Abstract
Anorexia Nervosa (AN) is a psychiatric illness with profound medical consequences. Among the many adverse physical sequelae of AN, bone health is impacted by starvation and can be permanently impaired over the course of the illness. In this review of skeletal complications associated with eating disorders, we discuss the epidemiology, neuroendocrine changes, adolescent vs. adult skeletal considerations, orthopedic concerns, assessment of bone health, and treatment options for individuals with AN. The focus of the review is the skeletal sequelae associated with anorexia nervosa, but we also briefly consider other eating disorders that may afflict adolescents and young adults. The review presents updates to the field of bone health in AN, and also suggests knowledge gaps and areas for future investigation.
Keywords: eating disorder, anorexia nervosa, bone health, bone mineral density
Introduction
Anorexia Nervosa (AN) is a psychiatric illness with profound medical consequences. Among the many adverse physical sequelae of AN, bone health is impacted by starvation and can be permanently impaired over the course of the illness.1,2,3 In this review of skeletal complications associated with eating disorders, we discuss both screening and treatment considerations. The focus of the review is the skeletal sequelae associated with anorexia nervosa, but we also briefly consider other eating disorders that may afflict adolescents and young adults.
Epidemiology
Under-nutrition is the primary cause of compromised bone health in AN. However, there are other factors that contribute to diminished bone mass and skeletal strength in this population. Lifestyle choices can be detrimental to bone health, including low physical activity, excess caffeine and/or carbonated beverages, a strict vegetarian diet, high salt diet, and regular cigarette or alcohol use.4,5 In addition, genetics explain approximately three quarters of skeletal phenotypic variance, an important point to consider in any patient who presents for care.4
The body draws on its nutrient-rich skeletal reserve to support physiologic function in the absence of adequate oral intake. Over time, this process leads to decreased bone mineral density (BMD), a common consequence of a restrictive eating disorder such as AN. Low BMD is most frequently detected using dual-energy X-ray absorptiometry (DXA). Among adolescent females with anorexia, decreased BMD at the lumbar spine is highly prevalent when compared with age-matched controls.6,7,8,9 The prevalence of males who present to our practice continues to rise, and among males low BMD has been shown to be more common at the hip, femoral neck, and trochanteric and intertrochanteric sites than among age-matched controls.10 Osteopenia (defined as −1.0 SD >/= T-score > −2.5 SD) is present in 92%, and osteoporosis (defined as T-score </= −2.5 SD) in 38% of adult women with AN;11 less than 15% of adult women with AN have been found to have normal BMD.12 In women with active AN, the rate of bone loss is approximately 2.5% per year.13 Collectively, these findings clearly establish the need for urgency in management of bone health among patients with AN. However, the literature in this area can be challenging to interpret as different groups use varying criteria to define skeletal losses and states of bone health, and preferred terminology also varies between children/adolescents and adults. It is important to note that the International Society for Clinical Densitometry (ISCD) recently identified “low bone mass or bone mineral density” (defined as BMD Z-score </= −2.0 SD) as the preferred term for identifying children and adolescents with compromised skeletal health. The term ‘osteopenia’ is not currently recommended for use in this age group because these terms can be confusing when applied to children and adolescents.14 Osteoporosis is reserved for those children and adolescents who have evidence of skeletal fragility such as vertebral compression fractures.14
Neuroendocrine Changes in Anorexia Nervosa
In the setting of prolonged nutritional restriction, multiple endocrine systems are negatively impacted, and hormonal regulation is altered to preserve essential body functions.
Hypothalamic-Pituitary-Adrenal Axis
In response to the ongoing physical stress of starvation, individuals with AN exhibit hypercortisolemia through both increased frequency of secretory bursts and a prolonged cortisol half-life.15 The overall effects of hypercorisolism, as in other clinical settings, is a contributory factor in the suppression of bone formation and stimulation of bone resorption.16 Many patients with AN exhibit comorbid depression, exacerbating the hypercortisolism, with consequent adverse effects on bone turnover. The hypoglycemia and hypoinsulinemia that occur in AN further promote cortisol release as the body strives to maintain blood glucose levels through gluconeogenesis. There is evidence to suggest that the elevated cortisol levels in AN contribute to the hypogonadotropic hypogonadism that often accompanies this disease through a negative effect on gonadotropin secretion.17, 18 Fortunately, the alterations in the hypothalamic-pituitary-adrenal axis that occur during a prolonged period of starvation appear to be reversible with weight restoration.19
Hypothalamic-Pituitary-Gonadal Axis
Secondary amenorrhea is a common finding among females with AN, and low testosterone is often present in males with the disorder.20 In the setting of undernourishment, decreased pulsatility of gonadotropin releasing hormone (GnRH) causes immature secretory patterns of both follicular stimulating hormone (FSH) and luteinizing hormone (LH). In females, insufficient LH stimulation prevents ovulation, leading to amenorrhea21 due to low levels of estradiol. In pubertal males, testosterone is aromatized to estrogen. Circulating testosterone levels are suppressed in underweight young men with restrictive eating disorders,10 decreasing levels of both testosterone and aromatized estrogen. This hormonal profile has the potential to affect bone formation and resorption in an adverse manner as androgens stimulate bone formation, and estrogens, skeletal resorption. Hypoestrogenism in both males and females leads to the arrest of skeletal development and increased bone resorption. Adolescent females with estrogen deficiency due to AN may experience a bone mass decline of up to 3-5 percent per year.5 The duration of amenorrhea is associated with decreased BMD among women with AN.6 While hypothalamic amenorrhea is a hallmark of AN, it is potentially reversible with weight restoration. Return of menses is expected as a sign of improved physiologic function during AN recovery. This clinical parameter appears to be highly correlated with body composition (i.e., percentage body fat).22
Hypothalamic-Pituitary Thyroid Axis
Individuals with AN have decreased thyroid volume likely due to chronic starvation.23 Thyroid function is often altered in AN as well, with low T3 and low-to-normal T4 thought to be related to diminished peripheral conversion;19 thyroid stimulating hormone is often low-to-normal.24 These findings are consistent with those seen in a ‘sick euthyroid’ state. As nutritional compliance and weight restoration occur, thyroid function typically improves.
