Abstract
The beneficial effects of anthocyanins consumption on cardiovascular risk are supported by mechanistic and epidemiologic evidence. In order to explore the effects of Vaccinium berries rich in anthocyanins on serum lipids, we conducted a meta-analysis of relevant randomized controlled trials (RCTs). Sixteen studies with 1109 subjects were included in this meta-analysis. Significant heterogeneity confirmed differential effects between Vaccinium subclasses. The whortleberry group is significantly superior to placebo in lipids improvement. Besides, bilberry groups show significant differences in reducing LDL-C and increasing HDL-C in comparison with other treatments. For many of the other subgroups and comparison arms, there was insufficient evidence to draw conclusions about efficacy.
1. Introduction
Cardiovascular diseases, especially coronary heart disease (CHD) and stroke, are the leading causes of death worldwide [1]. One of the known risk factors for CHD is elevated serum lipid [2, 3]. Previous studies suggest that increasing high density lipoprotein cholesterol (HDL-C) and reducing triglycerides and small low density lipoprotein cholesterol (LDL-C) particles may have positive impact in prevention of CHD [4]. Current guidelines support LDL-C as a primary target of therapy [5]. General recommendation for lowering elevated lipid is developing a healthy lifestyle, including quitting smoking, exercising regularly, and low-oil, low-salt, low-fat diet. In reality, few people can strictly follow the above requirements, so drug therapy is of great necessity. As we know, statins are the first choice prescribed to achieve the goal of lipid-lowering effect [6]. Considering residual cardiovascular risk that remains after statin therapy, such as declines in hepatic function [7], muscle toxicity [8], and increasing risks of diabetes [9, 10], there is a strong demand for novel lipid-modifying agents that can be easily implemented by the majority of the population. The substitute should be safe without any toxic or side effect and rich in nutrients and have a prevention effect on hyperlipidemia. One of the promising alternatives is Vaccinium berry.
Vaccinium is a genus of shrubs or dwarf shrubs in the plant family Ericaceae. The fruits of many species are eaten by humans and some are of medicinal value, including cranberry, blueberry, bilberry, and whortleberry. The common characteristic of the Vaccinium berries is the abundant polyphenols content [11, 12], such as flavonols, phenolic acids, and anthocyanins. Anthocyanins have been reported to have a positive impact on inflammation, hypertension, hyperglycemia, oxidative damage, obesity, and lipid metabolism disorders [13–20]. In recent years, human and animal experiments have gradually found the lipids-lowering effects of extracts from different plants rich in anthocyanins [15, 21–25]. However it is still controversial, because the results of reported randomized controlled trials (RCTs) appear contradictory. Besides, different species of plants may have different effects on lipids metabolism. We cannot conclude which source of anthocyanins is having the most significant effect. In order to make clear the effect of Vaccinium berries, we conduct a meta-analysis of randomized controlled trials. We also selected Vaccinium berries based on the fact that these berries are commercially available all over the world, and therefore, our study findings may have guiding significance to promote public health.
2. Methods
2.1. Search Strategy
The Cochrane Library, MEDLINE, EMBASE, Science Citation Index, The China Journal Full-Text Database, Chinese Scientific Journals Full-Text Database, and Chinese Biomedical Literature Databases were searched from their earliest record to December 2014 with the terms (cranberr∗ or whortleberr∗ or bilberr∗ or lingonberr∗ or Blueberry Plant or Huckleberry Plant or Vaccinium macrocarpon or Vaccinium myrtillus or Vaccinium vitis-idaea), in combination with the medical subject headings. The related article function also was used to expand the search results. We did not restrict any languages during the searching. Hand searching was made by retrieving the reference lists of every obtained study for additional studies. Unpublished data were obtained through contacting authors. We identified ongoing trials by searching https://clinicaltrials.gov/, the UK National Research Register and Meta-Register of controlled trials on the Internet.
2.2. Study Selection
Randomized controlled clinical trials (irrespective of language, date of trial, blinding, or publication status) were included in meta-analysis as long as they were conducted in adult subjects with a duration equal to or over two weeks and contained a true control group. Trials only with baseline and after treatment values for synthesizing risk (mean) differences were included. The outcome measures were differences of serum total cholesterol (TC), HDL-C, LDL-C, and triglycerides (TG) between postrandomization baselines and after treatments. Eligible interventions were capsules of single isolated component or mixtures of different kinds of anthocyanins from Vaccinium berries. Interventions in forms of diets were also included as long as they compared Vaccinium berries containing treatments with Vaccinium berries depleting controls. Trials were excluded from meta-analysis if data required for pooling were missing (i.e., baseline mean and standard deviation [SD], end mean and SD, or change by group) or if studies involved children or pregnant participants or patients with conditions that required cholesterol-lowering medical treatment.
2.3. Data Extraction and Quality Assessment
All abstracts identified by the above search strategies were assessed for subject relevance. The full text of all relevant abstracts was downloaded from databases and meticulously assessed for inclusion. Data abstraction form was introduced to record details of study design, participants, setting and timing, interventions, patient characteristics, and outcomes. Data abstraction was strictly performed independently by two reviewers, with disagreement solved by discussion with the third researcher.
