that are visible by electron microscopy. In normal 4 month old saphenous nerves 60% of unmyelinated Schwann cells ensheath >6 axons (Fig. 2A, while in vehicle treated Nf1flox/flox; DhhCre mice at the same age only 5% of non-myelinating Schwann cells ensheathed >6 axons (Figure 2C). To determine if this aspect of neurofibroma formation can be rescued by MEK inhibition, drug treated mice (n=3/group) were sacrificed at 4 months of age (Fig. 2B). The percentage of Schwann cells ensheathing >6 axons was not significantly improved by treatment with MEK inhibitor for 3 months, and remained significantly different from wild type mouse saphenous nerve (vehicle or PD-0325901 treated; p<0.0001 and p<0.005 respectively (n=3 per group) (Fig. 2D). However, the results trended towards a slight improvement for the treatment group, and rare Remak bundles showed complete wrapping of small axons (Figure 4C, enlargement). Thus preventative PD-0325901 treatment, despite slowing neurofibroma growth, does not rescue the ensheathment defect characteristic of Nf flox/flox; Dhh-Cre nerves.
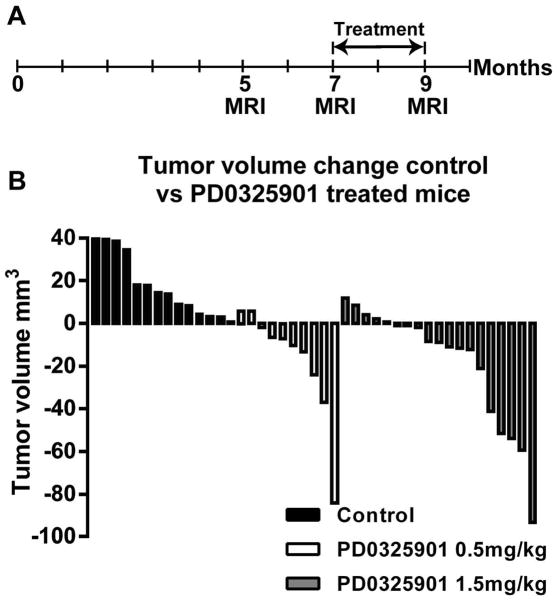
Fig. 4. Low Dose PD-0325901 shrinks neurofibromas.
(A) Mice were MRI scanned at 5 and 7 months of age to determine neurofibroma growth rate. Mice were then treated for 2 months with vehicle, or PD-0325901 (0.5 mg/kg or 1.5 mg/kg), followed by a final scan at 9 months of age. (B) Change in neurofibroma volume from 7 to 9 months of age in mice exposed to vehicle, PD-0325901 0.5 mg/kg or PD-0325901 1.5 mg/kg. Each bar displays the absolute difference in neurofibroma volume between 7 and 9 months in a single mouse based on serial MRI imaging. A positive value indicates neurofibroma size increase; a negative value indicates neurofibroma shrinkage. Neurofibroma volume remained significantly lower both PD-0325901 treatment groups compared to vehicle treated controls.
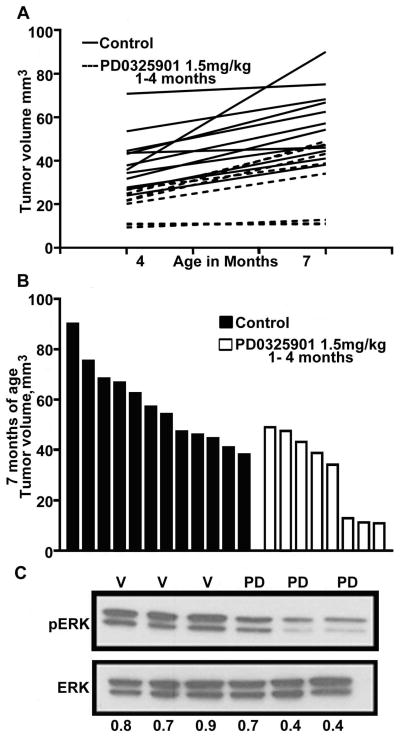
To determine whether there are rebound effects on neurofibroma volume after removal of PD-0325901, 8 mice treated with PD-0325901 from 1 – 4 months of age were held off drug for three months, and then re-imaged by MRI at 7 months of age. We compared the growth rates of neurofibromas in the PD-0325901 group to neurofibroma growth rates of untreated control mice. Growth rates remained the same (p=0.9576) between the controls and the PD-0325901-treated group, signifying that there was no rebound i.e. no acceleration in growth; in other words, tumors did not grow faster than expected for this model system, but rather reverted to their normal trajectories. In addition, we observed that a subset of mice (n=3) in the PD-0325901 treatment group never developed measureable neurofibromas; the significance of this result is unclear based on the small numbers analyzed (Fig. 3A). Overall, neurofibroma volumes in the PD-0325901 group remained significantly smaller (p=0.0008) than expected for mice in this model system at 7 months of age (Fig. 3B).