Thyroid hormones are integrally involved in the activity of osteoblasts, osteoclasts, and in bone health maintenance, as evidenced by the changes in linear growth patterns and bone health in patients with depressed or elevated thyroid function. One study found total T3 to explain some of the longitudinal variance in BMD in a cohort of young women with AN.25
Growth Hormone-Insulin-like Growth Factor I Axis
In the starvation state, insulin-like growth factor I (IGF-I) secretion from the liver decreases as body mass index (BMI) and body fat decline.26,27 Growth hormone (GH) levels increase in this setting due to decreased IGF-I feedback in the pituitary and hypothalamus.27 Over time, GH resistance leads to acquired GH deficiency and related consequences, including decreased linear growth.27,28 Adequate circulating IGF-I has implications for bone mineral density, trabecular bone structure, bone marrow adiposity, and adipose tissue homeostasis.29 Depressed IGF-I levels among adolescents with anorexia is thought to be one of the contributing factors to compromised bone health in this population.26,27
Bone Marrow Composition and Adipokines
Mesenchymal stem cells give rise to both osteoblasts and adipocytes within bone marrow. Prompted by a growing body of literature, there has been increasing interest over recent years in the role that adipocytes play in the maintenance of bone health.30 Bone marrow adipocytes secrete adipokines including leptin and adiponectin.31 Of interest, leptin levels in AN are low, contributing to diminished reproductive function due to a hypogonadal state and play a role in alterations of bone turnover.32 Adiponectin has been shown to diminish gonadotropin secretion,33 thereby also potentially contributing to hypogonadotropic hypogonadism in patients with AN. In this disease, neuroendocrine changes include increased levels of ghrelin and peptide YY which contribute to alterations energy balance, feeding behaviors, and bone formation in this disease. Individuals with AN have been shown to have higher bone marrow fat content, despite diminished subcutaneous and visceral fat, and there is an inverse correlation observed between bone marrow fat content and BMD.34,35 These findings suggest that increased bone marrow fat may contribute to low BMD in patients with AN,30,35 and this relationship remains an area of active research.
Adolescent vs. Adult Skeletal Considerations
Although bone mineral accumulation takes place throughout childhood, much of the overall growth in skeletal structure occurs during the second decade of life. An estimated 26% of calcium deposition present in adults is established in early adolescence, with peak rates occurring at 12.5 years for girls and 14.0 years for boys;36 most skeletal growth is complete by early in the third decade of life with the achievement of peak bone mass.4 Thus, establishment of healthy bones and attainment of peak bone mass requires adequate, balanced nutritional intake during adolescence, the highest risk decade for development of AN.37 In fact, adolescents with AN show rapid skeletal compromise due to decreased bone mass accrual.6,8,38,39 In addition to decreased bone density, linear growth potential may be diminished in the setting of chronic nutritional restriction during a period of critical growth.28,29
Although skeletal maturity has been achieved by adulthood, the effects of AN on bone health are also profound in older populations due to erosion of established bone mass and increased resorption;39,40 over 90% of women with AN have diminished bone density.11 Among adult women with AN, those who developed the disease as adolescents have lower bone density measures than those who developed the disease later in life, supporting the critical relationship between adolescent nutritional intake, peak bone mass accrual and life-long bone health.41 Although skeletal losses that occur during the course of AN can improve with recovery,42 full recovery cannot always be expected.1,2,3
Orthopedic Considerations
Cartilage
To our knowledge, no studies to date have examined the direct effect of an eating disorder on the risk of sustaining a cartilaginous injury. However, several reports have examined components of the female athlete triad, such as menstrual irregularity on risk of musculoskeletal injuries, which have included anterior cruciate ligament (ACL) tears, patellofemoral disorders, and other diagnoses.43 One study examined the difference in serum concentrations of testosterone, 17-β estradiol and progesterone between female patients, with and without ACL rupture.44 Lower levels of each hormone were found in the group with ACL rupture, important as this is the hormonal profile seen commonly in young women with anorexia nervosa. Studies that examine the prevalence and mechanisms underlying cartilaginous injuries in adolescent girls and young women with eating disorders will represent an important contribution to this field.