All studies that met the selection criteria were assessed for methodological quality to determine the risk of bias for each outcome. Two reviewers independently assessed the risk of bias according to the criteria stated in the Cochrane Collaboration Handbook [26], with disagreements resolved by discussion with the third researcher. The following methodological domains were considered: sequence generation, allocation concealment, blinding of participants, incomplete outcome data, selective outcome reporting, and other potential risk factors.
2.4. Statistical Analysis
We conducted the meta-analysis to determine the effect of Vaccinium berries on TC, HDL-C, LDL-C, and TG after summarizing available data from all trials reporting results. Blood lipid levels were unified in mmol/L. If cholesterol levels (TC, HDL, and LDL) or triglyceride levels were published in mg/dL, amounts were multiplied by a factor of 0.02586 for cholesterol and 0.0113 for triglycerides to convert to mmol/L. Results for continuous outcomes were expressed as weighted mean difference. All statistical analyses were performed with Review Manager (RevMan version 5.1.6) [27] by inputting the number of participants and the means and SDs of lipid concentrations at endpoint in the two comparison groups. For groups with four treatment arms, we grouped together all the experimental groups and compared them with the control group, respectively [28].
Chi-squared statistic and I 2 statistic were used to assess heterogeneity between trials and the extent of inconsistency apart. If there was a significant heterogeneity, a random-effects statistical model was introduced to confirm the summary results. A fixed-effect model was also applied to merge case estimates and their 95% CIs, unless there was a significant heterogeneity. Subgroup analysis was introduced by Vaccinium subclasses to explore obvious therapeutic differences among trials. Sensitivity analyses were also performed by removing one study at a time to assess any impact of study quality on the effect estimates.
3. Results
3.1. Trial Flow
From Figure 1 we can see the flowchart studies from the initial results of publication searches to the final inclusion. Sixteen trials of Vaccinium berries versus control for serum lipids with 19 comparison arms including 1109 patients were recruited in this meta-analysis. Reasons for exclusion mostly were nonrandomization, lack of control, insufficient original data, or baseline values.
Figure 1.
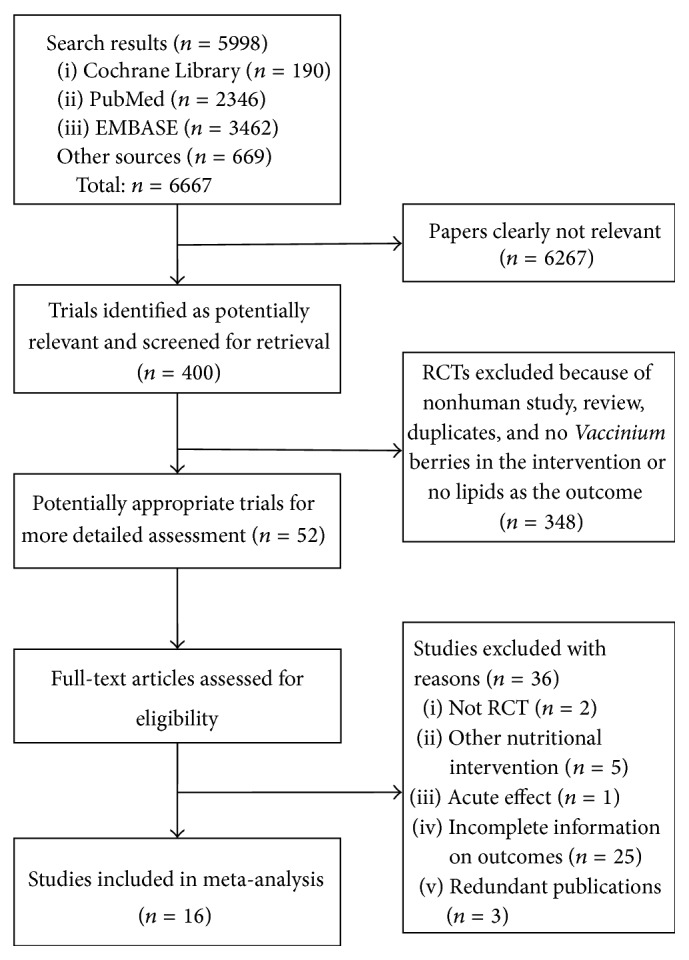
Flow diagram of trial selection.
3.2. Study Characteristics
Characteristics of each trial were given in Table 1. The population being studied were adults with or without some chronic diseases. Means of interventions varied from berry juice to capsules containing berry extracts. Cranberry is introduced in 7 trials, blueberry in 3 trials, bilberry in 4 trials, and whortleberry in 2 trials. The average intake of anthocyanins was up to 742 mg and length of treatment was ranging from 2 to 24 weeks. Three trials recruited healthy subjects while thirteen included participants with cardiovascular risk factors. Two studies just recruited female, one recruited male, and ten recruited subjects with both genders.
Table 1.