Fig. 3. Neurofibromas resume growing when PD-01325901 is removed.
(A) Neurofibroma volumes at 4 and 7 months of age in mice either treated with PD-0325901 from 1 – 4 months of age (n=8), or vehicle treated/control mice (n=8). (B) Neurofibroma volume at 7 months of age, from mice treated from 1 – 4 months of age. Each bar represents the volume of neurofibromas in an individual mouse. Volumes of treated mice differ from those of untreated mice (p=0.0001). (C) Pharmacodynamic readout. Tumors lysates were prepared from mice treated with PD-0325901 for 90 days at 1.5 mg/kg, taken off drug for 90 days and subsequently treated daily for 12 days. Tumors were harvested 3–4 hours after final dose. Top: phosphorylated ERK (pERK), bottom: total Erk (ERK), V= vehicle treated tumor, PD = PD-0325901 1.5 mg/kg treated tumors.
We determined whether mice would decrease P-ERK on treatment with a MEK inhibitor after being off drug for 3 months. Mice were put back on treatment PD-0325901 at 1.5 mg/kg/day; 4 mice were treated with vehicle, while another 5 mice were treated with PD-0325901. Mice were sacrificed after 12 days of treatment and tumor lysates analyzed by immunohistochemistry (not shown) or western blotting (Fig. 3C). Down regulation of phosphorylated Erk in the PD-0325901 group was detectable 3 – 4 hours after the final dose when compared to the vehicle treated mice (Fig. 3C). Thus mice do not become resistant to MEK inhibition after one course of therapy.
Low dose treatment with PD-0325901 significantly reduces neurofibroma volume
Clinical trials have shown side effects with prolonged high-dose administration of MEK inhibitors (29). It is unknown whether toxicity will be problem with prolonged administration of lower doses. We sought to determine if lower doses than those being used in current studies might be effective in shrinking neurofibromas. Nf flox/flox; DhhCre mice (n=18) were treated with either vehicle (n=4), PD-0325901 0.5 mg/kg (n=10) or PD-0325901 1.5 mg/kg (n=4). The 1.5 mg/kg group served as an internal positive control group, allowing for comparison with previous treatment trials (27). Mice were scanned using MRI at 5 months of age, and re-scanned at 7 months of age to establish growth rates in individual mice. Mice were then treated for 2 months and finally re-imaged 9 months of age (Fig. 4A). All mice survived the treatment period without significant weight loss or other morbidity.
Eight of 10 mice treated with PD-0325901 at 0.5 mg/kg daily had neurofibromas that shrank over the treatment period, as compared to in-group vehicle treated mice or historical controls, in which neurofibromas continued to grow. This result was significant (Fig. 4B); p<0.001). The extent of shrinkage seen in the 0.5 mg/kg PD-0325901 group is comparable to the shrinkage of tumors from mice treated in-experiment with PD-0325901 at 1.5 mg/kg, and those treated with PD-0325901 1.5 mg/kg in Jessen et al. 2013 (Fig. 4B). We found no difference (p=0.69) in tumor growth trajectory between 7 and 9 months when we compared mice exposed to 0.5, 1.5, 5 or 10 mg/kg PD-0325901.