Bone
Not only does AN impact bone density, but it also may affect skeletal function and strength. DXA hip structural analysis provides geometric measures of bone strength, which can be informative regarding fracture risk.45,46 Among adolescent and young adult females with AN, decreased bone resistance to axial and bending loads is present, suggesting decreased bone strength in these individuals.47 Fracture risk is higher among females with AN;48,49 this elevated risk is present even among AN patients who do not have documented low BMD as assessed by DXA or imaging modalities.49 Among adolescent males with AN, the index of susceptibility to local cortical buckling under compressive loads was higher than that of age-matched controls,50 suggesting increased risk for fracture in this population, as well. It is known that female runners are particularly susceptible to stress fractures and other injuries, especially in the setting of the female athlete triad, as has been the subject of several reviews.51,52,53 However, data on fracture risk are mixed, with other studies suggesting that disordered eating behavior is not associated with compression fracture among an adolescent and young adult population,54 or stress fracture among adolescent females <17 years old.55 The discrepancy among findings from different studies suggests that there are multiple factors contributing to fracture risk in this population, and that counseling patients about BMD should include a discussion of the uncertainty regarding the relationship between a given BMD and future fracture risk.
Screening for bone health
Any individual with an eating disorder, and in particular AN, should be considered at risk for poor bone health. The initial evaluation of bone health in an individual with AN includes a thorough history, exploring the extent of the patient’s nutritional deficiency, past orthopedic injury, and careful review of dietary intake. Genetics is an important determinant of bone health;4 thus an assessment of family history for fracture or osteoporosis can help to identify AN patients at particularly high risk for skeletal compromise. Presentation of fracture is especially concerning, and poor underlying bone health is likely in cases of fracture involving the axial skeleton such as the hip or spine36,56 or in cases of low-impact fractures.56
Laboratory evaluation of bone health is generally limited to obtaining a serum 25-hydroxyvitamin D (25OHD) level. Due to the 3-4 week half-life, this metabolite most accurately reflects the body’s vitamin D stores. Vitamin D deficiency is defined as a serum 25OHD level <20 ng/mL; insufficiency is defined as 21-29 ng/mL.57 It should be noted that among bone health experts there is inconsistency regarding the ‘optimal’ threshold for 25OHD; recently, 2 national guidelines were published that offer differing recommendations.57,58 Serum 25OHD levels may vary with seasonal changes and geographic latitude: a patient who is deficient during the winter may rebound to normal levels in the summer. Providers should measure a serum 25OHD level in any patient with AN in order to ensure timely and adequate treatment of vitamin D deficiency/insufficiency.57 Measures of total or free thyroxine, triiodothyronine, thyroid stimulating hormone, follicle stimulating hormone and prolactin represent a simple screening panel to evaluate the amenorrhea associated with restrictive eating disorders. However, each of these hormones can play a role in modulating bone turnover and should be considered a component of the bone health evaluation.
Structural Evaluation of Bone Health
The most widely used imaging tool for bone health in AN is DXA, a measure of bone mineral content (in grams) in the bone area scanned (in square centimeters). A BMD T-score (for older adults) or Z-score (for adolescents) is generated which is adjusted for age, gender and often ethnicity. Reference data for Z-scores were developed using DXA data among healthy individuals; data for adolescents with chronic illness are less available. Thus, interpretation of DXA Z-scores—which provide a two-dimensional assessment of bone density and therefore can be confounded by bone size in the developing adolescent—can be challenging.59 For example, in the case of an adolescent with growth deficits and short stature due to chronic AN, DXA results may overestimate skeletal losses. However, DXA is a safe, rapid, and widely used densitometric technique.59 Individuals with AN should undergo DXA scans annually during the period of restrictive eating due to high risk for low BMD and ongoing skeletal losses.56 As noted above, past research has not clearly demonstrated the relationship between low bone density and future fracture risk. Therefore, in counseling patients with AN, fracture risk should be reviewed as a consequence of the disease, but cannot necessarily be attributed to a particular finding on a DXA assessment.56 It is also important to recognize that these young women may respond differently to results of bone density testing, depending on where they are in the course of their illness. Clinicians need to be sensitive when presenting these results, as they may elicit unhealthy thoughts (e.g., “If my bone density is normal, I am not restricting enough”), as well as positive behavioral changes that result in long-term improvement, especially if the testing occurs later in the course of the illness.60
Recent technology has improved a clinician’s ability to evaluate bone health with greater accuracy in the form of axial and peripheral quantitative computed tomography (QCT). These assessment tools provide a measurement of volumetric bone mineral density (in grams per cubic centimeter) and are more accurate than DXA, particularly in adolescent patients whose bone size changes over time, and for individuals with chronic illness.61 However, the high radiation dose associated with axial QCT has limited its utility in general clinical practice. In contrast, peripheral QCT—especially high-resolution peripheral QCT—examines bone micro-architecture in a more precise way and can identify skeletal changes that occur with physical activity, pubertal development, and/or significant illness. Quantitative ultrasound is generally not recommended for bone assessments in growing adolescents as there is debate over what skeletal properties are captured by this methodology, and the presence of growth plates can complicate the data generated. Peripheral QCT is preferred over bone ultrasound for measurements of the peripheral skeleton for children and adolescents.62 Although these newer technologies are currently available only at academic centers, they continue to be explored as diagnostic tools for clinical practice, and ongoing research considers whether computerized tomography may enhance diagnostic precision in the future.