Characteristics of the 16 included studies.
| Trials | Country | Subject | Design | Sample size (I/C dropouts) |
Cranberry form | Dosage of anthocyanins | Control | Length of study |
|---|---|---|---|---|---|---|---|---|
| Duthie et al. 2006 [29] | UK | Healthy subjects | Parallel | 11/9 (0) | Cranberry juice | 2.1 mg/d | Placebo | 2 w |
|
| ||||||||
| Valentová et al. 2007 [28] | Czech Republic | Healthy women | Parallel | 21/20/16 (8) | Dried cranberry juice | 2.6 mg/d and 7.8 mg/d | Placebo | 4 w and 8 w |
|
| ||||||||
| Wang et al. 2007 [30] | Taiwan | Healthy subjects | Parallel | 20/20 | Cranberry vinegar | Not stated | Placebo | 10 w |
|
| ||||||||
| Lee et al. 2008 [31] | Taiwan China |
Type 2 diabetes | Parallel | 15/15 (0) | Cranberry capsule | Not stated | Placebo | 12 w |
|
| ||||||||
| Basu et al. 2011 [32] | USA | Women with metabolic syndrome | Parallel | 15/16 (5) | Cranberry juice | 24.8 mg/d | Placebo | 8 w |
|
| ||||||||
| Dohadwala et al. 2011 [33] | USA | Coronary artery disease | Crossover | 44 (3) | Cranberry | 94 mg/d | Placebo | 4 w |
|
| ||||||||
| Flammer et al. 2013 [34] | USA | Cardiovascular risk factors | Parallel | 32/37 (15) | Cranberry juice cocktail | 69.5 mg/d | Placebo | 8 w |
|
| ||||||||
| Riso et al. 2013 [35] | Italy | Men with cardiovascular risk factors | Crossover | 18 (2) | Blueberry drink | 375 mg | Placebo | 6 w |
|
| ||||||||
| Stull et al. 2010 [36] | USA | Obese, insulin-resistant | Parallel | 15/17 | Blueberry smoothie | 668 mg/d | Placebo | 6 w |
|
| ||||||||
| Basu et al. 2010 [37] | USA | Obese, metabolic syndrome | Parallel | 25/23 (18) | Blueberry beverage | 742 mg/d | Placebo | 8 w |
|
| ||||||||
| Erlund et al. 2008 [38] | USA | Cardiovascular risk factors | Parallel | 35/36 (1) | Bilberry, lingonberry | 299 mg/d | Placebo | 8 w |
|
| ||||||||
| Qin et al. 2009 [25] | China | Dyslipidemic subjects | Parallel | 60/60 | Bilberry, blackcurrant | 320 mg/d | Placebo | 12 w |
|
| ||||||||
| Zhu et al. 2013 [39] | China | Hypercholesterolemia | Parallel | 73/73 (4) | Bilberry, blackcurrant | 320 mg/d | Placebo | 24 w |
|
| ||||||||
| Lankinen et al. 2014 [40] | Finland | Metabolic syndrome | Parallel | 37/34 | Bilberry whole grain, fish | Not stated | Whole grain | 12 w |
|
| ||||||||
| Kianbakht et al. 2014 [41] | Iran | Hyperlipidemia | Parallel | 40/40 (25) | Whortleberry | 7.35 mg/d | Placebo | 8 w |
|
| ||||||||
| Soltani et al. 2014 [42] | Iran | Hyperlipidemia | Parallel | 25/25 (4) | Whortleberry | 90 mg/d | Placebo | 4 w |
I: intervention group; C: control group.
3.3. Risk of Bias in Included Studies
The assessment of risk of bias is presented in Figure 2. All sixteen trials were claimed as randomized, but only five trails clearly described how randomization was achieved. The attempts to mask participants and researchers were reported in 5 studies and 4 studies, respectively, but none of the trials reported masking the outcome assessors. Allocation concealment was clearly adequate in 8 trials. None of the trials carried out ITT analysis. The dropout rates for the trials ranged from 0 to 27.3%. We considered two trials [37, 41] to have unclear risk of bias for this domain, as we could not determine whether the high dropout of more than 20% could have affected the treatment estimates.
Figure 2.
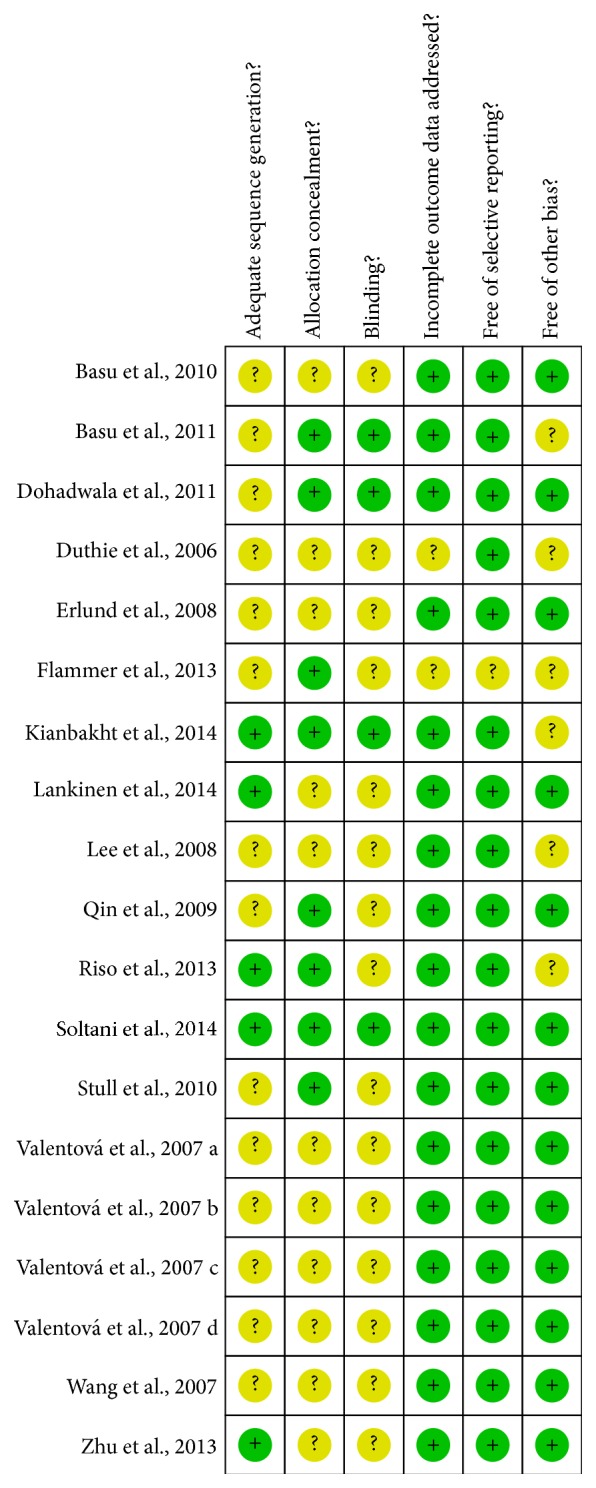
Methodological quality of included studies.