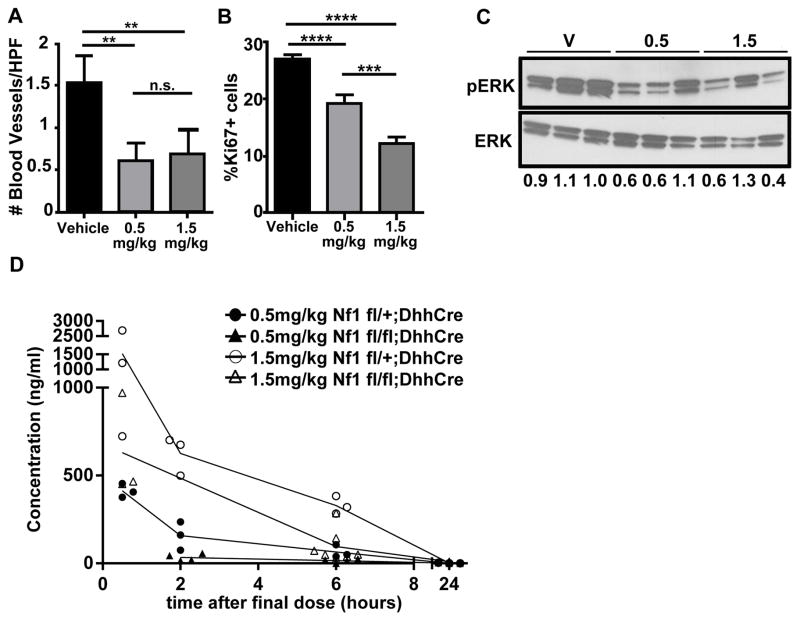
We analyzed tissue sections to define cellular effects in mice treated with various levels of PD-0325901. Numbers of blood vessels were highest in the vehicle-treated group and lower in both 0.5 and 1.5 mg/kg group, confirming previous studies using this paradigm (27). Importantly, there was no significant difference on blood vessels/area between the PD-0325901 groups (Fig. 5A). Ki67-positive proliferating cells were significant different between controls and PD-0325901 treated mice (p<0.0001; vehicle vs. 0.5 mg/kg and 1.5 mg/kg respectively). Mice exposed to 1.5 mg/kg dose showed fewer proliferating cells than the 0.5 mg/kg treated group (p<0.0005) (Fig. 5B). Thus, PD-0325901 effects on blood vessels trend with efficacy (tumor shrinkage), and cell proliferation trends with the administered dose of PD-0325901. We also confirmed that MEK remained inhibited in tumor bearing mice after the final dose of PD-0325901. Pharmacodynamic (PD) analysis used western blotting as a read out for the MEK substrate ERK. We probed protein lysates from tumors (n=3/group) collected 6 hours after final dose of PD-0325901 for phosphorylated ERK (pERK) and total ERK. P-ERK, quantified as a fraction of total ERK, was similarly decreased in tumors harvested from mice treated with 0.5 mg/kg and 1.5 mg/kg PD-0325901 (Fig. 5F). Thus PD-0325901 inhibits MEK in neurofibromas, even at low doses.
Fig. 5. MEK inhibitor retains efficacy at low doses.
(A) Blood vessels per field. Immunohistochemistry with the Meca 32 antibody was used to highlight blood vessels. Vessels per field were reduced in the 0.5 mg/kg, showing a significant difference from wild type (p<0.05); a reduction in the 1.5 mg/kg group did not reach significance in this experiment. (B) Percentage of Iba1+ macrophages. (C) Mast cell number counting. (D) Percentage of Ki67+ proliferating cells. (E) Pharmakodynamic analysis. Blood from PD-0325901 (0.5 mg/kg and 1.5 mg/kg) treated non-tumor bearing (Nf fl/+; DhhCre) mice collected at 0.5, 2, 6, and 24 hours after a single dose of drug (n=3 per time point/dose). Blood was also collected from tumor bearing (Nf1 flox/flox; DhhCre) mice 0.5, 2, 6, and 24 hours after a final dose of drug (n=3 per time point/dose). Differences in the PK values between wild type and knockout mice may be explained by the number of doses received or genotype (1 vs. 60 respectively). (F) Pharmacodynamic Analysis. Phosphorylated ERK (pERK): total Erk (ERK), V= vehicle treated tumor, 0.5 and 1.5 correspond to the doses in mg/kg. Numbers are the ratio of P-ERK to total ERK for each sample.