Treatment considerations
Treatment of eating disorders is best accomplished by an interdisciplinary team of experienced providers. Eating disorders are primarily psychiatric illnesses and thus long-term recovery will only be achieved through intensive therapeutic intervention by an experienced therapist. The goal of therapy is to address underlying body dissatisfaction, which motivates the eating disordered patient’s under-nourished state. For some patients, psychopharmacology may play an important role in treating co-morbid conditions such as anxiety, depression, obsessive-compulsive disorder; maximizing management of these conditions will help in treatment of the eating disorder as well. The most effective and available treatment option for poor bone health (and other physiologic compromise) among individuals with AN is rapid, sustained weight restoration through nutritional change. A range of nutrient deficiencies impact bone health among eating disordered patients, including calcium, phosphorus, magnesium, silicon, vitamins D, A, C and K, iron, fluoride copper, zinc and boron.63,64 Nutritional rehabilitation is best accomplished with the input of a registered dietitian in order to maximize healthy weight restoration, and the recovery of bone health. These patients often recognize the dietitian as the most influential member of the treatment team, and the most helpful in achieving ultimate recovery. Finally, medical supervision ensures ongoing monitoring of physiologic function during the period of recovery, and may direct inpatient or residential care if outpatient treatment fails to achieve treatment goals.
Prevalence of AN is highest among adolescents; sufficient calcium intake during the second decade of life is critical to maximize skeletal growth during the pubertal growth spurt4,65 and help decrease fracture risk during adulthood.66 According recent guidelines from the Institute of Medicine, the recommended dietary allowance per day for calcium is 700 mg for children 1-3 years old; 1,000mg for 4-8 years old; 1,300mg for 9-18 years old; 1,000 for 19-70 years old; and 1,200 for 70 years and older.58 Reaching these nutritional goals can be challenging in the setting of active AN. Supplementation is often an easier way to ensure success in the setting of active restricting behavior. Calcium supplements are associated with constipation, hypercalciuria and other negative side effects; therefore, calcium-rich foods should be emphasized during nutritional rehabilitation, and any supplement dose should be carefully monitored.
Vitamin D treatment should be pursued for any individual who is found to be either insufficient or deficient, including patients with AN, and supplementation prescribed for all adolescents and young adults with a potential eating disorder. Treatment for vitamin D deficiency (25OHD level of <20 ng/mL) for children and adolescents includes either 2000 IU daily or 50,000 IU weekly for 6 weeks, followed by maintenance therapy of 1000 IU per day; for adults treatment includes either 6000 IU daily or 50,000 IU weekly for 8 weeks, followed by maintenance therapy of 2000 IU per day.57 Treatment for vitamin D insufficiency (25OHD < 21-29 ng/mL) includes 1000 IU daily.57 Due to the risk for poor bone health in AN, all individuals with this disease should be prescribed ongoing supplementation of 400-1,000 IU daily once normalization of 25OHD is achieved.57 Either vitamin D2 or vitamin D3 is effective at raising blood levels to the desired range, although recent research suggests that vitamin D3 is more potent and supports cortical bone mass and strength better than vitamin D2.57,67 Bioavailability of oral ergocalciferol is not impaired in AN, and therefore dosing recommendations are similar in this population to those in the general public.68 Milk is the most available vitamin D-fortified food product in the US, but fortification can be inconsistent between different products and milk labels do not always accurately reflect a product’s vitamin D content.57
Once an individual with AN weight restores, bone health can be supported by incorporating weight-bearing physical activity into the treatment plan. Biomechanical forces exerted on the bone during such activity help to promote healthy growth, especially among adolescents.4,69,70 There is no current recommendation to restrict athletics to non-contact sports as a method of minimizing fracture risk; however, the risk of fracture should be discussed with patients who are found to have a low Z-score (< −2.0), especially when there is a past history of fracture.