For other potential sources of bias, we focused on two aspects, namely, baseline comparability and the financial support on trials. The intervention and control groups in all trials were reported or appeared to be comparable at baseline for the lipid levels. Seven trials reported that the studies received financial support from nonprofitable organization such as university research grant.
3.4. Effects of Interventions
3.4.1. Outcome: Total Cholesterol
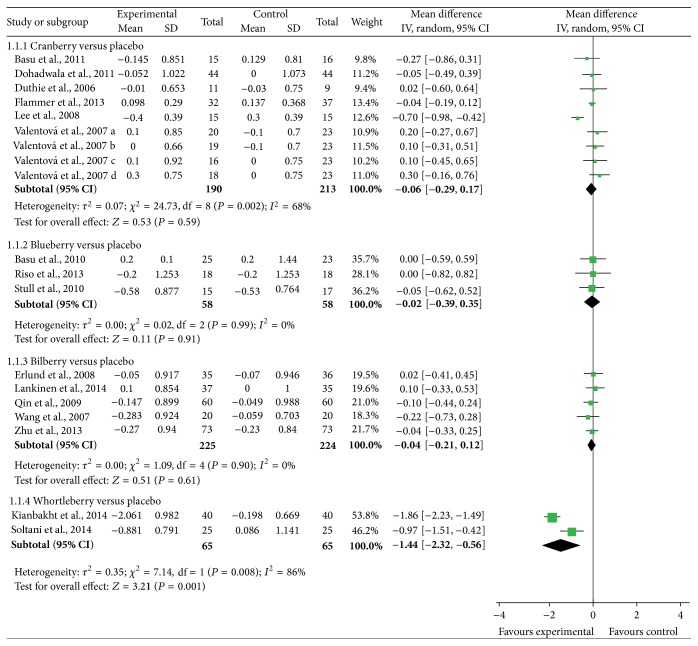
Figure 3 shows no significant differences between intervention and control groups in total cholesterol were found for comparisons between cranberry, blueberry, bilberry, and controls. However, two trials [41, 42] that compared whortleberry with placebo show significant differences between the treatments favouring whortleberry (mean difference = −1.44 (95% CI: −2.32, −0.56) mmol/L; P = 0.001).
Figure 3.
Forest plot of comparisons of Vaccinium berries versus control (outcome: total cholesterol).
Sensitivity analyses revealed that the heterogeneity of included studies in cranberry group on total cholesterol was highly affected by the study performed by Lee et al. When this study was removed from the analysis, the heterogeneity changed from 68% to 0%. However, it showed no significant difference on the total effect in the cranberry group.
3.4.2. HDL-C
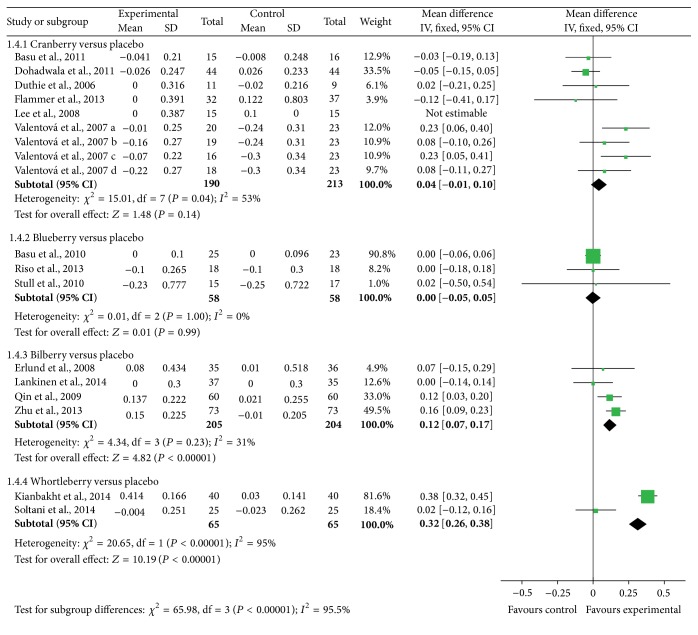
Significant differences were found in HDL-C among studies in bilberry groups (mean difference = 0.12 (95% CI: 0.07, 0.17) mmol/L; P < 0.001) and whortleberry groups (mean difference = 0.32 (95% CI: 0.26, 0.38) mmol/L; P < 0.001) while no obvious differences were observed in HDL-C levels among cranberry and blueberry groups (shown in Figure 4).