PK analysis was carried out on non-tumor bearing (Nf1 flox/+;DhhCre) and tumor bearing (Nf flox/flo; DhhCre) mice. Non tumor bearing mice were given a onetime dose of drug, and blood was collected at 0.5, 2, 6 and 24 hours after final dose (n=3/time point/dose). PD-0325901 was cleared by 24 hours; thus, the maximum area under the curve was achieved with a single dose (Figure 5D). Maximum concentrations of PD-0325901 (Cmax) in non-tumor-bearing mice treated with either 0.5 mg/kg/day or 1.5 mg/kg/day PD-0325901 were reached by 30 min. after dosing (tmax), the earliest time point studied. Cmax levels and area under the curve (AUC0-t) were 3 times as high for the 1.5 mg/kg dose in this setting, correlating with reduction in the administered dose. We also analyzed PD-0325901 plasma levels from tumor bearing mice, after 60 days of daily drug treatment (Fig. 5D). Blood levels of PD-0325901 were slightly lower in tumor bearing mice at individual time points as compared to non-tumor bearing mice. This may be explained by the duration of the experiment; 60 days vs. 1 day, and/or by differences caused by mouse genotype. Importantly, in spite of low blood levels and therefore low AUC0-t, daily dosing in mice of 0.5 mg/kg PD-0325901 retained efficacy in the preclinical neurofibroma model system.
Discussion
Here we sought to identify effective strategies to prevent and treat neurofibromas, using a mouse model that accurately mimics the kinetics of human neurofibroma growth and the histology of the human tumors. Our results show that inhibition of the Ras-Raf-Mek-Erk pathway in the Nf1fl/fl;DhhCre mouse model, using a clinically relevant MEK inhibitor at 1.5 mg/kg/day, significantly delays neurofibroma growth when administered prior to tumor development, and that taking mice off drug does not lead to a deleterious rebound effect. We also dose de-escalated the MEK inhibitor to a level at which efficacy was retained using (0.5 mg/kg/day), ERK remained dephosphorylated, and blood vessels per area remained at low levels, even though cell proliferation was intermediate between untreated mice and mice exposed to higher levels of drug PD-0325901, and blood levels of PD-0325901 were low (under 200ng/ml by 2 hours after dosing). Therefore, daily dosing in mice of 0.5 mg/kg PD-0325901 retains efficacy and MEK targeting in the preclinical neurofibroma model system.
MEK inhibition has not previously been tested for ability to prevent neurofibroma formation. Based on the strong anti-tumor effect of PD-0325901 in mice with established neurofibromas, we hypothesized that PD-0325901 might serve as a preventative agent. However, while PD-0325901 significantly inhibited cell proliferation when given prior to tumor formation, tumor formation was not completely inhibited. There was also no effect of 3 months of MEK inhibition on blood vessel number in 4-month-old mice. This may be because vessels per area are few at this time point. In contrast, the robust effect of MEK inhibition in animals treated later correlates with treatment times when vessels per area of nerve. In addition, nerves remained disrupted in mice treated with MEK inhibitor from 1 to 4 months of age. We cannot exclude the possibility that even earlier treatment with MEK inhibitor, and/or a different dosing schedule, would provide improved efficacy. We prefer the notion that combinatorial treatments will be necessary to completely block neurofibroma formation, at least in this model system, because at one month of age, when treatment began in our study, nerves are not yet disrupted. It is possible that tumor cell death will be necessary to achieve complete remission. Treating NF1 deficient cells, that are resistant to MEK-inhibitors, with the dual PI3K/mTOR inhibitor PI-103, sensitized melanoma cells to MEK inhibitors (30, 31). Additional treatment with this or other inhibitors might sensitize neurofibromas to tumor cell death.