Treatment of bone health in AN primarily consists of treating the primary illness with weight restoration and return to physical activity; even modest weight gain with subsequent return of menses will maximize bone recovery.3 There are no current recommendations for general use of any pharmacologic regimen to support bone health, although there is some evidence that certain medications may improve bone density in AN patients. In the past, it was thought that using combined estrogen/progestin pills would enhance bone density in females with AN; more recent studies do not support this regimen and thus such treatment is not recommended.71,72 However, there is promising evidence to suggest that transdermal estrogen may lead to prevention of bone loss in adolescents with AN.73 Other recent contributions indicate that combined treatment with a low-dose estrogen oral contraceptive and dehydroepiandrosterone (DHEA) together results in cessation of bone loss among older adolescents,74 and can improve bone density, cross sectional area, and cortical thickness in adolescents and young adults.54 Oral DHEA alone has proven minimally effective at altering bone density among patients with AN.72
A combined regimen of oral contraceptives and IGF-I has shown to increase spine BMD in adult women,75 but it is less widely used in adolescents in part due to lack Food and Drug Administration (FDA) approval and the need for twice-daily injection dosing of the medication.40 Use of another anabolic agent, teriperatide, recently was shown to improve spine BMD by 6-10% in a randomized, controlled trial of 21 adult women with AN,76 however, this promising finding has yet to be more rigorously assessed with further evaluation.
A recent randomized controlled trial of oral bisphosphonates found a 3-4% increase in spine and 2% increase in hip BMD in adult women with AN,77 though prior study of bisphosphonates among adolescents has not demonstrated similar BMD change with use.78 At this time, therefore, bisphosphonates are not recommended for treatment of low BMD in AN patients. Of note, bisphosphonates should be considered as a therapeutic option for premenopausal women using extreme caution given the prolonged bone retention of these agents, and potential for adverse skeletal effects in offspring.
Lastly, leptin is one of several metabolic modulators thought to impact reproductive function and bone health in malnourished patients through its role as a mediator between GnRH secretion and kisspeptin neurons. The role of kisspeptins in physiologic energy balance remains an area of active research. However, the administration of recombinant leptin has been explored as a potential treatment option to improve poor bone health in low weight women with hypothalamic amenorrhea.79,80
Future Directions and Considerations
Bone health in individuals with AN may deteriorate substantially during the course of this debilitating disease. Threats to skeletal health can be minimized with early identification of illness, aggressive weight restoration, and ongoing interdisciplinary management of the disease through psychiatric, medical, and nutritional support. However, new contributions to bone assessment technologies and both anabolic and anti-resorptive skeletal agents may help to preserve bone health during the period of malnutrition, and provide promise for improved skeletal support in this vulnerable population. Further research is needed to clearly establish treatment options that might best preserve bone density and skeletal strength during the often slow re-nourishment process.
Of note, research to date in this area is overwhelmingly female-focused; the impact of AN on male bone health should be further examined, particularly as the disease prevalence in young men continues to increase. The International Olympic Committee (IOC) recently acknowledged this historical bias in the literature in a revision of their consensus statement on the Female Athlete Triad, establishing the replacement term “Relative Energy Deficit in Sport” (RED-S).81 By expanding the descriptive terminology for the energy deficit that can occur in under-nourished athletes, the IOC is leading the way in acknowledging the need to assess males, as well as females for nutritional deficits in the setting of intense physical activity. Though the diagnostic criteria for RED-S are distinct from AN, there are a number of shared physiologic consequences, including changes in bone health, metabolism, hormonal function (including menstruation in females), immunity, protein synthesis, and cardiovascular health.
Finally, there is also a critical need to understand how eating disorders affect soft tissue including the potential increased risk for cartilaginous injuries and peripheral neuropathies in these patients. To date, most of the musculoskeletal research carried out in this patient group has focused on bone. Thus, there remain many gaps in knowledge when considering the musculoskeletal system more broadly, including effects of these disorders on bone, cartilage, and soft tissue. Peripheral neuropathy related to severe, chronic under-nutrition is an area that has not been extensively explored, although it has been suggested that compression neuropathies may be more likely to occur in patients with AN due to subcutaneous tissue loss.82
Translational research in the field of bone health among eating disordered patients should focus on gaining a more thorough understanding of the pathophysiology of the complex disease process, to allow for targeted interventions to counter disease-associated bone loss. In order to capture the scope of the skeletal consequences of under-nutrition in this population, both short-term and long-term sequelae will need to be assessed and ultimately, better understood. As more male patients present to our practices, it will also be important to understand how these adverse sequelae vary by gender.
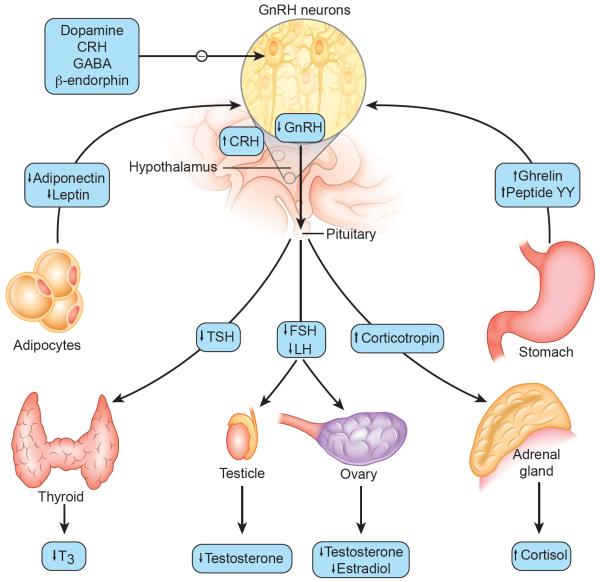
Figure 1. Hormonal and other changes in patients with restrictive eating disorders.
