Abstract
MicroRNAs (miRNAs) are key components of a broadly conserved post-transcriptional mechanism that controls gene expression by targeting mRNAs. miRNAs regulate diverse biological processes, including the growth and differentiation of stem cells as well as the regulation of both endogenous tissue repair that has critical implications in the development of regenerative medicine approaches. In this review, we first describe key features of miRNA biogenesis and their role in regulating self-renewal, and then discuss the involvement of miRNAs in the determination of cell fate decisions. We highlight the role of miRNAs in the emergent field of reprogramming and trans-differentiation of somatic cells that could further our understanding of miRNA biology and regenerative medicine applications. Finally, we describe potential techniques for proper delivery of miRNAs in target cells.
Keywords: microRNAs, regenerative medicine, self-renewal, reprogramming, differentiation, trans-differentiation
Graphical Abstract
Roles of microRNAs in modulating stem cells.
1. Introduction
Cell therapy has been successfully used to improve the function of various types of damaged tissues in preclinical studies. These studies have been mainly performed using two classes of stem cells: (1) pluripotent stem cells (PSCs) that include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) and (2) adult stem cells. Stem cells are unique cells found in all multicellular organisms which can self-renew to produce more stem cells or differentiate into diverse cell types.
Embryonic stem cells (ESCs) are obtained from the inner mass of blastocysts and can be cultured indefinitely in vitro with their pluripotency intact [1]. These cells possess the ability to differentiate into lineage-specific cell types. For example, ESCs cultured in vitro can form three-dimensional aggregates (embryoid bodies) followed by subsequent spontaneous differentiation. ESCs are able to form all three primary germ layers: ectoderm, mesoderm, and endoderm, giving rise to more than 220 adult cell types. Despite the immense capability of ESCs, the use of these cells for regenerative therapies for clinical purpose is extremely controversial because of their immunogenicity, propensity to form teratomas, and more importantly the ethical issues associated with harvesting human embryos for deriving ESCs.
This conundrum has led to much excitement and anticipation when Yamanaka and colleagues first described the reprogramming of adult cells to a pluripotent state resembling that of ESCs [2]. The authors demonstrated that the introduction of several pluripotency-related transcription factors was sufficient to reprogram embryonic and adult fibroblasts into induced pluripotent stem cells (iPSCs). To date, successful maintenance and differentiation of both ESCs and iPSCs have been reported with different labs adopting different protocols. Because of space limitation, interested readers are advised to consult various published reviews with a focus on stem cell differentiation [3].
Unlike the aforementioned cells, most adult stem cells are multipotent and can only form a limited number of cell types. Nevertheless, these cells remain attractive for regenerative strategies due to their high plasticity and the use of such autologous cells lessen the risk of rejection in recipients. For example, human mesenchymal stem cells (MSCs) which can be isolated from various tissues are known to be highly plastic and are routinely used in stem cell–based therapeutics.
The ultimate aim of regenerative medicine is to develop cell-based approaches for repairing, replacing, or regenerating tissues and organs that have been damaged by disease, injury, or aging. In this context, a thorough understanding of lineage determination that controls cell fate is essential as regenerative medicine strategies largely depend on both PSCs and adult stem cells. The molecular programs underpinning cell fate are diverse, with differentiation, de-differentiation, trans-differentiation, and proliferation all being important contributors [4]. These processes are thought to be controlled by transcription factors and epigenetic regulation. Transcription factors that directly or indirectly bind DNA elements at specific genomic loci are capable of controlling the expression of numerous genes to execute whole cellular programs, and also to modulate epigenetic regulation such as DNA methylation and histone modification [5]. This is perhaps best illustrated by the work of Weintraub and colleagues, who demonstrated that the addition of a single transcription factor, in this case MyoD, was sufficient to convert fibroblasts, pigment, nerve, fat and liver cells into a myogenic developmental pathway [6, 7]. Although the transcriptional modulation of cell fate decisions has been an active area of investigation for many years, recent findings have shown that gene regulation at post-transcriptional levels is equally important. Indeed, a class of small non-coding RNAs, namely microRNAs (miRNAs), has emerged as critical post-translational regulators that determine cell linage fate.
miRNAs are a novel class of non-coding regulatory RNAs that are widely expressed in various species. To date, more than 2,500 mature miRNAs have been described in humans [8, 9]. The first known miRNA, lin-4, was discovered over two decades ago in a screen of Caenorhabditis elegans heterochronic genes, which distinguished one larval developmental stage from another [10]. Subsequent analysis of the heterochronic pathways revealed the first miRNA target, lin-14, whose translation could be inhibited by lin-4 via direct binding to the 3′ untranslated region (3′UTR) of lin-14 [11]. As each miRNA targets a large number of mRNAs, and multiple miRNAs can bind to one specific mRNA, the potential impact of miRNA on the expression of a large number of proteins and regulation over the transcriptome is increasingly being investigated for their crucial role in developmental events and stem cell biology. In this review, we focus on the current progress in understanding the biological function of miRNAs in regulating cell fate decisions, and also their potential role in regulating trans-differentiation, which is increasingly an emerging field in regenerative medicine.
2.0 Overview of the biogenesis and function of miRNAs
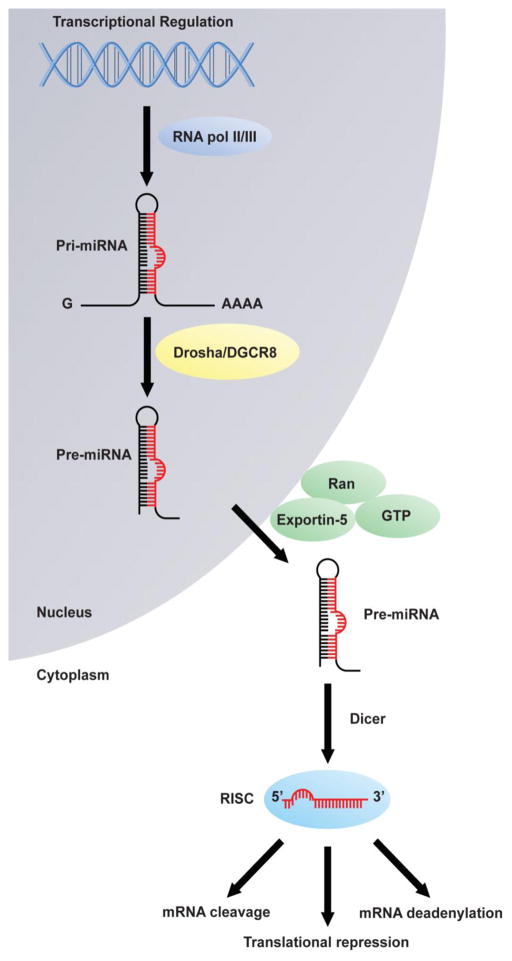
Mature miRNAs are ~22 nucleotides (nt) in length, and regulate gene expression at the post-transcriptional level by mRNA sequestration, translation repression, or miRNA-mediated mRNA decay [12]. Around half of miRNA genes are located within intergenic regions, and can be regulated from their own promoters or as polycistronic clusters from a shared promoter. The remaining miRNAs are encoded within protein-coding genes, and co-transcribed with their host genes or from miRNA-specific promoters [13, 14]. Mature miRNAs are generated by multiple processing steps of sequential endonucleolytic cleavages. Initially, miRNAs are transcribed by RNA polymerase II from the genome into primary transcripts (pri-miRNAs) that contain a 5′7-methyl-guanosine cap and a 3′ polyadenylation signal [13, 15]. These transcripts are several kilobases long and possess local hairpin secondary structures [16]. RNA polymerase III can also generate pri-miRNAs from clusters interspersed among repetitive Alu (5′-AG/CT-3′) sequences [17]. Pri-miRNAs are then cleaved in the nucleus by a microprocessing complex into a 60–70-nt stem loop precursor (pre-miRNAs) and then exported into the cytoplasm by nuclear transport receptor exportin-5, which acts by recognizing a 2- to 3-bp overhang of the pre-miRNA stem-loop structure. The microprocessing complex consists of the RNAse III-like enzyme, Drosha, and its cofactor, DiGeorge syndrome critical region gene 8 (DGCR8) [16, 18–22]. Pre-miRNAs are further cleaved by Dicer, a RNAse III enzyme, and the transactivator RNA-binding protein at the terminal loop end of the pre-miRNA to form an 22–24 nt mature miRNA/miRNA* duplex [23]. Following processing, the strand of the duplex with a less thermodynamically stable 5′-end (the guide strand) is recruited by Argonaute proteins (AGOs) and incorporated into the RNA-induced silencing complex (RISC) to function as miRNA [24, 25]. The passenger strand (miRNA*) is then degraded [26], although in some cases, both strands are incorporated into the RISC (Figure 1).
Figure 1.
Current model of microRNAs (miRNAs) biogenesis and function. miRNAs are endogenously transcribed within the nucleus forming pre-miRNAs. After cleavage into hairpin-shaped pre-miRNAs by Drosha/DGCR8, they are exported into the cytoplasm by exportin-5. Pre-miRNAs are then cleaved by Dicer and loaded onto the RISC complex to function as mature miRNAs for downstream mRNA targeting.
