Cardiovascular diseases (CVD) represent a significant cause of death and disability worldwide.1 The decline of CV mortality as a result of modern medicine and surgery has in turn led to a rapid increase of patients suffering from heart failure, with the only definite cure being heart transplantation. However, many patients are unable to undergo transplantation surgery due to complications from existing comorbidities, and among suitable patients, the procedure is plagued by limited donor supply, high costs, and the need for chronic immunosuppressant therapy. Hence, recent advances in cardiac regenerative therapy have emerged as an attractive alternative.
Currently, there are several methods to achieve cardiac regeneration. Endogenous cardiac repair that involves generation of new cardiomyocytes from differentiation of cardiac progenitor cells or renewal of pre-existing adult cardiomyocytes is one such approach, albeit a rare and inefficient process to cope with the loss of cardiomyocytes after myocardial infarction or other cardiac diseases.2 Alternatively, functional myocardium may be salvaged or replenished through transplantation of exogenous stem cells.3, 4 However, the poor long-term engraftment and survival in current transplantation has largely precluded substantial cell replacement, and instead supports the paracrine hypothesis that the release of external factors contributes to myocardial salvage or repair.
Historically, the paracrine hypothesis is thought to be mediated primarily by chemical and physical signals such as soluble proteins, gene products, lipids, and gases. Indeed, various studies have demonstrated that stem cells produce and secrete a broad range of cytokines, chemokines, and growth factors that are involved in cardiac repair.5 Strong support of a paracrine hypothesis came from experimental studies in which the administration of conditioned medium (CM) from stem cells was able to confer beneficial effects without the physical presence of stem cells within the infarcted heart.5, 6 There is a growing body of evidence showing that stem cells are also able to release membranous vesicles into extracellular space that can contribute to cell-to-cell communication, including microparticles, microvesicles, and exosomes.7-9
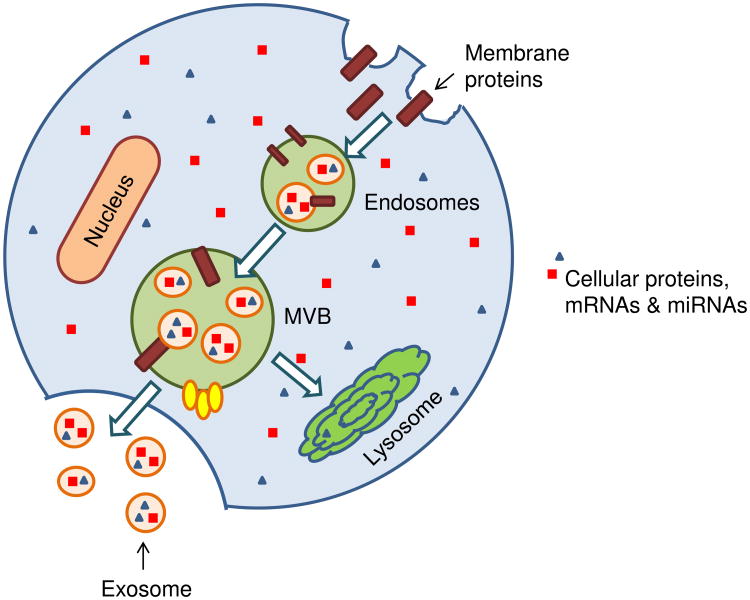
Exosomes are phospholipid bilayer microvesicles released from the endocytic compartment of live cells, typically ranging between 30-100 nm in size.10 Early endosomes form by the fusion of small vesicles of different sizes that originate from invaginations of the plasma membrane. As the endosomes mature, exosomes form multivesicular bodies (MVBs) by accumulating intraluminal vesicles through the invagination of the limiting membrane of the endosomes. MVBs that are not degraded through fusion with lysosomes subsequently fuse with the plasma membrane and release intraluminal vesicles into the extracellular environment as exosomes.11 A wide range of cargo is transported within exosomes, including mRNA, miRNA, proteins, molecular chaperones, and signaling molecules (Figure 1). The ability of exosomes to mediate the cross-talk between different cell types has been increasingly documented since the seminal 2002 study which demonstrated that dendritic cell-derived exosomes were capable of activating naïve CD4+ T cell12; this was further corroborated by another landmark study that validated exosomes as a natural carrier system capable of transporting mRNA, miRNA, and proteins among cells.13 The growing role of exosomes in stem cell biology has been demonstrated over the past few years, primarily based on their potential utility as cell-free therapeutic candidates that can mediate cardiac regeneration.7, 14-16 In addition, exosomes have been implicated in mediating cardioprotection through distinct mechanisms such as enhancement of angiogenesis15, less fibrosis17, and reduced apoptosis16 highlighting the broad potential of exosomes.