In patients with restrictive eating disorders there are alterations of hormones and other factors that affect the secretion of gonadotropin-releasing hormone (GnRH), including low levels of leptin and high levels of both ghrelin and peptide YY. β-endorphin, corticotropin-releasing hormone (CRH), dopamine, and γ-aminobutyric acid (GABA) are factor that negatively influence GnRH secretion. Some of these factors may also serve as hunger signals from the peripheral to the central nervous system and as links between nutrition and reproduction. Hallmark findings in adolescents and young adults with restrictive eating disorders include over-activity of the hypothalamic-pituitary-adrenal axis, suppression of the hypothalamic-pituitary-gonadal axis, and alterations of thyroid hormone regulation. FSH denotes follicle-stimulating hormone, LH lutenizing hormone, TSH thyrotropin, and T3 triiodothyronine.
Adapted from Gordon CM. Functional hypothalamic amenorrhea. N Engl J Med 2010; 363: 365-71. Copyright © 2010 Massachusetts Medical Society. Reprinted with permission.
Acknowledgements
Dr. Gordon is supported by NIH grant R01 AR060829.
Abbreviations
- (AN)
Anorexia Nervosa
- (BMD)
bone mineral density
- (DXA)
dual-energy X-ray absorptiometry
- (ISCD)
International Society for Clinical Densitometry
- (GnRH)
gonadotropin releasing hormone
- (FSH)
follicular stimulating hormone
- (LH)
luteinizing hormone
- (IGF-I)
insulin-like growth factor I
- (BMI)
body mass index
- (GH)
Growth hormone
- (ACL)
anterior cruciate ligament
- (25OHD)
serum 25-hydroxyvitamin D
- (QCT)
quantitative computed tomography
- (DHEA)
dehydroepiandrosterone
- (FDA)
Food and Drug Administration
- (IOC)
International Olympic Committee
- (RED-S)
Relative Energy Deficit in Sport
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Both authors materially contributed to the article preparation, and have approved the final article.
The authors declare no conflicts of interest or financial disclosures.
References
- [1].Herzog W, Minne H, Deter C, et al. Outcome of bone mineral density in anorexia nervosa patients 11.7 years after first admission. J Bone Miner Res. 1993;8:597–605. doi: 10.1002/jbmr.5650080511. [DOI] [PubMed] [Google Scholar]
- [2].Hartman d, Crisp A, Rooney B, Rackow C, Atkinson R, Patel S. Bone density of women who have recovered from anorexia nervosa. Int J Eat Disord. 2000;28:107–112. doi: 10.1002/(sici)1098-108x(200007)28:1<107::aid-eat13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [3].Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231–1237. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- [5].Mehler PS, Anderson AE. Eating Disorders: a guide to medical care and complications. 2nd ed Johns Hopkins University; Baltimore, MD: 2010. pp. 144–155. [Google Scholar]
- [6].Soyka L, Grinspoon S, Levitsky L, Herzog D, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- [7].Soyka L, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- [8].Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, Herzog DB, Klibanski A. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114:1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- [9].Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, Meenaghan E, Goldstein MA, Bouxsein ML, Misra M, Klibanski A. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength in the distal radius. J Clin Endocrinol Metab. 2013;98:1923–1929. doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Misra M, Katzman DK, Cord J, Mendes N, Herzog DB, Miller KK, Klibanski A. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3029–3036. doi: 10.1210/jc.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miller K, Grinspoon S, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- [13].Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, Gleysteen S, Mickley D, Herzog D, Klibanski A. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931–2937. doi: 10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hagg Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- [15].Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- [16].Vergely N, Lafage-Proust MH, Caillot-Augusseau A, Millot L, Lang F, Estour B. Hypercorisolism blunts circadian variations in osteocalcin regardless of nutritional status. Bone. 2002;30:428–435. doi: 10.1016/s8756-3282(01)00677-9. [DOI] [PubMed] [Google Scholar]
- [17].Padmanabhan V, Keech C, Convey EM. Cortisol inhibits and adrenocorticotropin has no effect on luteinizing hormone-releasing hormone-induced release of luteinizing hormone from bovine pituitary cells in vitro. Endocrinology. 1983;112:1782–1787. doi: 10.1210/endo-112-5-1782. [DOI] [PubMed] [Google Scholar]
- [18].Gordon CM. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363:365–71. doi: 10.1056/NEJMcp0912024. [DOI] [PubMed] [Google Scholar]
- [19].Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055–1063. doi: 10.4158/EP.14.8.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Misra M, Prabhakaran R, Miller KK, Tsai P, Lin A, Lee N, et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res. 2006;59:598–603. doi: 10.1203/01.pdr.0000203097.64918.63. [DOI] [PubMed] [Google Scholar]
- [21].Loucks AB, Heath Em. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78:910–915. doi: 10.1210/jcem.78.4.8157720. [DOI] [PubMed] [Google Scholar]