Notably, early intervention, before visible neurofibroma onset, did significantly delay tumor formation. These effects correlated with a significant inhibition of tumor blood vessels. These findings may be important therapeutically. In children and adolescents neurofibromas grow most rapidly when patients are young (32, 33), and it may be beneficial to use MEK inhibitors early to prevent tumor growth during the period of most robust growth to prevent severe disfigurement or compromised organ function.
Unlike oncology trials in which some toxicity is often deemed tolerable, NF1 patients remain on treatment for extended periods and may find the same side effects intolerable over the long term (34). Given that a dose comparable to 10 mg/kg PD-0325901 showed some long term toxicities in humans (29), the possibility of further lowering dose may be of importance for NF1 patients. Long term toxicities of MEK inhibitors at the doses now in human clinical trials are not yet known. We recognize that detection of toxicities may require extended monitoring. Depending on the plexiform neurofibroma, treatment may be given only during the years of active tumor growth; growth of plexiform neurofibroma slows down or stops in adolescence or adulthood (32, 33).
Results from oncology trials (35) and preclinical data (36) were used to estimate that 85% of MEK activity needs to be blocked to identify effects on cellular proliferation. It is therefore surprising that in mice treated with 0.5 mg/kg/day PD-0325901 in which the concentration of PD-0325901 in plasma was low, neurofibroma cell proliferation remained decreased [although reduced less than that observed at higher doses of PD-0325901], levels of P-ERK remained decreased, and MEK inhibition continued to shrink neurofibromas. Importantly, 0.5 mg/kg is a dose more than three times lower than that planned to enter clinical trials. In a mouse JMML model, the most effective dose of PD-0325901 was 5 mg/kg/day PD-0325901, so it is possible that individual cell and tumor types will require different amounts of drug for optimal results (37)—that is, neurofibromas may be especially sensitive to MEK inhibition. We support the idea that clinicians define the lowest dose possible efficacious dose MEK inhibitor in NF1 patients with neurofibromas and other manifestations, to achieve maximum patient safety. However, at lower doses, the value of Erk as a biomarker for activity may be reduced. Our goal was to define doses at which neurofibromas continue to shrink, or are prevented from increasing in size. We hope that these studies in mice will accommodate in designing human trials in which potential dangerous side effects from long term treatment are minimized.
Our studies in genetically engineered mice are carried out in an effort to inform clinical trials. Some evidence suggests that our model is predictive. Treatment of Nf flox/flox;DhhCre mice with RAD001 did not alter neurofibroma growth in mice (14), nor did rapamycin shrink tumors NF1 human patients (Brian Weiss, personal communication). Conversely, treatment of mice with PD-0325901 shrank tumors, and in humans some efficacy is being reported in Phase I trial of AZ-D6244 (38). The interfacing of genetically engineered mouse models and clinical trials is a strategy that, while relatively unproven, is gaining popularity (39). Nonetheless, it will be wise to consider our results with some caution pending additional results and comparisons to human trials.
Acknowledgments
We thank the Children’s Tumor Foundation for support of this work, through the Neurofibromatosis Therapeutic Consortium, and Pfizer for providing PD-0325901. We thank the members of the NFTC, especially Kevin Shannon and Karen Cichowski, and the NFTC advisory board for important comments and criticism, and Brigitte Widemann for support of Dr. Dombi. Analytical work was performed by the Clinical Pharmacology Analytical Core laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center supported by the National Cancer Institute grant P30 CA082709.