The RISC helps to mediate miRNA-mRNA interactions through binding of miRNAs to the 3′ UTRs of their targets based on the complementarity between each other. Nucleotides 2–8 (from the 5′ end) of the mature miRNA (the “seed region”) are critical for target recognition, because perfect complementarity leads to the miRNA-induced degradation of the target mRNA through AGO2 endonuclease activity [27–29]. Partial pairing is also known to result in repression of target mRNA translation or sequestration of target mRNAs into cytoplasmic processing bodies (P-bodies), which act by recruiting poly(A) nucleases to degrade mRNA through de-adenylation [30, 31]. Given their short lengths, it remains challenging to determine the specificity between miRNAs and their target mRNAs. Accordingly, current algorithmic databases that have been designed for miRNA target prediction usually generate hundreds of target transcripts for each annotated miRNA, but the majority of these interactions have not been fully validated experimentally. Principles underlying these computational algorithms are mainly based on: (a) base pairing pattern; (b) comparative sequence analysis to check conservation; (c) thermodynamic stability of miRNA:mRNA complex; and (d) examination of the presence of multiple target binding sites in 3′ UTR [32].
3.0 miRNAs and stem cells properties
Importantly, miRNAs have been shown to be implicated in a broad range of stem cell roles in both healthy and diseased states and their involvement will be discussed in the following sections (Table 1).
Table 1.
Involvement of miRNAs in stem cell differentiation and outcomes.
| Differentiation lineage | Original cell type | Means of delivery | miRNA | OE | KD | Outcomes | Ref |
|---|---|---|---|---|---|---|---|
| Neuronal | mESC | Lipoplex transfection | miR-134 | x | Enhances differentiation to ectodermal lineages | [63] | |
| mESC | Lipoplex transfection | miR-34a miR-100 miR-137 |
x | x | Inhibition of these miRNAs blocks ESCs differentiation |
[165] | |
| mNSC | Viral transduction | miR-106b-25 | x | Enhances neuronal differentiation | [166] | ||
| hNPC | Lipoplex transfection | miR-9 | x | ||||
| mNSC | Lipoplex transfection | miR-9 | x | Enhances neuronal differentiation | [76] | ||
| Human fibroblast | Viral transduction | miR-9/9* miR-124 |
x | Enhances neuronal differentiation | [82] | ||
| Cortical neurons | Lipoplex transfection | miR-124a | x | miR-124 is required for neuronal differentiation | [81] | ||
| hNES | Viral transduction Lipoplex transfection |
miR-153 miR-324-5p/3p miR-181a/a* |
x | Ectopic expression of these miRs enhances neuronal differentiation | [167] | ||
| Hematopoietic | HPCs | Viral transduction | miR-181 miR-223 |
x | Increases lymphoid lineage cells Increases myeloid lineage cells |
[87] | |
| HPCs | Lipoplex transfection | miR-223 | x | x | miR-223 promotes granulopoiesis and impairs erythroid and monocytic differentiation | [89] | |
| Lymphoid progenitors | Viral transduction | miR-150 | x | miR-150 promotes T cell differentiation | [168] | ||
| Musculoskeletal | MG-63 Osteoblast-like osteosarcoma cell line | Lipoplex transfection | miR-31 | x | Inhibition of miR-31 promotes osteogenesis | [169] | |
| MC3T3 Osteoblastic cell | Lipoplex transfection | miR-29 | x | Inhibition of miR-29 increased osteonectin protein levels | [97] | ||
| 3T3-L1 pre-adipocytes | Viral transduction | miR-103 miR-143 |
x | Accelerated adipogenesis | [102] | ||
| 3T3-L1 pre-adipocytes | Nucleofection | Let-7 | x | Overexpression of let-7 inhibits adipogenesis | [103] | ||
| hASC | Viral transduction | miR-21 | x | miR-21 decreased proliferation of hASCs | [106] | ||
| hMSC | Lipoplex transfection | miR-449 | x | x | miR-449 negatively regulates chondrogenesis | [170] | |
| Cardiovascular | mESC | Viral transduction | miR-1 miR-133 |
x | Promotes mesoderm formation. miR-1 promotes cardiac differentiation | [124] | |
| hESC | Viral transduction | miR-1 miR-499 |
x | Promotes cardiac differentiation | [126] | ||
| hCPC | Lipoplex transfection | miR-1 miR-499 |
x | x | Increases cardiac differentiation at an earlier rate | [125] |
mESC, mouse embryonic stem cells; mNSC, mouse neural stem cells; hNPC, human neural progenitor cells; hNES, human neuroepithelial-like stem cells; HPCs, hematopoietic progenitor cells; hASC, human adipose stromal cells; hMSC, human mesenchymal stem cells; hCPCs, human cardiac progenitor cells, OD: overexpression; KD: knockdown
3.1 Overall role of miRNAs in stem cells
The overall function of the miRNA pathway in development has been evaluated in mice by examining the phenotypes of Dicer (involved in both miRNA and small interfering RNA pathways) and DGCR8 mutants (involved in miRNA pathway), respectively. Deletion of Dicer in mice led to embryonic lethality, indicating an essential role for miRNAs in development [33, 34]. Dicer-null mouse ESCs exhibited severe growth and differentiation defects and prolonged G1 and G0 phases of the cell cycle. The expression of Oct4, a pluripotency marker that is only partially decreased following the induction of differentiation in these cells, and of endodermal and mesodermal markers, typical of differentiated ESCs, is undetectable.
As Dicer is involved in the biogenesis and function of other small RNA species, it is possible that the loss of miRNAs in the Dicer-null ESCs was not solely responsible for the phenotype observed. However, Dgcr8-deficient ESCs also exhibit delayed or reduced expression of differentiation markers, as well as delayed cell cycle progression, similar to those seen in Dicer-null ESCs [35]. The majority of Dgcr8-null ESCs accumulated in G1 phase, and failed to repress expression of pluripotency markers such as Oct4, Rex1, Nanog, and Sox2. Dgcr8-null ESCs also failed to form teratomas when injected into host mice, which is in contrast with wild-type ESCs. Collectively, these results from Dicer- and Dgcr8-mutants suggest that miRNAs play an important role in regulating pluripotency and differentiation capacity in stem cells.
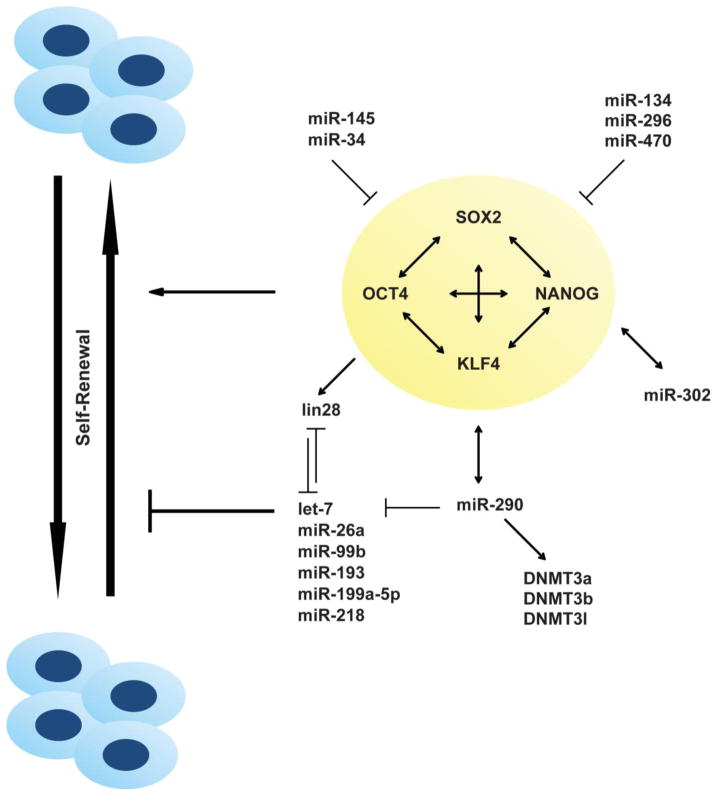
3.2 miRNAs regulating self-renewal
The molecular basis of stem cell self-renewal has been best explored using ESCs (Figure 2). In ESCs, a core transcriptional network that includes the transcription factors Oct4, Sox2, Nanog, Tcf3, and the Myc family of proteins regulates the self-renewal program that is poised to either activate or repress the transcription of genes of any lineage from the three germ layers upon differentiation [36, 37]. ESCs have an abbreviated G1 phase compared to somatic cells that promotes their rapid proliferation, leading to a rapid G1/S transition [38]. In a typical somatic cell, the G1/S restriction point prevents the initiation of S phase and DNA replication. This series of signaling events is achieved through regulated cell cycle components, including the cyclins, the cyclin dependent kinases (CDKs), cdk inhibitors, the Rb family of proteins, and the E2F transcription factors [39, 40].
Figure 2.
miRNAs that are involved in regulating stem cell self-renewal. ESCs express a unique set of miRNA signature, which is regulated by core transcription factors (Oct4, Sox2, Nanog, and Klf4). Positive and negative regulators that modulate these transcription factors are illustrated by an arrow and a T line, respectively.