Figure 4.
Forest plot of comparisons of Vaccinium berries versus control (outcome: HDL-C).
3.4.3. LDL-C
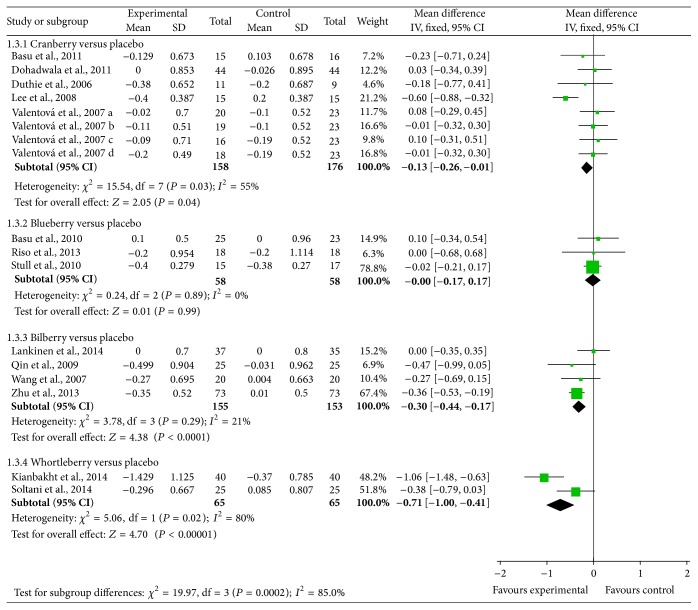
Statistical differences were found in comparisons of Vaccinium berries versus control in LDL-C levels (mean difference = −0.20 (95% CI: −0.28, −0.12); P < 0.001). Particularly, bilberry (mean difference = −0.30 (95% CI: −0.44, −0.17) mmol/L; P < 0.001) and whortleberry (mean difference = −0.71 (95% CI: −1.00, −0.41) mmol/L; P < 0.001) groups show more benefit comparing with other treatments. Changes are also observed in cranberry groups (mean difference = −0.13 (95% CI: −0.26, −0.01) mmol/L; P = 0.04). However, results pooled for three placebo-controlled trails in blueberry groups show no significant differences between intervention and control groups (shown in Figure 5).
Figure 5.
Forest plot of comparisons of Vaccinium berries versus control (outcome: LDL-C).
Sensitivity analysis revealed that the heterogeneity of included studies in cranberry group on LDL-C was highly affected by the study performed by Lee et al. When this study was removed from the analysis, the heterogeneity changed from 55% to 0%. It showed significant difference on the total effect in the cranberry group (P value changed from 0.04 to 0.99). Considering the study performed by Lee et al. is of medium quality and does not match the exclusion standard, we can only suggest that cranberry may have some effect on reducing LDL-C.
3.4.4. Triglycerides
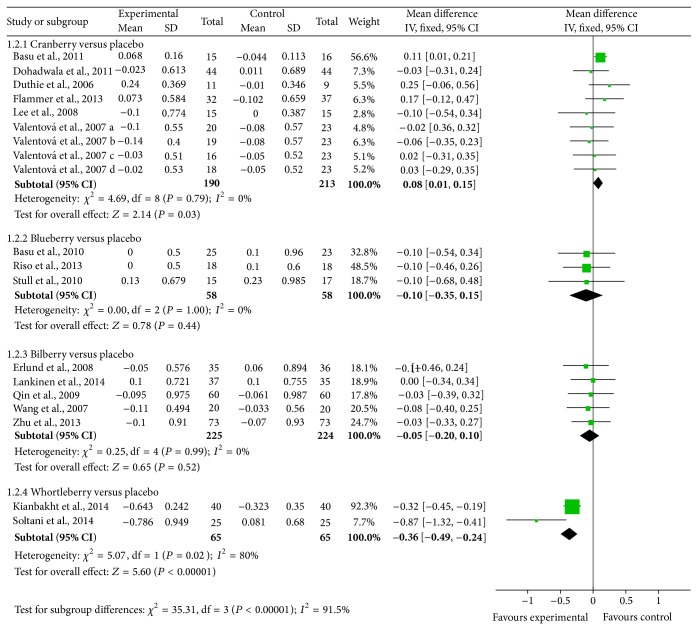
No significant differences were found in TG between groups for all comparisons except for the comparisons between whortleberry and control groups (mean difference = −0.36 (95% CI: −0.49, −0.24)) (shown in Figure 6).
Figure 6.
Forest plot of comparisons of Vaccinium berries versus control (outcome: triglycerides).