- [22].Pitts SAB, Blood E, DiVasta A, Gordon CM. Percentage body fat by dual-energy X-ray absorptiometry is associated with menstrual recovery in adolescents with anorexia nervosa. J Adolesc Health. 2014;54:739–41. doi: 10.1016/j.jadohealth.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stoving RK, Bennedback FN, Hegedus L, Hagen C. Evidence of diffuse atrophy of the thyroid gland in patients with anorexia nervosa. Int J Eat Disord. 2001;29:230–235. doi: 10.1002/1098-108x(200103)29:2<230::aid-eat1013>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [24].Miyai K, Yamamoto T, Azukizawa M, Ishibashi K, Kumahara Y. Serum thyroid hormones and thyrotropin in anorexia nervosa. J Clin Endocrinol Metab. 1975;40:334–338. doi: 10.1210/jcem-40-2-334. [DOI] [PubMed] [Google Scholar]
- [25].Legroux-Gérot I, Vignau J, d’Herbomez M, Flipo RM, Cortet B. Predictive factors of change in BMD at 1 and 2 years in women with anorexia nervosa: a study of 146 cases. Osteoporos Int. 2012 Dec;23:2855–61. doi: 10.1007/s00198-012-1919-8. [DOI] [PubMed] [Google Scholar]
- [26].Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, Gundberg CM, Leboff MS. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141:64–70. doi: 10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- [27].Misra M, Miller K, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- [28].Katzman DK. Medical complications in adolescents with anorexia nervosa: A review of the literature. Int J Eat Disord. 2005;37:52–59. doi: 10.1002/eat.20118. [DOI] [PubMed] [Google Scholar]
- [29].Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111:14–9. doi: 10.1002/jcb.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hardouin P, Pansini V, Cortet B. Bone marrow fat. Joint Bone Spine. 2014;81:313–319. doi: 10.1016/j.jbspin.2014.02.013. [DOI] [PubMed] [Google Scholar]
- [31].Misra M, Klibanski A. Endocrine Consequences of Anorexia Nervosa. Lancet Diabetes Endocrinol. 2014;2:581–92. doi: 10.1016/S2213-8587(13)70180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Welt CK, Chan LJ, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Eng J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- [33].Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LBT2 gonadotropes. Mol Endocrinol. 2008;22:760–771. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- [37].Mehler PS, Anderson AE. Eating Disorders: a guide to medical care and complications. 2nd ed Johns Hopkins University; Baltimore, MD: 2010. p. 7. [Google Scholar]
- [38].Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72:602–606. doi: 10.1210/jcem-72-3-602. [DOI] [PubMed] [Google Scholar]
- [39].Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, et al. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84:2049–2055. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- [40].Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- [41].Biller B, Saxe V, Herzog D, Rosenthal D, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- [42].Schulze UME, Schuler S, Schlamp D, Schneider P, Mehler-Wex C. Bone mineral density in partially recovered early onset anorexic patients—a follow-up investigation. Child Adolesc Psychiatry Ment Health. 2010;4:20. doi: 10.1186/1753-2000-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thien-Nissenbaum, et al. Menstrual irregularity and musculoskeletal injury in female high school athletes. J Athl Train. 2012;47:74–82. doi: 10.4085/1062-6050-47.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ireland ML, Ott SM. Special concerns of the female athlete. Clin Sports Med. 2004;23:281–98. doi: 10.1016/j.csm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [45].Beck TJ, Ruff CB, Warden KE, Scott WW, Jr, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- [46].Beck TJ, Ruff CB, Mourtada FA, Shaffer RA, Maxwell-Wililams K, Kao GL, et al. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11:645–653. doi: 10.1002/jbmr.5650110512. [DOI] [PubMed] [Google Scholar]
- [47].DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29:151–157. doi: 10.1002/jbmr.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, Cummings SR, Diez-Perez A, Eisman JA, Fujiwara S, Gluer CC, Goltzman D, Hans D, Khaw KT, Krieg MA, Kroger H, Lacroix AZ, Lau E, Leslie WD, Mellstrom D, Melton LJ, 3rd, O’Neill TW, Pasco JA, Prior JC, Reid DM, Rivadeneira F, van Stass T, Yoshimura N, Zillikens MC. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29:223–233. doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- [49].Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Klibanski A. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47:458–66. doi: 10.1002/eat.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Misra M, Katzman DK, Clarke H, Snelgrove D, Brigham K, Miller KK, Klibanski A. Hip structural analysis in adolescent boys with anorexia nervosa and controls. J Clin Endocrinol Metab. 2013;98:2952–2958. doi: 10.1210/jc.2013-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stijak L, Kadija M, Djulejić V, Aksić M, Petronijević N, Marković B, Radonjić V, Bumbaširević M, Filipović B. The influence of sex hormones on anterior cruciate ligament rupture: female study. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-014-3077-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [52].Hoch AZ, Pepper M, Akuthota V. Stress fractures and knee injuries in runners. Phys Med Rehabil Clin N Am. 2005;16:749–77. doi: 10.1016/j.pmr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [53].Boles CA, Ferguson C. The female athlete. Radiol Clin North Am. 2010;48:1249–66. doi: 10.1016/j.rcl.2010.07.015. [DOI] [PubMed] [Google Scholar]