As discussed earlier, Dgcr8-null ESCs exhibit cell cycle defects with pronounced accumulation in G1 phase [35]. By screening a library of miRNAs for their ability to enhance proliferation in a Dgcr8-knockout ESCs background, Wang et al. determined that a subset of miRNAs (mir-290/371 cluster), referred to as ESC-specific cell cycle-regulating miRNAs (ESCC miRNAs), not only could accelerate proliferation of these mutant ESCs, but also could decrease the number of cells accumulated in the G1 phase [39]. In mice, the miR-290 family comprises miR-290, miR-291a, miR-291b, miR-292, miR-293, miR-294, and miR-295, and the human ortholog, miR-371 family, includes miR-371, miR-372, miR-373, and miR-373* [41]. In mouse ESCs, the miR-290-295 cluster is regulated by Oct4, and binds to the promoter region of Oct4, Sox2, Nanog, and Tcf3 [42]. Increased expression of the miR-290 family promotes G1/S transition and mediates the suppression of several negative regulators of the cell cycle in ESCs, including Cdkn1a (p21), Rbl2, and Lats2. Also, a separate study demonstrated that the overexpression of the miR-290 cluster in ESCs prevents early differentiation by directly targeting two cell cycle regulating genes, Wee1 and Fbx15, [43].
Intriguingly, the regulation of Rbl2 by the miR-290 family is linked to DNA methylation defects [44, 45]. ESCs have a relatively open chromatin, indicative of their pluripotency, which undergoes de novo DNA methylation during differentiation. Suppression of Rbl2 in Dicer-null ESCs results in decreased levels of DNA methyltransferases, leading to impaired de novo DNA methylation. As a result, Oct4 is incompletely and reversibly silenced, leading to failure of differentiation in these ESCs. Transfection of miR-290 family was shown to partially salvage this DNA methylation phenotype through restoration of Dnmt3a and Dnmt3b levels, but not that of Dnmt1 [44, 45].
The miR-290 family also has an antagonizing effect on the pro-differentiation let-7 family in regulating ESC self-renewal and differentiation. Although mature let-7 is not expressed in ESCs, pri-let-7 transcripts are abundant in ESCs [42]. However, conversion of pri-let-7 into the mature form is prevented by RNA-binding protein Lin28 [46–49]; and conversely, mature let-7 inhibits the expression of Lin28, forming a negative feedback loop [49]. Transcriptional activation of Lin28 indirectly involves c-Myc, a transcription factor that promotes proliferation and is required for ESC self-renewal [50]. Also, c-Myc is known to be involved in a negative feedback regulatory loop with let-7 [51–53]. Let-7 is known to have inhibitory effects on the expression of pluripotency factors, in particular Sox2, Oct4, and Nanog [53]. Interestingly, while the forced expression of let-7 induced Dgcr8-null ESCs to differentiate, ectopic expression of let-7 into wild-type ESCs failed to induce differentiation, suggesting an antagonistic function of miR-290 family on let-7 [53]. The importance of miR-290 in regulating ESC fate is further supported by a positive feedback loop between miR-290 family and c-Myc as ChIP-seq data showed that c-Myc binds to the promoter of the miR-290 cluster, indicating a direct activation of expression of these miRNAs [54]. Interestingly, the overexpression of miR-290 in ESCs failed to maintain pluripotency under differentiation conditions although inhibition of it resulted in earlier downregulation of Oct4 [55] demonstrating the complexity of miRs that needs to be taken into consideration when used for tissue engineering and regenerative medicine.
Similar to mir-290 family, the miR-302 family is highly expressed in human ESCs and iPSCs, and includes a cluster of eight miRNAs (miR-302a/a*/b/b*/c/c*/d, and miR-367) [41]. In human ESCs and iPSCs, miR-302 is the predominant miRNA species [41]. The transcription factors Oct4, Sox2, and Nanog are required for the transcription regulation of miR-302 through binding to its promoter [42, 56]. Conversely, miR-302-376 is required for the expression of these pluripotency factors forming an autoregulatory positive loop in pluripotent cells. The expression of miR-302 is known to promote G1/S transition by repressing the translation of cyclin D1, and inhibition of miR-302 led to an accumulation of pluripotent human ESCs in the G1 phase [56]. The importance of miR-302 in inducing pluripotency has been explored by various studies by overexpressing miR-302 alone or in conjunction with other factors in inducing reprogramming of somatic cells to pluripotent stem cells [57–59], representing a significant potential for regenerative medicine.
In contrast to their role in human ESCs, the role of miRNAs in the self-renewal of somatic stem cells is not well understood. miR-205 was identified as the most highly expressed miRNA in mammary gland progenitor cells [60]. The overexpression of miR-205 in a mammary epithelial cell line was found to enhance proliferation and to expand the population of progenitor cells, effects which were linked to modulation of PTEN, a tumor-suppressor gene, by miR-205 [61]. However, these findings are contrary to a recent study reporting that knockdown, not overexpression of miR-205 in mammary epithelial cells, promoted epithelial-mesenchymal transition (EMT), disrupted epithelial cell polarity, and enhanced symmetric division to expand the stem cell population [62].
3.3 miRNAs regulating ESC differentiation
Recent studies have shown that miRNAs promote the transition from self-renewal to differentiation by either directly suppressing ESC self-renewal state or stabilizing the differentiated state. In mouse ESCs, miRNA-134, miR-296, and miR-470 have been reported to induce differentiation by directly targeting the core pluripotency genes, Pou5f1 (also known as Oct4), Nanog, and Sox2 [63, 64]. As these miRNAs are upregulated during retinoic acid-induced neuronal differentiation, it suggests that they may be involved in lineage specific suppression of ESCs self-renewal. In a separate study, miR-200c, miR-203, and miR-183 were found to synergistically repress Sox2 and Klf4 [65]. In human ESCs, miR-145 is significantly upregulated during differentiation and directly suppresses self-renewal by targeting OCT4, SOX2, and KLF4 [66]. miR-145 itself is repressed by OCT4 in human ESCs by a negative feedback loop [66]. These studies which involved the use of pre-miRNAs for overexpression of candidate miRNAs demonstrate the attractiveness of such approaches for promoting differentiation in tissue engineering. Recently, the miR-125 and miR-181 families have also been reported to facilitate the differentiation of mouse ESCs through repression of the Polycomb ortholog Cbx7, which is crucial for maintaining the self-renewal and pluripotency state in these cells [67]. The ectopic expression of both these miRNAs led to acceleration of ESCs differentiation and may potentially be used in tissue engineering for differentiation of ESCs to desired lineages as shown in this recent report by Boissart et al demonstrating the role of miR-125 in determining the neural lineage commitment of human ESCs [68].
In contrast to these miRNAs that repress the self-renewal program, the let-7 family of miRNAs functions as stabilizers of the differentiated cell fate by reducing the expression of factors that is required for the pluripotency state of ESCs previously activated by transcription factors Oct4, Nanog, and Sox2 [53]. Let-7 miRNAs are also responsible in promoting the somatic cell cycle by targeting multiple activators of the G1/S transition, leading to an increase in the number of cells in the G1 phase [69, 70]. It has been reported recently that inhibition of the let-7 family promotes de-differentiation of somatic cells to iPSCs [71]. Based on these observations, it may be possible to harness let-7 for improving the differentiation efficiency of stem cells and future work is needed to support this notion.
3.4 miRNAs regulating differentiation in somatic stem cells
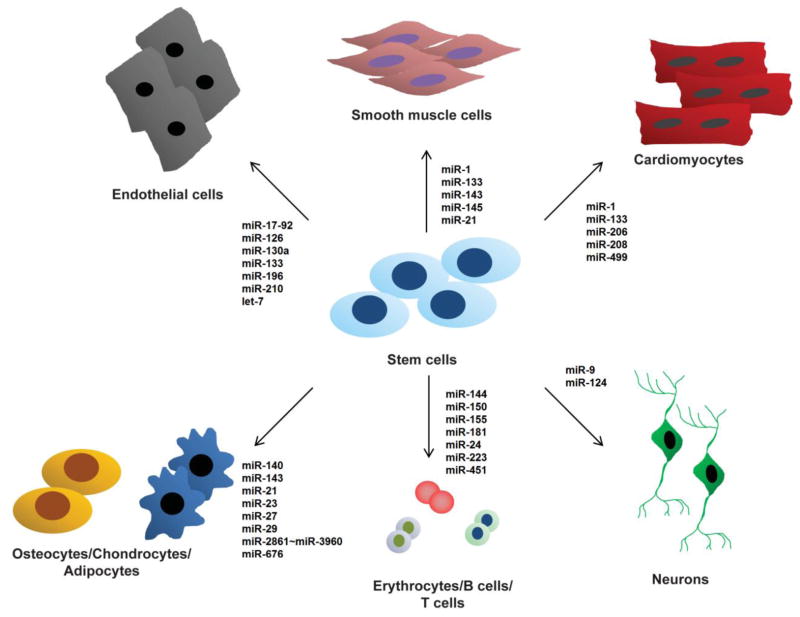
The roles of miRNAs in somatic tissue stem cells are also receiving increasing attention. This section reviews the miRNAs that have been identified in various somatic cells/tissues to have a role in regulating adult stem cell proliferation and differentiation (Figure 3).
Figure 3.
miRNAs are capable of regulating proliferation and differentiation in various somatic stem cells. Several lineage-specific cells are shown here which are regulated by selected miRNAs.
3.4.1 miRNAs regulating neuronal differentiation
Neuronal stem cells (NSCs) are capable of self-renewing or differentiating into neurons, astrocytes, or oligodendrocytes of the central nervous system. The role of miRNAs in early neurogenesis has been studied in Dicer-null mouse models. Deletion of Dicer1 in Purkinje cells resulted in neuronal cell death, indicating a role for miRNAs in maintaining survival of differentiated neurons [72]. The study of miRNAs expression profile in E11 and E13 neuronal progenitor cells (NPCs) revealed miRNAs that were differentially expressed at the onset of neurogenesis [73]. Among the two most enriched miRNAs are miR-9 and miR-124, which have been reported as up-regulated during brain development and also during the lineage progression of neuronal progenitor cells [74].