3.4.5. Side Effects
Two trials [32, 33] reported side effects of nausea or dyspepsia in a small number of participants in the intervention groups (1 and 2 people, resp.). Basu et al. [37] reported a dropout of 27% in intervention group due to nausea, vomiting, constipation, or diarrhea. However, the number was appreciably similar to that in placebo groups (28% dropouts due to personal reasons). Four trials stated no healthy complaints of participants. Four trials [25, 28, 35, 42] investigated the biomarkers of hepatic and renal functions or hematology. All reported no changes in liver function, biochemistry, or hematology. The rest of nine trials did not have adequate information about side effects.
3.4.6. Publication Bias
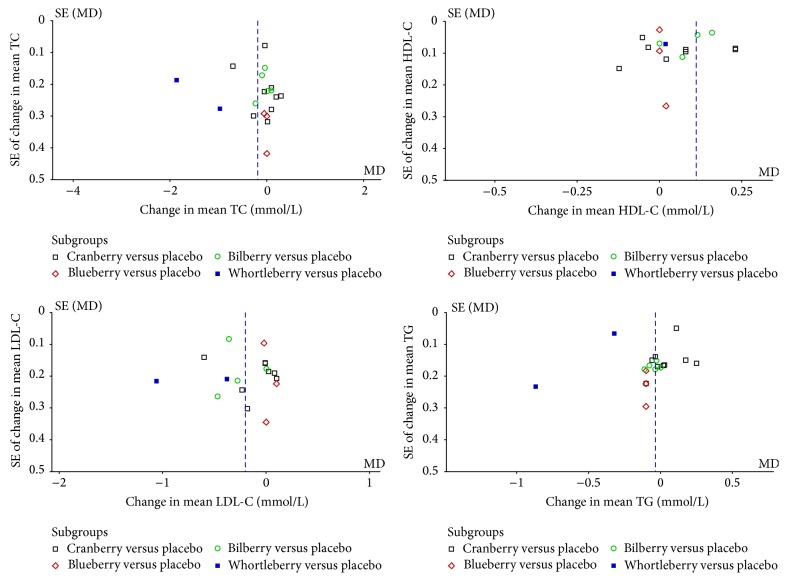
Funnel plots use Begg's test [43] of trials to investigate the effect of Vaccinium berries on cholesterol (TC, LDL, and HDL) and triglyceride levels, indicating no publication bias except for total cholesterol (shown in Figure 7).
Figure 7.
Funnel plots of trials included in the meta-analysis on the effect of Vaccinium berries on TC, HDL-C, LDL-C, and TG.
4. Discussion
4.1. Main Summary of Findings
Sixteen RCTs with nineteen comparison arms involving 1109 patients were included in this review. The findings from two trials [41, 42] clearly show that whortleberry is significantly superior to placebo in lipid reduction, decreasing the TC, TG, and LDL-C and increasing HDL-C at the same time. However, given that the I 2 values were high (86% in TC, 80% in TG, 80% in LDL-C, and 95% in HDL-C), the results should be interpreted with caution. Differences in the daily doses and sources of anthocyanins, age of subjects, and lipids baseline values as well as the different durations of the trials might contribute to some extent to the observed statistical heterogeneity. Besides, bilberry groups show significant differences in reducing LDL-C and increasing HDL-C in comparison with other treatments. The lipids-lowering properties of anthocyanins have been linked to the inhibition of cholesteryl ester transfer protein and the suppression of LDL oxidation, as well as improvement in HDL-associated paraoxonase 1 activity [15, 25, 39, 44]. For many other Vaccinium subclasses or other comparison groups, there was insufficient evidence to draw conclusions about efficacy.
Valentová et al. [28] compared dried cranberry juice with placebo in lipids reducing effects in two different anthocyanins doses (400 mg/d and 1200 mg/d) and two different durations (4 weeks and 8 weeks). However, no significant changes were found in four comparison arms, showing no dose-response and time-response effects. These may be due to the poor absorption of anthocyanins. Various berry (but nor cranberry) anthocyanin glycosides have been found to be absorbed and excreted into urine unmetabolized by both human beings and animals. Only 0.1% of the amount ingested was excreted into the urine [45]. Ohnishi et al. [46] recently found that cranberry anthocyanins are excreted into urine at a total amount of 5% of the dose consumed within 24 h with a maximum excretion period between 3 and 6 h after consumption. Additional speculation is that the most abundant active material may not necessarily produce the highest concentrations of biologically active ingredients. Future studies should focus on the acute effect of anthocyanins, trying to find its clinical relevant endpoint. Besides, as for bilberry and whortleberry, we need to explore the dose-dependent effect of anthocyanins and verify whether synergistic effects are necessary with some other nutrients.
4.2. Strengths and Limitations
The importance of anthocyanins as a part of heart healthy diet has been widely proved. Purified anthocyanins mixture reduced the inflammatory response in hypercholesterolemic subjects [39] and consumption of the wild blueberry drink for 6 weeks significantly reduced the levels of oxidized DNA bases and increased the resistance to oxidatively induced DNA damage [35]. Epidemiology studies support the protective effect of cranberry and blueberry on urinary tract infection [47–49]. As far as we know, this report is the first systematic review assessing the effectiveness of the range of Vaccinium subclasses rich in anthocyanins on lipid improvement within RCTs. This systematic review, the most comprehensive to date, includes a quantitative pooling of results and assessment of risk of bias of included studies.