Figure 1. Biogenesis of exosomes.
Invaginations of the plasma membrane lead to formation of early endosomes which may include transfer of plasma membrane proteins. Inward budding of multivesicular bodies (MVBs) form small internal vesicles containing proteins, mRNA, and miRNAs from the cytoplasm. These internal vesicles are released as exosomes when MVBs fuse with the cell membrane, and can participate in cell-cell communication upon release. Alternatively, MVBs fuse with lysosomes leading to degradation.
In this issue of Circulation Research, Khan et al. presented results showing that mouse embryonic stem cells-derived exosomes (mES-Exo) are capable of promoting endogenous repair and preserving cardiac function in a mouse model of myocardial infarction (MI), effects which are mediated at least in part by the transfer of miR-294. Initial in vitro experiments revealed that mES-Exo-treated H9c2 myoblasts experienced decreased caspase-3 expression upon exposure to H2O2 compared to cells treated with mouse embryonic fibroblasts-derived exosomes (mEF-Exo). Consistently, intramyocardial injection of mES-Exo into infarcted mouse myocardium was associated with preserved cardiac function 4-weeks post-surgery when compared to mEF-Exo or saline control. Although the transplantation of undifferentiated ES cells is often associated with tumor formation18, the authors did not observe any tumor formation in mice treated with mES-Exo, indicating that exosomes are an attractive option for avoiding the tumorigenic potential of ES cells while preserving their therapeutic modality. The preserved cardiac function seen in mES-Exo-treated hearts was associated with increased neovascularization, decreased apoptosis, and enhanced myocyte proliferation. Moreover, mES-Exo were found to increase the number of proliferating cardiac progenitor cells (CPCs) in the infarcted heart for up to 4 weeks post-infarct.
To complement their in vivo studies, Khan et al. designed in vitro experiments to investigate the mechanisms associated with mES-Exo that could contribute to enhanced survival and proliferation of endogenous CPCs. Mirroring the results seen in mice, the authors found that CPCs treated with mES-Exo had better survival in response to H2O2 challenge compared to mEF-Exo or non-treated CPCs, which was attributed to increased proliferation and metabolic activity. The authors went on to demonstrate that following MI, mice that received transplantation of CPCs pretreated with mES-Exo had improved LV function compared to those that received cells pretreated with mEF-Exo. CPCs pretreated with mES-Exo had better survival and proliferation, resulting in increased angiogenesis and to a certain extent differentiating into new myocytes, indicating the potential usefulness of mES-Exo as a therapeutic candidate for enhancing cell survival and function. Since exosomes are known to harbor a plethora of biological molecules that can be transferred to target cells leading to phenotypic modulation, Khan et al. sought to investigate miRNAs that are enriched in mES-Exo as potential modulators of cardiac regenerative mechanisms. The authors showed that mES-Exo were enriched for ES cell-specific miR-290 family, and subsequent gain-of-function studies revealed miR-294 as a primary candidate that accelerated cell cycle in CPCs treated with miR-294 mimics, suggesting a central role in mediating the effects of mES-Exo in promoting cardiac regeneration.