The expression of miR-9 is induced in differentiated NPCs derived from human ESCs in vitro. Loss of miR-9 suppressed proliferation, but promoted the migration of NPCs by targeting Stathmin that increases microtubule instability [75]. In another study, miR-9 promoted the neuronal differentiation and suppressed NSC self-renewal by targeting TLX, a nuclear receptor involved in NSC renewal [76]. Conversely, the promoter region of miR-9 is occupied by TLX and the corepressor HDAC5 that regulates miR-9 expression, forming a negative feedback regulatory network to balance both proliferation and differentiation of NSCs [76]. A similar phenomenon was described with another miR-9 target, FOXG1, which is involved in the generation of Caja1-Retzius (CR+) neurons [77]. Overexpression of miR-9 resulted in premature neuronal differentiation in the subventricular zone, as evidenced by the early appearance of CR+ cells [78]. These results suggest that manipulation of miR-9 may be an attractive option to be considered during differentiation of stem cells along the neuronal lineages although care must be taken as overexpression of miR-9 might result in premature neuronal differentiation.
Overexpression of miR-124 in HeLa cells resulted in these non-neuronal cells displaying a gene expression profile toward a neuronal phenotype [79]. Similarly, inhibition of miR-124 in terminally differentiated cortical neurons resulted in the increase of non-neuronal transcripts, indicating a role of miR-124 in regulating neuronal differentiation [80]. miR-124 was found to have an impact on the splicing of neuronal genes through regulation of the splicing factor PTBP1, culminating in the transition from non-neuron to neuron-specific alternative splicing patterns [81]. During neural development, an exchange of the BAF53A (also known as ACTL6a), a component of the chromatin remodeling SWI/SNF-like complex) for the homologous BAF53B (ACTLl6b), occurs in post-mitotic neurons; this switch was found to be mediated by miR-9* and miR-124, which target Baf53a [82]. miR-124 has also been shown to target the 3′ UTR of small carboxy-terminal domain phosphatase 1 (SCP1), which is critical for inducing neurogenesis, as SCP1 is an anti-neural factor that binds to a conserved response element and prevents the expression of neural genes [83, 84]. Sox9, a transcription factor which has antagonizing effects on neural differentiation, has also been identified as a direct target of miR-124 [85]. Sox9 overexpression prevented neuronal differentiation, whereas Sox9 knockdown led to increased neuron formation. Hence, miR-124-mediated repression of Sox9 is essential for progression along the subventricular zone stem cell lineage to neurons. Increasing studies have looked at improving neuronal differentiation through overexpression of miR-124 although more in-depth studies are needed for better understanding of the resulting cells’ subtype specifications.
3.4.2 miRNAs regulating hematopoiesis
The hematopoietic system is defined by self-renewing divisions of hematopoietic stem cells (HSCs), giving rise to various cell types of myeloid and lymphoid lineages. An integrative approach that combines measurement of miRNA expression using microarray and bioinformatics prediction of miRNA targets has revealed distinct miRNA signatures at each stage of hematopoiesis. For example, miR-128 and miR-181 are expressed in early HSCs and prevent the differentiation of all hematopoietic lineages [86]. Likewise, some miRNAs act by restricting differentiation of later progenitor cells (miR-16, miR-103, and miR-107), while others act only on a single specific lineage (miR-221 and miR-222 in erythroid development, and miR-223 in granulocytic development) [86]. In cases where hematopoiesis is desired, inhibition of these miRNAs which inhibit hematopoiesis may aid in the induction of hematopoiesis and remains to be tested.
Chen and colleagues reported that miR-223 and miR-181 are expressed at low levels in the hematopoietic progenitor cells, but their expression was increased following differentiation into the myeloid and lymphoid lineages, respectively [87]. Forced expression of miR-181 using retrovirus in undifferentiated hematopoietic progenitor cells led to a doubling of cells committed to the B-lymphoid lineage cells, but not of myeloid lineage cells. Opposite effects were seen in overexpression of miR-223, which promoted an increase in the T-lymphoid lineage without affecting B lymphoid lineage [87]. miR-223 has also been reported to interact with various transcription factors in regulating lineage-specification and differentiation of HSCs. During granulopoiesis in myeloid precursor cells, transcription factor NFI-A represses miR-223 expression, whereas its displacement by C/EBPα upon retinoic acid-induced differentiation upregulated miR-223 [88]. The replacement of NFI-A by C/EBPα and the ensuing granulocytic differentiation are further reinforced by a feedback loop regulation of miR-223, which represses NFI-A. Interestingly, a separate study using CD34+ human hematopoietic progenitor cells (HPCs) showed that the promoter of miR-223 is bound by different transcription factors in a lineage-dependent manner, in which miR-223 is up-regulated during granulopoiesis and monocytic differentiation but remains low during erythropoiesis [89]. Using transfection of synthetic oligonucleotides (mimics and inhibitors), overexpression of miR-223 in CD34+ HPCs promoted granulopoiesis, whereas silencing of it facilitated the erythroid and monocytic pathways. Hence, overexpression of miR-223 presents itself as an attractive option in tissue engineering and regenerative medicine for enhancing granulopoiesis.
Erythropoiesis is essential for supplying red blood cells and is also tightly regulated by miRNAs. miR-144 and miR-451 were found to be regulated by a critical hematopoietic transcription factor, GATA-1, and depletion of miR-451 in zebrafish embryos led to impaired maturation of erythroid cells into circulating red blood cells [90]. miR-451 was also shown to be up-regulated during erythropoietic differentiation from ESCs, but down-regulated during megakaryoiesis [91]. In contrast, miR-24 is known to inhibit erythroid lineage differentiation through repression of ALK4, a type I activin receptor [92]. Activin, along with erythropoietin, promotes erythroid differentiation by binding to ALK4. miR-221 and miR-222 have also been reported to prevent erythropoiesis by targeting Kit (also known as CD117), a cytokine receptor that is enriched in HSCs [93]. These results demonstrate that manipulation of miRNAs may potentially improve erythroid differentiation and novel studies have emerged in support of this concept including a recent study by Kouhkan et al which modulated miR-451 and miR-150 to stimulate CD133+ differentiation into erythroid lineage [94].
3.4.3 miRNAs regulating osteogenic, adipogenic, and chondrogenic differentiation
Multiple miRNAs have been found to regulate osteogenic differentiation at different stages. miR-29b activates the osteogenic pathway by repressing genes that are known to inhibit bone formation, which include HDAC4, TGFβ3, AcvR2a, and DUSP2 [95]. In addition, miR-29 targets collagen type I (COL1A1) and osteonectin (SPARC), extracellular matrix proteins that contribute to osteoblast differentiation and bone mass [96, 97]. Human MSCs upon osteoblast differentiation had increased miR-96 and miR-199a levels while maintaining a low expression of miR-124. Yang et al reported that overexpression of miR-96 in bone marrow-derived MSCs using agomirs enhanced osteogenic differentiation as indicated by alkaline phosphatase mRNA and its activity [98]. Likewise, miR-31 was found to be downregulated upon human MSCs differentiation toward osteoblastic lineage suggesting that targeting of miRNAs might help to improve osteoblastic differentiation [99]. Inhibition of selected miRNAs such as miR-338-3p [100] and miR-542-3p [101] were shown to enhance osteoblast differentiation and manipulation of such miRNAs which inhibit osteogenesis may be beneficial in the field of tissue engineering to enhance differentiation.
Adipogenesis can be divided into two main phases: the determination phase that mesenchymal stem cells (MSCs) commit to the pre-adipocyte fate, followed by the terminal differentiation phase in which the pre-adipocyte matures into adipocytes. Mature adipocytes are involved in lipid transport and synthesis, insulin sensitivity, and the secretion of adipocyte-specific proteins. miR-143 has been shown to increase during human and mouse pre-adipocyte differentiation, and its ectopic expression using retrovirus enhanced adipogenesis [102–104]. Inhibition of miR-143 using antisense oligonucleotides (~60% knockdown efficiency) repressed differentiation in cultured human pre-adipocytes, although the underlying mechanism remains unknown. In addition, miR-21 has been shown to enhance adipogenesis through modulation of the endogenous transforming growth factor β (TGFβ) signaling pathway [105]. Overexpression of miR-21 by lentiviral vectors also decreased proliferation of human adipose tissue-derived stromal cells by targeting STAT3 [106]. The suppression of several miRNAs, including miR-27a and 27-b, has also been linked to adipogenic differentiation of MSCs. Ectopic expression of miR-27 reduces adipogenesis in 3T3-L1 cells and hASCs by targeting the 3′ UTR of PPARγ [107, 108]. A combinatorial approach of enhancing expression of pro-adipogenesis miRNAs and decreasing expression of anti-adipogenesis miRNAs may be useful in tissue engineering to achieve effective adipogenesis.