The shortcomings of the sixteen trials are represented in Figure 2. Several trials failed to contain adequate methodological information, such as method of randomization, allocation concealment, blinding, funding, and dropouts, which are essential for assessing risk of bias. In conclusion, the included studies have moderate risk of bias. In addition, since purified anthocyanins extracted from berries used by Qin et al. [25] and Zhu et al. [39] and berry diets in other researches both show the lipids reducing effect, it is not clear whether the anthocyanins themselves (rather than other bioactive substances) are solely or partially responsible for the observed effects, since the specific biological active ingredients mediating the lipids improvement of the berries belonging to the Vaccinium genus are not yet characterized. In addition, even though we have undertaken extensive searches for published material, we still could not exclude the possibility that studies with negative findings remain unpublished.
5. Conclusion
5.1. Implications for Practice
Results from this review provide some evidence of the beneficial effects of bilberry and whortleberry on lipids reduction. However, recommending bilberry and whortleberry for lowering lipids levels is not justifiable on current evidence because of the limited data. As objects in whortleberry group are diagnosed hyperlipidemia patients whereas not all of the objects in other groups exhibit dyslipidemia, we cannot draw the conclusion that other types of Vaccinium berries have no effect on lipids lowering. In addition, although whortleberry can reduce serum lipids, it cannot always lower the lipids to the normal level as statins do, so it can be just recommended as an adjunct instead of replacement. More studies are needed before these berries can be widely recommended for cardiovascular health. Anyway, adding some berries in our daily diets is good for human health.
5.2. Suggestion to Future Trails
The included trials were all small and had methodological problems. Further trials should be designed rigorously with large sample sizes to confirm the effectiveness of Vaccinium berries for lipids improvement. Besides, further researches need to assess dose-response effects, be of adequate duration, and report all primary outcomes. Additionally, anthocyanins are commonly consumed as part of a normal diet, and a future focus on purified anthocyanins extracted from different subclasses of Vaccinium is needed to determine their specific lipids-lowering effects.
Acknowledgments
This study was supported by grants from Natural Science Foundation of China (81300933) and Science and Technology Commission of Shanghai Municipality (13JC1401504). The authors thank Hongliang Tian (from Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University) for his generous advice.
Disclosure
Yitong Zhu, Ya Miao, and Zheying Meng are co-first authors.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 1990;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker P. M., Cook N. R. Statins: new American guidelines for prevention of cardiovascular disease. The Lancet. 2013;382(9907):1762–1765. doi: 10.1016/s0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S., Whitlock G., Clarke R., et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. The Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 4.Gotto A. M., Jr., Moon J. E. Management of cardiovascular risk: the importance of meeting lipid targets. The American Journal of Cardiology. 2012;110(1):3A–14A. doi: 10.1016/j.amjcard.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Wierzbicki A. S., Hardman T. C., Viljoen A. New lipid-lowering drugs: an update. International Journal of Clinical Practice. 2012;66(3):270–280. doi: 10.1111/j.1742-1241.2011.02867.x. [DOI] [PubMed] [Google Scholar]
- 6.Moser A., Segars L. W. Assessment of antihyperlipidemic therapy in US patients with coronary heart disease. Journal of the American Osteopathic Association. 2010;110(6):331–339. [PubMed] [Google Scholar]
- 7.de Denus S., Spinler S. A., Miller K., Peterson A. M. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24(5):584–591. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 8.Taha D. A., De Moor C. H., Barrett D. A., Gershkovich P. Translational insight into statin-induced muscle toxicity: from cell culture to clinical studies. Translational Research. 2014;164(2):85–109. doi: 10.1016/j.trsl.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J. S., Lee H. W. Diabetogenic effect of statins: a double-edged sword? Diabetes and Metabolism Journal. 2013;37(6):415–422. doi: 10.4093/dmj.2013.37.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preiss D., Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Current Opinion in Lipidology. 2011;22(6):460–466. doi: 10.1097/mol.0b013e32834b4994. [DOI] [PubMed] [Google Scholar]
- 11.Bagchi D., Sen C. K., Bagchi M., Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 2004;69(1):75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 12.Hou D.-X., Kai K., Li J.-J., et al. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25(1):29–36. doi: 10.1093/carcin/bgg184. [DOI] [PubMed] [Google Scholar]
- 13.Lynn A., Mathew S., Moore C. T., et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods for Human Nutrition. 2014;69(2):122–127. doi: 10.1007/s11130-014-0409-x. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki R., Nishimura N., Hoshino H., et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochemical Pharmacology. 2007;74(11):1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y., Huang X., Zhang Y., et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. The Journal of Clinical Endocrinology & Metabolism. 2014;99(2):561–569. doi: 10.1210/jc.2013-2845. [DOI] [PubMed] [Google Scholar]
- 16.Titta L., Trinei M., Stendardo M., et al. Blood orange juice inhibits fat accumulation in mice. International Journal of Obesity. 2010;34(3):578–588. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- 17.Riso P., Visioli F., Gardana C., et al. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. Journal of Agricultural and Food Chemistry. 2005;53(4):941–947. doi: 10.1021/jf0485234. [DOI] [PubMed] [Google Scholar]
