Abstract
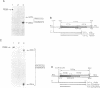
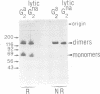
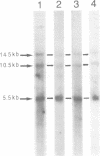
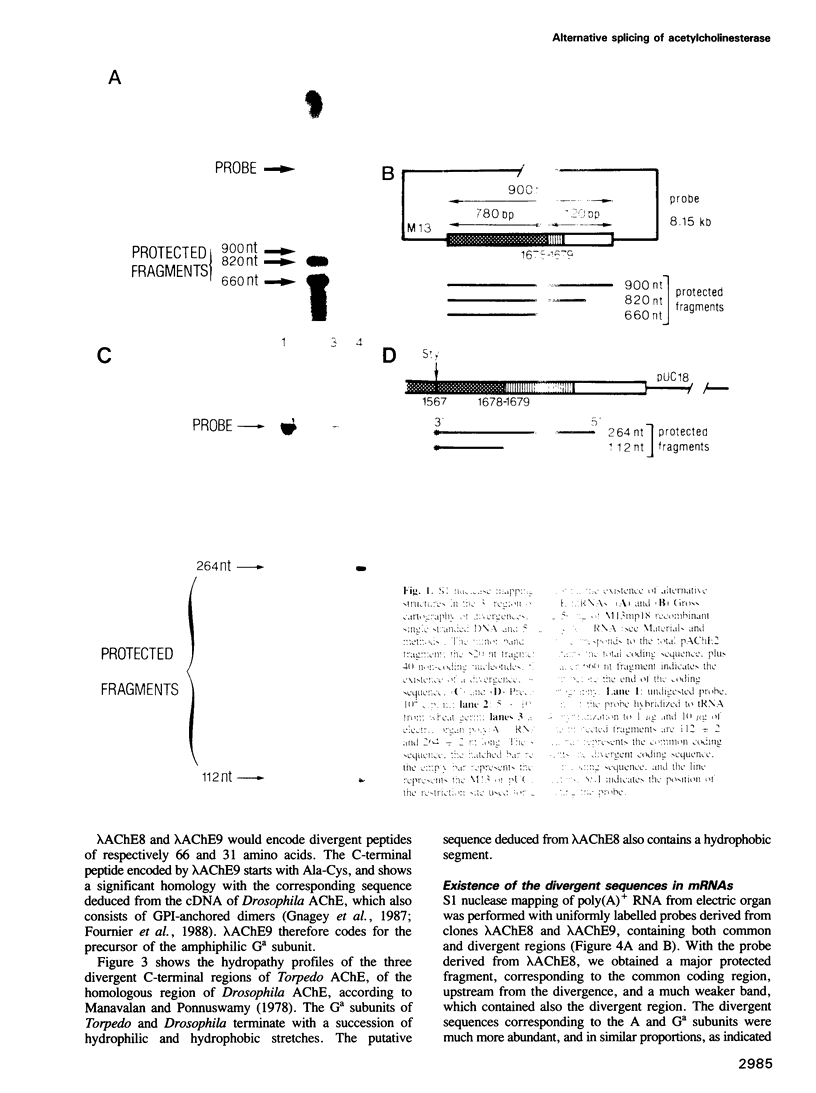
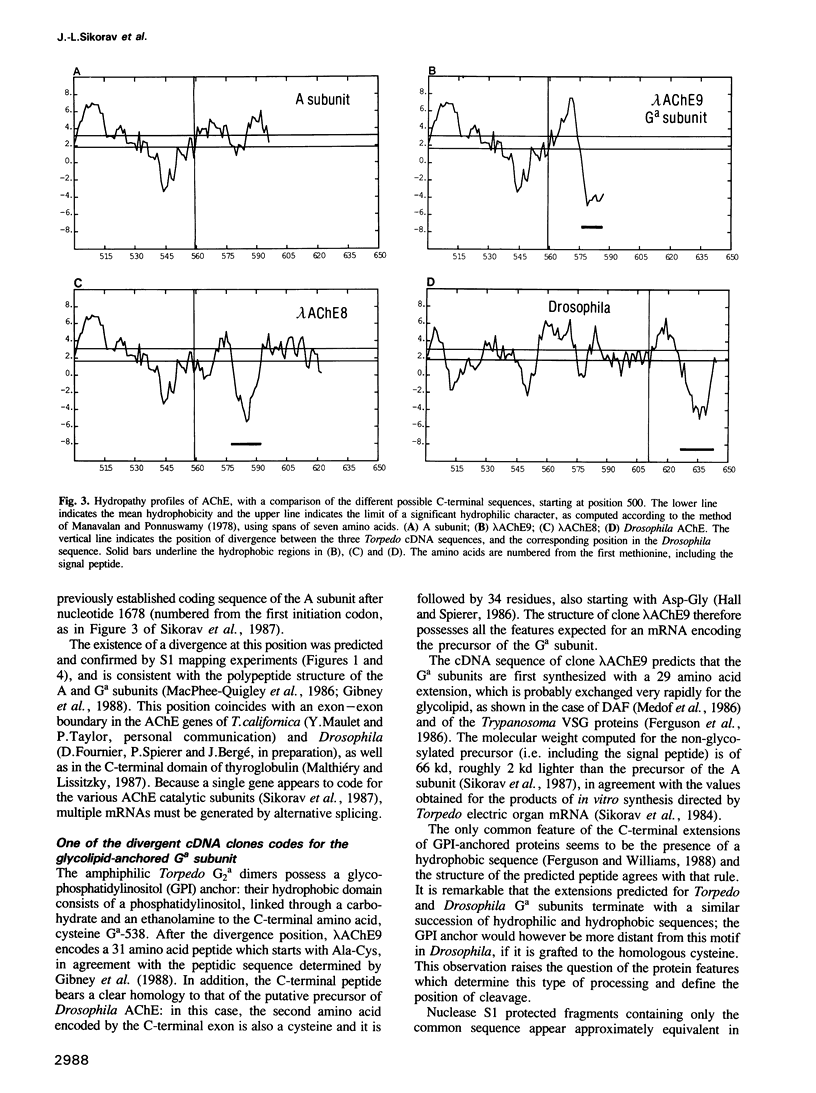
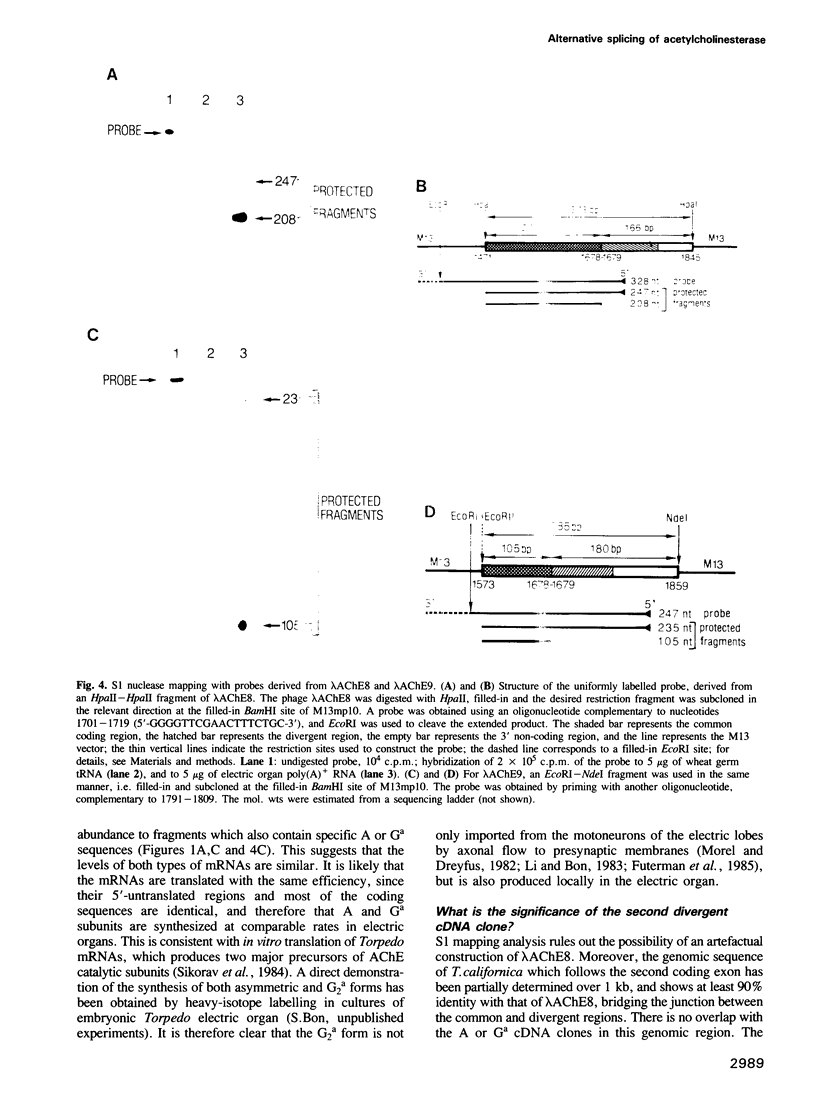
In this paper, we show the existence of alternative splicing in the 3' region of the coding sequence of Torpedo acetylcholinesterase (AChE). We describe two cDNA structures which both diverge from the previously described coding sequence of the catalytic subunit of asymmetric (A) forms (Schumacher et al., 1986; Sikorav et al., 1987). They both contain a coding sequence followed by a non-coding sequence and a poly(A) stretch. Both of these structures were shown to exist in poly(A)+ RNAs, by S1 mapping experiments. The divergent region encoded by the first sequence corresponds to the precursor of the globular dimeric form (G2a), since it contains the expected C-terminal amino acids, Ala-Cys. These amino acids are followed by a 29 amino acid extension which contains a hydrophobic segment and must be replaced by a glycolipid in the mature protein. Analyses of intact G2a AChE showed that the common domain of the protein contains intersubunit disulphide bonds. The divergent region of the second type of cDNA consists of an adjacent genomic sequence, which is removed as an intron in A and Ga mRNAs, but may encode a distinct, less abundant catalytic subunit. The structures of the cDNA clones indicate that they are derived from minor mRNAs, shorter than the three major transcripts which have been described previously (14.5, 10.5 and 5.5 kb). Oligonucleotide probes specific for the asymmetric and globular terminal regions hybridize with the three major transcripts, indicating that their size is determined by 3'-untranslated regions which are not related to the differential splicing leading to A and Ga forms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bon S., Chang J. Y., Strosberg A. D. Identical N-terminal peptide sequences of asymmetric forms and of low-salt-soluble and detergent-soluble amphiphilic dimers of Torpedo acetylcholinesterase. Comparison with bovine acetylcholinesterase. FEBS Lett. 1986 Dec 15;209(2):206–212. doi: 10.1016/0014-5793(86)81112-7. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctor B. P., Camp S., Gentry M. K., Taylor S. S., Taylor P. Antigenic and structural differences in the catalytic subunits of the molecular forms of acetylcholinesterase. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5767–5771. doi: 10.1073/pnas.80.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fournier D., Bergé J. B., Cardoso de Almeida M. L., Bordier C. Acetylcholinesterases from Musca domestica and Drosophila melanogaster brain are linked to membranes by a glycophospholipid anchor sensitive to an endogenous phospholipase. J Neurochem. 1988 Apr;50(4):1158–1163. doi: 10.1111/j.1471-4159.1988.tb10587.x. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Michaelson D. M., Silman I. Solubilization of membrane-bound acetylcholinesterase by a phosphatidylinositol-specific phospholipase C. J Neurochem. 1985 Nov;45(5):1487–1494. doi: 10.1111/j.1471-4159.1985.tb07217.x. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Silman I. A hydrophobic dimer of acetylcholinesterase from Torpedo californica electric organ is solubilized by phosphatidylinositol-specific phospholipase C. Neurosci Lett. 1983 Sep 19;40(1):85–89. doi: 10.1016/0304-3940(83)90097-6. [DOI] [PubMed] [Google Scholar]
- Gibney G., MacPhee-Quigley K., Thompson B., Vedvick T., Low M. G., Taylor S. S., Taylor P. Divergence in primary structure between the molecular forms of acetylcholinesterase. J Biol Chem. 1988 Jan 25;263(3):1140–1145. [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Auffray C., Rougeon F., Changeux J. P. Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1982;1(6):713–717. doi: 10.1002/j.1460-2075.1982.tb01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnagey A. L., Forte M., Rosenberry T. L. Isolation and characterization of acetylcholinesterase from Drosophila. J Biol Chem. 1987 Sep 25;262(27):13290–13298. [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey N. D., Noonan D. J., Mixter K. S., Claudio T., Davidson N. Structure and expression of genomic clones coding for the delta-subunit of the Torpedo acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):79–82. doi: 10.1101/sqb.1983.048.01.010. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Roberts W. L., Marshall T. L., Rosenberry T. L. Acetylcholinesterase from bovine caudate nucleus is attached to membranes by a novel subunit distinct from those of acetylcholinesterases in other tissues. J Biol Chem. 1987 Apr 5;262(10):4441–4444. [PubMed] [Google Scholar]