Chondrogenesis involves the differentiation of MSCs into chondrocytes and have also been shown to be regulated by miRNAs. The miRNA miR-140 is a positive regulator of chondrogenesis that contributes to craniofacial development and endochondral bone formation in mice through repression of HDAC4 that inhibits BMP signaling [109–112]. Both HDAC4 and BMP signaling pathways contribute to chondrocyte hypertrophy and osteoblast differentiation. Chondrocyte differentiation of MSCs was also found to be negatively regulated by miR-449 by targeting lymphoid enhancer-binding factor-1 (LEF1), a critical component of the Wnt signaling pathway. As a result of miR-449 inhibiting LEF1, these MSCs had reduced expression of chondrocytes markers, such as Col2a1 and Sox9, as well as reduced proteoglycan production. Hence, it may be possible to enhance chondrogenesis by knockdown of miR-449 in target cells and further work is required to explore this notion. In a separate study, it was reported that downregulation of miR-29a is essential for the differentiation of MSCs into chondrocytes suggesting that miR-29a should be inhibited for successful chondrogenesis. It should be noted that careful consideration should be taken in manipulating miRNAs for enhancing differentiation as various differentiation lineages are intricately linked as seen in the case of miR-21 which accelerates osteogenesis and impairs adipogenesis and chondrogenesis in MSCs when induced whereas its inhibition leads to accelerated proliferation [113].
3.4.4 miRNAs regulating endothelial differentiation
Angiogenesis is characterized by the formation of new blood vessels from existing vessels, a process which is increasingly associated with miRNA regulation. Endothelial cells (ECs) derived from ESCs in vitro have enriched expression of miR-146b, miR-197, and miR-625, a profile that mirrors ECs derived from mouse embryos in vivo [114, 115]. In a separate study, directed endothelial differentiation of human ESCs demonstrated upregulation of miR-126, miR-130a, miR-133, miR-210, miR-196, and the let-7 family of miRNAs [116]. The increased expression of let-7 family members is consistent with observations that let-7 miRNAs are involved in repressing pluripotency factors and increased upon ESC differentiation [53]. Huang et al also reported that MSCs transfected with lentiviral vectors containing miR-126 had increased propensity towards endothelial differentiation indicating the additive effects of pro-endothelial differentiation miRNAs [117]. A recent study revealed that although miR-17-92 is important for regulating vascular integrity and angiogenesis, none of the members had a significant effect on the endothelial differentiation of mouse ESCs and iPSCs [118]. In a separate study involving differentiation of rat bone marrow mononuclear cells into endothelial progenitor cells, lentiviral-mediated upregulation of miR-107 was associated with suppression of differentiation, while its downregulation promoted differentiation [119].
The role of miRNAs in regulating endothelial lineage differentiation has also been investigated in iPSCs. Ectopic overexpression of miR-21 in pre-differentiated iPSCs induced further EC marker upregulation and these effects were abolished upon inhibition of miR-21 [120] by miR-21 antagomirs. Chen et al. also demonstrated that overexpression of miR-199b using pre-miRs enhanced expression of endothelial markers through targeting Jagged1 in iPSCs [121]. Fine-tuning of endothelial differentiation with suitable addition of pro-endothelial differentiation miRNAs may provide additional improvement to current available endothelial differentiation protocols.
3.4.5 miRNAs regulating cardiac differentiation
miR-1 is among the highly expressed miRNAs in the mammalian heart and was also the first miRNA to be implicated in cardiac development [122]. In vertebrates, members of the miR-1 and miR-133 families are clustered and generated from common bicistronic transcripts [123]. Both miR-1 and miR-133 are highly expressed in human ESC-derived cardiomyocytes and suppress differentiation towards endodermal and ectodermal lineages, while promoting mesoderm specification in vitro [124]. Overexpression of miR-1 and miR-499 in human cardiac progenitor cells (CPCs) is associated with early occurrence of spontaneous beating areas upon differentiation. This is mediated through inhibition of cell proliferation by miR-1 and induction of cardiac differentiation by miR-499 [125]. We have previously demonstrated using human ESCs that miR-1 is associated with electrophysiological maturation whereas miR-499 promotes ventricular specification of human ESCs providing further proof that miRNAs are capable of inducing differentiation effectively [126]. Likewise, human cardiac stem cells overexpressing miR-499 in vivo was found to have increased myocyte differentiation when transplanted into infarcted rat hearts [127]. Similarly, Lee et al reported that MSCs transfected with miR-133a were observed to express cardiac-specific markers [128]. On the contrary, forced expression of miR-124 resulted in a significant downregulation of cardiac specific markers in MSCs co-cultured with cardiomyocytes whereas inhibition of endogenous miR-124 with antagomirs had opposite effects [129]. Collectively, these results indicate that miRNAs may play a role in cardiac lineage differentiation and may potentially contribute to enhance cardiac differentiation protocols.
4.0 The role of miRNAs in reprogramming
The iPSCs were first derived from mouse embryonic fibroblasts (MEFs) by overexpression of four reprogramming factors OCT4, SOX2, KLF4, and C-MYC (OSKM, Yamanaka factors) [2]. Apart from the Yamanaka factors, another set of four factors can also induce the generation of iPSCs, namely OCT4, SOX2, LIN28, and NANOG [130]. Generated iPSCs are similar but not identical to ESCs and show a comparable miR expression profile [131]. Current iPSC generation methods remain inefficient and the use of some of the pluripotency factors have safety concerns, and several strategies have been proposed as alternatives for generating iPSCs, including the use of miRNAs. As the miR-290 family of miRNAs is highly enriched in human ESCs, initial attempts at reprogramming of MEFs were performed by overexpression of miR-291-3p, miR-294, and miR-295 in combination with the reprogramming factors OSK [132]. While all three miRNAs enhanced the reprogramming efficiency significantly, the improvement in reprogramming efficiency was the highest by overexpressing miR-294, which increased the efficiency by 75% of that achieved with the three reprogramming factors alone. miR-294 was shown to substitute, but not enhance, the contribution of c-Myc to reprogramming efficiency, suggesting that miR-294, miR-291-3p, miR-294, and miR-295 function as downstream effectors of c-Myc during reprogramming.
The same group also demonstrated that addition of synthetic mimics of the human miR-302 and miR-372 family and together with the reprogramming factors, OSKM, enhanced the reprogramming of human fibroblasts by targeting genes involved in cell cycle, EMT, epigenetic regulation, and vesicular transport [59]. Recently, Anokye-Danso and colleagues reported that overexpression of miR-302/367 directly reprogrammed mouse and human somatic cells without the need of additional factors, with greater speed and a nearly two orders of magnitude greater efficiency compared to lentiviral transfection of OSKM [133]. Efficient reprogramming was also achieved by direct transfection of mature double-stranded miRNAs (miR-200c, miR-302, and miR-369) in mouse and human cells, but the overall reprogramming efficiency was low [58].
Another group of miRNAs, namely miR-93 and miR-106b, were shown to modulate reprogramming. Highly induced during reprogramming, overexpression of the miR-106b/25 cluster members, miR-93 and miR-106b, enhanced the reprogramming efficiency of MEFs by retroviral transfection with OSK or OSKM factors, in part by targeting the TGFβ receptor II that is involved in mesenchymal-epithelial transition (MET), a hallmark during the initial stages of reprogramming [134]. Furthermore, a library screen of murine miRNAs identified miR-130/301/721 to be capable of enhancing early reprogramming of MEFs. These miRNAs target Meox2, a transcription factor modulating TGFβ signaling, and hence promoting MET [135]. miR-181 is another family of miRNA that has recently been identified as an enhancer of the initiation phase of reprogramming, as knockdown of miR-181 suppressed iPSCs formation from MEFs [136].
Downregulating expression of miRNAs that are tissue-specific may also contribute to reprogramming efficiency. For example, depletion of miR-21 and miR-29a, which are among the most abundant miRNAs in MEFs, enhances reprogramming efficiency through reduced p53 expression [137]. It should also be noted that miR-34 is also a target of p53 during iPSC reprogramming, providing a barrier for reprogramming of somatic cells [138]. Genetic ablation of miR-34 in MEFs promoted iPSC generation without affecting self-renewal or differentiation. In addition, transient transfection of antisense inhibitors against the pro-differentiation let-7 in MEFs also led to a slight improvement in reprogramming efficiency together with the OSK (~4.3-fold) or OSKM (~1.75-fold) factors [53, 71], suggesting that increased reprogramming in response to let-7 inhibition is mediated by let-7 target genes, such as c-Myc. Collectively, these results illustrate the important roles of miRNAs in regulating reprogramming of somatic cells into iPSCs.
5.0 The role of miRNAs in direct lineage reprogramming (trans-differentiation)
Direct conversion or trans-differentiation refers to the direct transformation of one differentiated cell type into another while bypassing the pluripotent state, and may hold great potential for regenerative medicine. Since the discovery 20 years ago that the expression of a single transcription factor (MyoD) in fibroblasts could coax them into adopting the morphology of skeletal myotubes [6, 7], transcription factors have been the most widely sought-after candidates for trans-differentiation as evidenced by several studies. Recent developments have also highlighted the potential of miRNAs as regulators of trans-differentiation.