- 18.Shaughnessy K. S., Boswall I. A., Scanlan A. P., Gottschall-Pass K. T., Sweeney M. I. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutrition Research. 2009;29(2):130–138. doi: 10.1016/j.nutres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Kalea A. Z., Clark K., Schuschke D. A., Klimis-Zacas D. J. Vascular reactivity is affected by dietary consumption of wild blueberries in the sprague-dawley rat. Journal of Medicinal Food. 2009;12(1):21–28. doi: 10.1089/jmf.2008.0078. [DOI] [PubMed] [Google Scholar]
- 20.Prior R. L., Wu X., Gu L., et al. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Molecular Nutrition and Food Research. 2009;53(11):1406–1418. doi: 10.1002/mnfr.200900026. [DOI] [PubMed] [Google Scholar]
- 21.Leahy M., Speroni J., Starr M. Latest developments in cranberry health research. Pharmaceutical Biology. 2002;40(supplement):50–54. doi: 10.1076/phbi.40.7.50.9172. [DOI] [Google Scholar]
- 22.Mahmoud M. Y. Natural antioxidants effect of mulberry fruits (Morus nigra and Morus alba L.) on lipids profile and oxidative stress in hypercholestrolemic rats. Pakistan Journal of Nutrition. 2013;12(7):665–672. doi: 10.3923/pjn.2013.665.672. [DOI] [Google Scholar]
- 23.Wu T., Qi X., Liu Y., et al. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chemistry. 2013;141(1):482–487. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 24.Graf D., Seifert S., Jaudszus A., Bub A., Watzl B. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in fischer rats. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066690.e66690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin Y., Xia M., Ma J., et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. American Journal of Clinical Nutrition. 2009;90(3):485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J. P. T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 27.The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.6. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- 28.Valentová K., Stejskal D., Bednář P., et al. Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: a pilot double-blind placebo-controlled trial. Journal of Agricultural and Food Chemistry. 2007;55(8):3217–3224. doi: 10.1021/jf0636014. [DOI] [PubMed] [Google Scholar]
- 29.Duthie S. J., Jenkinson A. M., Crozier A., et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. European Journal of Nutrition. 2006;45(2):113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang C.-K., Fu H.-Y., Chiang M. Cardiovascular disease prevention of cranberry vinegar. Nutritional Sciences Journal. 2007;32(4):129–132. [Google Scholar]
- 31.Lee I. T., Chan Y. C., Lin C. W., Lee W. J., Sheu W. H.-H. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabetic Medicine. 2008;25(12):1473–1477. doi: 10.1111/j.1464-5491.2008.02588.x. [DOI] [PubMed] [Google Scholar]
- 32.Basu A., Betts N. M., Ortiz J., Simmons B., Wu M., Lyons T. J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutrition Research. 2011;31(3):190–196. doi: 10.1016/j.nutres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dohadwala M. M., Holbrook M., Hamburg N. M., et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. The American Journal of Clinical Nutrition. 2011;93(5):934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flammer A. J., Martin E. A., Gössl M., et al. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. European Journal of Nutrition. 2013;52(1):289–296. doi: 10.1007/s00394-012-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riso P., Klimis-Zacas D., Del Bo' C., et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. European Journal of Nutrition. 2013;52(3):949–961. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 36.Stull A. J., Cash K. C., Johnson W. D., Champagne C. M., Cefalu W. T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. Journal of Nutrition. 2010;140(10):1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu A., Du M., Leyva M. J., et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. Journal of Nutrition. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erlund I., Koli R., Alfthan G., et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. The American Journal of Clinical Nutrition. 2008;87(2):323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y., Ling W., Guo H., et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutrition, Metabolism and Cardiovascular Diseases. 2013;23(9):843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Lankinen M., Kolehmainen M., Jääskeläinen T., et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet) PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0090352.e90352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kianbakht S., Abasi B., Dabaghian F. H. Improved lipid profile in hyperlipidemic patients taking Vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytotherapy Research. 2014;28(3):432–436. doi: 10.1002/ptr.5011. [DOI] [PubMed] [Google Scholar]
- 42.Soltani R., Hakimi M., Asgary S., Ghanadian S. M., Keshvari M., Sarrafzadegan N. Evaluation of the effects of Vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evidence-Based Complementary and Alternative Medicine. 2014;2014:6. doi: 10.1155/2014/217451.217451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 44.Lau B. H. S. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. Journal of Nutrition. 2006;136(supplement 3):765S–768S. doi: 10.1093/jn/136.3.765S. [DOI] [PubMed] [Google Scholar]
- 45.McGhie T. K., Ainge G. D., Barnett L. E., Cooney J. M., Jensen D. J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. Journal of Agricultural and Food Chemistry. 2003;51(16):4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- 46.Ohnishi R., Ito H., Kasajima N., et al. Urinary excretion of anthocyanins in humans after cranberry juice ingestion. Bioscience, Biotechnology and Biochemistry. 2006;70(7):1681–1687. doi: 10.1271/bbb.60023. [DOI] [PubMed] [Google Scholar]
- 47.Jepson R. G., Williams G., Craig J. C. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews. 2012;(10) doi: 10.1002/14651858.CD001321.pub5.CD001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eke A. C., Akarolo-Anthony S. N., Enumah A. P. Cranberries for treating asymptomatic bacteriuria during pregnancy. Cochrane Database of Systematic Reviews. In press. [Google Scholar]
- 49.Jepson R. G., Craig J. C. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Molecular Nutrition and Food Research. 2007;51(6):738–745. doi: 10.1002/mnfr.200600275. [DOI] [PubMed] [Google Scholar]