- Kanehisa M. Use of statistical criteria for screening potential homologies in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):203–213. doi: 10.1093/nar/12.1part1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kim B. H., Rosenberry T. L. A small hydrophobic domain that localizes human erythrocyte acetylcholinesterase in liposomal membranes is cleaved by papain digestion. Biochemistry. 1985 Jul 2;24(14):3586–3592. doi: 10.1021/bi00335a029. [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Bon C. Presence of a membrane-bound acetylcholinesterase form in a preparation of nerve endings from Torpedo marmorata electric organ. J Neurochem. 1983 Feb;40(2):338–349. doi: 10.1111/j.1471-4159.1983.tb11288.x. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- MacPhee-Quigley K., Taylor P., Taylor S. Primary structures of the catalytic subunits from two molecular forms of acetylcholinesterase. A comparison of NH2-terminal and active center sequences. J Biol Chem. 1985 Oct 5;260(22):12185–12189. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987 Jun 15;165(3):491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Ponnuswamy P. K. Hydrophobic character of amino acid residues in globular proteins. Nature. 1978 Oct 19;275(5681):673–674. doi: 10.1038/275673a0. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- McTiernan C., Adkins S., Chatonnet A., Vaughan T. A., Bartels C. F., Kott M., Rosenberry T. L., La Du B. N., Lockridge O. Brain cDNA clone for human cholinesterase. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6682–6686. doi: 10.1073/pnas.84.19.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Kim B. H., Rosenberry T. L. Differences in the glycolipid membrane anchors of bovine and human erythrocyte acetylcholinesterases. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7817–7821. doi: 10.1073/pnas.84.22.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Rosenberry T. L. Selective radiolabeling and isolation of the hydrophobic membrane-binding domain of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3091–3098. doi: 10.1021/bi00359a004. [DOI] [PubMed] [Google Scholar]