5.1 miRNA in direct reprogramming to neuronal cells
Neurons are among one of the most therapeutically relevant cell types and tightly regulated by miRNA expression as discussed above. miR-9 and miR-124 are among the most enriched miRNAs in neuronal cells and may have the potential to direct somatic cells to become neurons. In one of the earliest studies investigating the role of miRNAs in trans-differentiation, forced expression of miR-124 along with two transcription factors, MYT1L and BRN2, was sufficient to directly reprogram adult human primary dermal fibroblasts (mesoderm) to functional neurons (ectoderm) in the absence of other cell types [139]. These trans-differentiated neurons exhibit typical neuronal morphology and fire action potentials, and form functional synapses. In a separate study, it was reported that the expression of miR-9/9* and miR-124 in human fibroblasts induced their direct conversion into neurons, a process which was facilitated by the addition of several transcription factors including NEUROD2, ASCL1 and MYT1L [82]. Importantly, the authors found that the expression transcription factors alone without the miRNAs was inefficient, demonstrating the critical role of miRNAs in neural fate determination. These effects were partly mediated by the widespread effects of miR-9/9* and miR-124 on multiple target genes, including BAF53a, REST, coREST, and PTBP-1 [82].
miR-124 is known to repress a splicing regulatory factor called pyrimidine-tract-binding protein (PTB), which is enriched in non-neuronal tissues and antagonizes the neuronal fate by targeting neuronal PTB [140] and miR-124 [81]. It was recently reported that the repression of PTB was sufficient to induce trans-differentiation of fibroblasts into functional neurons [141]. As PTB is a repressor of miR-124, the removal of PTB relieves the blockage on miR-124 and enables miR-124 to target multiple components of the REST complex, which represses a large array of neuronal-specific genes, including miR-124 itself, hence forming an autoregulatory loop.
5.2 miRNA in direct reprogramming to myocardial cells
Repopulation of the injured heart with new, functional cardiomyocytes remains a major challenge in regenerative medicine. One potential method to address this is through direct reprogramming of resident fibroblasts into cardiomyocytes, obviating an intermediate pluripotent stage. It has been reported that induced cardiomyocyte-like cells (iCMs) can be directly generated from mouse fibroblasts by the combination of transcription factors, Gata4, Mef2c, and Tbx5 (GMT), GMT plus Hand2 (GHMT), or Mef2c, Myocd, and Tbx5 in vitro [142–144]. These findings were extended to generation of iCMs from human fibroblasts in vitro and from mouse cardiac fibroblasts in vivo [145–147]. In addition, miRNAs have been used to improve the direct reprogramming efficiency of fibroblasts into cardiomyocytes. Recent studies by Dzau and colleagues demonstrated that transient transfection of four miRNAs (miR-1, miR-133a, miR-208a, and miR-499) could induce direct cellular reprogramming of mouse cardiac fibroblasts into cardiomyocytes, both in vitro and in vivo [148]. Interestingly, while miR-1 alone was sufficient to induce cardiac reprogramming, the effects were more pronounced when applied in conjunction with the miR-133a, miR-208a, and miR-499. Treatment with JAK inhibitor 1 further enhanced the reprogramming efficiency mediated by these four miRNAs by ~10-fold. These reprogrammed cells displayed a cardiomyocyte-like phenotype characterized by expression of cardiac-specific genes and proteins, sarcomere organization, spontaneous calcium oscillations, as well as mechanical contractions. These promising in vitro observations were extended in vivo when lentiviral delivery of miR-1 or all four miRNAs in the border zone of post-infarct mouse heart led to the reprogramming of mouse cardiac fibroblasts into cardiomyocytes, albeit at a very low efficiency (~1%).
Another study demonstrated that neonatal and adult human fibroblasts could be directly reprogrammed towards a cardiac lineage by a combination of four transcription factors (Gata4, Hand2, Tbx5, and Myocd) when combined with miR-1 and miR-133 [146]. Notably, this combination of transcription factors and miRNAs was different from the previously documented combination for cardiac reprogramming of mouse fibroblasts, which included Mef2c instead of Myocd. Two weeks after retroviral infection, reprogrammed human fibroblasts had increased expression of cardiac markers coupled with suppression of non-myocyte genes. Extended cultures up to 4–11 weeks displayed sarcomere-like structures and calcium transients, with a small subset of cells having spontaneous contractility. Ieda and colleagues showed that the addition of miR-133a in combination with GATA4, MEF2C, and TBX5 (GMT), or GMT plus MESP1 and MYOCD, improved cardiac reprogramming by directly suppressing SNAI1, a key regulator of EMT [149] in several types of cells, including MEFs, adult mouse cardiac fibroblasts, and human cardiac fibroblasts. Although it remains inconclusive whether miRNAs alone are sufficient for cardiac reprogramming, current evidence has clearly shown the role of miRNAs as important regulators of it and may be further manipulated for effective cardiac reprogramming.
6.0 Delivery of miRNAs into target cells
To fully capitalize on the efficacy of miRNAs in mediating cell fate, successful delivery of miRNAs is a prerequisite especially in the case of stem cells, which have a low transfection efficiency compared to immortalized cell lines. Here we will discuss several methods that can potentially be adapted when considering the use of miRNAs in modulating cell fate.
6.1 Non-viral delivery
Lipid-based transfection systems are the most common methods currently used to deliver miRNAs in vitro and in vivo. However, safe and efficacious delivery of miRNAs in vivo is rare and infrequent due to potential toxicity issues, non-specificity, and immunological issues. There are various commercially available reagents based on this technology including Lipofectamine, siPORT™, HiPerfect, DOTAP, and Oligofectamine [150]. The use of lipoplex transfection for delivering miRNAs have been reported in several stem cell types including ESCs [63], hematopoietic stem cells [151], and CPCs [125], although the efficiencies are highly variable. Care should be exercised in using liposomes for delivery in vivo as a recent study reported that liposome delivery of miR-145 in MSCs triggered an immunological response as opposed to electroporation delivery of the same miR [152].
Polyethylenimine (PEI) is among one of the most commonly used and studied polymers for gene delivery and can potentially be used for delivering miRNAs. Under physiological conditions, PEI is positively charged due to protonation of the amine groups, and thus can condense nucleic acids [153]. Polyplexes formed by PEI and targets such as nucleic acids or miRNAs typically retain a net positive charge enabling them to be able to interact with negatively-charged cell surface. The complexes can then undergo endocytosis, before being released from the endosome, and eventually disintegration allowing the release of cargo to cytoplasm. Schade et al. demonstrated that PEI bound to magnetic nanoparticles could be used for delivery of miRNAs in human MSCs with uptake rates around 60% [154].
Electroporation involves the use of an electric field to introduce foreign DNA/RNA into target cells through permeabilization of the cell membrane. This method has been reported in several different studies for delivering shRNA in neural progenitor cells with knockdown efficiencies of ~60–90% [155, 156]. A recent study that involved human ESCs-derived myeloid progenitor cells reported on the use of electroporation to deliver 466 human miRNA mimics for screening purposes, demonstrating the feasibility of this method with an efficiency of ~50–80% [157].
Exosomes and microvesicles (collectively named extracellular vesicles, EVs) are circulating lipid vesicles that have emerged as potential candidates for mediating cell-cell communication in various physiological or pathological conditions through transfer of protein, mRNAs, and miRNAs. Exosomes are released through fusion of multivesicular bodies with the plasma membrane, while microvesicles bud directly from the plasma membrane. We have previously shown that exosomes released from cardiac endothelial cells can confer phenotypic modulation of neighboring CPCs through transfer of miR-126 and miR-210 [158]. Likewise, other groups have reported exosomes released from various stem cell types can induce tissue regeneration or elicit protective effects suggesting that these EVs are an attractive option that may be harnessed for delivery of miRNAs [159, 160]. Efforts are currently ongoing to have a deeper understanding of biological EV synthesis, including understanding how certain biological molecules are specifically uptaken into EVs which will enable enrichment at a later point. Synthetic EVs that come in the form of liposomes have also been looked at as an alternative to naturally occurring EVs [161, 162], although further understanding is required. For instance, the lipid and protein composition of EVs, which may be critical for their cellular interactions, is complex compared to the current process of manufacturing synthetic EVs which is relatively straightforward involving simple synthetic lipid mixture without taking into account other components within the bilayer.
The choice of which method to use depends on the needs of the experiments as each method has different transfection efficiency and transgene expression duration. For example, although liposomes have lesser transfection efficiency compared to electroporation, the latter is associated with a higher cell death which is not desirable in certain applications where a lower transfection efficiency is adequate and preferable over increased toxicity [163, 164]. Likewise, in certain applications such as differentiation into specific lineage, transient rather than sustained expression may be more desirable and this may potentially be achieved using the aforementioned non-viral delivery methods as opposed to methods that result in longer expression profiles such as viral delivery methods which will be discussed in the ensuing section.
6.2 Viral Delivery
The use of viral vectors for delivery foreign nucleic acids to cells has been investigated for decades. When selecting a viral system for modulating miRNAs, it is important to consider vector capacity, replication in target cells, and integration within the host genome.
Lentivirus and adeno-associated virus (AAV) are two commonly used viral packaging systems that can be used for miRNA delivery although there are distinct differences between both systems. Both are capable of transfecting dividing and non-dividing cells. As the size of miRNA mimics or inhibitors is usually small in size, these viral vectors can be adapted to incorporate fluorescent tags and antibiotic resistance genes that allow for in vitro and in vivo selection and identification of stably infected cells. While lentivirus can integrate into the host genome and its associated effects are transmissible onto progeny cells, AAV cannot replicate without a helper virus and its effects are lost through cell division. Transfection efficiency is typically high for both systems especially when stably transfected cells can be selected for subsequent passaging. The use of viral vectors remains challenging in vivo due to safety issues despite its high transduction efficiency and may be more beneficial for in vitro regenerative medicine or preclinical proof-of-concept studies.