The study by Khan et al. provides important and novel insights into the potential application of exosomes as cell-free therapeutic agents in place of autologous or allogeneic cell administration, which is often hampered by issues such as poor cell survival, electrical/mechanical coupling, and immunogenicity. Importantly, this study paves the way for expansion of exosomes beyond ES cells, showing that they could be harnessed for other cell types such as induced pluripotent stem cells (iPSCs).19 However, some concerns must be addressed before the immense potential of exosomes as a biomedical tool in stem cell-based cardiovascular therapeutics can be fully capitalized. Although stem cell-derived exosomes have generally been found to be less immunogenic than parental cells, mainly due to lesser membrane-bound proteins such as MHC complex20, there is still an inherent risk of exosomes triggering an immune response, especially in the infarcted myocardium. Notably, the authors emphasized the role of miR-294 as one of the contributing factors that underlie the beneficial effects of mES-Exo. Given that the cargo of exosomes is extremely complex, focusing on miRNAs is likely only part of the equation and it would be interesting to perform in-depth characterization of mES-Exo's full content in future studies using RNA-sequencing or proteomics. Furthermore, since previous studies have demonstrated that cells secrete exosomes differentially under physiological and maladaptive conditions21, it would be illuminating to perform additional characterization of mES-Exo when ES cells are exposed to hypoxic conditions to mimic the ischemic heart. While it is conceivable that the transfer of ES cell-specific miR-294 from exosomes into the heart can stimulate pre-existing cardiomyocyte proliferation due to its inherent role in accelerating G1-S transition, it is somewhat intriguing to contemplate how miR-294 can promote the switch of CPCs into cardiomyocytes given that the miR-290 cluster has been reported to actually inhibit differentiation of cells, which is in line with its role of accelerating the cell cycle.22, 23 Along the same line, given that miRNAs are capable of affecting multiple targets, future efforts should be made to ensure proper targeting of exosomes to specific tissues to prevent any undesirable off-target effects.
Taken together, the work of Khan et al. shows that exosomes can be harnessed as an extremely useful tool for cardiac regenerative strategies. Although the molecular mechanisms of exosomal-mediated cardiac repair are not fully understood, the fact that exosomes are capable of mediating such effect is extremely encouraging. Future work will undoubtedly shed more light on the biology of these natural carriers of biological molecules, such as in-depth systems biology for characterizing exosomes24, paving the way for novel and exciting possibilities for the use of exosomes in regenerative medicine.
Acknowledgments
Sources of Funding: We are grateful for the funding support by American Heart Association Postdoctoral Fellowship 15POST22940013 (S-GO), and National Institutes of Health P01 GM099130, U01 HL107393, R01 HL093172, American Heart Association Established Investigator Award, and Fondation Leducq (JCW).
Footnotes
Disclosures: None
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circulation Research. 2011;108:1226–1237. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nature Medicine. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American Journal of Physiology Heart and Circulatory Physiology. 2006;291:H886–893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34(+) stem cells mediate their proangiogenic paracrine activity. Circulation Research. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mrna and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 9.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-micrornas. Nucleic Acids Research. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nature reviews Immunology. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 11.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. The Journal of Cell Biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive cd4+ t cells by dendritic cell-derived exosomes. Nature Immunology. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog-modified human cd34+ cells preserve cardiac function after acute myocardial infarction. Circulation Research. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC. Cross talk of combined gene and cell therapy in ischemic heart disease: Role of exosomal microrna transfer. Circulation. 2014;130:S60–69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting mecp2 via mir-22. PloS One. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsa E, Burridge PW, Wu JC. Human stem cells for modeling heart disease and for drug discovery. Science Translational Medicine. 2014;6:239ps236. doi: 10.1126/scitranslmed.3008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo R, Lai R, Tan K, Lim S. Exosome: A novel and safer therapeutic refinement of mesenchymal stem cell. Exosomes Microvesicles. 2013;1:1–12. [Google Scholar]
- 21.Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Reviews. 2013;27:31–39. doi: 10.1016/j.blre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Zovoilis A, Smorag L, Pantazi A, Engel W. Members of the mir-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation. 2009;78:69–78. doi: 10.1016/j.diff.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Jakob P, Landmesser U. Role of micrornas in stem/progenitor cells and cardiovascular repair. Cardiovascular Research. 2012;93:614–622. doi: 10.1093/cvr/cvr311. [DOI] [PubMed] [Google Scholar]
- 24.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microrna clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation Research. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]