- Rogers J., Choi E., Souza L., Carter C., Word C., Kuehl M., Eisenberg D., Wall R. Gene segments encoding transmembrane carboxyl termini of immunoglobulin gamma chains. Cell. 1981 Oct;26(1 Pt 1):19–27. doi: 10.1016/0092-8674(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Scoggin D. M. Structure of human erythrocyte acetylcholinesterase. Characterization of intersubunit disulfide bonding and detergent interaction. J Biol Chem. 1984 May 10;259(9):5643–5652. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Grassi J., Bon S. Synthesis in vitro of precursors of the catalytic subunits of acetylcholinesterase from Torpedo marmorata and Electrophorus electricus. Eur J Biochem. 1984 Dec 17;145(3):519–524. doi: 10.1111/j.1432-1033.1984.tb08587.x. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Krejci E., Massoulié J. cDNA sequences of Torpedo marmorata acetylcholinesterase: primary structure of the precursor of a catalytic subunit; existence of multiple 5'-untranslated regions. EMBO J. 1987 Jul;6(7):1865–1873. doi: 10.1002/j.1460-2075.1987.tb02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Vallette F., Grassi J., Massoulié J. Isolation of a cDNA clone for a catalytic subunit of Torpedo marmorata acetylcholinesterase. FEBS Lett. 1985 Dec 2;193(2):159–163. doi: 10.1016/0014-5793(85)80142-3. [DOI] [PubMed] [Google Scholar]
- Silman I., Futerman A. H. Modes of attachment of acetylcholinesterase to the surface membrane. Eur J Biochem. 1987 Dec 30;170(1-2):11–22. doi: 10.1111/j.1432-1033.1987.tb13662.x. [DOI] [PubMed] [Google Scholar]
- Stieger S., Brodbeck U. Amphiphilic detergent-soluble acetylcholinesterase from Torpedo marmorata: characterization and conversion by proteolysis to a hydrophilic form. J Neurochem. 1985 Jan;44(1):48–56. doi: 10.1111/j.1471-4159.1985.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Stieger S., Brodbeck U., Witzemann V. Inactive monomeric acetylcholinesterase in the low-salt-soluble extract of the electric organ from Torpedo marmorata. J Neurochem. 1987 Aug;49(2):460–467. doi: 10.1111/j.1471-4159.1987.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Boustead C. Structural differences in the catalytic subunits of acetylcholinesterase forms from the electric organ of Torpedo marmorata. EMBO J. 1983;2(6):873–878. doi: 10.1002/j.1460-2075.1983.tb01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]