- [54].DiVasta AD, Feldman HA, Gordon CM. Vertebral fracture assessment in adolescents and young women with anorexia nervosa: a case series. J Clin Densitom. 2014;17:207–211. doi: 10.1016/j.jocd.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Loud K, Gordon CM, Micheli LJ, Field AE. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;115:e399. doi: 10.1542/peds.2004-1868. [DOI] [PubMed] [Google Scholar]
- [56].Bishop N, Braillon P, Burnham, et al. Dual-energy X-ray absorptiometry assessment in children and adolescents with disease that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:29–42. doi: 10.1016/j.jocd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [57].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- [58].Institute of Medicine . Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- [59].Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [60].Stoffman N, Schwartz B, Austin SB, Grace E, Gordon CM. Influence of bone density results on adolescents with anorexia nervosa. Int J Eat Disord. 2005;37:250–255. doi: 10.1002/eat.20131. [DOI] [PubMed] [Google Scholar]
- [61].Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr. 2005;146:776–779. doi: 10.1016/j.jpeds.2005.01.028. [DOI] [PubMed] [Google Scholar]
- [62].Adams JE, Engelke K, Zemel BS, Ward KA, Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions International Society of Clinical Densitometry. J Clin Densitom. 2014;17:258–74. doi: 10.1016/j.jocd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [63].Ilich JZ, Kerstetter JE. Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr. 2000;19:715–737. doi: 10.1080/07315724.2000.10718070. [DOI] [PubMed] [Google Scholar]
- [64].Price CT, Langford JR, Liporace FA. Essential nutrients for bone health and a review of their availability in the average North American diet. Open Orthop J. 2012;6:143–149. doi: 10.2174/1874325001206010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Greene DA, Naughton GA. Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: a randomised controlled trial. Osteoporos Int. 2011;22:489–498. doi: 10.1007/s00198-010-1317-z. [DOI] [PubMed] [Google Scholar]
- [66].Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2:740–750. doi: 10.1016/j.pmrj.2010.04.020. [DOI] [PubMed] [Google Scholar]
- [67].Sayers A, Fraser WD, Lawlor DA, Tobias JH. 25-Hydroxyvitamin-D(3) levels are positively related to subsequent cortical bone development in childhood: findings from a large prospective cohort study. Osteoporos Int. 2012;23:2117–2128. doi: 10.1007/s00198-011-1813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].DiVasta AD, Feldman HA, Brown JN, Giancaterino C, Holick MF, Gordon CG. Bioavailability of vitamin d in malnourished adolescents with anorexia nervosa. J Clin Endocrinol Metab. 2011;96:2575–2580. doi: 10.1210/jc.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dias Quiterio AL, Carnero EA, Baptista FM, Sardinha LB. Skeletal mass in adolescent male athletes and nonathletes: relationships with high-impact sports. J Strength Cond Res. 2011;25:3439–3447. doi: 10.1519/JSC.0b013e318216003b. [DOI] [PubMed] [Google Scholar]
- [70].Farr JN, Blew RM, Lee VR, Lohman TG, Going SB. Associations of physical activity, duration, frequency and load with volumetric BMD, geometry, and bone strength in young girls. Osteoporos Int. 2011;22:1419–1430. doi: 10.1007/s00198-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Strokosch GR, Friedman AJ, Wu S, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health. 2006;39:819–827. doi: 10.1016/j.jadohealth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- [72].Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- [73].Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].DiVasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61:1010–1020. doi: 10.1016/j.metabol.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Meatab. 2002;87:2883–2991. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- [76].Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99:1322–1329. doi: 10.1210/jc.2013-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2081–2088. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, et al. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, doubleblind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:3179–3185. doi: 10.1210/jc.2004-1659. [DOI] [PubMed] [Google Scholar]
- [79].Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- [80].Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [81].Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A. The IOC consensus statement: beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S) Br J Sports Med. 2014;48:491–497. doi: 10.1136/bjsports-2014-093502. [DOI] [PubMed] [Google Scholar]
- [82].Mackenzie JR, LaBan MM, Sackeyfio AH. The prevalence of peripheral neuropathy in patients with anorexia nervosa. Arch Phys Med Rehabil. 1989;70:827–830. [PubMed] [Google Scholar]