References
- 1.Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. Journal of medical genetics. 1989;26(11):712–21. doi: 10.1136/jmg.26.11.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12(1):1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- 3.Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Human mutation. 2000;15(6):541–55. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Donovan S, Shannon KM, Bollag G. GTPase activating proteins: critical regulators of intracellular signaling. Biochimica et biophysica acta. 2002;1602(1):23–45. doi: 10.1016/s0304-419x(01)00041-5. [DOI] [PubMed] [Google Scholar]
- 5.Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, De Wever I, Vermeesch JR, de Raedt T, De Paepe A, Speleman F, van Oosterom A, Messiaen L, Legius E. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Human molecular genetics. 2006;15(6):1015–23. doi: 10.1093/hmg/ddl016. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM, Birch PH. Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1,728 patients. Am J Med Genet. 1997;70(2):138–43. doi: 10.1002/(sici)1096-8628(19970516)70:2<138::aid-ajmg7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Upadhyaya M, Cooper DN, editors. Neurofibromatosis Type 1. Springer; 2012. p. 211. [Google Scholar]
- 8.Prada CE, Rangwala FA, Martin LJ, Lovell AM, Saal HM, Schorry EK, Hopkin RJ. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–7. doi: 10.1016/j.jpeds.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Beert E, Brems H, Daniels B, De Wever I, Van Calenbergh F, Schoenaers J, Debiec-Rychter M, Gevaert O, De Raedt T, Van Den Bruel A, de Ravel T, Cichowski K, Kluwe L, Mautner V, Sciot R, Legius E. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50(12):1021–32. doi: 10.1002/gcc.20921. [DOI] [PubMed] [Google Scholar]
- 10.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, Barker FG, Connor S, Evans DG, Fisher MJ, Goutagny S, Harris GJ, Jaramillo D, Karajannis MA, Korf BR, Mautner V, Plotkin SR, Poussaint TY, Robertson K, Shih CS, Widemann BC, Collaboration REI. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–40. doi: 10.1212/01.wnl.0000435744.57038.af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakacki RI, Dombi E, Potter DM, Goldman S, Allen JC, Pollack IF, Widemann BC. Phase I trial of pegylated interferon-alpha-2b in young patients with plexiform neurofibromas. Neurology. 2011;76(3):265–72. doi: 10.1212/WNL.0b013e318207b031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson KA, Nalepa G, Yang FC, Bowers DC, Ho CY, Hutchins GD, Croop JM, Vik TA, Denne SC, Parada LF, Hingtgen CM, Walsh LE, Yu M, Pradhan KR, Edwards-Brown MK, Cohen MD, Fletcher JW, Travers JB, Staser KW, Lee MW, Sherman MR, Davis CJ, Miller LC, Ingram DA, Clapp DW. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. The Lancet Oncology. 2012;13(12):1218–24. doi: 10.1016/S1470-2045(12)70414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss B, Widemann BC, Wolters P, Dombi E, Vinks A, Cantor A, Perentesis J, Schorry E, Ullrich N, Gutmann DH, Tonsgard J, Viskochil D, Korf B, Packer RJ, Fisher MJ. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a Neurofibromatosis Clinical Trials Consortium phase II study. Neuro-oncology. 2014;(61):982–6. doi: 10.1093/neuonc/nou235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Dombi E, Jousma E, Scott Dunn R, Lindquist D, Schnell BM, Kim MO, Kim A, Widemann BC, Cripe TP, Ratner N. Preclincial testing of sorafenib and RAD001 in the Nf(flox/flox); DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatric blood & cancer. 2012;58(2):173–80. doi: 10.1002/pbc.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing Nf1+/-- and c-kit-dependent bone marrow. Cell. 2008;135(3):437–48. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brossier NM, Carroll SL. Genetically engineered mouse models shed new light on the pathogenesis of neurofibromatosis type I-related neoplasms of the peripheral nervous system. Brain research bulletin. 2012;88(1):58–71. doi: 10.1016/j.brainresbull.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prada CE, Jousma E, Rizvi TA, Wu J, Dunn RS, Mayes DA, Cancelas JA, Dombi E, Kim MO, West BL, Bollag G, Ratner N. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta neuropathologica. 2013;125(1):159–68. doi: 10.1007/s00401-012-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13(2):105–16. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessen WJ, Miller SJ, Jousma E, Rizvi TA, Eaves D, Wu J, Widemann B, Kim MO, Dombi E, Dudley AH, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N. MEK inhibition exhibits efficacy in human and mouse Neurofibromatosis tumors, despite transcriptional feedback onto ERK. The Journal of clinical investigation. 2012 doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26(32):4609–16. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick F. Ras signaling and NF1. Current opinion in genetics & development. 1995;5(1):51–5. doi: 10.1016/s0959-437x(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 22.Haura EB, Ricart AD, Larson TG, Stella PJ, Bazhenova L, Miller VA, Cohen RB, Eisenberg PD, Selaru P, Wilner KD, Gadgeel SM. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(8):2450–7. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 23.LoRusso PM, Krishnamurthi SS, Rinehart JJ, Nabell LM, Malburg L, Chapman PB, DePrimo SE, Bentivegna S, Wilner KD, Tan W, Ricart AD. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(6):1924–37. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 24.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature reviews Cancer. 2004;4(12):937–47. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 25.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. The Biochemical journal. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boasberg PD, Redfern CH, Daniels GA, Bodkin D, Garrett CR, Ricart AD. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer chemotherapy and pharmacology. 2011;68(2):547–52. doi: 10.1007/s00280-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 27.Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim MO, Dombi E, Sabo J, Hardiman Dudley A, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. The Journal of clinical investigation. 2013;123(1):340–7. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monk KR, Wu J, Williams JP, Finney BA, Fitzgerald ME, Filippi MD, Ratner N. Mast cells can contribute to axon-glial dissociation and fibrosis in peripheral nerve. Neuron Glia Biol. 2007;3(3):233–44. doi: 10.1017/S1740925X08000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Yang AH, Matsumoto D, Collette W, Marroquin L, Ko M, Aguirre S, Younis HS. PD0325901, a mitogen-activated protein kinase kinase inhibitor, produces ocular toxicity in a rabbit animal model of retinal vein occlusion. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2009;25(6):519–30. doi: 10.1089/jop.2009.0060. [DOI] [PubMed] [Google Scholar]
- 30.Maertens O, Johnson B, Hollstein P, Frederick DT, Cooper ZA, Messiaen L, Bronson RT, McMahon M, Granter S, Flaherty K, Wargo JA, Marais R, Cichowski K. Elucidating distinct roles for NF1 in melanomagenesis. Cancer discovery. 2013;3(3):338–49. doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer research. 2012;72(13):3350–9. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen R, Dombi E, Widemann BC, Solomon J, Fuensterer C, Kluwe L, Friedman JM, Mautner VF. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet journal of rare diseases. 2012;7:75. doi: 10.1186/1750-1172-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dombi E, Solomon J, Gillespie AJ, Fox E, Balis FM, Patronas N, Korf BR, Babovic-Vuksanovic D, Packer RJ, Belasco J, Goldman S, Jakacki R, Kieran M, Steinberg SM, Widemann BC. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68(9):643–7. doi: 10.1212/01.wnl.0000250332.89420.e6. [DOI] [PubMed] [Google Scholar]
- 34.Kim A, Gillespie A, Dombi E, Goodwin A, Goodspeed W, Fox E, Balis FM, Widemann BC. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73(16):1273–9. doi: 10.1212/WNL.0b013e3181bd1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Molecular cell. 2013;49(2):249–61. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO, Kogan S, Braun BS, Shannon K. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. The Journal of clinical investigation. 2013;123(1):335–9. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widemann BCML, Fisher MJ, Weiss BD, Kim A, Dombi E, Baldwin A, Whitcomb P, Martin S, Gillespie A, Doyle A. Phase I study of the MEK1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs). 2014 ASCO Annual Meeting; 2014. [Google Scholar]
- 39.Lunardi A, Ala U, Epping MT, Salmena L, Clohessy JG, Webster KA, Wang G, Mazzucchelli R, Bianconi M, Stack EC, Lis R, Patnaik A, Cantley LC, Bubley G, Cordon-Cardo C, Gerald WL, Montironi R, Signoretti S, Loda M, Nardella C, Pandolfi PP. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nature genetics. 2013;45(7):747–55. doi: 10.1038/ng.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]