7.0 Conclusions and Future Perspectives
Although the field of miRNA-mediated differentiation and trans-differentiation is relatively young, it has the potential to further our understanding of the regulation of cell fate decision and the development novel therapies for regenerative medicine. The majority of information pertaining to the roles of miRNAs has been derived from developmental biology in studying tissue development, where temporal expression of miRNAs is required for proper development. Hence, a better understanding of the miRNAs expression and their targets will allow future endeavors in harnessing miRNAs to modulate reprogramming and differentiation of stem cells. Although the use of miRNAs in mediating stem cell fate has been increasing, we believe that current strategies involving genes and proteins are more consistent, and are widely adopted in laboratories worldwide. Care should also be taken in terms of off-target effects of miRNAs to prevent undesirable outcomes. In this context, improving bioinformatics tools are useful in accurately predicting the specific targets of miRNAs, and equally important is the development of novel delivery systems to ensure proper uptake of miRNAs in desired cells/organs. In addition, the functionality of miRNAs in non-human cells has to be tested in human cells as miRNAs shown to work in non-human cells (e.g. fibroblasts) might not lead to similar outcomes when translated to human cells. In conclusion, we foresee an increasing involvement of miRNA biology in the differentiation of stem cells and regenerative therapies in the near future and as such, in-depth understanding of how these molecules work is imperative.
Acknowledgments
We thank Dr. Ioannis Karakikes for his careful reading of the manuscript. We are also grateful for the funding support by American Heart Association Postdoctoral Fellowship 15POST22940013 (S-GO), and National Institutes of Health P01 GM099130, U01 HL107393, R01 EB009689, R01 HL093172, American Heart Association Established Investigator Award, and Fondation Leducq (JCW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8.0 References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell stem cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nature reviews Molecular cell biology. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 5.Lunyak VV, Rosenfeld MG. Epigenetic regulation of stem cell fate. Human molecular genetics. 2008;17:R28–36. doi: 10.1093/hmg/ddn149. [DOI] [PubMed] [Google Scholar]
- 6.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic acids research. 2008;36:D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic acids research. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 11.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 12.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circulation research. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome research. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. Rna. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. The EMBO journal. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nature structural & molecular biology. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 19.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nature reviews Molecular cell biology. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y, Cullen BR. Sequence requirements for micro RNA processing and function in human cells. Rna. 2003;9:112–123. doi: 10.1261/rna.2780503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & development. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 24.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 26.Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes & development. 2005;19:2979–2990. doi: 10.1101/gad.1384005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS biology. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nature cell biology. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezzeddine N, Chang TC, Zhu W, Yamashita A, Chen CY, Zhong Z, Yamashita Y, Zheng D, Shyu AB. Human TOB, an antiproliferative transcription factor, is a poly(A)-binding protein-dependent positive regulator of cytoplasmic mRNA deadenylation. Molecular and cellular biology. 2007;27:7791–7801. doi: 10.1128/MCB.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min H, Yoon S. Got target? Computational methods for microRNA target prediction and their extension. Experimental & molecular medicine. 2010;42:233–244. doi: 10.3858/emm.2010.42.4.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nature genetics. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nature genetics. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature genetics. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 38.Fluckiger AC, Marcy G, Marchand M, Negre D, Cosset FL, Mitalipov S, Wolf D, Savatier P, Dehay C. Cell cycle features of primate embryonic stem cells. Stem cells. 2006;24:547–556. doi: 10.1634/stemcells.2005-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nature genetics. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Current opinion in cell biology. 1997;9:768–772. doi: 10.1016/s0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- 41.Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Developmental biology. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, Calabrese JM, Dennis LM, Volkert TL, Gupta S, Love J, Hannett N, Sharp PA, Bartel DP, Jaenisch R, Young RA. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z, Gocza E. The miR-290-295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation; research in biological diversity. 2011;81:11–24. doi: 10.1016/j.diff.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Benetti R, Gonzalo S, Jaco I, Munoz P, Gonzalez S, Schoeftner S, Murchison E, Andl T, Chen T, Klatt P, Li E, Serrano M, Millar S, Hannon G, Blasco MA. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nature structural & molecular biology. 2008;15:268–279. doi: 10.1038/nsmb.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P, Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nature structural & molecular biology. 2008;15:259–267. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 46.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. Rna. 2008;14:1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Molecular cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature cell biology. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 50.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 51.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nature genetics. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nature genetics. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 53.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Zovoilis A, Smorag L, Pantazi A, Engel W. Members of the miR-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation; research in biological diversity. 2009;78:69–78. doi: 10.1016/j.diff.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Molecular and cellular biology. 2008;28:6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu S, Wilson KD, Ghosh Z, Han L, Wang Y, Lan F, Ransohoff KJ, Burridge P, Wu JC. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem cells. 2013;31:259–268. doi: 10.1002/stem.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, Saito T, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell stem cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nature biotechnology. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes & development. 2007;21:3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene SB, Gunaratne PH, Hammond SM, Rosen JM. A putative role for microRNA-205 in mammary epithelial cell progenitors. Journal of cell science. 2010;123:606–618. doi: 10.1242/jcs.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao CH, Chang CC, Wu MJ, Ko HW, Wang D, Hung MC, Yang JY, Chang CJ. MicroRNA-205 signaling regulates mammary stem cell fate and tumorigenesis. The Journal of clinical investigation. 2014;124:3093–3106. doi: 10.1172/JCI73351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, Liu R, George J, Ng HH, Perera RJ, Lufkin T, Rigoutsos I, Thomson AM, Lim B. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem cells. 2008;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- 64.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 65.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature cell biology. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 66.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 67.O’Loghlen A, Munoz-Cabello AM, Gaspar-Maia A, Wu HA, Banito A, Kunowska N, Racek T, Pemberton HN, Beolchi P, Lavial F, Masui O, Vermeulen M, Carroll T, Graumann J, Heard E, Dillon N, Azuara V, Snijders AP, Peters G, Bernstein E, Gil J. MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation. Cell stem cell. 2012;10:33–46. doi: 10.1016/j.stem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boissart C, Nissan X, Giraud-Triboult K, Peschanski M, Benchoua A. miR-125 potentiates early neural specification of human embryonic stem cells. Development. 2012;139:1247–1257. doi: 10.1242/dev.073627. [DOI] [PubMed] [Google Scholar]
- 69.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer research. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 70.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell research. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 71.Worringer KA, Rand TA, Hayashi Y, Sami S, Takahashi K, Tanabe K, Narita M, Srivastava D, Yamanaka S. The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell stem cell. 2014;14:40–52. doi: 10.1016/j.stem.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. The Journal of experimental medicine. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nielsen JA, Lau P, Maric D, Barker JL, Hudson LD. Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC neuroscience. 2009;10:98. doi: 10.1186/1471-2202-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. Rna. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell stem cell. 2010;6:323–335. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nature structural & molecular biology. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 78.Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10415–10421. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 80.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes & development. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005;307:596–600. doi: 10.1126/science.1100801. [DOI] [PubMed] [Google Scholar]
- 85.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature neuroscience. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Georgantas RW, 3rd, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 88.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 89.Vian L, Di Carlo M, Pelosi E, Fazi F, Santoro S, Cerio AM, Boe A, Rotilio V, Billi M, Racanicchi S, Testa U, Grignani F, Nervi C. Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell death and differentiation. 2014;21:290–301. doi: 10.1038/cdd.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dore LC, Amigo JD, Dos Santos CO, Zhang Z, Gai X, Tobias JW, Yu D, Klein AM, Dorman C, Wu W, Hardison RC, Paw BH, Weiss MJ. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3333–3338. doi: 10.1073/pnas.0712312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin HL, Kim JS, Kim YJ, Kim SJ, Broxmeyer HE, Kim KS. Dynamic expression of specific miRNAs during erythroid differentiation of human embryonic stem cells. Molecules and cells. 2012;34:177–183. doi: 10.1007/s10059-012-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, Chen YG. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111:588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 93.Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kouhkan F, Hafizi M, Mobarra N, Mossahebi-Mohammadi M, Mohammadi S, Behmanesh M, Soufi Zomorrod M, Alizadeh S, Lahmy R, Daliri M, Soleimani M. miRNAs: a new method for erythroid differentiation of hematopoietic stem cells without the presence of growth factors. Applied biochemistry and biotechnology. 2014;172:2055–2069. doi: 10.1007/s12010-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 95.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nature reviews Endocrinology. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. The Journal of biological chemistry. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. Journal of cellular biochemistry. 2009;108:216–224. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang M, Pan Y, Zhou Y. miR-96 promotes osteogenic differentiation by suppressing HBEGF-EGFR signaling in osteoblastic cells. FEBS letters. 2014;588:4761–4768. doi: 10.1016/j.febslet.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y, Gu P, Fan X. Effects of a miR-31, Runx2, and Satb2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells. Stem cells and development. 2013;22:2278–2286. doi: 10.1089/scd.2012.0686. [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Sun Q, Wan C, Li L, Zhang L, Chen Z. MicroRNA-338-3p regulates osteogenic differentiation of mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2. Journal of cellular physiology. 2014;229:1494–1502. doi: 10.1002/jcp.24591. [DOI] [PubMed] [Google Scholar]
- 101.Kureel J, Dixit M, Tyagi AM, Mansoori MN, Srivastava K, Raghuvanshi A, Maurya R, Trivedi R, Goel A, Singh D. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell death & disease. 2014;5:e1050. doi: 10.1038/cddis.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Molecular endocrinology. 2009;23:925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. The Journal of biological chemistry. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 105.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 106.Kim YJ, Hwang SH, Cho HH, Shin KK, Bae YC, Jung JS. MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21 expression in white adipose tissues. Journal of cellular physiology. 2012;227:183–193. doi: 10.1002/jcp.22716. [DOI] [PubMed] [Google Scholar]
- 107.Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochemical and biophysical research communications. 2010;392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochemical and biophysical research communications. 2009;390:247–251. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 109.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, Takada S, Lotz MK, Ueno-Kudo H, Asahara H. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes & development. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pei M, Chen D, Li J, Wei L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation; research in biological diversity. 2009;78:260–268. doi: 10.1016/j.diff.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 111.Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalmay T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS letters. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 112.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 113.Trohatou O, Zagoura D, Bitsika V, Pappa KI, Antsaklis A, Anagnou NP, Roubelakis MG. Sox2 suppression by miR-21 governs human mesenchymal stem cell properties. Stem cells translational medicine. 2014;3:54–68. doi: 10.5966/sctm.2013-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Developmental cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, Houslay MD, Mountford JC, Emanueli C, Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 117.Huang F, Fang ZF, Hu XQ, Tang L, Zhou SH, Huang JP. Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biological chemistry. 2013;394:1223–1233. doi: 10.1515/hsz-2013-0107. [DOI] [PubMed] [Google Scholar]
- 118.Treguer K, Heinrich EM, Ohtani K, Bonauer A, Dimmeler S. Role of the microRNA-17-92 cluster in the endothelial differentiation of stem cells. Journal of vascular research. 2012;49:447–460. doi: 10.1159/000339429. [DOI] [PubMed] [Google Scholar]
- 119.Meng S, Cao J, Wang L, Zhou Q, Li Y, Shen C, Zhang X, Wang C. MicroRNA 107 partly inhibits endothelial progenitor cells differentiation via HIF-1beta. PloS one. 2012;7:e40323. doi: 10.1371/journal.pone.0040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Bernardini E, Campagnolo P, Margariti A, Zampetaki A, Karamariti E, Hu Y, Xu Q. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor beta2 (TGF-beta2) pathways. The Journal of biological chemistry. 2014;289:3383–3393. doi: 10.1074/jbc.M113.495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen T, Margariti A, Kelaini S, Cochrane A, Guha ST, Hu Y, Stitt AW, Zhang L, Xu Q. MicroRNA-199b modulates vascular cell fate during iPS cell differentiation by targeting the notch ligand jagged1 and enhancing VEGF signaling. Stem cells. 2014 doi: 10.1002/stem.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 123.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature genetics. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell stem cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sluijter JP, van Mil A, van Vliet P, Metz CH, Liu J, Doevendans PA, Goumans MJ. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 126.Wilson KD, Hu S, Venkatasubrahmanyam S, Fu JD, Sun N, Abilez OJ, Baugh JJ, Jia F, Ghosh Z, Li RA, Butte AJ, Wu JC. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circulation Cardiovascular genetics. 2010;3:426–435. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Lee SY, Ham O, Cha MJ, Song BW, Choi E, Kim IK, Chang W, Lim S, Lee CY, Park JH, Lee J, Bae Y, Seo HH, Choi E, Jang Y, Hwang KC. The promotion of cardiogenic differentiation of hMSCs by targeting epidermal growth factor receptor using microRNA-133a. Biomaterials. 2013;34:92–99. doi: 10.1016/j.biomaterials.2012.09.069. [DOI] [PubMed] [Google Scholar]
- 129.Cai B, Li J, Wang J, Luo X, Ai J, Liu Y, Wang N, Liang H, Zhang M, Chen N, Wang G, Xing S, Zhou X, Yang B, Wang X, Lu Y. microRNA-124 regulates cardiomyocyte differentiation of bone marrow-derived mesenchymal stem cells via targeting STAT3 signaling. Stem cells. 2012;30:1746–1755. doi: 10.1002/stem.1154. [DOI] [PubMed] [Google Scholar]
- 130.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 131.Wilson KD, Venkatasubrahmanyam S, Jia F, Sun N, Butte AJ, Wu JC. MicroRNA profiling of human-induced pluripotent stem cells. Stem cells and development. 2009;18:749–758. doi: 10.1089/scd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nature biotechnology. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell stem cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. The EMBO journal. 2011;30:823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pfaff N, Fiedler J, Holzmann A, Schambach A, Moritz T, Cantz T, Thum T. miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO reports. 2011;12:1153–1159. doi: 10.1038/embor.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Judson RL, Greve TS, Parchem RJ, Blelloch R. MicroRNA-based discovery of barriers to dedifferentiation of fibroblasts to pluripotent stem cells. Nature structural & molecular biology. 2013;20:1227–1235. doi: 10.1038/nsmb.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang CS, Li Z, Rana TM. microRNAs modulate iPS cell generation. Rna. 2011;17:1451–1460. doi: 10.1261/rna.2664111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, Ozturk A, Hicks GG, Hannon GJ, He L. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature cell biology. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell stem cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boutz PL, Stoilov P, Li Q, Lin CH, Chawla G, Ostrow K, Shiue L, Ares M, Jr, Black DL. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes & development. 2007;21:1636–1652. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. Journal of molecular and cellular cardiology. 2012;53:323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 144.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State. Stem cell reports. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circulation research. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, Nakashima H, Akiyama M, Wada R, Inagawa K, Nishiyama T, Kaneda R, Fukuda T, Takeda S, Tohyama S, Hashimoto H, Kawamura Y, Goshima N, Aeba R, Yamagishi H, Fukuda K, Ieda M. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. The EMBO journal. 2014;33:1565–1581. doi: 10.15252/embj.201387605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yau WW, Rujitanaroj PO, Lam L, Chew SY. Directing stem cell fate by controlled RNA interference. Biomaterials. 2012;33:2608–2628. doi: 10.1016/j.biomaterials.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 151.Liao R, Sun J, Zhang L, Lou G, Chen M, Zhou D, Chen Z, Zhang S. MicroRNAs play a role in the development of human hematopoietic stem cells. Journal of cellular biochemistry. 2008;104:805–817. doi: 10.1002/jcb.21668. [DOI] [PubMed] [Google Scholar]
- 152.Karlsen TA, Brinchmann JE. Liposome delivery of microRNA-145 to mesenchymal stem cells leads to immunological off-target effects mediated by RIG-I. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:1169–1181. doi: 10.1038/mt.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schade A, Muller P, Delyagina E, Voronina N, Skorska A, Lux C, Steinhoff G, David R. Magnetic Nanoparticle Based Nonviral MicroRNA Delivery into Freshly Isolated CD105(+) hMSCs. Stem cells international. 2014;2014:197154. doi: 10.1155/2014/197154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J, Chopiuk G, Demas J, Charette B, Halder R, Weerapana E, Cravatt BF, Cline HT, Peters EC, Zhang J, Walker JR, Wu C, Chang J, Tuntland T, Cho CY, Schultz PG. A small molecule accelerates neuronal differentiation in the adult rat. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16542–16547. doi: 10.1073/pnas.1010300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim YH, Chung JI, Woo HG, Jung YS, Lee SH, Moon CH, Suh-Kim H, Baik EJ. Differential regulation of proliferation and differentiation in neural precursor cells by the Jak pathway. Stem cells. 2010;28:1816–1828. doi: 10.1002/stem.511. [DOI] [PubMed] [Google Scholar]
- 157.Kamat V, Paluru P, Myint M, French DL, Gadue P, Diamond SL. MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells. Stem cells. 2014;32:1337–1346. doi: 10.1002/stem.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation. 2014;130:S60–69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation research. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem cell reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. Journal of controlled release: official journal of the Controlled Release Society. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 162.Maas SL, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, Schiffelers RM, Wauben MH, Broekman ML, Nolte-’t Hoen EN. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. Journal of controlled release: official journal of the Controlled Release Society. 2015;200:87–96. doi: 10.1016/j.jconrel.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gheisari Y, Soleimani M, Azadmanesh K, Zeinali S. Multipotent mesenchymal stromal cells: optimization and comparison of five cationic polymer-based gene delivery methods. Cytotherapy. 2008;10:815–823. doi: 10.1080/14653240802474307. [DOI] [PubMed] [Google Scholar]
- 164.Santos JL, Oramas E, Pego AP, Granja PL, Tomas H. Osteogenic differentiation of mesenchymal stem cells using PAMAM dendrimers as gene delivery vectors. Journal of controlled release: official journal of the Controlled Release Society. 2009;134:141–148. doi: 10.1016/j.jconrel.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 165.Tarantino C, Paolella G, Cozzuto L, Minopoli G, Pastore L, Parisi S, Russo T. miRNA 34a, 100, and 137 modulate differentiation of mouse embryonic stem cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:3255–3263. doi: 10.1096/fj.09-152207. [DOI] [PubMed] [Google Scholar]
- 166.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging. 2011;3:108–124. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stappert L, Borghese L, Roese-Koerner B, Weinhold S, Koch P, Terstegge S, Uhrberg M, Wernet P, Brustle O. MicroRNA-based promotion of human neuronal differentiation and subtype specification. PloS one. 2013;8:e59011. doi: 10.1371/journal.pone.0059011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 170.Paik S, Jung HS, Lee S, Yoon DS, Park MS, Lee JW. miR-449a regulates the chondrogenesis of human mesenchymal stem cells through direct targeting of lymphoid enhancer-binding factor-1. Stem cells and development. 2012;21:3298–3308. doi: 10.1089/scd.2011.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]